Fermentation Performance Evaluation of Lactic Acid Bacteria Strains for Sichuan Radish Paocai Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Source of LAB and Indicator Strains
2.3. Determination of LAB Strain Growth Characteristics
2.3.1. Gompertz Model Fitting of LAB Strain Growth Rates
2.3.2. Dynamic Monitoring of the pH and TTA of the Fermentation Liquid
2.3.3. NaCl Tolerance of the LAB Strains
2.3.4. MIC Determination of Fermentation Supernatant
2.4. Preparation of Radish Paocai
2.4.1. Stater Culture Preparation
2.4.2. Radish Samples Production
2.5. Acidification of Radish Paocai
2.6. HPLC Measurement of Organic Acids
2.7. FAAs in Radish Paocai
2.8. Analysis of Taste Contour Using E-Tongue
2.9. Texture Profile Analysis
2.10. Color Analysis
2.11. Volatile Organic Compounds (VOCs) Analyzed Using GC–IMS
2.12. Sensory Evaluation and Acceptability
2.13. A Comprehensive Evaluation of the LAB Strains Quantified Using TOPSIS
2.14. Data Analysis
3. Results and Discussion
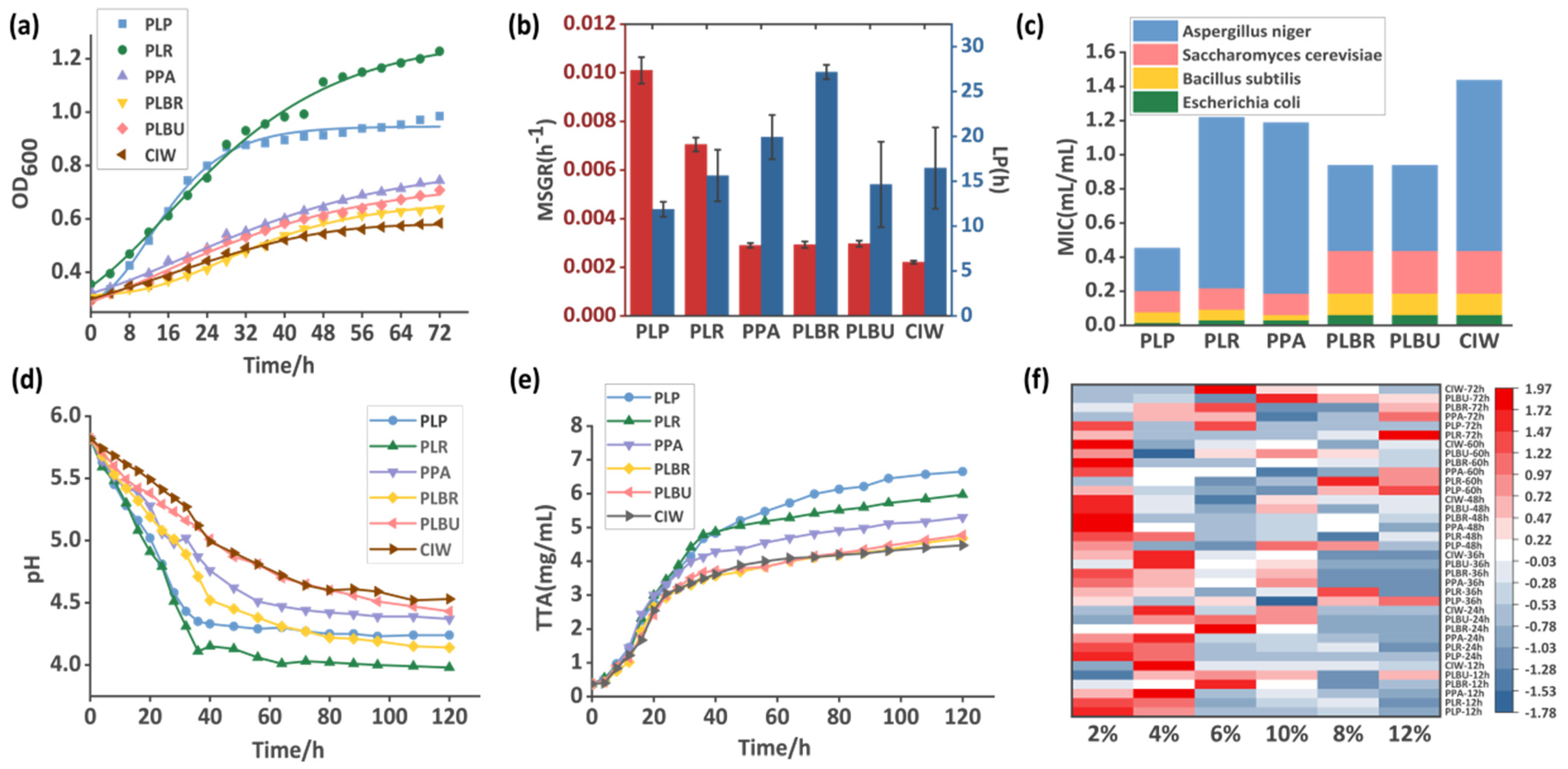
3.1. Growth Characteristics of the LAB Strains
3.2. Dynamic Measurement of Acidification in Radish Paocai
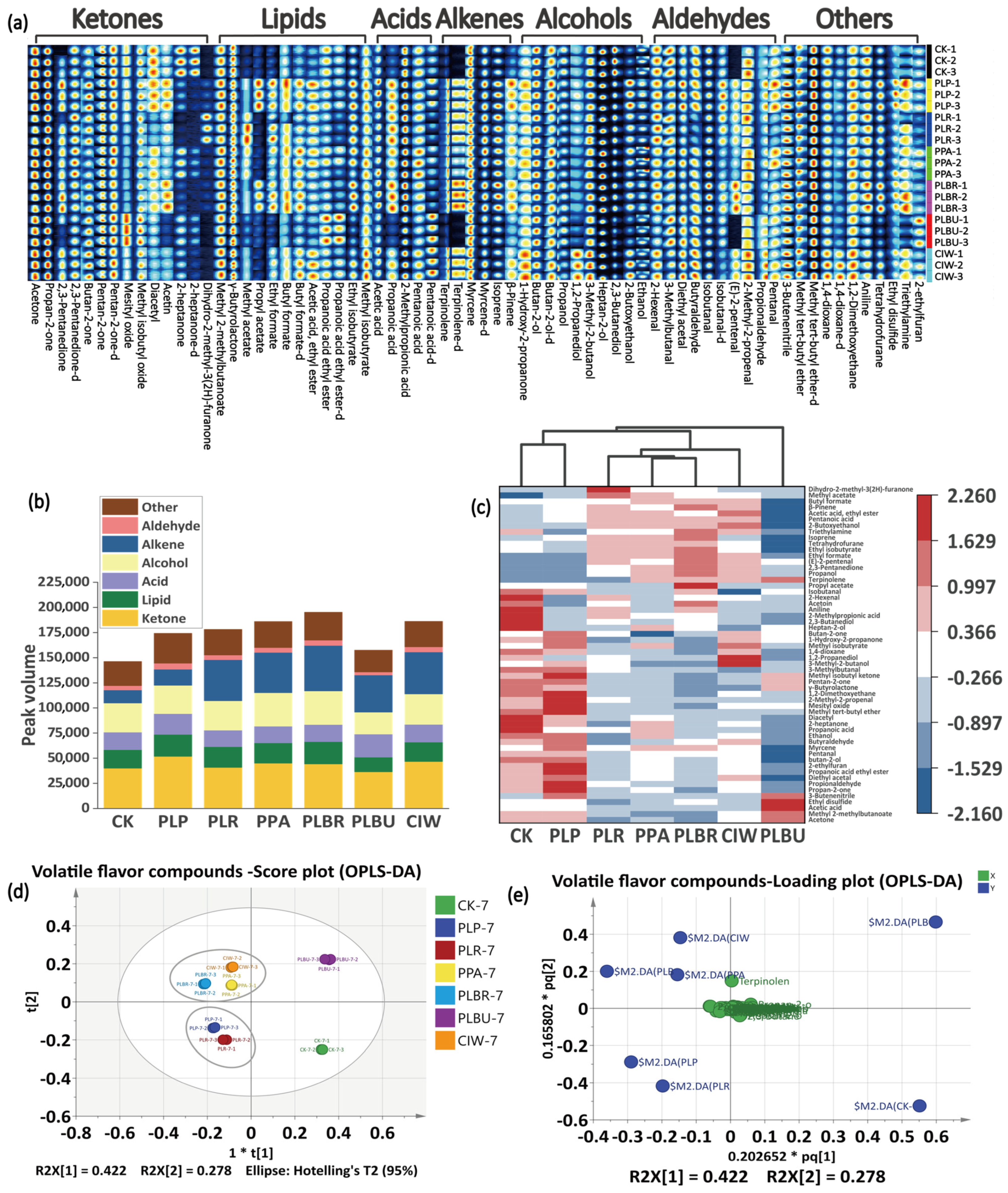
3.3. VOCs Identification
3.4. Taste Contour and FAAs Analysis
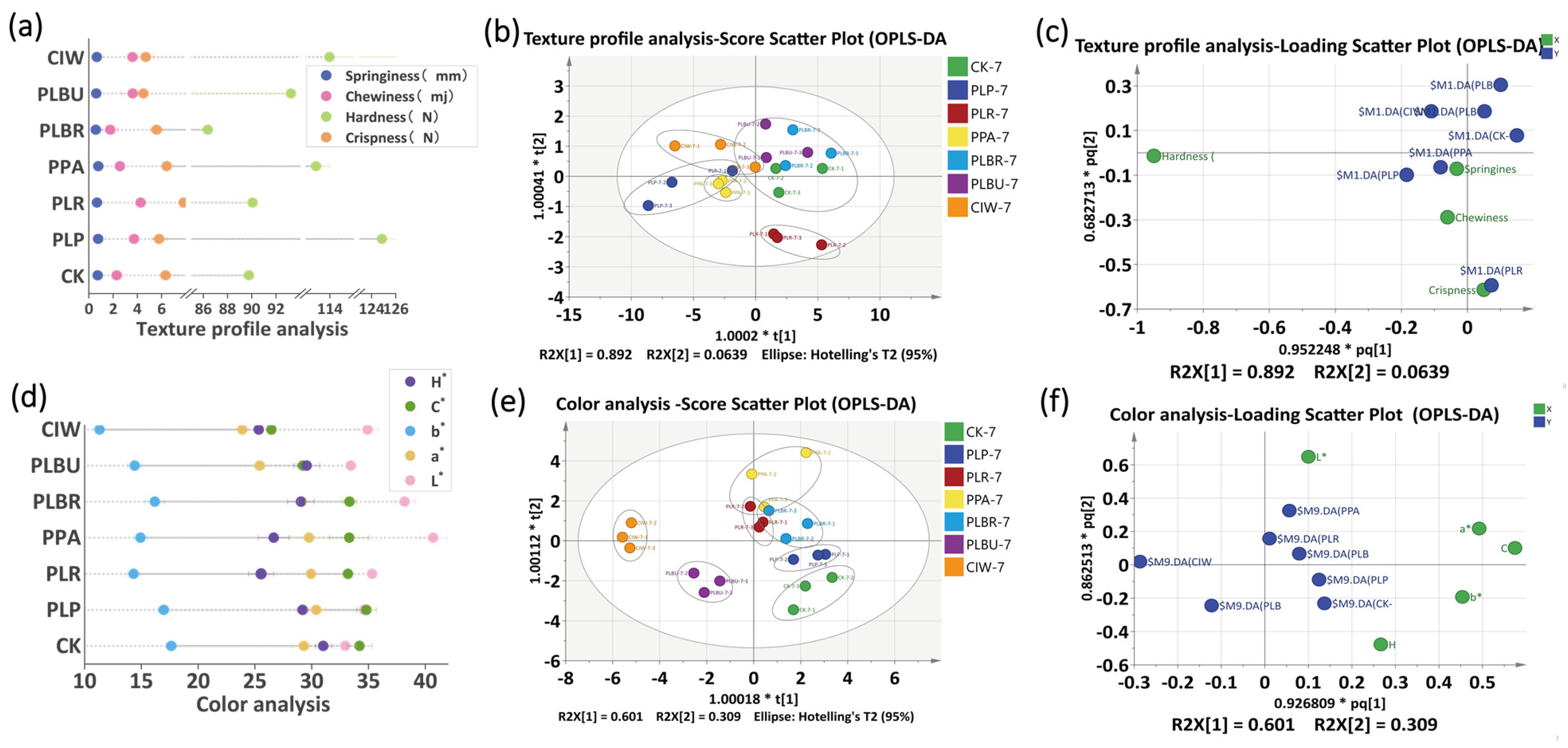
3.5. Texture Analysis of Radish Paocai
3.6. Determination of Color Indicators
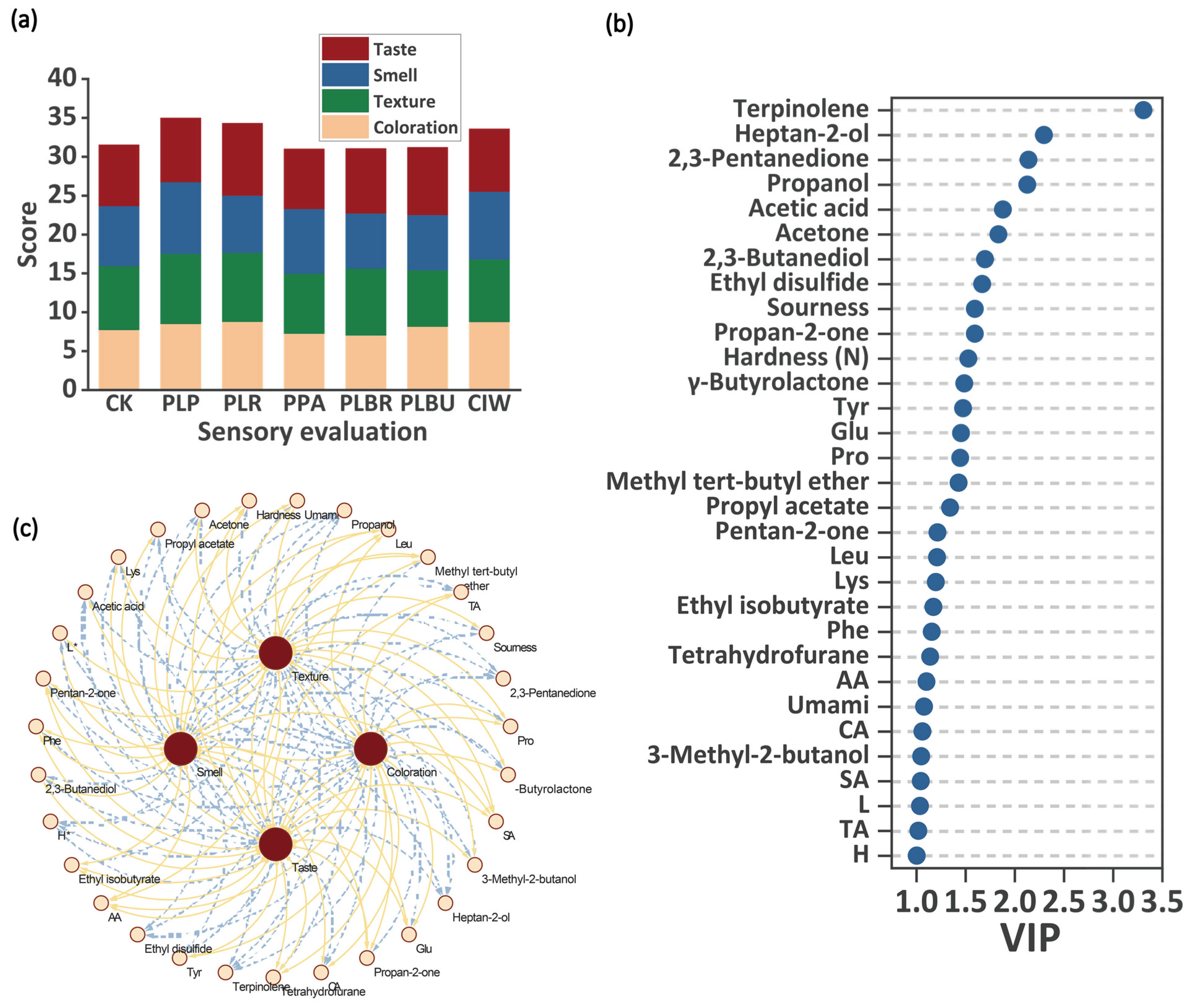
