Abstract
Mycotoxins are well-known secondary metabolites produced by several fungi that grow and occur in different crops during both pre-harvest and post-harvest conditions. The contamination and occurrence of mycotoxins currently represent some of the major issues in the entire agri-food system. The quantification of mycotoxins in different feeds and foodstuffs is extremely difficult because of the low concentration ranges; therefore, both sample collection and preparation are essential to providing accurate detection and reliable quantification. Currently, several analytical methods are available for the detection of mycotoxins in both feed and food products, and liquid chromatography coupled with high-resolution mass spectrometry (LC-HRMS) represents the most reliable instrumental approach. In particular, the fast development of high-throughput methods has made it possible to screen and analyze, in the same analytical run and with high accuracy, multiple mycotoxins, such as those regulated, masked, or modified, and emerging ones. Therefore, the aim of this review is to provide an overview of the state of the art of mycotoxins occurrence, health-related concerns, and analyses, discussing the need to perform multi-screening approaches combined with omics technologies to simultaneously analyze several mycotoxins in different feed and food matrices. This approach is expected to provide more comprehensive information about the profile and distribution of emerging mycotoxins, thus enhancing the understanding of their co-occurrence and impact on the entire production chain.
1. Introduction
Mycotoxins are secondary metabolites produced by filamentous fungi belonging to different genera, including (among others) Aspergillus, Penicillium, Fusarium, and Alternaria [1,2,3,4]. These compounds have been the focus of research in several countries and are considered one of the major risk factors in agricultural products [5]. Among the main environmental factors contributing to the development of fungi-producing mycotoxins, it is possible to list humidity and temperature [6,7]. Tropical and subtropical climates represent optimal conditions for fungi growth; however, climate change is starting to dramatically affect continental climates, thus representing a great challenge for mitigation activities [8]. Mycotoxin contamination is one of the biggest threats in the agri-food chain, able, on the one side, to impact the global economy, and, on the other side, to force farmers to destroy infested crops [9]. Additionally, it can reduce animal performance and profitability, causing several health damage to animals, with corresponding economic losses [10,11]. Also, the simultaneous occurrence of mycotoxins in both feed and foodstuffs could potentially determine some synergistic effects in terms of toxicity to animals and humans [12,13].
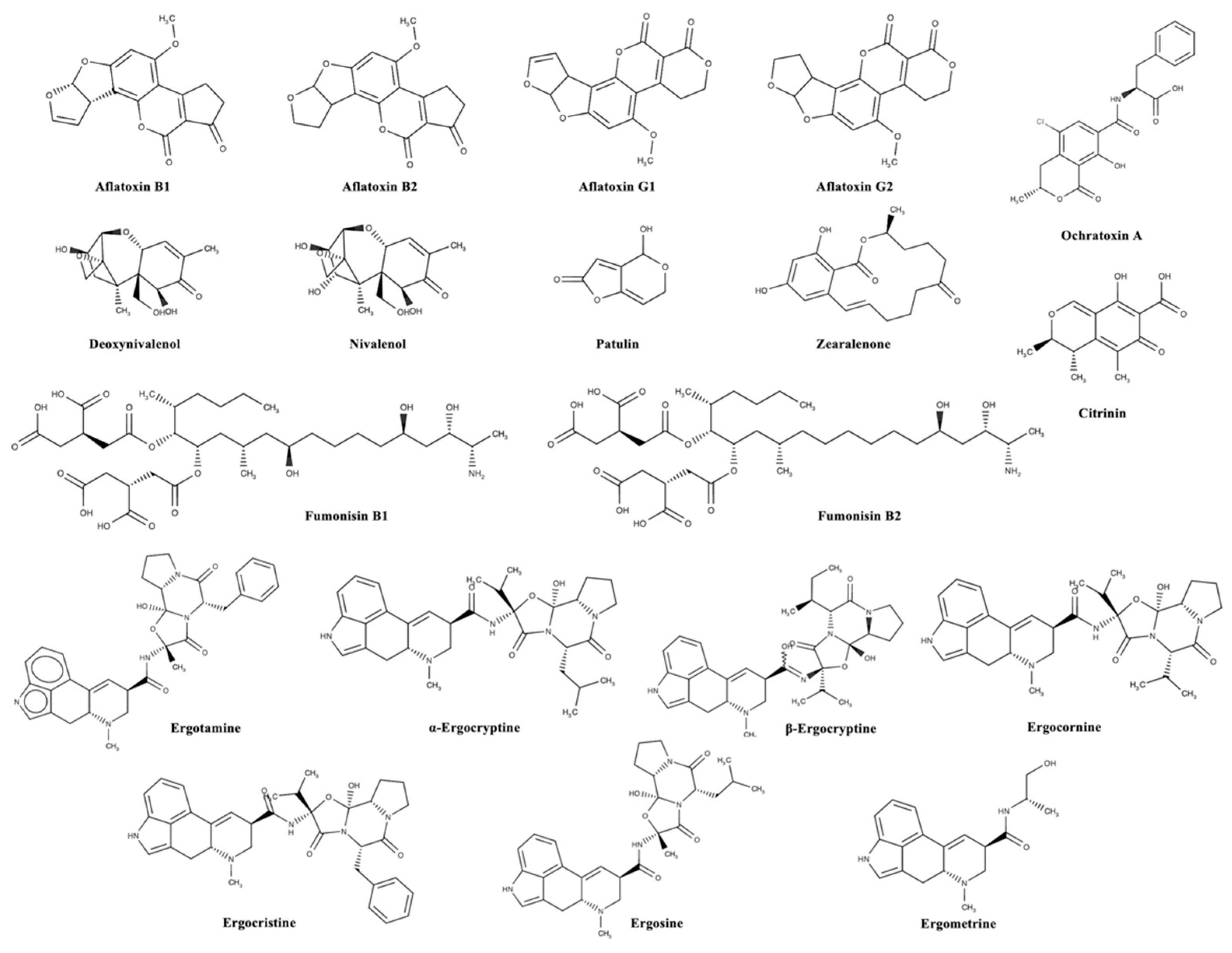
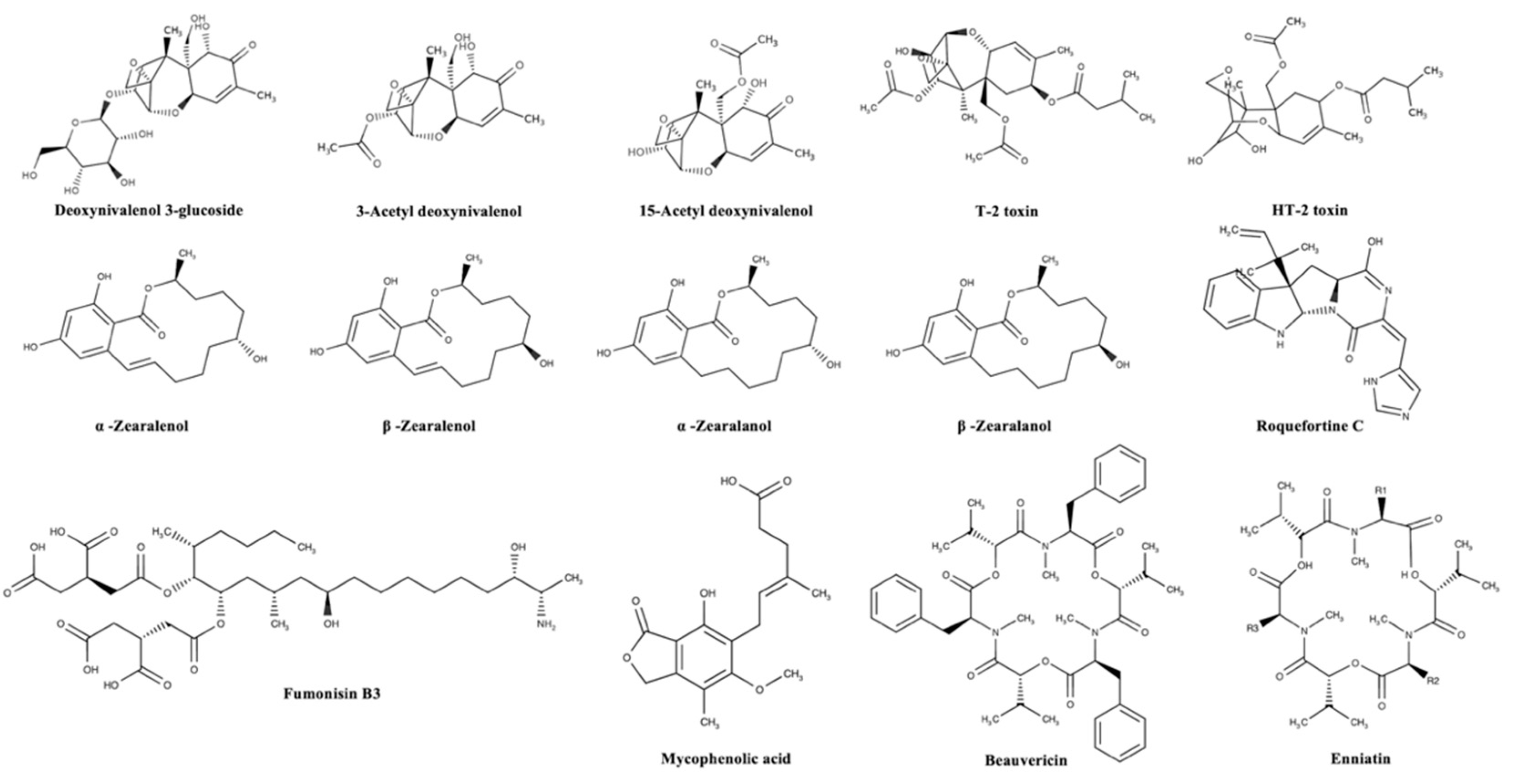
Mycotoxins can be categorized according to substantial structural variation rather than their different origins; this huge diversity results in marked differences in their physical, chemical, and biological properties [14]. The chemical structures of the most important regulated and emerging mycotoxins are reported in Figure 1 and Figure 2, respectively.
