Phytosterols: Physiological Functions and Potential Application
Abstract
:1. Introduction
2. Extraction of Phytosterols
2.1. Sources of Phytosterols and Extraction Techniques
2.2. Analytical Identification of Phytosterols
3. Physiological Function of Phytosterols
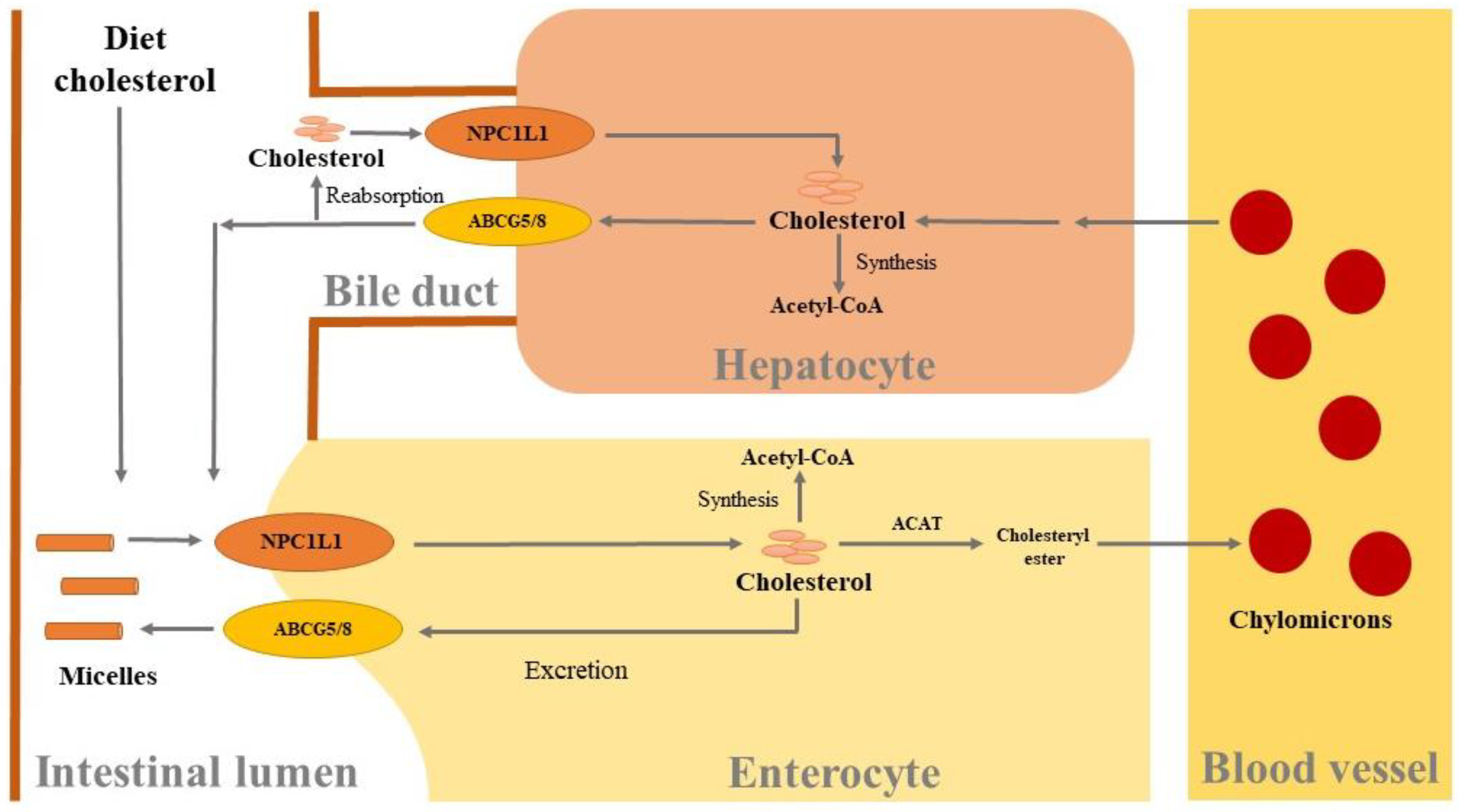
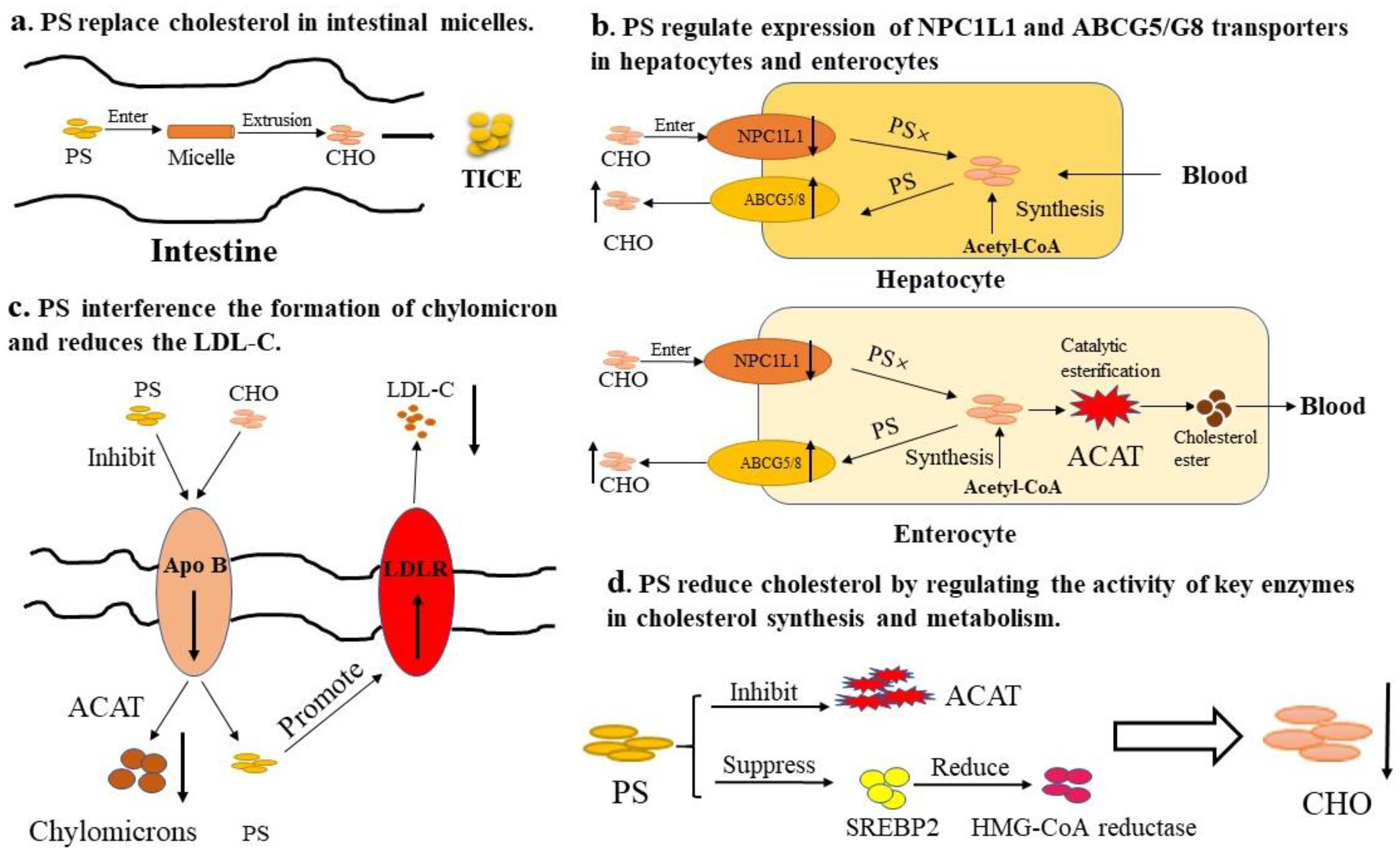
3.1. Cholesterol-Lowering Effects
3.2. Anticancer Effects
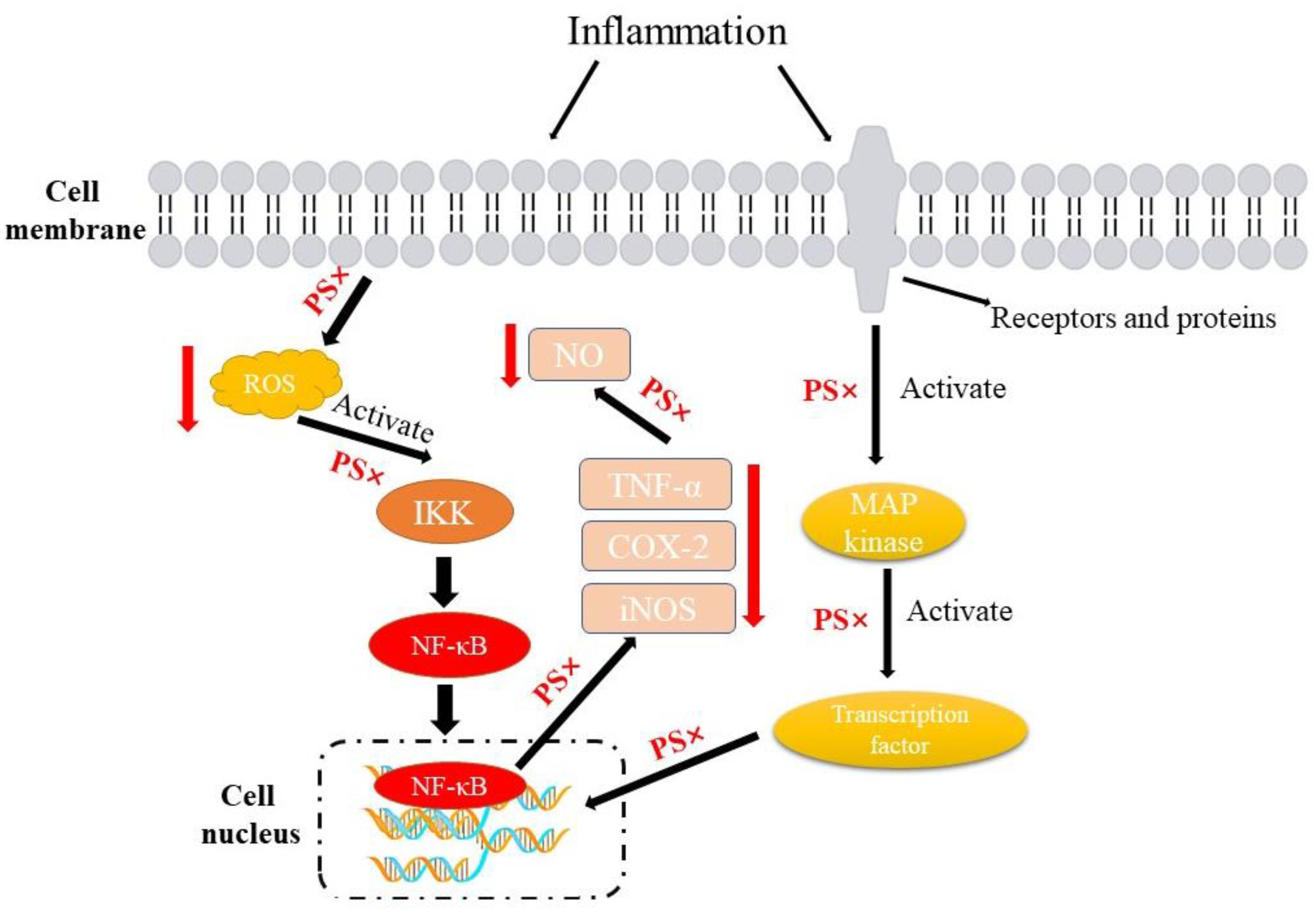
3.3. Anti-Inflammatory Effects
3.4. Antioxidation Activities
3.5. Immunomodulatory Effects
3.6. Other Physiological Functions
4. Application of Phytosterols
4.1. Application in Functional Foods
4.2. Problems and Solutions in the Application of Phytosterols
4.2.1. Phytosterol Oxidation Products (POPs)
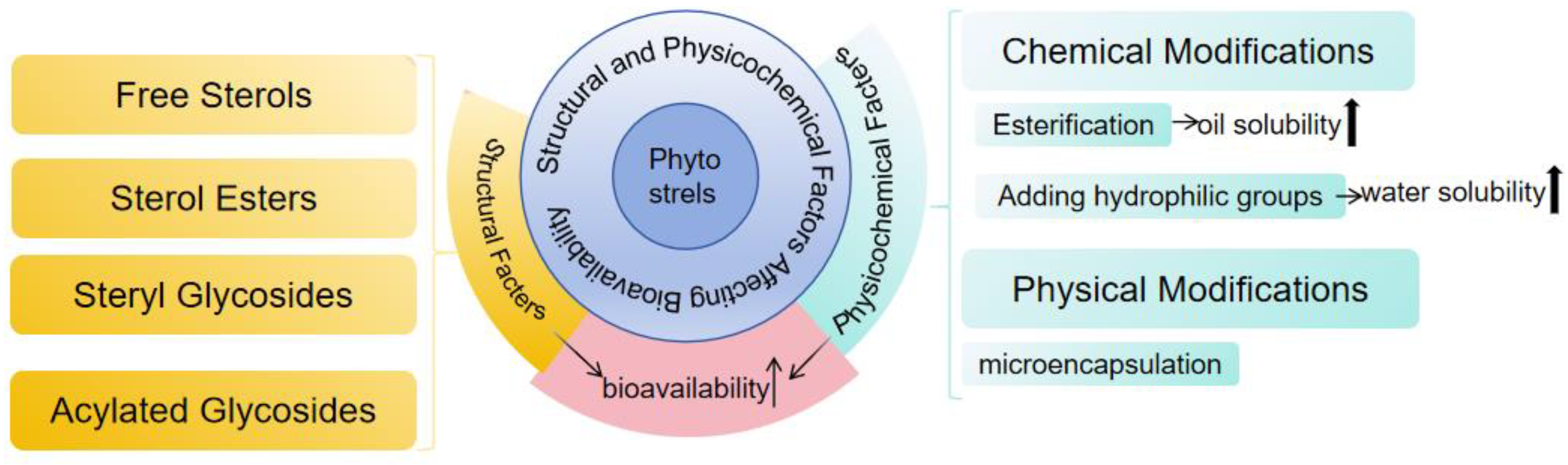
4.2.2. Bioaccessibility and Bioavailability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nattagh, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef] [PubMed]
- MacKay, D.S.; Jones, P.J.H. Phytosterols in human nutrition: Type, formulation, delivery, and physiological function. Eur. J. Lipid Sci. Tech. 2011, 113, 1427–1432. [Google Scholar] [CrossRef]
- Moreau, R.; Nyström, L.; Whitaker, B.; Winkler-Moser, J.; Baer, D.; Gebauer, S.; Hicks, K. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef] [PubMed]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015, 7, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Hu, Y.; Huang, W.; Wang, M.; Jiang, Y.; Lou, T. Effect of transition metal ions on the B ring oxidation of sterols and their kinetics in oil-in-water emulsions. Sci. Rep. 2016, 6, 27240. [Google Scholar] [CrossRef] [PubMed]
- Ramprasath, V.; Awad, A. Role of phytosterols in cancer prevention and treatment. J. Aoac Int. 2015, 98, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, N.; Khan, W.; Shadab, M. Phytosterols as a natural anticancer agent: Current status and future perspective. Biomed. Pharmacother. 2017, 88, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, M.; Pallarés, N.; Ferrer, E.; Berrada, H.; Barba, F.J. Sterols and fat-soluble vitamins. In Food Lipids; Lorenzo, J.M., Munekata, P.E.S., Pateiro, M., Barba, F.J., Domínguez, R., Eds.; Academic Press: New York, NY, USA, 2022; pp. 323–348. [Google Scholar]
- Feng, S.; Wang, L.; Shao, P.; Sun, P.; Yang, C.S. A review on chemical and physical modifications of phytosterols and their influence on bioavailability and safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 5638–5657. [Google Scholar] [CrossRef] [PubMed]
- Honcharov, D.; Tkachenko, N.; Nikolaieva, V. Transeterification of a Mixture of Vegetable Fats with the Addition of Phytosterols. Eur. J. Agric. Food Sci. 2021, 3, 45–48. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, cholesterol control, and cardiovascular disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef]
- Maniet, G.; Jacquet, N.; Richel, A. Recovery of sterols from vegetable oil distillate by enzymatic and non-enzymatic processes. Comptes Rendus Chim. 2019, 22, 347–353. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Khamitova, G.; Angeloni, S.; Sempere, A.N.; Tao, J.; Maggi, F.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G. Spent coffee grounds: A potential commercial source of phytosterols. Food Chem. 2020, 325, 126836. [Google Scholar] [CrossRef] [PubMed]
- Ferdosh, M.; Akanda, S.; Ghafoor, M.; Ali, K.; Sarker, M. Techniques for the extraction of phytosterols and their benefits in human health: A review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar]
- Younas, R.; Sahar, A.; Sameen, A. A narrative review on extraction techniques of phytosterols and their applications in food industry. Biomass Convers. Bior. 2023, 1–15. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ghafoor, K.; Al Juhaimi, F.; Ahmed, I.A.M.; Babiker, E.E. Efect of cold-press and soxhlet extraction on fatty acids, tocopherols and sterol contents of the Moringa seed oils. S. Afr. J. Bot. 2019, 124, 333–337. [Google Scholar] [CrossRef]
- Fithriani, D.N. Optimization of the condition of phytosterol extraction conditions from microalgae nannochloropsis using ethanol of different purity levels. J. Bio-Sci. 2019, 27, 143–148. [Google Scholar] [CrossRef]
- Norhazlindah, M.F.; Jahurul, M.H.A.; Norliza, M.; Shihabul, A.; Islam, S.; Nyam, K.L.; Zaidul, I.S.M. Techniques for extraction, characterization, and application of oil from sacha inchi (Plukenetia volubilis L) seed: A review. J. Food Meas. Charact. 2023, 17, 904–915. [Google Scholar] [CrossRef]
