(-)-Gallocatechin Gallate Mitigates Metabolic Syndrome-Associated Diabetic Nephropathy in db/db Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Body Composition Measurements
2.3. Glucose Tolerance Test and Insulin Resistance Test
2.4. Lipid Profile Measurements
2.5. Blood Pressure Measurement
2.6. Determination of Renal Functions
2.7. Histological Examination
2.8. Transcriptome Analysis of Renal Tissues
2.9. Statistical Analysis
3. Results
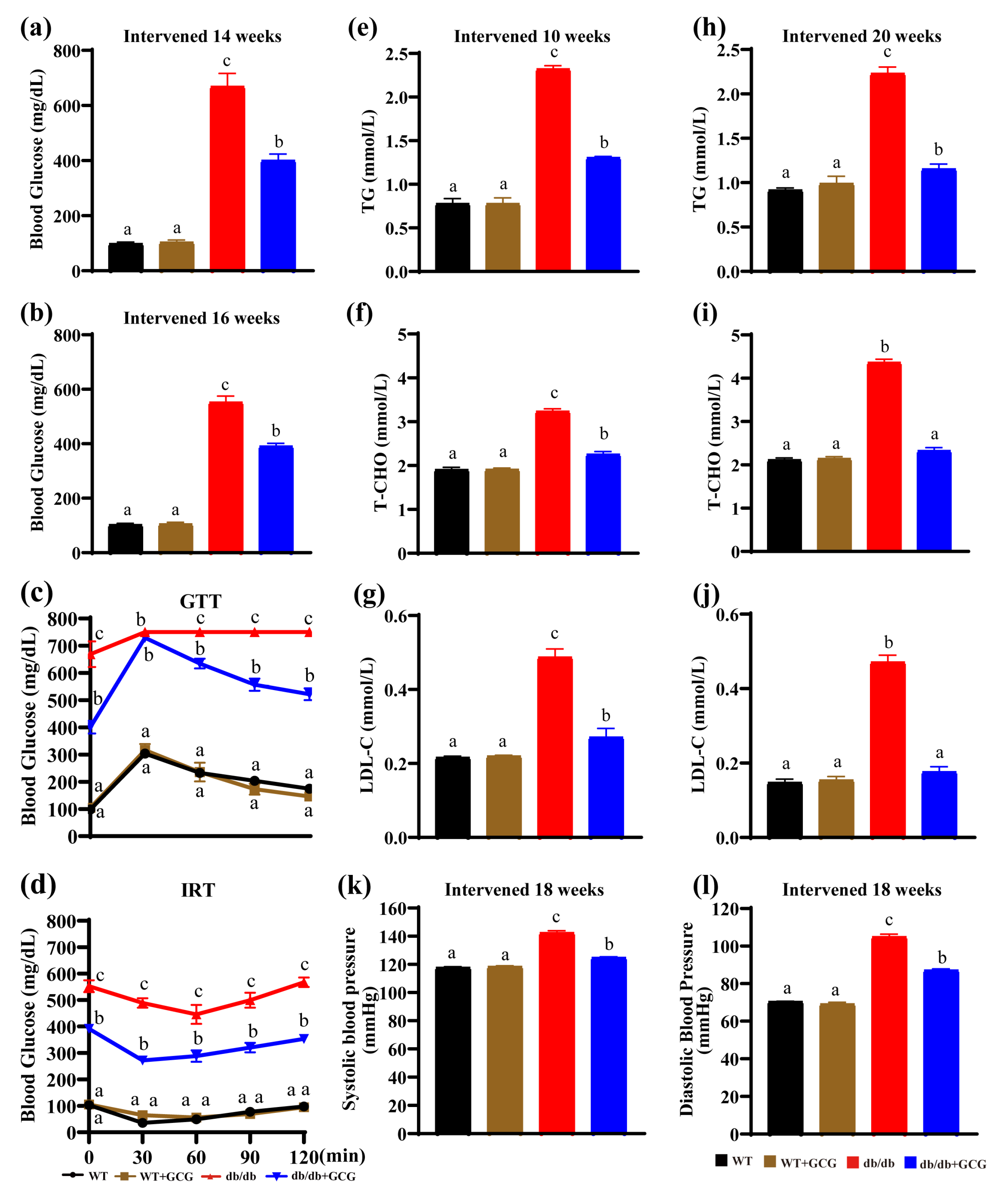
3.1. GCG Alleviating Type 2 Diabetes Sign in db/db Mice
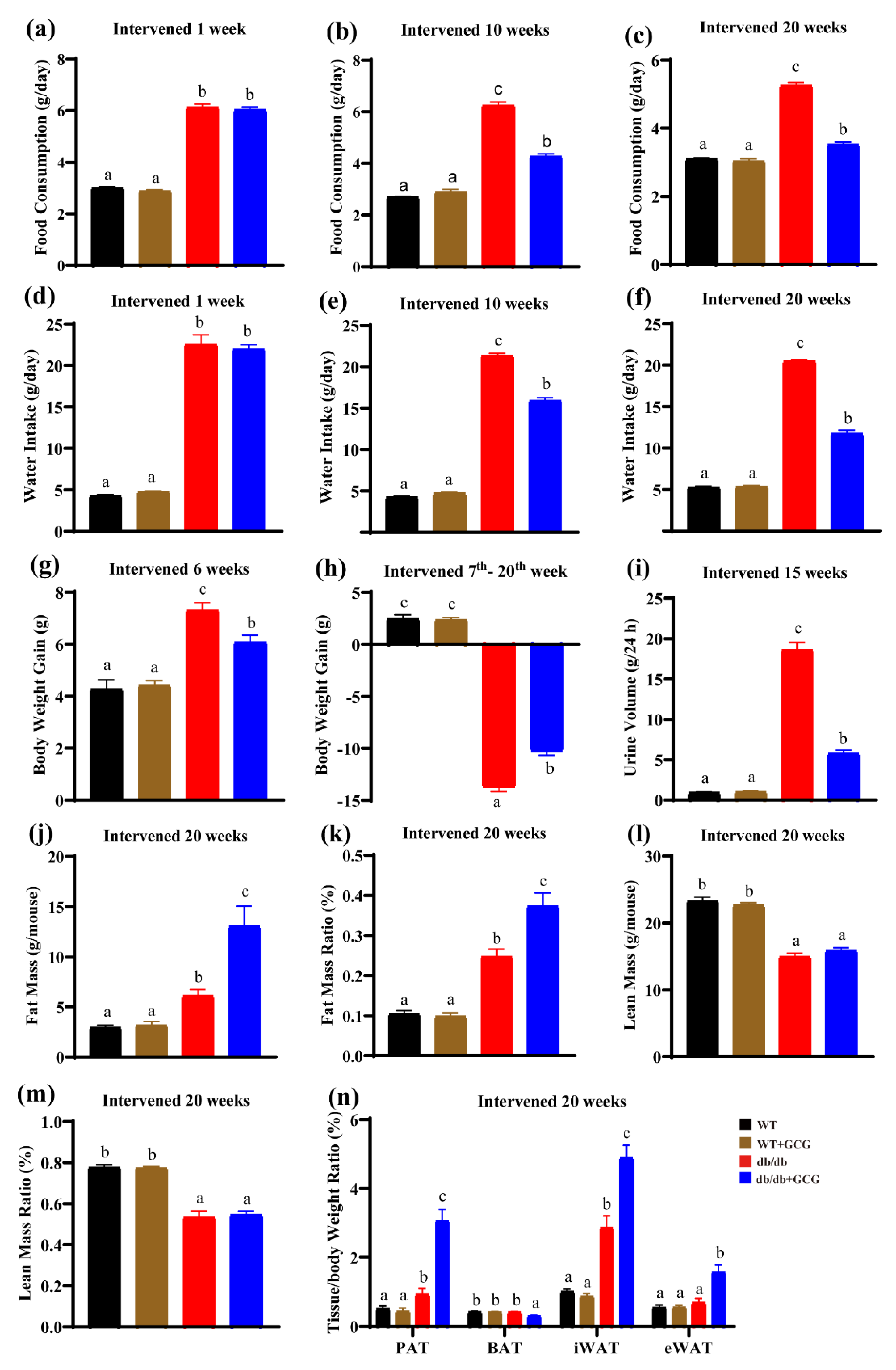
3.2. GCG Prevents Metabolic Syndrome in db/db Mice
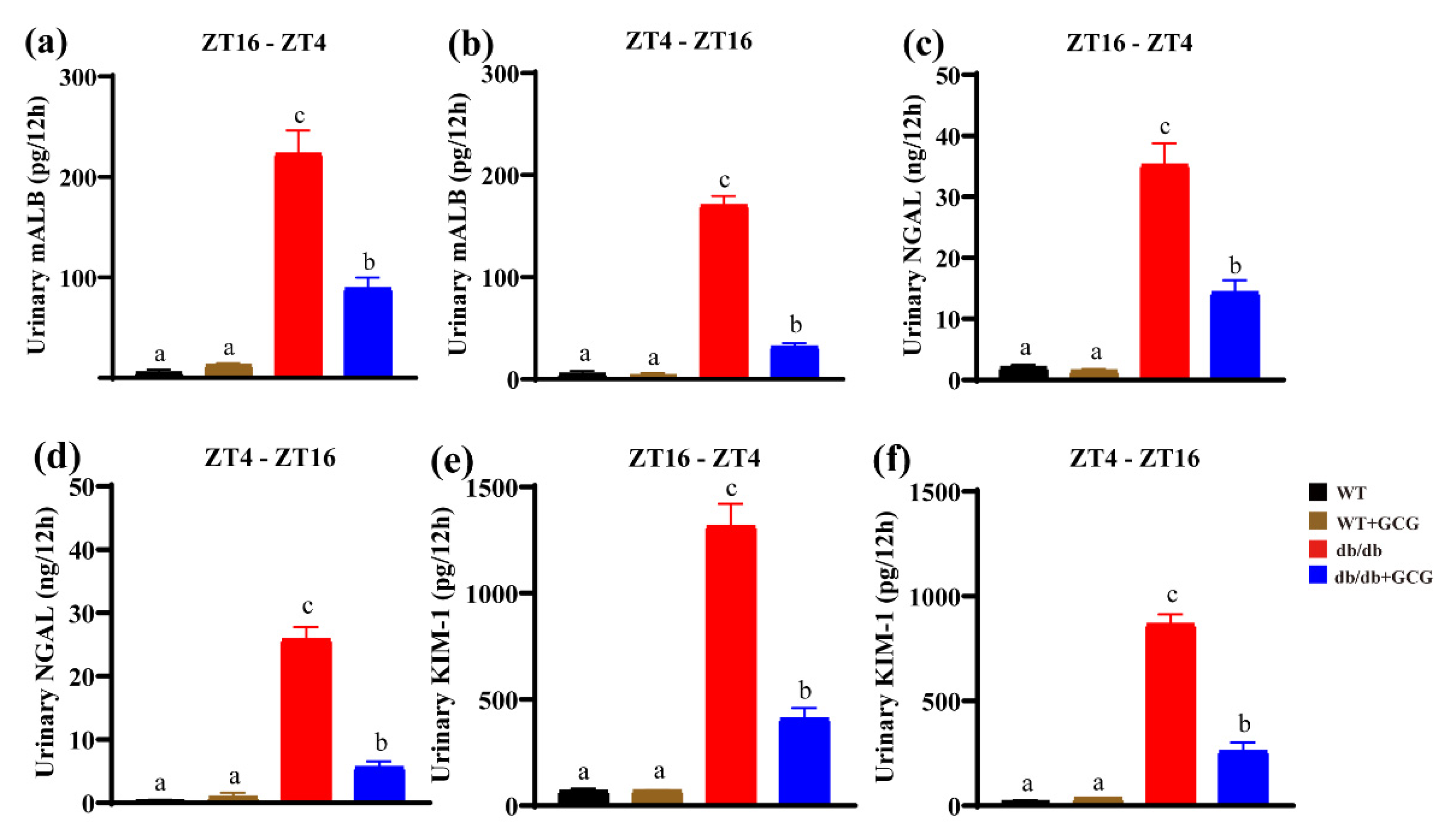
3.3. GCG Improves Urinary Renal Function Biomarkers in db/db Mice
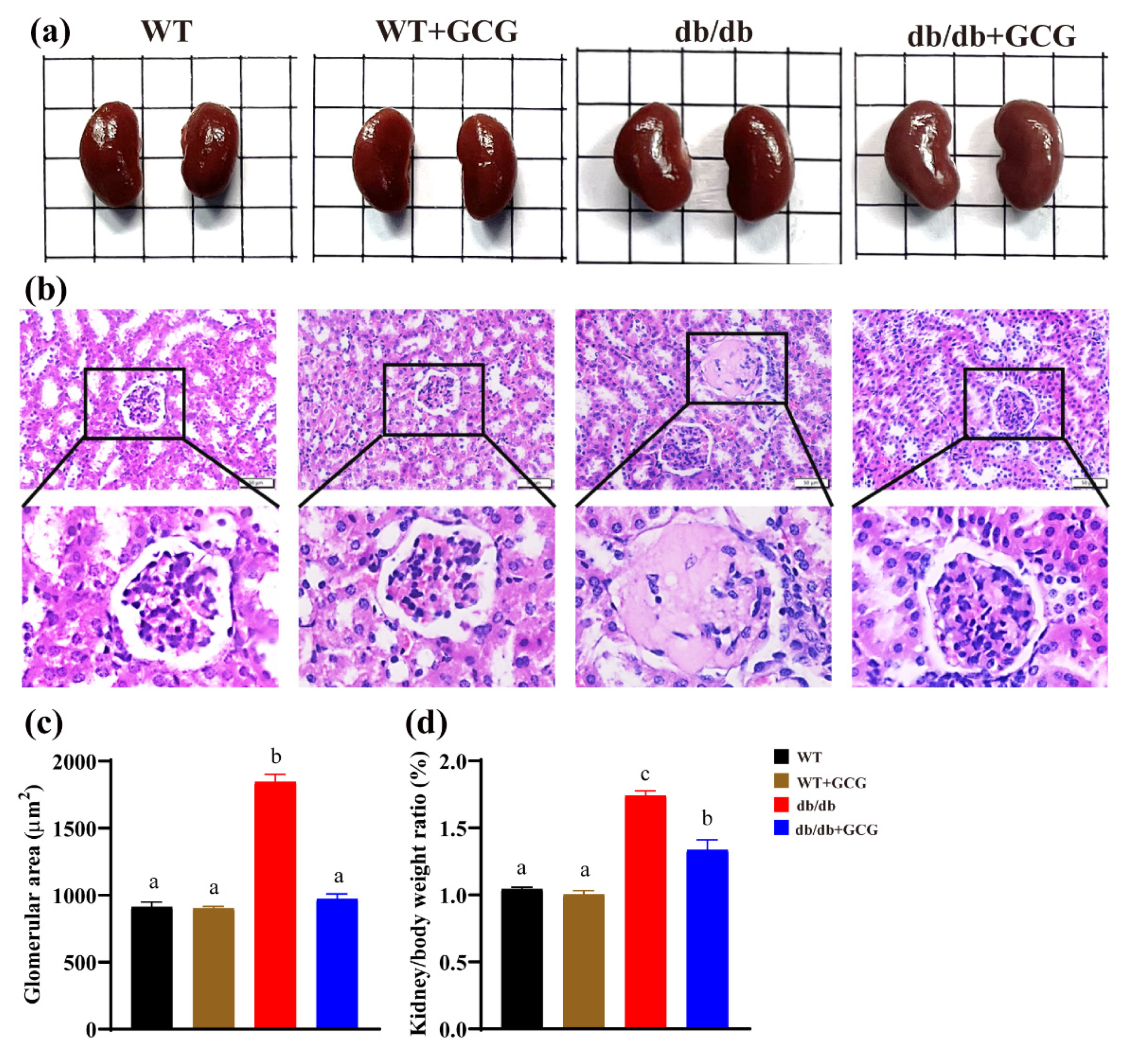
3.4. GCG Improved the Renal Structure in db/db Mice
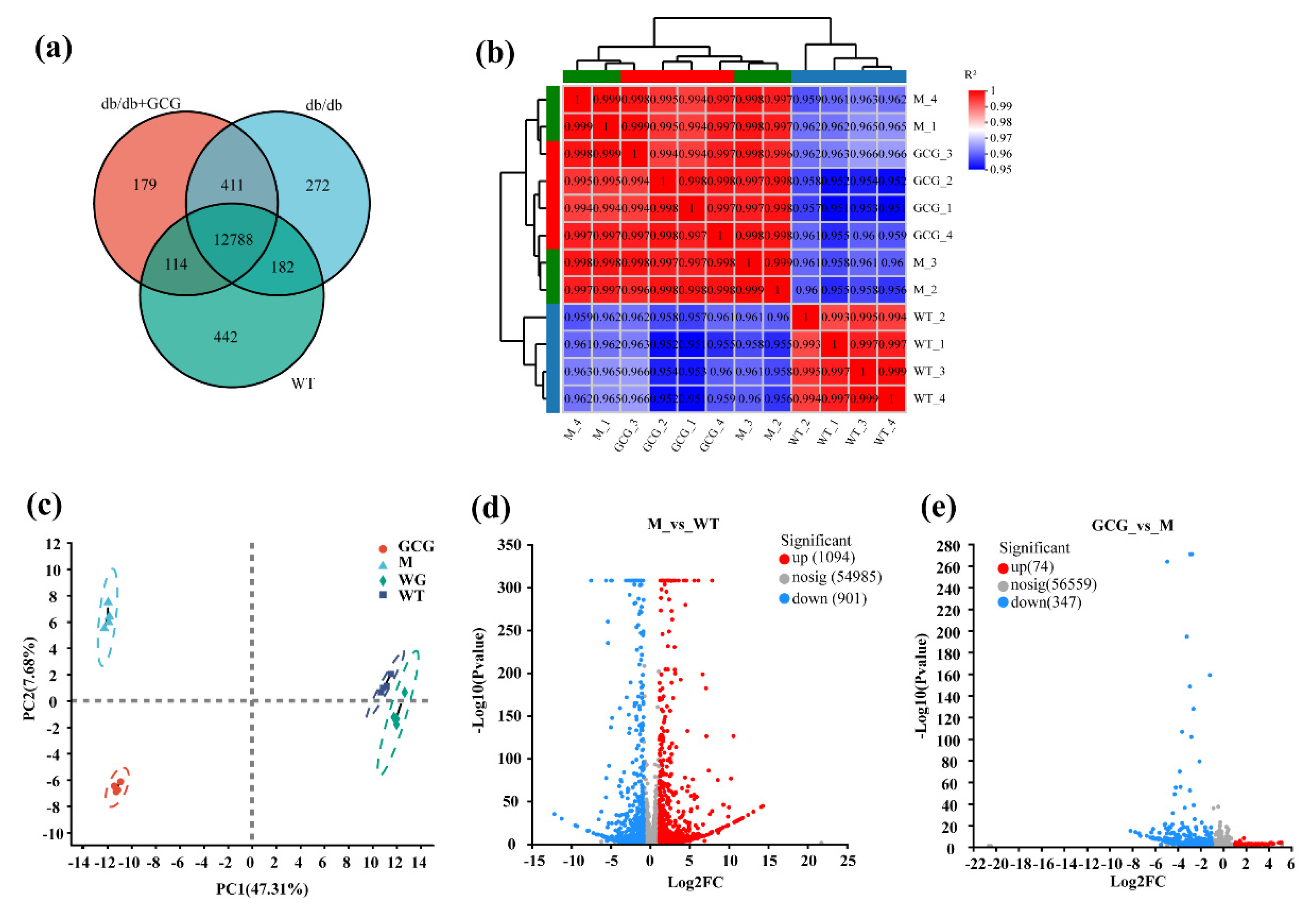
3.5. Evaluation of Gene Expression and Identification of DEGs among Sample Sets
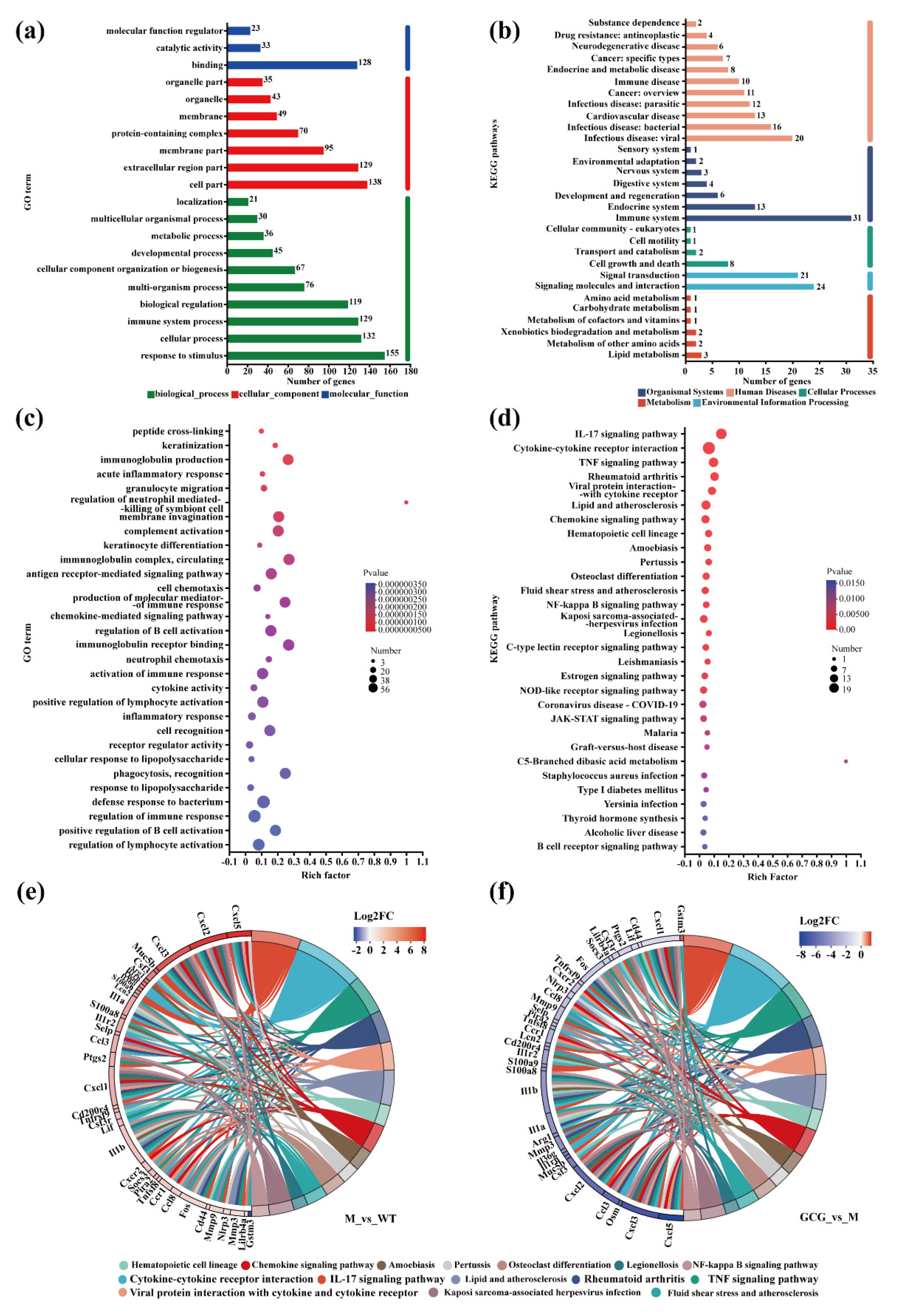
3.6. Gene Set Functional Annotation and Enrichment Analysis
3.7. Mechanisms of GCG in Preventing Diabetic Kidney Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahed, G.; Aoun, L.; BouZerdan, M.; Allam, S.; BouZerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Zafar, U.; Khaliq, S.; Ahmad, H.U.; Manzoor, S.; Lone, K.P. Metabolic syndrome: An update on diagnostic criteria, pathogenesis, and genetic links. Hormones 2018, 17, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, M. Hypertension with diabetes mellitus: Physiology and pathology. Hypertens. Res. 2018, 41, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E.; Adler, A.I.; Flyvbjerg, A.; Nelson, R.G.; So, W.Y.; Wanner, C.; Kasiske, B.L.; Wheeler, D.C.; de Zeeuw, D.; Nsen, C.E. Diabetic kidney disease: A clinical update from kidney