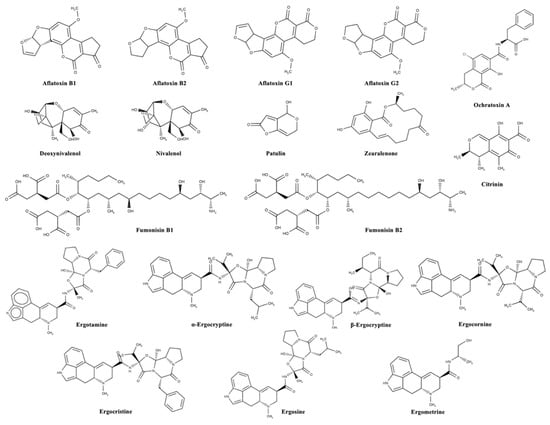
Figure 1.
Chemical structures of the most important regulated mycotoxins in the agro-food chain.
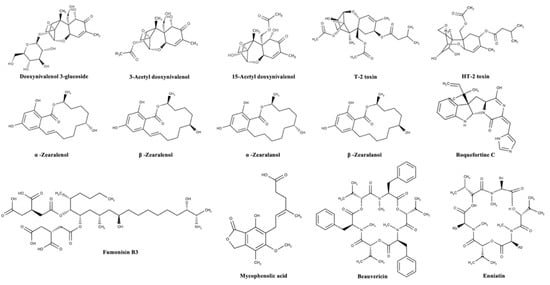
Figure 2.
Chemical structures of the most important emerging mycotoxins in the agro-food chain.
The most relevant groups of mycotoxins for health concerns are represented by aflatoxins (AFs), fumonisins (FUMs), trichothecenes (TCs), zearalenone (ZEN), and ochratoxins (OTs) [15].
AFs have been defined as potent carcinogenic compounds by IARC [16]. These toxins are able to damage different organs, causing, among other things, hepatotoxicity, immunosuppression, and the severe alteration of coagulation processes [17,18]. In this regard, AFB1 can be listed as the most potent and harmful aflatoxin, mainly produced by A. flavus and A. parasiticus fungi [19,20]. The damaging effects triggered by AFs occur in both humans and animals. AFB1 exerts dangerous effects mainly on the liver, besides causing great challenges in terms of altered immune responses and gut health. On the other hand, FUMs are produced by F. verticilliodes and F. proliferatum and are mainly studied for their nephrotoxic and hepatotoxic activities [21].
Additionally, group A T-2 and HT-2 toxins can be categorized within the trichothecenes group, with the latter being characterized by more than 200 known compounds. T-2 and HT-2 toxins are produced by many species of fungi (such as Fusarium, Trichoderma, Cephalosporium, and others) and can negatively affect, among other things, heart muscle functionality and the immune system [22,23]. On the other hand, among the group B trichothecenes, it is possible to list DON. F. graminearum represents the main producer of this toxin, previously studied for its ability to inhibit protein synthesis; furthermore, other studies evaluated its high toxicity at the intestinal level and its ability to cause immune deficiency [24].
As far as ZEN is concerned, this toxin is mainly produced by F. graminearum. However, other species produce this toxin in lower quantities, such as (among others) F. culmorum and F. verticillioides. This toxin has been widely studied for its estrogenic properties, inducing reproductive disorders and immunotoxicity. This has been observed, especially in swine and rabbits [25,26]. Going into detail, ZEN promotes the release of different hormones, such as prolactin and luteinizing hormones, interfering with the normal estrus cycle, ovulation, and embryo implantation [27].
Finally, Aspergillus and Penicillium strains are reported to produce ochratoxins. The complete and accurate mechanisms of action of these toxins seem to be based on structural similarity with phenylalanine, determining an antagonist effect on the same biological targets. Overall, the most observed imbalance conditions are related to calcium homeostasis and affect protein synthesis [28]. OTA (belonging to the ochratoxins group) is a toxin first isolated from A. ochraceus. The most dangerous effects of this toxin are correlated with its nephrotoxic, immunotoxic, and genotoxic activities [29].
Starting from these background conditions, in this comprehensive review, we briefly describe the impact of mycotoxins on animal and human health and then we critically discuss the main findings on the available multi-screening methods to profile regulated and emerging mycotoxins in food, feed, and biological fluids. For the aim of this comprehensive review on mycotoxins, a search in the database Scopus was first carried out in order to identify related published reviews using the primary keywords “mycotoxins and masked mycotoxins”, “mass spectrometry”, and “multi-screening”. Afterwards, primary studies were searched in this database for studies with different combinations of the primary keywords to cover food, feed, and biological fluids in order to provide a comprehensive review of the most recent literature in this area. Additional searches in the Google Scholar engine were also carried out to enrich the references. Therefore, a total of 100 papers were finally considered in the context of this review.
2. Impact of Mycotoxins on Animal and Human Health
When talking about clinical and subclinical mycotoxicosis in animals, major mycotoxin exposure is usually related to the ingestion of contaminated feed [30]. In this regard, AFB1, DON, Fumonisins B (FBs), and ZEN are commonly found in grains and animal feeds. These toxins are widespread in agricultural products and animal feed and usually co-occur in the product, thus creating a major challenge in terms of toxicity [31]. Mycotoxin exposure determines different animal responses (depending on species), with sheep being described as relatively resistant when compared to poultry, piglets, and cattle [32,33]. As a general consideration, young poultry, rabbits, and swine are recognized as more susceptible to AFs [33], which are usually associated with anorexia, hemorrhages, edema, and jaundice. Swine are described as the most susceptible species to ZEN exposure, especially when considering young specimens and sows, while cattle and sheep are the most resistant to estrogenic side effects [34]. Equine and swine are highly susceptible to FUMs, with jaundice, edema, and severe dyspnea representing some of the most prominent and toxic effects. Ochratoxin exposure was previously reported to induce nephrotoxicity in laboratory animals, recording a maximum LD50 of 30.3 mg/kg bw, depending on the species [30]. As far as the group of ochratoxins is concerned, swine and poultry are extremely sensitive. Interestingly, swine may develop an exclusive condition known as porcine nephropathy [35]; in this regard, it is worth mentioning the Balkan endemic nephropathy, which has high relevance for Serbia, Bulgaria, Romania, Croatia, and Bosnia [36]. On the other hand, the main effect on poultry is related to an alteration in the intestinal microbiota, affecting meat pigmentation and eggshell fragility [30,37]. Several studies have reported that poultry, swine, and rabbits are the most susceptible species to trichothecene mycotoxins [30,38,39]. Farming animals are reported to suffer from vomiting, refusing feed, and changing feeding behavior. Finally, poultry, swine, and rabbits represent the most susceptible species to DON, with the latter being associated with oral ulcers and melena [40].
As comprehensively reviewed by Franchino et al. [30], acute mycotoxicosis on animal farms is rare because of the introduction of severe and improved animal feed hygiene standards. On the other hand, an opposite trend is followed by subclinical mycotoxicosis because of the low amount of mycotoxins in the feeding system, causing detrimental economic losses. Furthermore, the immunosuppressive effects of these fungal metabolites can potentially expose animals to other pathologies, thus reducing their ability to face less aggressive on-farm conditions. Mycotoxins can decrease animal resistance to environmental and microbial stressors, reducing their resilience and making them more susceptible to diseases [30]. Additionally, in the meat production area, mycotoxins can significantly compromise the overall quality of final products. Finally, all known mycotoxins are characterized by different levels of absorption, metabolism, and kinetics. Therefore, legislation connected with the mycotoxin contamination of animal feeds must consider all these parameters to properly identify and recommend limits for each mycotoxin (Table 1) [41,42,43].

Table 1.
Legal limits for the most important mycotoxins in animal feeds, according to the European Commission recommendations. Adapted from [30].
Recently, Franchino and co-authors [30] showed some very interesting findings of a five-year monitoring plan dealing with the evaluation of AFB1, ZEN, DON, OTA, FUMs, and T-2/HT-2 toxins in 722 samples of animal feed. The authors revealed that fourteen samples were characterized by mycotoxin concentrations higher than related maximum residue limits (MRLs). In particular, non-compliant samples were associated with DON, AFB1, and ZEN, respectively, with maize being the most frequent raw material in these samples [30]. Also, the authors highlighted a potential correlation between mycotoxin type and structure and animal susceptibility.
According to both the European Food Safety Authority (EFSA) and the International Agency for Research on Cancer (IARC), some of these mycotoxins are classified as potentially dangerous for human health [44], with most of them causing toxic responses at low concentrations. As recently reported by Sdogati et al. [45], approximately 300 mycotoxins have been identified and characterized so far, but a very low number of them have relevance for both animal and human health. Mycotoxins can promote the generation of reactive oxygen species (ROS), thus inducing oxidative stress and the oxidation of DNA, proteins, and lipids [46]. For example, aflatoxins produced by A. flavus and A. parasiticus (such as AFB1, AFB2, AFG1, and AFG2) are bioactivated by cytochrome metabolism into exo-aflatoxin B1-8,9-epoxide (a highly reactive intermediate), leading to DNA damage during replication [47]. Also, aflatoxins can dramatically affect the liver and its overall functionality [48].