- Eller, F.J.; Moser, J.K.; Kenar, J.A.; Taylor, S.L. Extraction and analysis of tomato seed oil. J. Am. Oil Chem. Soc. 2010, 87, 755–762. [Google Scholar] [CrossRef]
- Bai, G.; Ma, C.; Chen, X. Phytosterols in edible oil: Distribution, analysis and variation during processing. Grain Oil Sci. Technol. 2021, 4, 33–44. [Google Scholar] [CrossRef]
- Schröder, M.; Vetter, W. High-speed counter-current chromatographic separation of phytosterols. Anal. Bioanal. Chem. 2011, 400, 3615–3623. [Google Scholar] [CrossRef]
- Azeez, R.; Abaas, I.; Kadhim, E. Isolation and characterization of β-sitosterol from elaeagnus angustifolia cultivated in iraq. Asian J. Pharm. Clin. Res. 2018, 11, 442–446. [Google Scholar] [CrossRef]
- Lucia, L.B.; Cenarro, A.; Cenarro, M.; Orea, I.; Barcelo-Batllori, S.; Pocovi, M.; Ros, E.; Civeira, F.; Nerin, C.; Domeno, C.; et al. Simultaneous determination of oxysterols, phytosterols and cholesterol precursors by high performance liquid chromatography tandem mass spectrometry in human serum. Anal. Methods 2013, 5, 2249–2257. [Google Scholar]
- Meiko, I.; Mami, I.; Toshiyuki, S.; Hideo, H.; Ryusuke, T. High-performance liquid chromatography with fluorescence detection for simultaneous analysis of phytosterols (stigmasterol, β-sitosterol, campesterol, ergosterol, and fucosterol) and cholesterol in plant foods. Food Anal. Method 2017, 10, 2692–2699. [Google Scholar]
- Gachumi, G.; El-Aneed, A. Mass Spectrometric Approaches for the Analysis of Phytosterols in Biological Samples. J. Agric. Food Chem. 2017, 65, 10141–10156. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.; Pasenkiewicz-Gierula, M.; Widomska, J.; Mainali, L.; Raguz, M. High cholesterol/low cholesterol: Effects in biological membranes: A review. Cell Biochem. Biophys. 2017, 75, 369–385. [Google Scholar] [CrossRef]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Deng, C.; Pan, J.; Zhu, H.; Chen, Z.Y. Effect of Gut Microbiota on Blood Cholesterol: A Review on Mechanisms. Foods 2023, 12, 4308. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Ma, K.Y.; Liang, Y.; Peng, C.; Zuo, Y. Role and Classification of Cholesterol-Lowering Functional Foods. J. Funct. Food. 2011, 3, 61–69. [Google Scholar] [CrossRef]
- Alphonse, P.A.S.; Jones, P.J.H. Revisiting Human Cholesterol Synthesis and Absorption: The Reciprocity Paradigm and Its Key Regulators. Lipids 2016, 51, 519–536. [Google Scholar] [CrossRef]
- Jing, J.; Wei, T.; Su, W.; Liu, Y.; Yao, W.; Zhu, H.; Fu, T. Structural related effects of natural steroid molecules on cholesterol crystallization in model bile and ethanol. ChemistrySelect 2018, 3, 3712–3717. [Google Scholar] [CrossRef]
- Afonso, M.; Machado, R.; Lavrador, M.; Quintao, E.; Moore, K.; Lottenberg, A. Molecular pathways underlying cholesterol homeostasis. Nutrients 2018, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Radhakrishnan, A.; Goldstein, J. Retrospective on cholesterol homeostasis: The central role of scap. Annu. Rev. Biochem. 2018, 87, 783–807. [Google Scholar] [CrossRef] [PubMed]
- Amir Shaghaghi, M.; Abumweis, S.; Jones, P. Cholesterol-lowering efficacy of plant sterols/stanols provided in capsule and tablet formats: Results of a systematic review and meta-analysis. J. Acad. Nutr. Diet. 2013, 113, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Knudsen, T.; Nielsen, A.; Duelund, L.; Christensen, M.; Hervella, P.; Needham, D.; Mouritsen, O. Inhibition of cholesterol transport in an intestine cell model by pine-derived phytosterols. Chem. Phys. Lipids 2016, 200, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, L.; Wang, X.; Ma, K.; Li, Y.; Wang, L.; Man, S.; Huang, Y.; Chen, Z. Plasma cholesterol-raising potency of dietary free cholesterol versus cholesteryl ester and effect of β-sitosterol. Food Chem. 2015, 169, 277–282. [Google Scholar] [CrossRef]
- Plat, J.; Nichols, J.; Mensink, R. Plant sterols and stanols: Effects on mixed micellar composition and LXR (target gene) activation. J. Lipid Res. 2005, 46, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gu, H.; Zhang, D. ATP-binding cassette transporters and cholesterol translocation. IUBMB Life 2013, 65, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Brufau, G.; Kuipers, F.; Lin, Y.; Trautwein, E.; Groen, A. A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS ONE 2011, 6, e21576. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Z.S.; Cheng, J.; Diao, C.; Yan, Y.; Liu, D.; Wang, H.; Zheng, F. Dietary supplementation of soybean-derived sterols regulates cholesterol metabolism and intestinal microbiota in hamsters. J. Funct. Foods 2019, 59, 242–250. [Google Scholar] [CrossRef]