disease: Improving global outcomes. Kidney Int. 2015, 87, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic kidney disease: Challenges, progress, and possibilities. Clin. J. Am. Soc. Physiol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Macisaac, R.J.; Ekinci, E.I.; Jerums, G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am. J. Kidney Dis. 2014, 63, S39–S62. [Google Scholar] [CrossRef]
- Vincent, A.M.; Hinder, L.M.; Pop-Busui, R.; Feldman, E.L. Hyperlipidemia: A new therapeutic target for diabetic neuropathy. J. Peripher. Nerv. Syst. 2009, 14, 257–267. [Google Scholar] [CrossRef]
- Chuang, S.M.; Shih, H.M.; Chien, M.N.; Liu, S.C.; Wang, C.H.; Lee, C.C. Risk factors in metabolic syndrome predict the progression of diabetic nephropathy in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2019, 153, 6–13. [Google Scholar] [CrossRef]
- Duran-Perez, E.G.; Almeda-Valdes, P.; Cuevas-Ramos, D.; Campos-Barrera, E.; Muñoz-Hernandez, L.; Gomez-Perez, F.J. Treatment of metabolic syndrome slows progression of diabetic nephropathy. Metab. Syndr. Relat. Disord. 2011, 9, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Du, Q.; Meng, X.; Zhang, Y. Natural polyphenols: A potential prevention and treatment strategy for metabolic ome. Food Funct. 2022, 13, 9734–9753. [Google Scholar] [CrossRef]
- Ntamo, Y.; Jack, B.; Ziqubu, K.; Mazibuko-Mbeje, S.E.; Nkambule, B.B.; Nyambuya, T.M.; Mabhida, S.E.; Hanser, S.; Orlando, P.; Tiano, L.; et al. Epigallocatechin gallate as a nutraceutical to potentially target the metabolic syndrome: Insights into therapeutic effects beyond its antioxidant and anti-inflammatory properties. Crit. Rev. Food Sci. Nutr. 2024, 64, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, P.; Feng, K. EGCG adjuvant chemotherapy: Current status and future perspectives. Eur. J. Med. Chem. 2023, 250, 115197. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Meng, Q.; Wang, Y.; Long, P.; Ho, C.T.; Cui, C.; Cao, L.; Li, D.; Wan, X. Roasting improves the glycemic effects of a large-leaf yellow tea infusion by enhancing the levels of epimerized catechins that inhibit α-glucose. Food Funct. 2018, 9, 5162–5168. [Google Scholar] [CrossRef]
- Xiao, X.; Feng, L.; Cheng, H.; Ge, H.; Wang, Y.; Wan, X.; Li, D.; Xie, Z. Unraveling the superiority of (−)-gallocatechingallate to (−)-epigallocatechin-3-gallate in protection of diabetic nephropathy of db/db mice. Food Front. 2024, 5, 771–788. [Google Scholar] [CrossRef]
- Sharma, K.; McCue, P.; Dunn, S.R. Diabetic kidney disease in the db/db mouse. Am. J. Physiol. Ren. Physiol. 2003, 284, F1138–F1144. [Google Scholar] [CrossRef]
- Fellmann, L.; Nascimento, A.R.; Tibiriça, E.; Bousquet, P. Murine models for pharmacological studies of the metabolic syndrome. Pharmacol. Ther. 2013, 137, 331–340. [Google Scholar] [CrossRef]
- Hur, J.; Dauch, J.R.; Hinder, L.M.; Hayes, J.M.; Backus, C.; Pennathur, S.; Kretzler, M.; Brosius, F.C., 3rd; Feldman, E.L. Metabolic syndrome and microvascular complications in a murine model of type 2 diabetes. Diabetes 2015, 64, 3294–3304. [Google Scholar] [CrossRef]
- Cil, O.; Chen, X.; Askew Page, H.R.; Baldwin, S.N.; Jordan, M.C.; MyatThwe, P.; Anderson, M.O.; Haggie, P.M.; Green, I.A.; Roos, K.P.; et al. A small molecule inhibitor of the chloride channel TMEM16A blocks vascular smooth le contraction and lowers blood pressure in spontaneously hypertensive rats. Kidney Int. 2021, 100, 311–320. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for ation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Berglund, E.D.; Yu, X.; Wang, M.Y.; Evans, M.R.; Scherer, P.E.; Holland, W.L.; Charron, M.J.; Roth, M.G.; Unger, R.H. Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proc. Natl. Acad. Sci. USA 2014, 111, 13217–13222. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.H.; Speakman, J.R.; Arch, J.R.; Auwerx, J.; Brüning, J.C.; Chan, L.; Eckel, R.H.; Farese, R.V., Jr.; Galgani, J.E.; Hambly, C.; et al. A guide to analysis of mouse energy metabolism. Nat. Methods 2011, 9, 57–63. [Google Scholar] [CrossRef]
- Cinti, S. The adipose organ at a glance. Dis. Model. Mech. 2012, 5, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, S.H.; AlZaim, I.; Al-Dhaheri, Y.; Eid, A.H.; El-Yazbi, A.F. Perirenal adipose tissue inflammation: Novel in linking metabolic dysfunction to renal diseases. Front. Endocrinol. 2021, 12, 707126. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.M.; Crislip, G.R.; Stowie, A.; Ellis, I.; Ramsey, A.; Castanon-Cervantes, O.; Gumz, M.L.; Davidson, A.J. Environmental circadian disruption suppresses rhythms in kidney function and accelerates excretion of renal injury markers in urine of male hypertensive rats. Am. J. Physiol. Ren. Physiol. 2021, 320, F224–F233. [Google Scholar] [CrossRef]
- Noh, J.Y.; Han, D.H.; Yoon, J.A.; Kim, M.H.; Kim, S.E.; Ko, I.G.; Kim, K.H.; Kim, C.J.; Cho, S. Circadian rhythms in urinary functions: Possible roles of circadian clocks? Int. Neurourol. J. 2011, 15, 64–73. [Google Scholar] [CrossRef]
- Su, W.; Xie, Z.; Guo, Z.; Duncan, M.J.; Lutshumba, J.; Gong, M.C. Altered clock gene expression and vascular smooth le diurnal contractile variations in type 2 diabetic db/db mice. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H621–H633. [Google Scholar] [CrossRef]
- Teng, Y.; Li, D.; Guruvaiah, P.; Xu, N.; Xie, Z. Dietary supplement of large yellow tea ameliorates metabolic syndrome and attenuates hepatic steatosis in db/db mice. Nutrients 2018, 10, 75. [Google Scholar] [CrossRef]