The consumption of mycotoxin-contaminated foods can lead to severe outbreaks [49]. Human exposure due to the transfer of mycotoxins from contaminated crops to the final food product represents one of the main exposure routes. Also, another possible route is related to the ability of animal metabolism to process and modify mycotoxins [45]. One of the most studied examples of the carry-over phenomenon is represented by the occurrence of AFM1, the hydroxylated metabolite of AFB1, in dairy products (i.e., milk and cheese). AFM1 presence in dairy products is a huge and severe health concern for humans; accordingly, this metabolite is characterized by high toxicity and carcinogenic properties. That is why the IARC classified AFB1 and AFM1 as human carcinogens belonging to Group 1 and Group 2B, respectively, because of their ability to form DNA adducts [50,51]. Animals consuming a diet potentially contaminated with mycotoxins are able to biochemically transform or process these toxins, distributing the unmetabolized forms in edible tissues. Therefore, milk, meat, liver, heart, and eggs could be highly contaminated. Taken together, this information paves the way towards the development of a thorough monitoring plan for food and feed products to better guarantee animal and human safety and avoid economic losses. Considering the risks for both animal and human health, the European Commission defined strict regulations, together with MRLs. In this regard, it is important to mention the Commission Regulation (EU) 2023/915 [52] on maximum levels for certain contaminants in food, repealing Regulation (EC) No. 1881/2006.
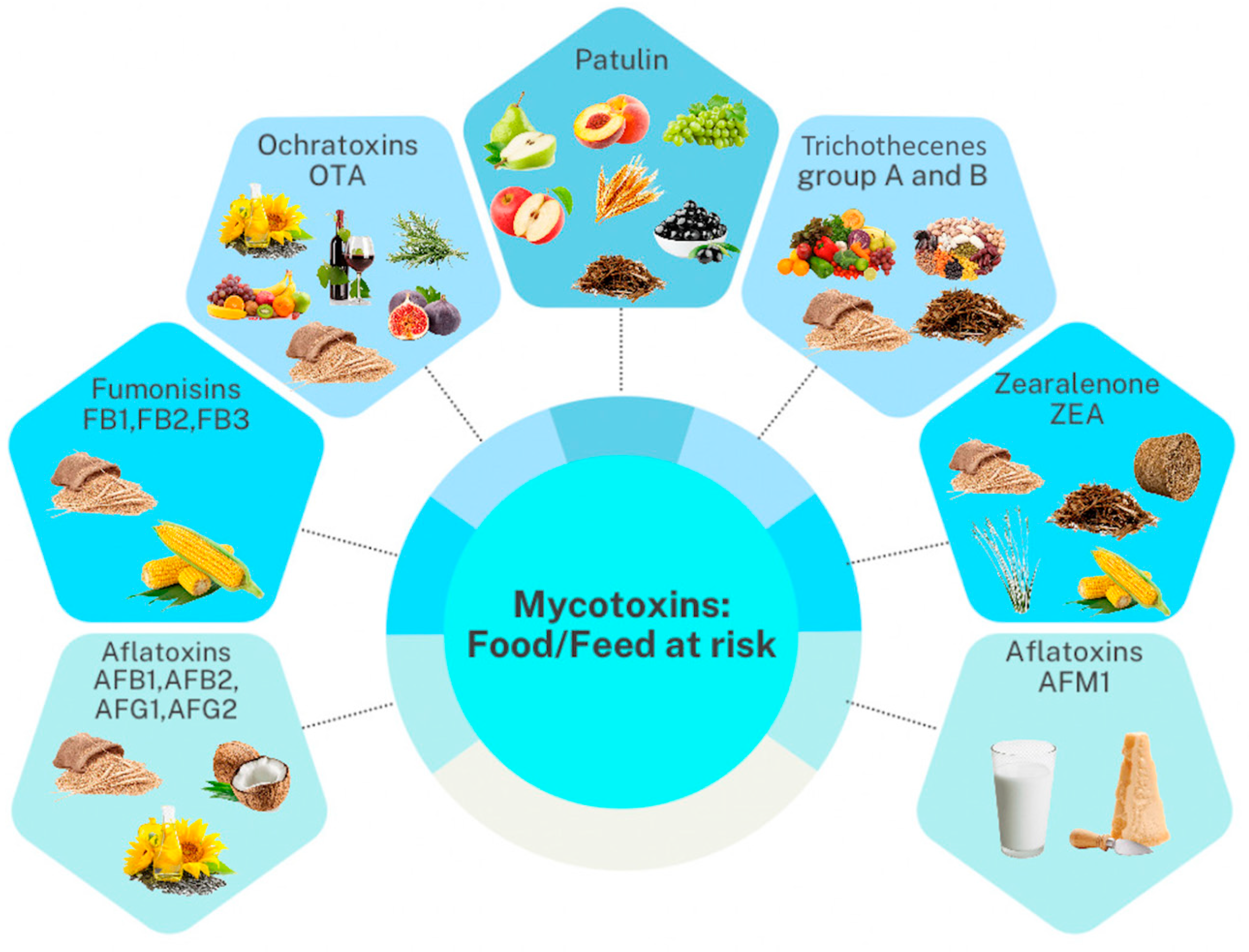
Mycotoxin occurrence and levels in diverse feeds and foodstuffs have been recently reviewed by Ali et al. [53] and El-Sayed et al. [54]. We recommend these two references for a comprehensive understanding of mycotoxin contamination and the quantitative levels in food and feed matrices from different countries. Therefore, in this review, we summarized only the most frequent mycotoxins and their metabolites per main food and feed category (Figure 3).
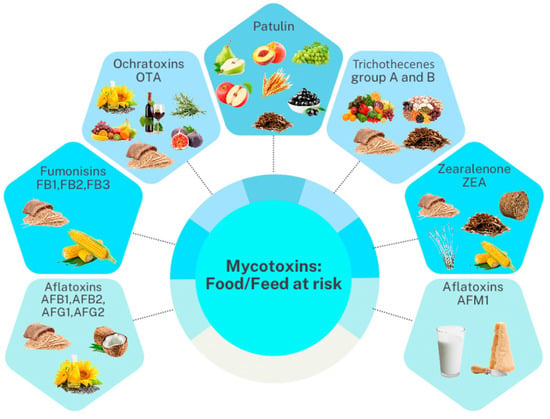
Figure 3.
Relevant mycotoxin groups that contaminate different feed and food products.
Overall, mycotoxins occur in multiple feeds and food items at very different levels. Our literature search outlined that cereal grains and nuts are the most contaminated sources [53]. Also, we found that a high frequency and level of selected mycotoxins can occur regardless of the geographical origin of the product category [53]. Food products that were mainly contaminated were cereals (i.e., wheat, maize, oats), nuts (i.e., peanuts, hazelnuts), animal-derived products (e.g., milk, meat, eggs), mixed food products, and others (such as cocoa and soybean meal). The most widespread and consumed cereals have been widely examined in previous scientific works for mycotoxin contamination. A comprehensive three-year survey (2018–2020) by Khodaei et al. [55] on the global occurrence of mycotoxins in cereals revealed that the hazard of AFB1 in wheat, maize, and rice is serious, considering that these mycotoxins were higher than the EU limit in most of the reviewed cases. Overall, the high stability of mycotoxins during different phases of the production chains (i.e., production, distribution, storage, and processing) of cereals poses a high concern about the risks associated with the consumption of this food category [56]. Therefore, practical control measures combined with proper management strategies are mandatory to ensure consumer safety.
3. Challenges in the Analysis of Emerging and Hidden Mycotoxins
Because of climate change and the corresponding increase in fungi contamination in several matrices, the official control of mycotoxins and fungal metabolites in both animal feed and human food has recently gained even more importance than in previous years [57]. Today, more than 300 mycotoxins are known, but only a very small number are subjected to legal regulations. Interestingly, previous studies carried out on commercial animal feed samples from European and Mediterranean vs. Asian and Pacific areas revealed that more than half of the commercial samples were definitely contaminated by mycotoxins at a higher level than the corresponding legal limits [30]. It is important to consider that regulated mycotoxins can be structurally modified by fungi, plant, and animal metabolic reactions, thus resulting in potentially toxic metabolites not considered in legislation. More important is the case of emerging mycotoxins; these are a group of compounds not included in regulations and scarcely analytically determined. However, several recent literature reports have highlighted their toxicity and high co-occurrence [58]. Emerging mycotoxins (such as nivalenol, enniatins, beauvericin, diacetoxyscirpenol, fusaric acid, patulin, moniliformin, and sterigmatocystin) are widely present in cereals and other feed commodities worldwide [58]. It is also important to mention the challenges associated with the determination and quantification of so-called masked or hidden mycotoxins. These are biologically modified metabolites of mycotoxins with improved or reduced toxicity compared with parent mycotoxins and are difficult to detect through classical and/or targeted analytical methods [59]. These can be attached to carbohydrates or proteins, thus hampering their extraction from the matrix of interest through the available extraction protocols designed for different classes of toxins [59]. The modified and/or altered physicochemical properties are related to different chromatographic behaviors and are difficult to identify because of the lack of authentic standard compounds. Taken together, the information collected suggests that, in this way, potential underestimations of the total mycotoxin content of samples occur. A high number of scientific publications are available and focused on the potential hydrolysis of these modified or hidden forms of mycotoxins to re-generate toxic parent compounds. This event is common during mammalian digestion, thus determining severe toxicological concerns because these toxins are highly bioavailable in the digestive system [60,61]. Again, a concrete monitoring plan for these mycotoxin metabolites is still a main task for guaranteeing both food safety and human and animal health. In this complex scenario, ultra-high-performance liquid chromatography coupled with tandem mass spectrometry (UHPLC–MS/MS) can overcome the issues raised by modified mycotoxins and be used as a selective detection and quantification-based approach for several compounds in different feed, food, and/or biological matrices [62]. Considering that, nowadays, no legal regulations on chemically differentiated or modified mycotoxins in food and feed exist, more studies and scientific knowledge are needed to protect human and animal health.