- Bao, X.; Yuan, X.; Li, X.; Liu, X. Flaxseed-derived peptide, IPPF, inhibits intestinal cholesterol absorption in Caco-2 cells and hepatic cholesterol synthesis in HepG2 cells. J. Food Biochem. 2022, 46, e14031. [Google Scholar] [CrossRef]
- Chen, J.; Jiao, R.; Jiang, Y.; Bi, Y.; Chen, Z. Algal sterols are as effective as β-sitosterol in reducing plasma cholesterol concentration. J. Agric. Food Chem. 2014, 62, 675–681. [Google Scholar] [CrossRef]
- Harding, S.; Rideout, T.; Jones, P. Hepatic nuclear sterol regulatory binding element protein 2 abundance is decreased and that of ABCG5 increased in male hamsters fed plant sterols. J. Nutr. 2010, 140, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Batta, A.; Xu, G.; Honda, A.; Miyazaki, T.; Salen, G. Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat. Metabolism 2006, 55, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Jesch, E.; Seo, J.; Carr, T.; Lee, J. Sitosterol reduces messenger RNA and protein expression levels of Niemann-Pick C1-like 1 in FHs 74 Int cells. Nutr. Res. 2009, 29, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Carr, T.P. Unsaturated fatty acids and phytosterols regulate cholesterol transporter genes in Caco-2 and HepG2 cell lines. Nutr. Res. 2013, 33, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Pal, S. Margarine phytosterols decrease the secretion of atherogenic lipoproteins from HepG2 liver and Caco2 intestinal cells. Atherosclerosis 2005, 182, 29–36. [Google Scholar] [CrossRef] [PubMed]
- El Kharrassi, Y.; Samadi, M.; Lopez, T.; Nury, T.; El Kebbaj, R.; Andreoletti, P.; El Hajj, H.I.; Vamecq, J.; Moustaid, K.; Latruffe, N.; et al. Biological activities of Schottenol and Spinasterol, two natural phytosterols present in argan oil and in cactus pear seed oil, on murine miroglial BV2 cells. Biochem. Biophys. Res. Commun. 2014, 446, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24 (S)-Saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective LXRβ agonist. J. Agric. Food Chem. 2014, 62, 6130–6137. [Google Scholar] [CrossRef] [PubMed]
- Calpe-Berdiel, L.; Escolà-Gil, J.C.; Benítez, S.; Bancells, C.; González-Sastre, F.; Palomer, X.; Blanco-Vaca, F. Dietary phytosterols modulate T-helper immune response but do not induce apparent anti-inflammatory effects in a mouse model of acute, aseptic inflammation. Life Sci. 2007, 80, 1951–1956. [Google Scholar] [CrossRef]
- Liang, Y.; Wong, W.; Guan, L.; Tian, X.; Ma, K.; Huang, Y.; Chen, Z. Effect of phytosterols and their oxidation products on lipoprotein profiles and vascular function in hamster fed a high cholesterol diet. Atherosclerosis 2011, 219, 124–133. [Google Scholar] [CrossRef]
- De Smet, E.; Mensink, R.; Konings, M.; Brufau, G.; Groen, A.; Havinga, R.; Schonewille, M.; Kerksiek, A.; Lütjohann, D.; Plat, J. Acute intake of plant stanol esters induces changes in lipid and lipoprotein metabolism-related gene expression in the liver and intestines of mice. Lipids 2015, 50, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gruber, H.; Pakenham, C.; Ratnayake, W.; Scoggan, K. Dietary phytosterols and phytostanols alter the expression of sterol-regulatory genes in SHRSP and WKY inbred rats. Ann. Nutr. Metab. 2009, 55, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, F.; Jia, S.; Xie, J.; Shen, M. Differences between phytosterols with different structures in regulating cholesterol synthesis, transport and metabolism in Caco-2 cells. J. Funct. Foods 2020, 65, 103715. [Google Scholar] [CrossRef]
- Xiao, J.; Mao, F.; Yang, F.; Zhao, Y.; Zhang, C.; Yamamoto, K. Interaction of dietary polyphenols with bovine milk proteins: Molecular structure–affinity relationship and influencing bioactivity aspects. Mol. Nutr. Food Res. 2011, 55, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Aherne, S.; O’Brien, N. Modulation of cytokine production by plant sterols in stimulated human Jurkat T cells. Mol. Nutr. Food Res. 2008, 52, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Yu, X.; Xu, J.; Kou, X.; Zheng, M.; Huang, F.; Huang, Q.; Wang, L. Single frequency intake of α-linolenic acid rich phytosterol esters attenuates atherosclerosis risk factors in hamsters fed a high fat diet. Lipids Health Dis. 2016, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhu, H.; Chen, Z. Plant sterols: Chemical and enzymatic structural modifications and effects on their cholesterol-lowering activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef] [PubMed]