- Wang, B.; Chandrasekera, P.C.; Pippin, J.J. Leptin- and leptin receptor-deficient rodent models: Relevance for human 2 diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Guo, Y.; Cai, B.; Yang, J. Epimerization of epigallocatechin gallate to gallocatechin gallate and its anti-diabetic activity. Med. Chem. Res. 2013, 22, 3372–3378. [Google Scholar] [CrossRef]
- Ansquer, J.C.; Foucher, C.; Rattier, S.; Taskinen, M.R.; Steiner, G. Fenofibrate reduces progression to microalbuminuria 3 years in a placebo-controlled study in type 2 diabetes: Results from the diabetes atherosclerosis intervention study (DAIS). Am. J. Kidney Dis. 2005, 45, 485–493. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, C.W.; Kim, J.K.; Shin, H.J.; Baik, J.H. GCG-rich tea catechins are effective in lowering cholesterol and triglyceride concentrations in hyperlipidemic rats. Lipids 2008, 43, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Jeong, H.W.; Kim, J.K.; Kim, A.Y.; Hong, Y.D.; Lee, J.H.; Choi, J.K.; Seo, D.B. Gallocatechin gallate-containing fermented green tea extract ameliorates obesity and hypertriglyceridemia through the modulation of lipid metabolism in adipocytes and myocytes. J. Med. Food 2019, 22, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Giunti, S.; Barit, D.; Cooper, M.E. Mechanisms of diabetic nephropathy: Role of hypertension. Hypertension 2006, 48, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.; Si, H.; Liu, D. Epigallocatechin gallate reduces vascular inflammation in db/db mice possibly through an NF-mediated mechanism. Mol. Nutr. Food Res. 2012, 56, 1424–1432. [Google Scholar] [CrossRef]