As comprehensively reviewed by Okasha et al. [59], the occurrence or co-occurrence of masked mycotoxins is higher in food than in feed. The majority of the available research studies are based on the evaluation of modified forms of ZEN resulting from phase I and phase II biotransformations. However, although the biochemical pathways dealing with biotransformation are known [63], very few studies are available on other modified forms, such as acetyl DON derivatives, hydrolyzed FBs, and phase I metabolites T2 and NIV3G. In particular, the correlation between in vitro data and in vivo situations necessitates further investigation [63]. It seems that one of the major weapons available now against the few and/or inconsistent data reported on masked or hidden mycotoxins (mainly in terms of their toxicity) could be represented by the recent advancements in analytical methods. In this regard, the ability of high-resolution mass spectrometry tools to simultaneously detect multiple mycotoxins (both in their original and masked forms) makes it possible to overcome the previously mentioned limitations [62]. On the other side, the utilization of these high-throughput analytical methods poses limitations in terms of costs, and, sometimes, data are difficult to compare because of the absence of standardized protocols (mainly when considering sample collection and preparation) [59]. Therefore, this literature overview suggests and invites researchers working in this field to dedicate a great deal of attention to both free and masked mycotoxins in terms of developing new targeted and untargeted screening methods able to provide a beneficial snapshot of the contamination levels of a food or feed item or the potential toxicity associated with the presence of potentially toxic metabolites and/or hidden forms.
4. Transition from Targeted Analysis to Targeted/Untargeted Multiscreening-Based HRMS Approaches
In the last few years, research on mycotoxins has moved quickly towards the utilization of LC-MSMS and LC-HRMS approaches to determine multiple mycotoxins. Therefore, so-called multi-screening methods (for both qualitative and quantitative purposes) have started to be rapidly developed [62]. One of the major advantages of using HRMS over targeted MS/MS techniques is represented by the possibility to perform both untargeted and retrospective data analysis, with the latter allowing the reconsideration of analytical results from stored data and already analyzed samples. In particular, these methods are based on the measurement of accurate MS and MS/MS spectra with a mass resolution lower than 5 ppm. These conditions usually allow the detection of several compounds, together with the possibility to perform a structural elucidation of unknowns [64]. All these aspects represent a valuable tool because of the lack of analytical standards for several known mycotoxins. Untargeted and retrospective data analyses are particularly useful when modified and/or hidden mycotoxins are targeted, especially if one wants to investigate combined toxic effects [65,66]. Therefore, the utilization of full-scan methods based on high-throughput platforms (exploiting resolving powers up to 100,000 FWHM), such as Time-of-Flight (TOF) and Orbitrap, has been explored as a robust and complementary alternative to triple–quadrupole-based methods [62]. Also, the use of LC or UHPLC coupled with different HRMS platforms is extremely advantageous because the high resolution of these instruments provides information about targeted compounds, non-targeted compounds, and novel compounds in a single analytical run [62,67]. Therefore, the main advantages arising from the utilization of HRMS in mycotoxin research can be exploited in the field of emerging and modified mycotoxins because of the lack of certain analytical standards (sometimes not commercially available), calibrants, and reference materials. Also, as previously mentioned, there is an urgent trend of collecting more data about the co-occurrence of different regulated and emerging mycotoxins in both food and feed matrices [1] in order to better define a risk assessment on the potential synergistic effects of different compounds in terms of toxicity. On the other hand, an important issue associated with LC-HRMS method development, validation, and routine utilization is represented by the matrix effect. This latter can alter the ionization efficiency of the selected analyte in the presence of coeluting compounds, thus affecting both accuracy and sensitivity. Overall, according to the scientific literature [68], there are several possibilities for evaluating matrix effects. One of them is represented by the post-extraction spike method comparison, where three sets of samples are considered, namely, a) a neat standard dissolved in a selected solvent, b) a post-extraction spiked sample, and c) a pre-extraction spiked sample to also calculate extraction efficiency.
Overall, the comprehensive information gathered by HRMS utilization is useful for developing and improving novel toxin-based libraries and databases [69] and for further exploiting retrospective analyses of full-scan data [70]. The availability of qualitative occurrence and co-occurrence data could be exploited to better define food quality controls, thus ensuring the food safety and health of consumers. However, as comprehensively reviewed by Righetti et al. [62], HRMS still presents some critical aspects to be faced for its routine application in mycotoxins and/or contaminants analyses, including (among others) the cost of instrumentation and analyses, the need to perform multivariate statistical analyses to better extrapolate the biological meaning, the utilization of software (sometimes not open-access), and the availability of a huge storage system. Another important point associated with the fast development of HRMS platforms and data management is associated with issues of isobaric co-eluting compounds and unknown compound identification; these points should always be considered and solved to allow for the effective use of HRMS for food safety purposes. Recently, the introduction and development of ion mobility-based mass spectrometry (IMS) allowed the addition of a third dimension of separation based on the size, shape, and charge of ions, thus implementing so-called levels of annotation of omics technologies [71,72]. Interestingly, a database for mycotoxins was previously established, containing more than 100 traveling wave IMS-derived CCS values [73]. Therefore, the actual and next frontier of multi-mycotoxin research seems represented by coupling IMS and UHPLC-HRMS platforms in order to improve the quality of annotated mass features. Also, besides the important issue of isobaric compounds (which can be resolved neither by MS nor by UHPLC), this strategy could enhance compound identification by adding structural information (i.e., size and shape) based on CCS measurements [62]. Some previous applications of LC–ESI–TWIMS–TOF–MS methods (including CCS-filtering in data processing) have been exploited for the analysis of mycotoxins in cereals [73] and other contaminants (such as pesticides) in different feed and food items [74].
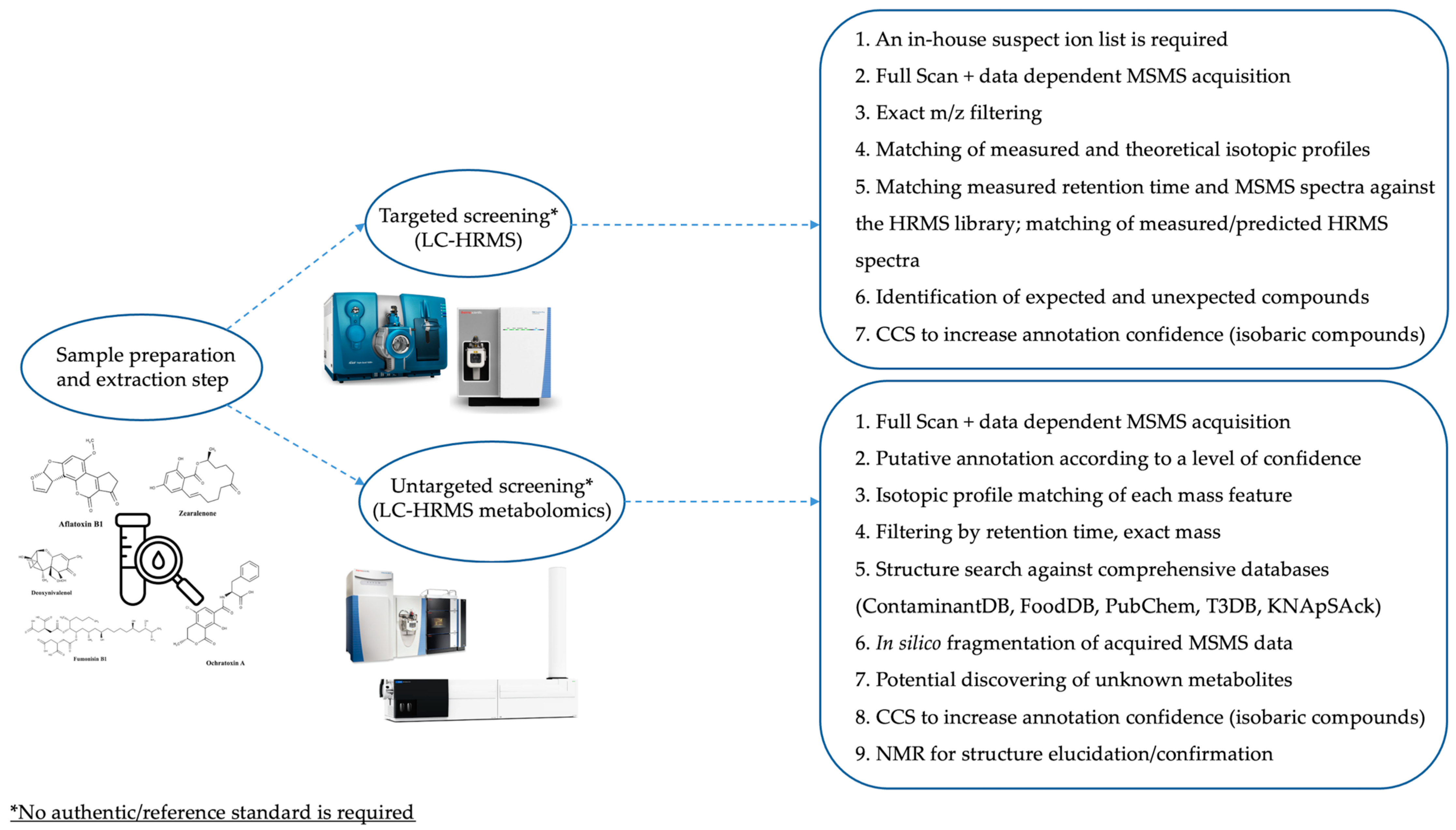
A schematic overview of the main differences and identification-based workflows of targeted, untargeted, and multiscreening/retrospective methods is reported in Figure 4.