3.7. Correlation Analysis of Sensory Evaluation and Important Indicators
3.8. Comprehensive Evaluation of the LAB Strains Using TOPSIS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larissa, P.M.; Bruna, A.K.; Ramon, P.B.; Verônica, O.A.; Alp, S.C.; Patrick, W.M.J.; Annereinou, D.; Marjo, J.C.; Starrenburg, R.S.S.; Adriano, G.C.; et al. High throughput screening of tchnological and biopreservation traits of a large set of wild lactic acid bacteria from Brazilian artisanal cheeses. Food Microbiol. 2021, 10, 103872. [Google Scholar] [CrossRef]
- Behera, S.S.; Aly, F.E.S.; Riadh, H.; Awanish, K. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Ajibola, O.O.; Raymond, T.; Babatunde, F.B. Selected fermented indigenous vegetables and fruits from Malaysia as potential sources of natural probiotics for improving gut health. Food Sci. Hum. Wellness 2023, 12, 1493–1509. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Z.; Miyao, M.; Zhang, W. Unraveling the flavor profile and microbial roles during industrial Sichuan radish paocai fermentation by molecular sensory science and metatranscriptomics. Food Biosci. 2022, 48, 101815. [Google Scholar] [CrossRef]
- Gamba, M.; Eralda, A.; Peter, F.R.; Marija, G.; Oscar, H.F.; Beatrice, M.; Weston, B.; Brandon, M.; Hua, K.; Taulant, M. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Tang, Y.; Ming, J.; Huang, R.; Li, J.; Ye, M.; Fan, Z.; Chi, Y.; Zhang, Q.; et al. Study of bacterial community succession and reconstruction of the core lactic acid bacteria to enhance the flavor of paocai. Int. J. Food Microbiol. 2022, 375, 109702. [Google Scholar] [CrossRef]