- Marisa, D.; Hayatie, L.; Juliati, S.; Suhartono, E.; Komari, N. Molecular docking of phytosterol compounds from kelakai (Stenochlaena palustris) as anti-breast cancer. Acta Biochim. Indones. 2021, 4, 59. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Wang, M.; Lin, Y.; Zhou, S. Stigmasterol simultaneously induces apoptosis and protective autophagy by inhibiting Akt/mTOR pathway in gastric cancer cells. Front. Oncol. 2021, 11, 629008. [Google Scholar] [CrossRef]
- Gajendran, B.; Durai, P.; Madhu Varier, K.; Chinnasamy, A. A novel phytosterol isolated from Datura inoxia, RinoxiaB is a potential cure colon cancer agent by targeting BAX/Bcl2 pathway. Bioorg Med. Chem. 2020, 28, 115242. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, W.; Sun, L.; Xiao, S.M.; Lin, S.; Zhao, J.; Xiao, H.; Xing, X.; Lao, X.Q.; Chen, Y.M.; et al. Independent and opposing associations of dietary phytosterols intake and PLCE1 rs2274223 polymorphisms on esophageal squamous cell carcinoma risk. Eur. J. Nutr. 2021, 60, 4357–4366. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Song, G.; Lim, W. Stigmasterol causes ovarian cancer cell apoptosis by inducing endoplasmic reticulum and mitochondrial dysfunction. Pharmaceutics 2020, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhao, X.; Xu, J.; Li, C.; Yu, Y.; Wang, W.; Zhu, L. The protective effect of dietary phytosterols on cancer risk: A systematic meta-analysis. J. Oncol. 2019, 2019, 7479518. [Google Scholar] [CrossRef] [PubMed]
- Cioccoloni, G.; Soteriou, C.; Websdale, A.; Wallis, L.; Zulyniak, M.A.; Thorne, J.L. Phytosterols and phytostanols and the hallmarks of cancer in model organisms: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 1145–1165. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Zhang, Z.; Liu, J.; Hong, L. β-sitosterol as a promising anticancer agent for chemoprevention and chemotherapy: Mechanisms of action and future prospects. Adv. Nutr. 2023, 14, 1085–1110. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, E.; Lee, H.; Kim, K.; Ahn, K.; Shim, B.; Kim, N.; Song, M.; Baek, N.; Kim, S. Identification of campesterol from Chrysanthemum coronarium L. and its antiangiogenic activities. Phytother. Res. 2007, 21, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Sadek, N.; Yuliana, N.; Prangdimurti, E.; Priosoeryanto, B.; Budijanto, S. Plant sterol esters in extruded food model inhibits colon carcinogenesis by suppressing inflammation and stimulating apoptosis. J. Med. Food. 2017, 20, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cheng, Y.; Nilsson, A.; Duan, R. Identification of one exon deletion of intestinal alkaline sphingomyelinase in colon cancer HT-29 cells and a differentiation-related expression of the wild-type enzyme in Caco-2 cells. Carcinogenesis 2004, 25, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- López-García, G.; Cilla, A.; Barberá, R.; Alegría, A. Antiproliferative effect of plant sterols at colonic concentrations on Caco-2 cells. J. Funct. Foods 2017, 39, 84–90. [Google Scholar] [CrossRef]
- Llaverias, G.; Escolà-Gil, J.; Lerma, E.; Julve, J.; Pons, C.; Cabré, A.; Cofán, M.; Ros, E.; Sánchez-Quesada, J.; Blanco-Vaca, F. Phytosterols inhibit the tumor growth and lipoprotein oxidizability induced by a high-fat diet in mice with inherited breast cancer. J. Nutr. Biochem. 2013, 24, 39–48. [Google Scholar] [CrossRef]
- Ifere, G.O.; Barr, E.; Equan, A.; Gordon, K.; Singh, U.; Chaudhary, J.; Igietseme, J.; Ananaba, G. Differential effects of cholesterol and phytosterols on cell proliferation, apoptosis and expression of a prostate specific gene in prostate cancer cell lines. Cancer Detect. Prev. 2009, 32, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Attanzio, A.; Barberá, R.; Tesoriere, L.; Livrea, M. Anti-proliferative effect of main dietary phytosterols and β-cryptoxanthin alone or combined in human colon cancer Caco-2 cells through cytosolic Ca2+-and oxidative stress-induced apoptosis. J. Funct. Foods 2015, 12, 282–293. [Google Scholar] [CrossRef]
- Alvarez-Sala, A.; Attanzio, A.; Tesoriere, L.; Garcia-Llatas, G.; Barberá, R.; Cilla, A. Apoptotic effect of a phytosterol-ingredient and its main phytosterol (β-sitosterol) in human cancer cell lines. Int. J. Food Sci. Nutr. 2019, 70, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kong, K.; Kim, Y.; Jung, K.; Kil, J.; Rhee, S.; Park, K. Induction of Bax and activation of caspases during β-sitosterol-mediated apoptosis in human colon cancer cells. Int. J. Oncol. 2003, 23, 1657–1662. [Google Scholar] [CrossRef]