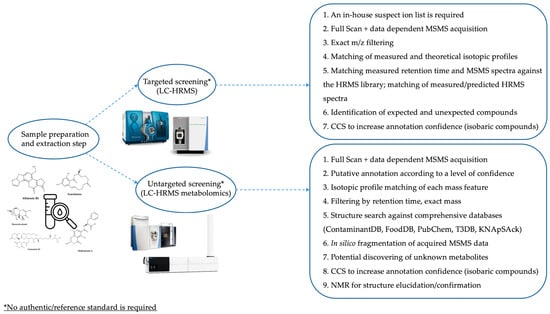
Figure 4.
Workflows based on targeted and untargeted multiscreening for the detection of mycotoxins in different food and feed products. Adapted from [61].
Some of the most recent applications dealing with the utilization of multi-LC-MSMS and multi-LC-HRMS for mycotoxin determination are reported in Table 2.

Table 2.
Most interesting studies of the last five years dealing with the development of multiscreening methods to simultaneously detect different mycotoxins in different feed and food matrices.
4.1. Applications of Multiscreening Methods for Biofluids
Lauwers et al. [75] developed an accurate multiscreening method for the quantification of mycotoxins in pig and chicken biofluids using LC-MSMS. The authors targeted mainly aflatoxins, ochratoxin A, and Fusarium mycotoxins, followed by emerging mycotoxins. Additionally, the same developed method was used under LC-HRMS conditions to qualitatively analyze phase I and II metabolites. Similarly, Tkaczyk and Jedzinak [76] proposed an LC-MS/MS method for the sensitive and selective analysis of 35 mycotoxins (as biomarkers of exposure) in pig urine samples. The authors revealed that DON, ZEN, and OTA were the main toxins detected in these samples.
4.2. Applications of Multiscreening Methods for Food Samples
Zhang et al. [77] developed a data-dependent acquisition-based HRMS (HPLC-Orbitrap) for tracing Alternaria mycotoxins and their sulfated metabolites in tomatoes, obtaining LOD and LOQ values of 0.009 and 0.030 μg/kg, respectively. A very interesting application of a multiscreening method for the simultaneous analysis of regulated, emerging, and modified mycotoxins was proposed by González-Jartín and co-authors [78] for milk; in particular, the authors developed a new method for the analysis of 40 mycotoxins in 31 cow milk samples using a QuEChERS extraction procedure to minimize the matrix effect. Overall, the authors demonstrated a high occurrence in these samples of Fusarium mycotoxins, namely, beauvericin and enniatins. BEA and ENNs are emerging mycotoxins frequently found in cereals and are mainly associated with adverse effects on the gastrointestinal tract. Similarly, looking at available data on milk samples, targeted analysis and retrospective screening of mycotoxins were carried out by Izzo et al. [79] using a UHPLC-HRMS approach. The authors analyzed 56 commercial milk samples from the Italian market, optimizing an alternative analytical tool for the simultaneous identification of 30 mycotoxins. Overall, no analyzed sample was contaminated by mycotoxins; however, the retrospective screening based on UHPLC-HRMS allowed for the tentative identification of other fungal and bacterial metabolites of interest. The potential for retrospective screening based on UHPLC-HRMS was also highlighted by Rocchetti et al. [80], who analyzed 45 bulk milk samples previously classified into different clusters according to the maize silage contamination profile. Generally, 14 compounds showed significant prediction ability when considering milk from contaminated and uncontaminated feeding systems, with antibiotic Y, bikaverin, and fumonisin B2 being the best predictive biomarkers. Among the discriminant metabolites, a high abundance of Fusarium mycotoxins was noticed, together with tetrapeptide tentoxin (an Alternaria toxin), α-zearalenol (a catabolite of zearalenone), mycophenolic acid, and apicidin. A multiscreening method was developed by Akinyemi and co-authors [81] to evaluate the mycotoxin contamination profile of raw milk from three different Nigerian animal species belonging to cow, goat, and camel. The authors analyzed 135 raw milk samples using an ultra-sensitive LC-MSMS method based on the determination of 36 different mycotoxins. The most abundant mycotoxins detected were aflatoxin P1, alternariol monomethyl ether, citrinin, dihydrocitrinone, enniatins, ochratoxin α, and sterigmatocystin, reported for the first time in animal milk. Interestingly, the most frequent mycotoxin detected in animal milk samples was beauvericin (87%), thus confirming the importance of emerging mycotoxins in the entire dairy food chain. In this scenario, Le et al. [82] developed a multiscreening method for mycotoxins in commercial cashew nut samples collected from Vietnam. The experimental conditions were based on QuEChERS extraction coupled with UPLC-MSMS to detect a total of 18 mycotoxins. The authors highlighted that the high heat resistance of mycotoxins represents a key factor facilitating their stability during conventional food processing. Also, cashews containing more additives had significantly higher FB1 concentrations, but not DON. Therefore, further studies are necessary to better correlate food processing conditions with mycotoxin formation in cashew nuts. Work by Li et al. [83] developed a method for the detection of mycotoxins in a complex matrix, such as vegetable oil. The comprehensive analysis of 20 commercial vegetable oils revealed that AFB1 and AFB2, followed by ZEN, were the most frequent mycotoxins observed. The authors stated that the method was simple and low-cost, thus promoting its wider applications for scanning mycotoxins in oil matrices. Recently, Sulyok et al. [84] reported the validation of an LC-MSMS method for the simultaneous quantification of more than 700 mycotoxins and other secondary fungal metabolites and plant toxins in pasta, biscuits, crackers, and muesli. Overall, application of the method to 147 samples from the EU market revealed the presence of enniatins and DON in the majority of samples.
4.3. Applications of Multiscreening Methods for Feed Samples
A comprehensive survey by Zhang et al. [85] was carried out on maize silage samples from the China region, known to be potentially contaminated with mycotoxins. The authors analyzed 200 maize silage samples collected in 2019 for regulated, masked, and some emerging mycotoxins. Moving to the main findings, the targeted multiscreening method revealed that DON and ZEN were the most frequent compounds in the analyzed samples, while the most commonly detected masked mycotoxin was 15-acetyldeoxynivalenol. Of interest, the results of this study pointed out the importance of beauvericin, which was detected in 99.5% of the samples analyzed with concentrations lower than 25 μg/kg. Also, the authors revealed that the accumulation of beauvericin was strongly correlated with DON and ZEA, thus indicating the urgent need to perform additional and comprehensive studies to better focus on masked and emerging mycotoxins. A previous application by González-Jartín et al. [86] allowed for the development of a multi-detection method for mycotoxins with modified QuEChERS extraction in feed. In particular, the authors outlined that maize and maize-based products were highly contaminated by toxins, although always below the legal limits. This might be of great concern since maize silage is one of the main components of milk cow feed in many regions of the world. Overall, the richness in protein and polysaccharides of maize cobs and leaves is reported to help fungi and other pathogens proliferate. Therefore, maize silage can be a dangerous source of AFB1, DON, and ZEN, with DON and ZEN being the most relevant ones in maize grains and whole maize. Another important and potential source of mycotoxins (because of their worldwide consumption) is represented by cashew nuts. A reliable, sensitive, and accurate multiple mycotoxin method was also developed by Nualkaw et al. [87] for the simultaneous determination of 17 mycotoxins in 300 feed samples (including swine, poultry, and dairy feeds) using stable isotope dilution (13C-ISTD) and UHPLC-MS/MS. The authors demonstrated that FUM, ZEN, AFB1, and DON highly contaminated the feed samples. Targeted analysis combined with retrospective screening allowed researchers to measure multiple mycotoxins in pet food; in particular, Castaldo et al. [88] used a comprehensive strategy based on a combination of quantitative methods for 28 mycotoxins and post-target screening for an additional 245 fungal and bacterial metabolites in dry pet food samples. Acetonitrile-based extraction was carried out, followed by the utilization of UHPLC-Q-Orbitrap (HRMS) as a detection method. The authors outlined the simultaneous occurrence of up to 16 toxins per sample.
5. Omics Technologies as a Valuable and Emerging Tool to Assess Mycotoxicity
As a recent research field, omics technologies, including metabolomics-based approaches, have been described as very useful in providing strong knowledge about mycotoxin metabolization in a host. Also, metabolomics is reported to provide a good level of knowledge of potential interactions with other biomolecules and targeted organs, allowing for the modeling of intraspecies variability and biochemical crosstalk [89]. In particular, omics-based approaches are valuable tools for the analysis of clinical samples, such as biofluids. This high-throughput technique can provide a comprehensive overview of the pathophysiological state mediated by mycotoxin exposure, thus helping to discover and define the most relevant exposure biomarkers related to adverse implications [90].
As previously stated, omics technologies based on HRMS allow researchers to consider all three potential forms of existing mycotoxins, namely, unmodified (parent) forms of mycotoxins biosynthesized by different fungi (e.g., AFs, OTA, ZEN, FBs, PAT, and DON), followed by matrix-bound mycotoxins such as those non-covalently complexed with proteins and polysaccharides, and chemically or biologically modified mycotoxins (masked or hidden) produced by fungi, bacteria, plants, or animals or derived from different food processing conditions [91]. Regarding biological samples, such as blood, it is therefore mandatory to consider in omics-based studies also those modified mycotoxins that have transformed back to their native forms in the digestive system and are potentially exerting similar toxic effects as the parent, thus contributing to final mycotoxin exposure.
Metabolomics is now considered a very robust and powerful technology to ensure the unbiased and overall determination of several metabolites (i.e., small metabolites with a mass range from 50 to 1500 Da). Therefore, this approach is finding wide application in different food matrices [89]. Targeted and untargeted metabolomics studies employ a combination of sample preparation, analytical tools, and computational analysis [92]. Among the most widespread analytical tools, it is possible to list NMR, followed by GC- and LC-HRMS. High-resolution mass analyzers allow for an extremely high putative identification level by, for example, using QTOF and Fourier transformation-based instruments (e.g., Orbitrap and ion cyclotron resonance). According to the available scientific literature, the most commonly analyzed biofluids for targeted and untargeted metabolomics profiling related to mycotoxin exposure are blood, serum, plasma, urine, and milk [88,89,90]. In this regard, targeted metabolomics is usually used for detecting different ranges of contamination by mycotoxins in foods, feeds, and agricultural products [93] as well as for checking biomarkers of exposure [94]. As recently reported by Owolabi et al. [90], untargeted metabolomics provides a robust and unbiased platform for mycotoxin research and a better evaluation of human and animal exposure, allowing for the identification of the best biomarkers to be monitored for guaranteeing food and feed safety.