- Huang, T.; Lu, Z.; Peng, M.; Liu, Z.; Chai, L.; Zhang, X.; Shi, J.; Qi, L.; Xu, Z. Combined effects of fermentation starters and environmental factors on the microbial community assembly and flavor formation of Zhenjiang aromatic vinegar. Food Res. Int. 2022, 152, 110900. [Google Scholar] [CrossRef]
- Sadeghi, M.; Panahi, B.; Mazlumi, A.; Hejazi, M.A.; Komi, D.E.A.K.; Nami, Y. Screening of potential probiotic lactic acid bacteria with antimicrobial properties and selection of superiorbacteria for application as biocontrol using machine learning models. LWT 2022, 162, 113471. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Tang, Y.; Li, J.; Huang, R.; Ye, M.; Ming, J.; Wu, Y.; Xu, F.; Lai, X.; et al. Correlation between autochthonous microbial communities and flavor profiles during the fermentation of mustard green paocai (Brassica juncea coss.), a typical industrial-scaled salted fermented vegetable. LWT 2022, 172, 114212. [Google Scholar] [CrossRef]
- An, F. Investigating the core microbiota and its influencing factors in traditional Chinese pickles. Food Res. Int. 2021, 147, 110543. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Gao, Y.; Yang, G.; Liu, X.; Huang, R.; Liang, W.; Li, S. Assessment of autochthonous lactic acid bacteria as starter culture for improving traditional Chinese Dongbei Suancai germentation. LWT 2023, 178, 114615. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Y.; Xiang, F.; Dong, Y.; Hou, Q.; Zhang, Z. Evaluating the flavor and divergent bacterial communities in corn-based Zha-Chili. Food Biosci. 2022, 46, 101563. [Google Scholar] [CrossRef]
- Xiao, M.; Huang, T.; Huang, C.; Hardie, J.; Peng, Z.; Xie, M.; Xiong, T. The microbial communities and fFlavour compounds of Jiangxi yancai, Sichuan paocai and Dongbei suancai: Three major types of traditional Chinese fermented vegetables. LWT 2020, 121, 108865. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhao, Y.; Guan, H.; Jin, C.; Gong, H.; Sun, X.; Wang, P.; Li, H.; Liu, W. Effect of Levilactobacillus brevis as a starter on the flavor quality of radish paocai. Food Res. Int. 2023, 168, 112780. [Google Scholar] [CrossRef] [PubMed]
- Lorn, D.; Thi-Kim-Chi, N.; Phu-Ha, H.; Reasmey, T.; Hélène, L.; Yves, W. Screening of lactic acid bacteria for their potential use as aromatic starters in fermented vegetables. Int. J. Food Microbiol. 2021, 350, 109242. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.H.; Zendo, T.; Kenji, S. Multiple Bacteriocin Production in Lactic Acid Bacteria. J. Biosci. Bioeng. 2022, 134, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Michelle, M.Z.T.; Ye, H.Z.; Li, D. Lactiplantibacillus plantarum 299v as starter culture suppresses Enterobacteriaceae more efficiently than spontaneous fermentation of carrots. Food Microbiol. 