- O’Callaghan, Y.; Kenny, O.; O’Connell, N.M.; Maguire, A.R.; McCarthy, F.O.; O’Brien, N.M. Synthesis and assessment of the relative toxicity of the oxidised derivatives of campesterol and dihydrobrassicasterol in U937 and HepG2 cells. Biochimie 2013, 95, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, F.; Li, L.; Rao, Y.; Ju, T.; Wong, W.; Hsieh, C.; Pivkin, M.; Hua, K.; Wu, S. Ergosterol peroxide from marine fungus Phoma sp. induces ROS-dependent apoptosis and autophagy in human lung adenocarcinoma cells. Sci. Rep. 2018, 8, 17956. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Boffetta, P.; Ronco, A.; Brennan, P.; Deneo-Pellegrini, H.; Carzoglio, J.; Mendilaharsu, M. Plant sterols and risk of stomach cancer: A case-control study in Uruguay. Nutr. Cancer 2000, 37, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’Acierno, L.; Giordano, R.; et al. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Vilahur, G.; Ben-Aicha, S.; Diaz-Riera, E.; Badimon, L.; Padró, T. Phytosterols and inflammation. Curr. Med. Chem. 2019, 26, 6724–6734. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- DiDonato, J.; Hayakawa, M.; Rothwarf, D.; Zandi, E.; Karin, M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 1997, 388, 548–554. [Google Scholar] [CrossRef]
- Martínez-Soto, D.; Ruiz-Herrera, J. Functional analysis of the MAPK pathways in fungi. Rev. Iberoam. Micol. 2017, 34, 192–202. [Google Scholar] [CrossRef]
- Peng, L.; Guo, F.; Pei, M.; Tsao, R.; Wang, X.; Jiang, L.; Sun, Y.; Xiong, H. Anti-inflammatory effect of lentil hull (Lens culinaris) extract via MAPK/NF-κB signaling pathways and effects of digestive products on intestinal barrier and inflammation in Caco-2 and Raw264. 7 co-culture. J. Funct. Foods 2022, 92, 105044. [Google Scholar] [CrossRef]
- Hunthayung, K.; Klinkesorn, U.; Hongsprabhas, P.; Chanput, W. Controlled release and macrophage polarizing activity of cold-pressed rice bran oil in a niosome system. Food Funct. 2019, 10, 3272–3281. [Google Scholar] [CrossRef]
- Jie, F.; Yang, X.; Yang, B.; Liu, Y.; Wu, L.; Lu, B. Stigmasterol attenuates inflammatory response of microglia via NF-κB and NLRP3 signaling by AMPK activation. Biomed. Pharmacother. 2022, 153, 113317. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.; Bui, H.; Tran, T.; Hoang, T.; Vu, T.; Do, D.; Kim, Y.; Song, S.; Nguyen, M. Dammarane triterpenes and phytosterols from Dysoxylum tpongense Pierre and their anti-inflammatory activity against liver X receptors and NF-κB activation. Steroids 2021, 175, 108902. [Google Scholar] [CrossRef]
- He, D.; Wang, S.; Fang, G.; Zhu, Q.; Wu, J.; Li, J.; Shi, D.; Lian, X. LXRs/ABCA1 activation contribute to the anti-inflammatory role of phytosterols on LPS-induced acute lung injury. J. Funct. Foods 2022, 89, 104966. [Google Scholar] [CrossRef]
- Rocha, V.; Ras, R.; Gagliardi, A.; Mangili, L.; Trautwein, E.; Santos, R. Effects of phytosterols on markers of inflammation: A systematic review and meta-analysis. Atherosclerosis 2016, 248, 76–83. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, F.; Shen, M.; Jia, S.; Xie, J. Phytosterols suppress phagocytosis and inhibit inflammatory mediators via ERK pathway on LPS-triggered inflammatory responses in RAW264. 7 macrophages and the correlation with their structure. Foods 2019, 8, 582. [Google Scholar] [CrossRef]
- Ortiz-Escarza, J.; Medina, M.; Trigos, A. On the peroxyl radical scavenging ability of β-sitosterol in lipid media: A theoretical study. J. Phys. Org. Chem. 2021, 34, e4123. [Google Scholar] [CrossRef]
- Hannan, M.; Sohag, A.; Dash, R.; Haque, M.; Mohibbullah, M.; Oktaviani, D.; Hossain, M.; Choi, H.; Moon, I. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, H.; Li, C. Antioxidant activities of novel galloyl phytosterols evaluated by human erythrocytes with the aid of confocal microscopy imaging. J. Funct. Foods 2016, 22, 224–231. [Google Scholar] [CrossRef]
- Lin, M.; Beal, M. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Chiang, S.; Kalinowski, D.; Bae, D.; Sahni, S.; Richardson, D. The role of the antioxidant response in mitochondrial dysfunction in degenerative diseases: Cross-talk between antioxidant defense, autophagy, and apoptosis. Oxid. Med. Cell Longev. 2019, 2019, 6392763. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Wu, Z.; Zhang, P.; Liu, R.; Chang, M.; Wang, X. The dopaminergic neuroprotective effects of different phytosterols identified in rice bran and rice bran oil. Food Funct. 2021, 12, 10538–10549. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Chen, N.; Leong, P.; Ko, K. β-Sitosterol Enhances Cellular Glutathione Redox Cycling by Reactive Oxygen Species Generated From Mitochondrial Respiration: Protection Against Oxidant Injury in H9c2 Cells and Rat Hearts. Phytother. Res. 2014, 28, 999–1006. [Google Scholar] [CrossRef]