An interesting application of an omics-based approach dealing with mycotoxins and biofluids was previously published by Rocchetti et al. [95], evaluating the metabolomics changes of milk due to mycotoxin-contaminated maize silage intake by dairy cows. The analysis was based on UHPLC coupled with Orbitrap-HRMS. Overall, 628 significant milk metabolites were strongly affected by the five levels of maize silage contamination. Interestingly, 78 metabolites showed a very high and significant prediction ability. Accordingly, sphingolipids, together with purine and pyrimidine-derived metabolites, were the most affected classes of compounds. Also, metabolomics revealed a pivotal role of oxidized glutathione in milk samples associated with the silage group contaminated by emerging Aspergillus toxins. Recently, the combination of stable isotopes with metabolomics allowed for the identification of newly identified biotransformation products in human cell models, such as ZEN-pyridoxine, DON-3-sulfate, DON-10-sulfonate 2, DON-10-glutathione, and DON-cysteine, thus improving the state-of-the-art and existing information about the biotransformation of ZEN and DON [96,97].
Besides LC-HRMS, a metabolomics approach can also be realized using different analytical techniques, such as NMR. Different samples, including rumen fluid, blood, and milk, collected from dairy cows were previously analyzed by Wang et al. [98] to obtain a comprehensive understanding of the metabolic changes resulting from AFB1 exposure. Particularly, the authors combined 1H-NMR spectroscopy with classical biochemical assays and outlined a significant impact of AFB1 exposure on amino acid metabolism (mainly phenylalanine) in all three biofluids. However, the authors concluded that mycotoxicity should be better assessed by using not only biomarkers of metabolomics but also some indicators of milk composition and production variables. Another interesting study was developed by Ogunade et al. [99] evaluating biomarkers of aflatoxin ingestion through a 1H-NMR-based metabolomics approach on dairy cows fed aflatoxin B1 with or without sequestering agents. The authors found that AFB1 greatly affected the plasma metabolomics of lactating Holstein dairy cows. In particular, a strong decrease in plasma amino acids (such as alanine, leucine, and arginine) and acetic acid was observed, while a corresponding increase in ethanol was detected. The latter was proposed as a good candidate biomarker of aflatoxin ingestion in dairy cows [99]. Another valuable example of a metabolomics-based approach dealing with mycotoxins and biofluids is represented by a study carried out by Gerdemann et al. [100] for the analysis of cellular alterations caused by 20 mycotoxins in HepG2 cells. Hydrophilic-based chromatography combined with targeted HRMS allowed for the selective and sensitive detection of more than 100 metabolites, easily associated with metabolic alterations caused by mycotoxins. In particular, the authors reported moniliformin and citrinin as the most significantly affected compounds for the citric acid cycle, also influencing glycolysis and energy metabolism. Penitrem A, ZEN, and T2 toxins mainly determined an imbalance of the urea cycle and amino acid homeostasis. The formation of ROS was associated with the presence of T2 toxins and gliotoxin, while OTA altered glycolysis. Finally, DNA synthesis was affected by several mycotoxins [100].
6. Conclusions and Future Perspectives
The presence of mycotoxins in food and feed is a severe problem affecting both quality and safety areas, as well as consumers’ health. The critical review of the available scientific literature here proposed suggests the need to switch from targeted to untargeted and/or retrospective screening analyses to better understand the exposure level and the potential toxicity of the very few studied mycotoxins and metabolites. The selection of samples, sampling, and extraction techniques are crucial for accurate and reliable results on mycotoxins. LC–HRMS is emerging, and it is today highly suitable for detecting multiple mycotoxins and modified mycotoxins in food and feed products, although the proper quantification and detection of masked/hidden mycotoxins in complex matrices is still one of the major obstacles. Additionally, the implementation of regulations for so-called emerging mycotoxins is still challenging. In this complex scenario, omics technologies are emerging as a valuable tool to comprehensively inspect the effect of mycotoxins on organisms by analyzing different biofluids (such as plasma and urine). Untargeted metabolomics allows researchers to check major changes in biochemical pathways together with the mode of action of different mycotoxins, although technical (due to sample preparation, the columns used, and their detection/analysis) and biological (due to different species targeted and/or different environmental/dietary factors) variability is still difficult to model in these studies. HRMS combined with an omics approach has the potential in the near future to be of great help to regulatory bodies to limit the presence of mycotoxins in food and feed; however, the lack of precise standard operating procedures makes the results of different laboratories difficult to compare. Therefore, this literature review supports the fast development of multi-omics approaches within a systems biology scenario; in this regard, genomics, transcriptomics, proteomics, and metabolomics have started to be integrated to create a more holistic comprehension of cells, organisms, and communities. The literature reviewed also shows that besides multi-omics evaluations of host–toxin interactions, another valuable strategy could be analyzing the multi-omics among host gut microbiomes–toxins in order to implement mitigation strategies. Finally, it is important to mention that machine learning approaches (such as convolutional neural networks) for both the detection and prediction of the presence of mycotoxins have seen a rise in recent years as an alternative to traditional detection methods (such as LC-HRMS), although there is an urgent need for reproducibility and transparency in machine learning research through open-access data and codes.
Author Contributions
Conceptualization, G.R. and A.G.; methodology, M.L. and M.E.; validation, G.R.; investigation, G.R., M.L. and M.E.; writing—original draft preparation, G.R. and M.L.; writing—review and editing, G.R. and A.G.; visualization, M.L. and G.R.; supervision, A.G.; project administration, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the NODES (“Nord Ovest Digitale e Sostenibile”) project, which received funding from the MUR—M4C2 1.5 of PNRR, grant agreement no. ECS00000036. The authors also thank the “Romeo ed Enrica Invernizzi” Foundation (Milan, Italy). M.L. was the recipient of a Ph.D. fellowship (AgriSystem) from the Università Cattolica del Sacro Cuore (Piacenza, Italy). The authors are grateful to Gold Standard Diagnostics (Trieste, Italy) for supporting M.L.’s research activity.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
Aflatoxins (AFs), Aflatoxin B1 (AFB1), Aflatoxin B2 (AFB2), Aflatoxin G1 (AFG1), Aflatoxin G2 (AFG2), Aflatoxin M1 (AFM1), Fumonisins (FUMs), Fumonisins B (FBs), Fumonisin B1 (FB1), Fumonisin B2 (FB2), Fumonisin B3 (FB3), Trichothecenes (TCs), Deoxynivalenol (DON), Nivalenol (NIV), Zearalenone (ZEN), Ochratoxins (OTs), Ochratoxin A (OTA), Beauvercin (BEA), Enniatins (ENNs), Patulin (PAT), International Agency for Research on Cancer (IARC), European Food Safety Authority (EFSA), Reactive Oxygen Species (ROS), European Commission (EC), Maximum Residue Levels (MRLs), Liquid Chromatography (LC), Mass Spectrometry (MS/MS), Ultra-High-Performance Liquid Chromatography (UHPLC), Ultra-High-Performance Liquid Chromatography Coupled Tandem Mass Spectrometry (UHPLC-MS/MS), High-Resolution Mass Spectrometry (HRMS), Liquid Chromatography Coupled High-Resolution Mass Spectrometry (LC-HRMS), Liquid Chromatography Coupled Tandem Mass Spectrometry (LC-MS/MS), Time-of-Flight (TOF), Ion Mobility-Based Mass Spectrometry (IMS), Cross Collisional Sections (CCs), Traveling Wave Ion Mobility Spectrometry (TWIMS), Liquid Chromatography Electron Spry Ionization Coupled High-Resolution Mass Spectrometry (LC-ESI-HRMS), High-Performance Liquid Chromatography Electrospray Ionization Coupled Tandem Mass Spectrometry (HPLC-ESI-MS/MS), Ultra-High-Performance Liquid Chromatography Electrospray Ionization Coupled Tandem Mass Spectrometry (UHPLC-ESI-MS/MS), Ultra-High-Performance Liquid Chromatography Electrospray Ionization Coupled Tandem Mass Spectrometry (UHPLC-ESI-MS/MS), Ultra-High-Performance Liquid Chromatography Electrospray Ionization Coupled Quadrupole and High-Resolution Mass Spectrometry (UHPLC-ESI-Q-Orbitrap HRMS), Limit of Detection (LOD), Limit of Quantification (LOQ), Quick-Easy-Cheap-Effective-Rugged-Safe (QuEChERS), Quadrupole Time-of-Flight (QTOF), Nuclear Magnetic Resonance (NMR), Proton Nuclear Magnetic Resonance (1H-NMR).