2022, 103, 103952. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, Y.; Li LChen, S.; An, K.; Yu, Y.; Xu, Z. Isolation of lactic acid bacteria from Chinese pickle and evaluation of fermentation characteristics. LWT 2023, 180, 114627. [Google Scholar] [CrossRef]
- Hu, C.; Yang, X.; Guo, Q.; Li, B.; Zheng, Y.; Huang, H.; Fan, Y. Isolation, identification, and fermentation characteristics of lactic acid bacteria from the radish pickles brines. Food Ferment. Ind. 2023, 49, 111–118. [Google Scholar] [CrossRef]
- Zhou, Q.; Zheng, Z.; Wu, Y.; Zhang, X.; Jia, Z.; Zhong, K.; Gao, H. Unraveling the core bacterial community responsible for quality and flavor improvement of the radish paocai during spontaneous fermentation. Food Biosci. 2023, 55, 102956. [Google Scholar] [CrossRef]
- Rabelo, J.S.; Dos Santos, E.A.; de Melo, E.I.; Vaz, M.G.M.V.; de Oliveira Mendes, G. Tolerance of Microorganisms to residual herbicides found in eucalyptus plantations. Chemosphere 2023, 329, 138630. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.239-2016; National Food Safety Standard. Determination of Acidity in Food [S]. Standards Press of China: Beijing, China, 2016.
- Le Lay, C.; Mounier, J.; Vasseur, V.; Weill, A.; Le Blay, G.; Barbier, G.; Coton, E. In vitro and in situ screening of lactic acid bacteria and propionibacteria antifungal activities against bakery product spoilage molds. Food Control 2016, 60, 247–255. [Google Scholar] [CrossRef]