- Brüll, F.; Mensink, R. Plant sterols: Functional lipids in immune function and inflammation? Clin. Lipidol. 2009, 4, 355–365. [Google Scholar] [CrossRef]
- Fraile, L.; Crisci, E.; Córdoba, L.; Navarro, M.A.; Osada, J.; Montoya, M. Immunomodulatory properties of beta-sitosterol in pig immune responses. Int. Immunopharmacol. 2012, 13, 316–321. [Google Scholar] [CrossRef]
- De Smet, E.; Mensink, R.; Boekschoten, M.; de Ridder, R.; Germeraad, W.; Wolfs, T.; Plat, J. An acute intake of plant stanol esters alters immune-related pathways in the jejunum of healthy volunteers. Br. J. Nutr. 2015, 113, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Kidani, Y.; Elsaesser, H.; Hock, M.; Vergnes, L.; Williams, K.; Argus, J.; Marbois, B.; Komisopoulou, E.; Wilson, E.; Osborne, T.; et al. Sterol regulatory element–binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 2013, 14, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Boukes, G.; Venter, M. In vitro modulation of the innate immune response and phagocytosis by three Hypoxis spp. and their phytosterols. S. Afr. J. Bot. 2016, 102, 120–126. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Angulo, C.; Cosío-Aviles, L.; López, M.; Calvo-Gómez, O. Cylindropuntia cholla aqueous root rich in phytosterols enhanced immune response and antimicrobial activity in tilapia Oreochromis niloticus leukocytes. Fish. Shellfish Immunol. 2022, 131, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, A.; Kim, S.; Jung, Y.; Lee, D.; Cho, E.; Lee, S. Antibacterial phytosterols and alkaloids from Lycoris radiata. Nat. Prod. Sci. 2014, 20, 107–112. [Google Scholar]
- Burčová, Z.; Kreps, F.; Greifová, M.; Jablonský, M.; Ház, A.; Schmidt, Š.; Šurina, I. Antibacterial and antifungal activity of phytosterols and methyl dehydroabietate of Norway spruce bark extracts. J. Biotechnol. 2018, 282, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kavita, K.; Singh, V.; Jha, B. 24-Branched Δ5 sterols from Laurencia papillosa red seaweed with antibacterial activity against human pathogenic bacteria. Microbiol. Res. 2014, 169, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Gao, J.; Wu, Z.; Sun, Z.; Hao, L.; Liu, S.; Tan, Z.; Cheng, Y.; Zhu, W. Multiomic Analyses Reveal the Effects of Supplementing Phytosterols on the Metabolic Function of the Rumen Microbiota in Perinatal Cows. Appl. Environ. Microbiol. 2022, 88, e00992-22. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Jiang, P.; Jiang, L.; Li, X.; Ye, X. Three phytosterols from sweet potato inhibit MCF7-xenograft-tumor growth through modulating gut microbiota homeostasis and SCFAs secretion. Food Res. Int. 2021, 141, 110147. [Google Scholar] [CrossRef]
- Misawa, E.; Tanaka, M.; Nomaguchi, K.; Nabeshima, K.; Yamada, M.; Toida, T.; Iwatsuki, K. Oral ingestion of Aloe vera phytosterols alters hepatic gene expression profiles and ameliorates obesity-associated metabolic disorders in Zucker diabetic fatty rats. J. Agric. Food Chem. 2012, 60, 2799–2806. [Google Scholar] [CrossRef]
- Qasimi, M.; Nagaoka, K.; Watanabe, G. The effects of phytosterols on the sexual behavior and reproductive function in the Japanese quail (Coturnix coturnix japonica). Poult. Sci. 2017, 96, 3436–3444. [Google Scholar] [CrossRef] [PubMed]
- Dumolt, J.; Rideout, T. The lipid-lowering effects and associated mechanisms of dietary phytosterol supplementation. Curr. Pharm. Des. 2017, 23, 5077–5085. [Google Scholar] [CrossRef] [PubMed]
- Dierckx, T.; Bogie, J.F.J.; Hendriks, J.J.A. The impact of phytosterols on the healthy and diseased brain. Curr. Med. Chem. 2019, 26, 6750–6765. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tan, M.A.; An, S.S.A. Phytosterols: Potential Metabolic Modulators in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 12255. [Google Scholar] [CrossRef] [PubMed]
- Poulose, N.; Sajayan, A.; Ravindran, A.; Chandran, A.; Priyadharshini, G.B.; Selvin, J.; Kiran, G.S. Anti-diabetic Potential of a Stigmasterol From the Seaweed Gelidium spinosum and Its Application in the Formulation of Nanoemulsion Conjugate for the Development of Functional Biscuits. Front. Nutr. 2021, 8, 694362. [Google Scholar] [CrossRef] [PubMed]