- Li, K.K.; Peng, J.M.; Zhu, W.; Cheng, B.H.; Li, C.M. Gallocatechin gallate (GCG) inhibits 3T3-L1 differentiation and lipopolysaccharide induced inflammation through MAPK and NF-κB signaling. J. Funct. Foods 2017, 30, 159–167. [Google Scholar] [CrossRef]
- Rowe, J.W.; Young, J.B.; Minaker, K.L.; Stevens, A.L.; Pallotta, J.; Landsberg, L. Effect of insulin and glucose infusions sympathetic nervous system activity in normal man. Diabetes 1981, 30, 219–225. [Google Scholar] [CrossRef]
- Mori, Y.; Ajay, A.K.; Chang, J.H.; Mou, S.; Zhao, H.; Kishi, S.; Li, J.; Brooks, C.R.; Xiao, S.; Woo, H.M.; et al. KIM-1 mediates fatty acid uptake by renal tubular cells to promote progressive diabetic kidney disease. Cell Metab. 2021, 33, 1042–1061. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Vogl, T.; Roth, J. S100 proteins expressed in phagocytes: A novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol. 2007, 81, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chen, Y.; Shi, J.; Yu, X.; Zhou, J.; Wang, X.; Xu, L.; Liu, J.; Gao, J.; Gu, X.; et al. Inhibition of S100A8/A9 ameliorates interstitial fibrosis in diabetic nephropathy. Metabolism 2022, 144, 155376. [Google Scholar] [CrossRef] [PubMed]
- Rudman-Melnick, V.; Adam, M.; Potter, A.; Chokshi, S.M.; Ma, Q.; Drake, K.A.; Schuh, M.P.; Kofron, J.M.; Devarajan, P.; Potter, S. Single-Cell profiling of AKI in a murine model reveals novel transcriptional signatures, profibrotic phenotype, epithelial-to-stromal crosstalk. J. Am. Soc. Nephrol. 2020, 31, 2793–2814. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, M.; Li, J.; Huang, J.; Zhou, S.; Hou, X.; Ye, H.; Liu, X.; Xiang, S.; Shen, W.; et al. β-catenin-controlled tubular cell-derived exosomes play a key role in fibroblast activation via the OPN-CD44 axis. J. Extracell. Vesicles 2022, 11, e12203. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.H.; Liu, B.Q.; Hao, M.J.; Fan, Y.X.; Qian, C.; Teng, P.; Zhou, X.W.; Hu, L.; Liu, W.T.; Yuan, Z.L.; et al. Paeoniflorinsuppressed high glucose-induced retinal microglia MMP-9 expression and inflammatory response via inhibition of TLR4/NF-κBpathway through upregulation of SOCS3 in diabetic retinopathy. Inflammation 2017, 40, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.B.; Ramos-Lobo, A.M.; Donato, J., Jr. SOCS3 as a future target to treat metabolic disorders. Hormones 2019, 18, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; López, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dong, Y.; Tian, X.; Tan, T.K.; Liu, Z.; Zhao, Y.; Zhang, Y.; Harris, D.; Zheng, G. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J. Nephrol. 2013, 2, 84–89. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Dell’agli, M.; Bellosta, S.; Rizzi, L.; Galli, G.V.; Canavesi, M.; Rota, F.; Parente, R.; Bosisio, E.; Romeo, S. A structure-activity study for the inhibition of metalloproteinase-9 activity and gene expression by analogues of gallocatechin-3-gallate. Cell. Mol. Life Sci. 2005, 62, 2896–2903. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Tang, L.Q. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol. Res. 2016, 114, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.W.; Yiu, W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Donate-Correa, J.; Martín-Núñez, E.; Muros-de-Fuentes, M.; Mora-Fernández, C.; Navarro-González, J.F. Inflammatory cytokines in diabetic nephropathy. J. Diabetes Res. 2015, 2015, 948417. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Komori, T.; Kohjimoto, Y.; Miyajima, A.; Hara, I.; Morikawa, Y. Essential roles of oncostatin M receptor β signaling in renal crystal formation in mice. Sci. Rep. 2020, 10, 17150. [Google Scholar] [CrossRef]