References
- Gallo, A.; Ghilardelli, F.; Atzori, A.S.; Zara, S.; Novak, B.; Faas, J.; Fancello, F. Co-Occurrence of regulated and emerging mycotoxins in corn silage: Relationships with fermentation quality and bacterial communities. Toxins 2021, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, A.; Jouany, J.-P. Mycotoxins in feeds and their fate in animals: A review. Anim. Res. 2002, 51, 81–99. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R.R.; Storm, I.M.L.D.; Rasmussen, P.H.; Smedsgaard, J.; Nielsen, K.F. Multi-mycotoxin analysis of maize silage by LC-MS/MS. Anal. Bioanal. Chem. 2010, 397, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Mehta, A. Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: A review. Food Sci. Nutr. 2020, 8, 2183–2204. [Google Scholar] [CrossRef] [PubMed]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; Khoury, A.E.; Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; et al. Mycotoxins: Factors influencing production and control strategies. Aims Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Kos, J.; Anić, M.; Radić, B.; Zadravec, M.; Janić Hajnal, E.; Pleadin, J. Climate change—A global threat resulting in increasing mycotoxin occurrence. Foods 2023, 12, 2704. [Google Scholar] [CrossRef] [PubMed]
- Casu, A.; Camardo Leggieri, M.; Toscano, P.; Battilani, P. Changing climate, shifting mycotoxins: A comprehensive review of climate change impact on mycotoxin contamination. Compr. Rev. Food Sci. Food. Saf. 2024, 23, e13323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef]
- Tsiouris, V.; Tassis, P.; Raj, J.; Mantzios, T.; Kiskinis, K.; Vasiljević, M.; Delić, N.; Petridou, E.; Brellou, G.D.; Polizopoulou, Z.; et al. Investigation of a novel multicomponent mycotoxin detoxifying agent in amelioration of mycotoxicosis induced by aflatoxin-B1 and ochratoxin A in broiler chicks. Toxins 2021, 13, 367. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in food and feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar] [PubMed]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aflatoxins, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 2012; Volume 100F.
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Dhakal, A.; Hashmi, M.F.; Sbar, E. Aflatoxin Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Eissa, F.; Sebaei, A.S. A comparative study between the top 10 origin countries involved in the EU RASFF notifications on aflatoxins from 1997 to 2022. Microb. Risk Anal. 2023, 25, 100277. [Google Scholar] [CrossRef]
- Alameri, M.M.; Kong, A.S.-Y.; Aljaafari, M.N.; Ali, H.A.; Eid, K.; Sallagi, M.A.; Cheng, W.-H.; Abushelaibi, A.; Lim, S.-H.E.; Loh, J.-Y.; et al. Aflatoxin contamination: An overview on health issues, detection and management strategies. Toxins 2023, 15, 246. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research progress on fumonisin B1 contamination and toxicity: A review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 toxins: Toxicity, occurrence and analysis: A Review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef]
- Wu, Q.; Qin, Z.; Kuca, K.; You, L.; Zhao, Y.; Liu, A.; Musilek, K.; Chrienova, Z.; Nepovimova, E.; Oleksak, P.; et al. An update on T-2 toxin and its modified forms: Metabolism, immunotoxicity mechanism, and human exposure assessment. Arch. Toxicol. 2020, 94, 3645–3669. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, D.; Cai, P.; Lin, H.; Ying, H.; Hu, Q.-N.; Wu, A. Elimination of Fusarium mycotoxin deoxynivalenol (DON) via microbial and enzymatic strategies: Current status and future perspectives. Trends Food Sci. Technol. 2022, 124, 96–107. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A.; Patyal, A.; Thakur, R.; Sharma, H.; Shakya, S. Mitigating the health risks of mycotoxins: Concerns and solutions vis-à-vis food web. Int. J. Livest. Res. 2020, 10, 1–14. [Google Scholar]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C.; Taranu, I. Zearalenone and the immune response. Toxins 2021, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, T.; Wang, P.; Yin, Q.; Liu, C.; Zhu, Q.; Lu, F.; Gao, T. Compound probiotics alleviating aflatoxin B1 and zearalenone toxic effects on broiler production performance and gut microbiota. Ecotoxicol. Environ. Saf. 2020, 194, 110420. [Google Scholar] [CrossRef] [PubMed]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef]
- Franchino, C.; Vita, V.; Iammarino, M.; De Pace, R. Monitoring of animal feed contamination by mycotoxins: Results of five years of official control by an accredited Italian laboratory. Microorganisms 2024, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, L.; Liu, M.; Su, Y.-T.; Xie, W.-M.; Zhang, N.-Y.; Dai, J.-F.; Wang, Y.; Rajput, S.A.; Qi, D.-S.; et al. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; González-Peñas, E. Co-occurrence of mycotoxins in feed for cattle, pigs, poultry, and sheep in Navarra, a region of Northern Spain. Toxins 2023, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Oswald, I.P.; et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, e04851. [Google Scholar] [PubMed]
- Stoev, S.D.; Gundasheva, D.; Zarkov, I.; Mircheva, T.; Zapryanova, D.; Denev, S.; Mitev, Y.; Daskalov, H.; Dutton, M.; Mwanza, M.; et al. Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and fumonisin B1. Exp. Toxicol. Pathol. 2012, 64, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, N.M. Balkan Endemic Nephropathy—Current Status and Future Perspectives. Clin. Kidney J. 2013, 6, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Battacone, G.; Nudda, A.; Pulina, G. Effects of ochratoxin A on livestock production. Toxins 2010, 2, 1796–1824. [Google Scholar] [CrossRef] [PubMed]
- Vörösházi, J.; Neogrády, Z.; Mátis, G.; Mackei, M. Pathological consequences, metabolism and toxic effects of trichothecene T-2 toxin in poultry. Poultry Sci. 2024, 103, 103471. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, G.S.; Pettersson, H. Toxicological evaluation of trichothecenes in animal feed. Anim. Feed Sci. Technol. 2004, 114, 205–239. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, Z.; Chen, L.; Nüssler, A.K.; Liu, L.; Yang, W. Deoxynivalenol, gut microbiota and immunotoxicity: A potential approach? Food Chem. Toxicol. 2018, 112, 342–354. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 574/2011 of 16 June 2011 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for nitrite, melamine, Ambrosia spp. and carry-over of certain coccidiostats and histomonostats and consolidating Annexes I and II thereto. Off. J. Eur. Union 2011, L159, 7–24. [Google Scholar]
- European Commission. Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, L91, 12–15. [Google Scholar]
- European Commission. Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sdogati, S.; Pacini, T.; Bibi, R.; Caporali, A.; Verdini, E.; Orsini, S.; Ortenzi, R.; Pecorelli, I. Co-occurrence of aflatoxin B1, zearalenone and ochratoxin A in feed and feed materials in central Italy from 2018 to 2022. Foods 2024, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ toxicological mechanisms involving humans, livestock and their associated health concerns: A review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.L.; Bren, U.; Stone, M.P.; Guengerich, F.P. Inherent stereospecificity in the reaction of aflatoxin B(1) 8,9-epoxide with deoxyguanosine and efficiency of DNA catalysis. Chem. Res. Toxicol. 2009, 22, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Li, W.; Lu, C.; et al. Contamination of aflatoxins induces severe hepatotoxicity through multiple mechanisms. Front. Pharmacol. 2020, 11, 605823. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Mortezazadeh, F.; Gholami-Borujeni, F. Review, meta-analysis and carcinogenic risk assessment of aflatoxin M1 in different types of milks in Iran. Rev. Environ. Health 2022, 38, 511–518. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Ali, S.; Freire, L.G.D.; Rezende, V.T.; Noman, M.; Ullah, S.; Abdullah; Badshah, G.; Afridi, M.S.; Tonin, F.G.; de Oliveira, C.A.F. Occurrence of mycotoxins in foods: Unraveling the knowledge gaps on their persistence in food production systems. Foods 2023, 12, 4314. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Fut. Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Khodaei, D.; Javanmardi, F.; Khaneghah, A.M. The global overview of the occurrence of mycotoxins in cereals: A three-year survey. Curr. Opin. Food Sci. 2021, 39, 36–42. [Google Scholar] [CrossRef]
- Milani, J.; Maleki, G. Effects of processing on mycotoxin stability in cereals. J. Sci. Food Agric. 2014, 94, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Ferrara, M.; Medina, A.; Pascale, M.; Magan, N. Toxigenic fungi and mycotoxins in a climate change scenario: Ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.; Siri-Anusornsak, W.; Petchkongkaew, A.; Elliott, C. A systematic review of global occurrence of emerging mycotoxins in crops and animal feeds, and their toxicity in livestock. Emerg. Contam. 2024, 10, 100305. [Google Scholar] [CrossRef]
- Okasha, H.; Song, B.; Song, Z. Hidden hazards revealed: Mycotoxins and their masked forms in poultry. Toxins 2024, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Falavigna, C.; Galaverna, G.; Dossena, A.; Marchelli, R. In vitro digestion assay for determination of hidden fumonisins in maize. J. Agric. Food Chem. 2010, 58, 12042–12047. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Russo, M.; La Rocca, C.; Pinto, O.; Mantovani, A. Modified mycotoxins, a still unresolved issue. Chemistry 2022, 4, 1498–1514. [Google Scholar] [CrossRef]
- Righetti, L.; Paglia, G.; Galaverna, G.; Dall’Asta, C. Recent advances and future challenges in modified mycotoxin analysis: Why HRMS has Become a key instrument in food contaminant research. Toxins 2016, 8, 361. [Google Scholar] [CrossRef]