- Jaski, J.M.; Barbosa Abrantes, K.K.; Zanqui, A.B.; Stevanato, N.; da Silva, C.; Barão, C.E.; Bonfm-Rocha, L.; Cardozo-Filho, L. Simultaneous extraction of sunfower oil and active compounds from olive leaves using pressurized propane. Curr. Res. Food Sci. 2022, 5, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, L.; Rantanen, J.; von Bonsdorff, A.; Karjalainen, M.; Yliruusi, J. A novel method of producing a microcrystalline β-sitosterol suspension in oil. Eur. J. Pharm. Sci. 2002, 15, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.; Grisi, C.; Vieira, E.; Ferreira, P.; Rodrigues, G.; Diniz, N. Light cream cheese spread of goat milk enriched with phytosterols: Physicochemical, rheological, and microbiological characterization. LWT-Food Sci. Technol. 2022, 157, 113103. [Google Scholar] [CrossRef]
- Santos, V.; Braz, B.; Silva, A.; Cardoso, L.; Ribeiro, A.; Santana, M. Nanostructured lipid carriers loaded with free phytosterols for food applications. Food Chem. 2019, 298, 125053. [Google Scholar] [CrossRef]
- Munawar, M.; Khan, M.; Saeed, M.; Younas, U.; Farag, M.; Di Cerbo, A.; El-Shall, N.; Loschi, A.; Dhama, K.; Alagawany, M. Phytosterol: Nutritional significance, health benefits, and its uses in poultry and livestock nutrition. Anim. Biotechnol. 2022, 34, 3206–3215. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Ma, Z.; Guan, T. Phytosterols in rice bran and their health benefits. Front. Nutr. 2023, 10, 1287405. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, M.; Zheng, M.; Guo, Q.; Wang, Y.; Wang, H.; Xie, X.; Huang, F.; Gong, R. Folate mediated self-assembled phytosterol-alginate nanoparticles for targeted intracellular anticancer drug delivery. Colloids Surf. B Biointerfaces 2015, 129, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Yamakawa, S.; Sugimoto, T.; Yoshizaki, Y.; Teranishi, R.; Hayashi, T.; Kotaka, A.; Shinde, C.; Kumei, T.; Sumida, Y.; et al. Carboxylated phytosterol derivative-introduced liposomes for skin environment-responsive transdermal drug delivery system. J. Liposome Res. 2018, 28, 275–284. [Google Scholar] [CrossRef]
- Miras-Moreno, B.; Sabater-Jara, A.; Pedreño, M.; Almagro, L. Bioactivity of phytosterols and their production in plant in vitro cultures. J. Agric. Food Chem. 2016, 64, 7049–7058. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, B.; Ansorena, D.; Poyato, C.; Astiasarán, I. Cholesterol and stigmasterol within a sunflower oil matrix: Thermal degradation and oxysterols formation. Steroids 2015, 99, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Knol, D.; Trautwein, E. Phytosterol oxidation products (POP) in foods with added phytosterols and estimation of their daily intake: A literature review. Eur. J. Lipid Sci. Technol. 2016, 118, 1423–1438. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; Theuwissen, E.; Husche, C.; Lütjohann, D.; Gijbels, M.; Jeurissen, M.; Shiri-Sverdlov, R.; van der Made, I.; Mensink, R. Oxidised plant sterols as well as oxycholesterol increase the proportion of severe atherosclerotic lesions in female LDL receptor+/− mice. Br. J. Nutr. 2014, 111, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. Unsaturated lipid matrices protect plant sterols from degradation during heating treatment. Food Chem. 2016, 196, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Knol, D.; Valk, I.; van Andel, V.; Friedrichs, S.; Lütjohann, D.; Hrncirik, K.; Trautwein, E. Thermal stability of plant sterols and formation of their oxidation products in vegetable oils and margarines upon controlled heating. Chem. Phys. Lipids. 2017, 207, 99–107. [Google Scholar] [CrossRef]
- Leal-Castañeda, E.; Inchingolo, R.; Cardenia, V.; Hernandez-Becerra, J.; Romani, S.; Rodriguez-Estrada, M.; Galindo, H. Effect of microwave heating on phytosterol oxidation. J. Agric. Food Chem. 2015, 63, 5539–5547. [Google Scholar] [CrossRef]
- Zhang, R.; Han, Y.; McClements, D.J.; Xu, D.; Chen, S. Production, Characterization, Delivery, and Cholesterol-Lowering Mechanism of Phytosterols: A Review. J. Agric. Food Chem. 2022, 70, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, C.; Hu, Y.; Guo, S.; Bai, G.; Yang, G.; Yang, R. Effects of food formulation on bioavailability of phytosterols: Phytosterol structures, delivery carriers, and food matrices. Food Funct. 2023, 14, 5465–5477. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J.; Yu, L.; Wang, X.; Zhang, W.; Zhang, Q.; et al. Phytosterol Contents of Edible Oils and Their Contributions to Estimated Phytosterol Intake in the Chinese Diet. Foods 2019, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sala, A.; Blanco-Morales, V.; Cilla, A.; Garcia-Llatas, G.; Sánchez-Siles, L.; Barberáa, R.; Lagarda, M. Safe intake of a plant sterol-enriched beverage with milk fat globule membrane: Bioaccessibility of sterol oxides during storage. J. Food Compos. Anal. 2018, 68, 111–117. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Moreau, R.; Rose, D.; Ciftci, O. Phytosterol nanoparticles with reduced crystallinity generated using nanoporous starch aerogels. J. Food Sci. 2016, 6, 108319–108327. [Google Scholar] [CrossRef]