- Chen, C.; Jing, L.; Cheng, G.; Liu, Y.; Yi, Y.; Chen, D.; Wang, X.; Cao, J. Flavor changes and microbial evolution in fermentation liquid of sour bamboo shoots. J. Food Compos. Anal. 2023, 120, 105273. [Google Scholar] [CrossRef]
- Rong, Y.; Xie, J.; Yuan, H.; Wang, L.; Liu, F.; Deng, Y.; Jiang, Y.; Yang, Y. Characterization of volatile metabolites in Pu-Erh teas with different storage years by combining GC-E-Nose, GC–MS, and GC-IMS. Food Chem. X 2023, 18, 100693. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Dingze, Y.; Zhao, Q.; Zhou, Y.; Hu, Y.; Sun, X.; Liu, X.; Ma, T. Comprehensive evaluation of the effect of fivesterilization methods on the quality of black carrot juice based on PCA, TOPSIS and GRA models. Food Chem. X 2023, 17, 100604. [Google Scholar] [CrossRef] [PubMed]
- GB/T 10220-2012; Sensory Analysis—Methodology—General Guidance[S]. Standards Press of China: Beijing, China, 2012.
- GB/T 21172-2007; Sensory Analysis—General Principles and Methods for Evaluating Food Colors [S]. Standards Press of China: Beijing, China, 2007.
- GB 16860-1997; Sensory Analysis Methods—Texture Profile Analysis [S]. Standards Press of China: Beijing, China, 1997.
- GB/T 12312-2012; Sensory Analysis—Determination of Taste Sensitivity [S]. Standards Press of China: Beijing, China, 2012.