- Tran, V.N.; Viktorová, J.; Ruml, T. Mycotoxins: Biotransformation and bioavailability assessment using Caco-2 cell monolayer. Toxins 2020, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; de Boevre, M.; Preußke, N.; de Saeger, S.; Birr, T.; Verreet, J.A.; Sönnichsen, F.D. Evaluation of high-resolution mass spectrometry for the quantitative analysis of mycotoxins in complex feed matrices. Toxins 2019, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Castaldo, L.; Narváez, A.; Gaspari, A.; Grosso, M.; Rodríguez-Carrasco, Y.; Ritieni, A. Target analysis and retrospective screening of contaminants in ready-to-eat cooked ham samples through UHPLC-Q-Orbitrap HRMS. Food Chem. 2023, 408, 135244. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Campillo, N.; López-García, I.; Hernández-Córdoba, M.; Vinas, P. High-resolution mass spectrometry for the determination of mycotoxins in biological samples. A review. Microchem. J. 2021, 166, 106197. [Google Scholar] [CrossRef]
- Izzo, L.; Narváez, A.; Castaldo, L.; Gaspari, A.; Rodríguez-Carrasco, Y.; Grosso, M.; Ritieni, A. Multiclass and multi-residue screening of mycotoxins, pharmacologically active substances, and pesticides in infant milk formulas through ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry analysis. J. Dairy Sci. 2022, 105, 2948–2962. [Google Scholar] [CrossRef] [PubMed]
- Panuwet, P.; Hunter, R.E.; D’Souza, P.E.; Chen, X.; Radford, S.A.; Cohen, J.R.; Marder, M.E.; Kartavenka, K.; Ryan, P.B.; Barr, D.B. Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring. Crit. Rev. Anal. Chem. 2016, 46, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Huang, X.; Liang, R.; Guo, T.; Xiao, Q.; Xia, B.; Wan, Y.; Zhou, Y. Determination of 63 mycotoxins in grain products by ultrahigh-performance liquid chromatography coupled with quadrupole-Orbitrap mass spectrometry. Food Cont. 2023, 150, 109772. [Google Scholar] [CrossRef]
- De Dominicis, E.; Commissati, I.; Gritti, E.; Catellani, D.; Suman, M. Quantitative targeted and retrospective data analysis of relevant pesticides, antibiotics and mycotoxins in bakery products by liquid chromatography-single-stage Orbitrap mass spectrometry. Food Addit. Contam Part A 2015, 32, 1617–1627. [Google Scholar] [CrossRef]
- Righetti, L.; Dreolin, N.; Celma, A.; McCullagh, M.; Barknowitz, G.; Sancho, J.V.; Dall’Asta, C. Travelling wave ion mobility-derived collision cross section for mycotoxins: Investigating interlaboratory and interplatform reproducibility. J. Agric. Food Chem. 2020, 68, 10937–10943. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef]
- Righetti, L.; Bergmann, A.; Galaverna, G.; Rolfsson, O.; Paglia, G.; Dall’Asta, C. Ion mobility-derived collision cross section database: Application to mycotoxin analysis. Anal. Chim. Acta 2018, 1014, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mesa, M.; Ropartz, D.; García-Campaña, A.M.; Rogniaux, H.; Dervilly-Pinel, G.; Le Bizec, B. Ion mobility spectrometry in food analysis: Principles, current applications and future trends. Molecules 2019, 24, 2706. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, M.; De Baere, S.; Letor, B.; Rychlik, M.; Croubels, S.; Devreese, M. Multi LC-MS/MS and LC-HRMS methods for determination of 24 mycotoxins including major phase I and II biomarker metabolites in biological matrices from pigs and broiler chickens. Toxins 2019, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Jedziniak, P. Development of a multi-mycotoxin LC-MS/MS method for the determination of biomarkers in pig urine. Mycotoxin Res. 2021, 37, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, J.; Dai, Z.; Lin, Y.; Lu, G.; Yang, S.; You, Y.; Liu, H.; Wu, Y.; Jiang, G.; et al. Data-dependent acquisition based high-resolution mass spectrum for trace Alternaria mycotoxin analysis and sulfated metabolites identification. Food Chem. 2021, 364, 130450. [Google Scholar] [CrossRef] [PubMed]
- González-Jartín, J.M.; Rodríguez-Canás, I.; Alfonso, A.; Sainz, M.J.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Multianalyte method for the determination of regulated, emerging and modified mycotoxins in milk: QuEChERS extraction followed by UHPLC–MS/MS analysis. Food Chem. 2021, 356, 129647. [Google Scholar] [CrossRef]
- Izzo, L.; Rodríguez-Carrasco, Y.; Tolosa, J.; Graziani, G.; Gaspari, A.; Ritieni, A. Target analysis and retrospective screening of mycotoxins and pharmacologically active substances in milk using an ultra-high-performance liquid chromatography/high-resolution mass spectrometry approach. J. Dairy Sci. 2020, 103, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Ghilardelli, F.; Masoero, F.; Gallo, A. Screening of regulated and emerging mycotoxins in bulk milk samples by high-resolution mass spectrometry. Foods 2021, 10, 2025. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, M.O.; Braun, D.; Windisch, P.; Warth, B.; Ezekiel, C.N. Assessment of multiple mycotoxins in raw milk of three different animal species in Nigeria. Food Cont. 2022, 131, 108258. [Google Scholar] [CrossRef]
- Le, L.H.T.; Tran-Lam, T.-T.; Nguyen, H.Q.; Quan, T.C.; Nguyen, T.Q.; Nguyen, D.T.; Dao, Y.H. A study on multi-mycotoxin contamination of commercial cashew nuts in Vietnam. J. Food Compos. Anal. 2021, 102, 104066. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Li, X.; Zhou, S.; Ma, J.; Zhao, X.; Zhang, Q.; Yin, X. Determination of multi-mycotoxins in vegetable oil via liquid chromatography-high resolution mass spectrometry assisted by a complementary liquid–liquid extraction. Food Chem. X 2023, 20, 100887. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Suman, M.; Krska, R. Quantification of 730 Mycotoxins and Other Secondary Metabolites of Fungi and Plants in Grain Products. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, T.; Chen, D.; Liang, W.; Lu, X.; Zhao, C.; Xu, G. Suspect and nontarget screening of mycotoxins and their modified forms in wheat products based on ultrahigh-performance liquid chromatography-high resolution mass spectrometry. J. Chromatogr. A 2023, 1708, 464370. [Google Scholar] [CrossRef] [PubMed]
- González-Jartín, J.M.; Ferreiroa, V.; Rodríguez-Canas, I.; Alfonso, A.; Sainz, M.J.; Aguín, O.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Occurrence of mycotoxins and mycotoxigenic fungi in silage from the north of Portugal at feed-out. Int. J. Food Microbiol. 2022, 365, 109556. [Google Scholar] [CrossRef] [PubMed]
- Nualkaw, K.; Poapolathep, S.; Zhang, Z.; Zhang, Q.; Giorgi, M.; Li, P.; Logrieco, A.F.; Poapolathep, A. Simultaneous determination of multiple mycotoxins in swine, poultry and dairy feeds using ultra high performance liquid chromatography-tandem mass spectrometry. Toxins 2020, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Graziani, G.; Gaspari, A.; Izzo, L.; Tolosa, J.; Rodríguez-Carrasco, Y.; Ritieni, A. Target analysis and retrospective screening of multiple mycotoxins in pet food using UHPLC-Q-Orbitrap HRMS. Toxins 2019, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Lanaridi, O.; Warth, B.; Ansari, K.M. Metabolomics as an emerging approach for deciphering the biological impact and toxicity of food contaminants: The case of mycotoxins. Crit. Rev. Food Sci. Nutr. 2023. [CrossRef] [PubMed]
- Owolabi, I.O.; Siwarak, K.; Greer, B.; Rajkovic, A.; Dall’asta, C.; Karoonuthaisiri, N.; Uawisetwathana, U.; Elliott, C.T.; Petchkongkaew, A. Applications of mycotoxin biomarkers in human biomonitoring for exposome-health studies: Past, present, and future. Expo. Health 2023. [Google Scholar] [CrossRef]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef]
- García-Pérez, P.; Becchi, P.P.; Zhang, L.; Rocchetti, G.; Lucini, L. Metabolomics and chemometrics: The next-generation analytical toolkit for the evaluation of food quality and authenticity. Trends Food Sci. Technol. 2024, 147, 104481. [Google Scholar] [CrossRef]
- Arce-López, B.; Lizarraga, E.; Vettorazzi, A.; González-Peñas, E. Human biomonitoring of mycotoxins in blood, plasma and serum in recent years: A review. Toxins 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Richard-Forget, F.; Atanasova, V.; Chéreau, S. Using metabolomics to guide strategies to tackle the issue of the contamination of food and feed with mycotoxins: A review of the literature with specific focus on Fusarium mycotoxins. Food Cont. 2021, 121, 107610. [Google Scholar] [CrossRef]
- Rocchetti, G.; Ghilardelli, F.; Bonini, P.; Lucini, L.; Masoero, F.; Gallo, A. Changes of milk metabolomic profiles resulting from a mycotoxins-contaminated corn silage intake by dairy cows. Metabolites 2021, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Flasch, M.; Bueschl, C.; Del Favero, G.; Adam, G.; Schuhmacher, R.; Marko, D.; Warth, B. Elucidation of xenoestrogen metabolism by non-targeted, stable isotope-assisted mass spectrometry in breast cancer cells. Environ. Int. 2022, 158, 106940. [Google Scholar] [CrossRef] [PubMed]
- Flasch, M.; Bueschl, C.; Woelflingseder, L.; Schwartz-Zimmermann, H.E.; Adam, G.; Schuhmacher, R.; Marko, D.; Warth, B. Stable isotope-assisted metabolomics for deciphering xenobiotic metabolism in mammalian cell culture. ACS Chem. Biol. 2020, 15, 970–981. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Zheng, N.; Guo, L.; Song, X.; Zhao, S.; Wang, J. Biological system responses of dairy cows to aflatoxin B1 exposure revealed with metabolomic changes in multiple biofluids. Toxins 2019, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.; Jiang, Y.; Adeyemi, J.; Oliveira, A.; Vyas, D.; Adesogan, A. Biomarker of aflatoxin ingestion: 1H NMR-based plasma metabolomics of dairy cows fed aflatoxin B1 with or without sequestering agents. Toxins 2018, 10, 545. [Google Scholar] [CrossRef]
- Gerdemann, A.; Behrens, M.; Esselen, M.; Humpf, H.U. Metabolic profiling as a powerful tool for the analysis of cellular alterations caused by 20 mycotoxins in HepG2 cells. Arch. Toxicol. 2022, 96, 2983–2998. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).