| Phytosterols | Dose | Model | Mechanisms | References |
|---|---|---|---|---|
| Sitosterol | 250 μmol/L | FHs 74 Int cells | Reduces the mRNA levels of NPC1L1 and HMG-CoA reductase. | [45] |
| Sitosterol and stigmasterol | 100 μmol/L | Caco-2 and HepG2 cells | Reduces the messenger RNA expression levels of NPC1L1, scavenger receptor class B type I, LDLR, and HMG-CoA reductase. | [46] |
| Stigmasterol, campesterol and β-sitosterol | 100 μmol/L | Caco-2 cells | Decreases apolipoprotein secretion of ApoB48. | [47] |
| Sitosterol, sitostanol and fucosterol | 125, 250 μmol/L | Caco-2 cells | Activates LXR and its target gene ABCA1 to increase reverse transport. | [37] |
| Schottenol and spinasterol | 2.5, 10 μmol/L | BV cells | Activates LXRα, LXRβ, and target genes ABCA1, ABCG1. | [48] |
| Saringosterol | 30 μmol/L | HEK293T, HepG 2, THP-1, RAW264.7 and Caco-2 cells | Activates the target gene of LXR (especially LXRβ). | [49] |
| Phytosterol mixture a | 2% of feed | Female ApoE-/- mice | Reduces cholesterol absorption in enterocytes. | [50] |
| β-Sitosterol and stigmasterol | 0.1% of feed | Male hamsters | Decreases mRNA levels of ACAT and MTP in intestine and reduces chylomicron assembly. | [51] |
| Phytosterol mixture b | 20 g/kg | Male hamsters | Inhibits the expression of SREBP-2 and increases the ABCG5 level in liver. | [43] |
| Stigmasterol | 0.5% of feed | Wistar and WKY rats | Inhibits activity of HMG-CoA reductase. | [44] |
| Phytosterol ester mixture c | 50 mg/every mouse | C57BL/6J mice | Reduces expression of SREBP2 and HMG-CoA reductase. | [52] |
| Phytosterol mixture d | 1%, 2%, 4% or 8% of feed | Wild-type and Abcg5-/- mice | Improves expression of ABCG5 to increase excretion of TICE. | [39] |
| Phytosterol mixture e | 2.13 g/kg | Male SHRSP and WKY inbred rats | Increases ABCG8 level of intestine, mRNA of ABCA1 and ABCG5 in liver, and increases the mRNA expression of 27α hydroxylase (CYP27A1). | [53] |
| Phytosterols | Dose | Types of Cancer | Mechanisms Related to Anticancer Effects | References |
|---|---|---|---|---|
| Phytosterols a | 115, 11, 6 μM | Colon cancer | Influence on cell viability and cell cycle. | [70] |
| Phytosterols b | 2% of feed | Breast cancer | Induction of lipoprotein oxidation. | [71] |
| Phytosterols c | 16 μM | Prostate cancer | Inhibition of the expression of caveolin-1. | [72] |
| Phytosterols d | 2.80–467.11 μg/mL | Leukemia | Inhibition of leukemic cell proliferation. | [4] |
| Phytosterols e | 2.5–25 μg/mL | Breast cancer | Suppression of tumor growth and the expression of tumor markers. | [64] |
| Phytosterols f | 0.1–2% of feed | Colon cancer | Slows tumor proliferation. | [70] |
| Phytosterols | 13.2 μM | Colon cancer | Increases the number of cells in sub-G1 phase. | [73] |
| β-Sitosterol | 13, 26, 52 μM | Breast, colon and cervical cancer | Induction of DNA fragmentation and apoptosis. | [74] |
| β-Sitosterol | 2.5–25 μM | Colon cancer | Induces apoptosis by increasing the sub-G1 cell population. | [75] |
| Campesterol | 30, 60, 120 μM | Lymphoma cancer | Enhancing cell apoptosis. | [76] |
| Ergosterol | 20 μM | Human lung adenocarcinoma cells | Inhibition of cancer growth (the oxidation products of ergosterol). | [77] |
| β-Sitosterol, stigmasterol | >56.0 mg/day or >9 mg/day | Gastric and stomach cancer | Affecting testosterone metabolism and enhancing apoptosis of cancer cells. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, M.; Yuan, L.; Zhang, J.; Wang, X.; Zhang, M.; Li, H.; Jing, Y.; Zeng, F.; Xie, J. Phytosterols: Physiological Functions and Potential Application. Foods 2024, 13, 1754. https://doi.org/10.3390/foods13111754
Shen M, Yuan L, Zhang J, Wang X, Zhang M, Li H, Jing Y, Zeng F, Xie J. Phytosterols: Physiological Functions and Potential Application. Foods. 2024; 13(11):1754. https://doi.org/10.3390/foods13111754
Chicago/Turabian StyleShen, Mingyue, Lanlan Yuan, Jian Zhang, Xufeng Wang, Mingyi Zhang, Haizhen Li, Ying Jing, Fengjiao Zeng, and Jianhua Xie. 2024. "Phytosterols: Physiological Functions and Potential Application" Foods 13, no. 11: 1754. https://doi.org/10.3390/foods13111754