- Andersen, B.V.; Per, B.B.; Grethe, H. The importance of liking of appearance, -Odour, -taste and -texture in the evaluation of overall Liking. A Comparison with the Evaluation of Sensory Satisfaction. Food Qual. Prefer. 2019, 71, 228–232. [Google Scholar] [CrossRef]
- Mehra, K.S.; Goel, V.; Singh, S.; Gaurav, P.; Singh, A.K. Experimental investigation of emission characteristics of CI engine using Biodiesel-Diesel Blends and Best Fuel Selection: An AHP-TOPSIS approach. Mater. Today Proc. 2023, in press. [CrossRef]
- Yi, L.; Qi, T.; Hong, Y.; Deng, L.; Zeng, K. Screening of bacteriocin-producing lactic acid bacteria in Chinese homemade pickle and dry-cured meat, and bacteriocin identification by genome sequencing. LWT 2020, 125, 109177. [Google Scholar] [CrossRef]
- Satora, P.; Skotniczny, M.; Strnad, S.; Piechowicz, W. Chemical composition and sensory quality of sauerkraut produced from different cabbage varieties. LWT 2021, 136, 110325. [Google Scholar] [CrossRef]
- Huynh, N.K.; Duyen, H.M.N.; Ha, V.H.N. Effects of processing on oxalate contents in plant foods: A review. J. Food Compos. Anal. 2022, 112, 104685. [Google Scholar] [CrossRef]
- Ji, G.; Liu, G.; Li, B.; Tan, H.; Zheng, R.; Sun, X.; He, F. Influence on the aroma substances and functional ingredients of apple juice by lactic acid bacteria germentation. Food Biosci. 2023, 51, 102337. [Google Scholar] [CrossRef]
- Tomita, S.; Watanabe, J.; Kuribayashi, T.; Tanaka, S.; Kawahara, T. Metabolomic evaluation of different starter culture effects on water-soluble and volatile compound profiles in nozawana pickle fermentation. Food Chem. Mol. Sci. 2021, 2, 100019. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, Z.J.; Chao, Y.Z.; Wu, Z.Q.; Zhou, M.X.; Xiao, S.T.; Zeng, J.; Zhe, J. Evaluation by electronic tongue and headspace-GC-IMS analyses of the flavor compounds in dry-cured pork with different salt content. Food Res. Int. 2020, 137, 109456. [Google Scholar] [CrossRef]
- Xiao, M.; Peng, Z.; Hardie, W.J.; Huang, T.; Liu, Z.; Zhang, Y.; Xie, M.; Xiong, T. Exploring the typical flavours formation by combined with metatranscriptomics and metabolomics during Chinese Sichuan paocai fermentation. LWT 2022, 153, 112474. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, Y.; Li, T.; Yang, Y.; Zeng, F.; Wang, H.; Suo, H.; Song, J.; Zhang, Y. Microbial composition and correlation between microbiota and quality-related physiochemical characteristics in chongqing radish paocai. Food Chem. 2022, 369, 130897. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, F. Candidate genes in red pigment biosynthesis of a red-fleshed radish cultivar (Raphanus sativus L.) as revealed by transcriptome analysis. Biochem. Syst. Ecol. 2019, 86, 103933. [Google Scholar] [CrossRef]
- Cui, H.; Chen, F.; Yu j Khizar, H.; Zhang, X. Accurate pH decrease induced inactivation of myrosinase and polyphenol oxidase in salted radish for color improvement. Food Biosci. 2023, 53, 102703. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of specific quantitative peptides of beef by LC–MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]
- Michalak, M.; Kubik-Komar, A.; Adam, W.; Polak-Berecka, M. Starter Culture for Curly Kale Juice Fermentation selected using principal component analysis. Food Biosci. 2020, 35, 100602. [Google Scholar] [CrossRef]
- Xu, Y.B.; Li, D.L.; Ding, X.Q.; Wang, Y.Y.; Liang, S.; Xie, L.Y.; Zhang, Y.F.; Fu, A.K.; Yu, W.Q.; Zhan, X.A. Probiotic characterization and comparison of broiler-derived lactobacillus strains based on technique for order preference by similarity to ideal solution analysis. Poult. Sci. 2023, 102, 102564. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Yang, X.; Hu, C.; Wei, B.; Xu, F.; Guo, Q. Fermentation Performance Evaluation of Lactic Acid Bacteria Strains for Sichuan Radish Paocai Production. Foods 2024, 13, 1813. https://doi.org/10.3390/foods13121813
Fan Y, Yang X, Hu C, Wei B, Xu F, Guo Q. Fermentation Performance Evaluation of Lactic Acid Bacteria Strains for Sichuan Radish Paocai Production. Foods. 2024; 13(12):1813. https://doi.org/10.3390/foods13121813
Chicago/Turabian StyleFan, Yiwen, Xu Yang, Cihai Hu, Banghong Wei, Fei Xu, and Quanyou Guo. 2024. "Fermentation Performance Evaluation of Lactic Acid Bacteria Strains for Sichuan Radish Paocai Production" Foods 13, no. 12: 1813. https://doi.org/10.3390/foods13121813