- Pollack, V.; Sarkozi, R.; Banki, Z.; Feifel, E.; Wehn, S.; Gstraunthaler, G.; Stoiber, H.; Mayer, G.; Montesano, R.; Strutz, F. Statin M-induced effects on EMT in human proximal tubular cells: Differential role of ERK signaling. Am. J. Physiol. Ren. Physiol. 2007, 293, F1714–F1726. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, R.; Hassouneh, R.; Hébert, R.L. PGE2, Kidney disease, and cardiovascular risk: Beyond hypertension and diabetes. J. Am. Soc. Nephrol. 2016, 27, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Fan, X.; Moeckel, G.W.; Harris, R.C. Podocyte COX-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. J. Am. Soc. Nephrol. 2011, 22, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, J.; You, L.; Dai, M.; Zhao, Y. GSTM3 Function and Polymorphism in Cancer: Emerging but Promising. Cancer Manag. Res. 2020, 12, 10377–10388. [Google Scholar] [CrossRef]
- Guardado, S.; Ojeda-Juárez, D.; Kaul, M.; Nordgren, T.M. Comprehensive review of lipocalin 2-mediated effects in lung inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 321, L726–L733. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Ge, H.; Wang, Y.; Wan, X.; Li, D.; Xie, Z. (-)-Gallocatechin Gallate Mitigates Metabolic Syndrome-Associated Diabetic Nephropathy in db/db Mice. Foods 2024, 13, 1755. https://doi.org/10.3390/foods13111755
Xiao X, Ge H, Wang Y, Wan X, Li D, Xie Z. (-)-Gallocatechin Gallate Mitigates Metabolic Syndrome-Associated Diabetic Nephropathy in db/db Mice. Foods. 2024; 13(11):1755. https://doi.org/10.3390/foods13111755
Chicago/Turabian StyleXiao, Xin, Huifang Ge, Yijun Wang, Xiaochun Wan, Daxiang Li, and Zhongwen Xie. 2024. "(-)-Gallocatechin Gallate Mitigates Metabolic Syndrome-Associated Diabetic Nephropathy in db/db Mice" Foods 13, no. 11: 1755. https://doi.org/10.3390/foods13111755




