Potential Prebiotic Effect of Inulin-Enriched Pasta after In Vitro Gastrointestinal Digestion and Simulated Gut Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
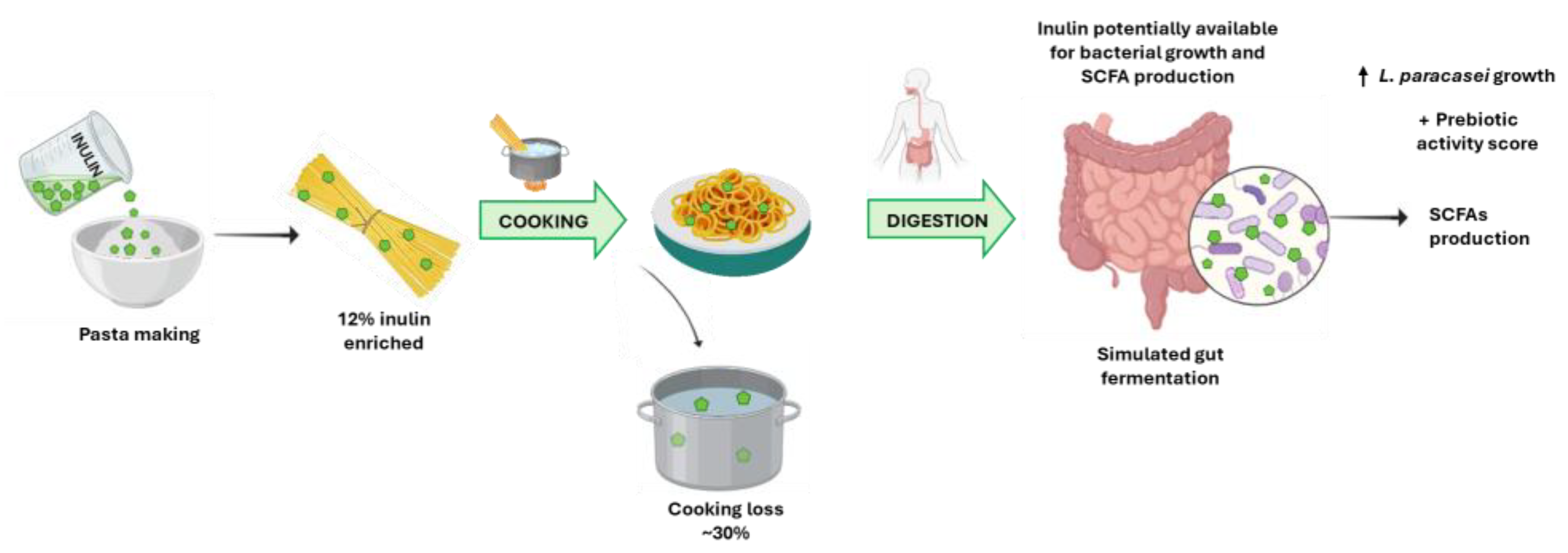
2.2. Inulin-Enriched Pasta
2.3. Determination of Total Protein Content and Amino Acid Content
2.4. Microorganisms and Growth Conditions for Preliminary Prebiotic Potential Evaluation
2.5. Preliminary Prebiotic Potential Evaluation
2.6. In Vitro Gastrointestinal Digestion
2.7. Inulin Quantification
2.8. Simulated Gut Fermentation
2.9. Microbiological Analysis after Simulated Gut Fermentation
2.10. SCFA Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Quality of Pasta
3.2. Prebiotic Potential of the Chicory Inulin
3.3. Inulin Quantification after In Vitro GI Digestion of Inulin-Enriched Pasta
3.4. Prebiotic Potential of Inulin-Enriched Pasta
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gur, J.; Mawuntu, M.; Martirosyan, D. FFC’s Advancement of Functional Food Definition. Funct. Foods Health Dis. 2018, 8, 385–397. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis. Fermentation 2022, 8, 303. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef]
- Yin, P.; Yi, S.; Du, T.; Zhang, C.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; Zhai, Q. Dynamic response of different types of gut microbiota to fructooligosaccharides and inulin. Food Funct. 2024, 15, 1402–1416. [Google Scholar] [CrossRef]
- Ji, K.; Zhang, M.; Du, L.; Wang, J.; Liu, Y.; Xu, C.; He, N.; Wang, Q.; Gu, Y.; Song, H.; et al. Exploring the Role of Inulin in Targeting the Gut Microbiota: An Innovative Strategy for Alleviating Colonic Fibrosis Induced by Irradiation. J. Agric. Food Chem. 2024, 72, 5710–5724. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Gut microbiota and pro/prebiotics in Alzheimer’s disease. Aging 2020, 12, 5539–5550. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Faseleh Jahromi, M.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2017, 206, 1–9. [Google Scholar] [CrossRef]
- Megur, A.; Daliri, E.B.-M.; Baltriukienė, D.; Burokas, A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 6097. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New Horizons for the Study of Dietary Fiber and Health: A Review. Plant Foods Hum. Nutr. 2016, 71, 1–12. [Google Scholar] [CrossRef]
- Hijova, E.; Bertkova, I.; Stofilova, J. Dietary fibre as prebiotics in nutrition. Cent. Eur. J. Public Health 2019, 27, 251–255. [Google Scholar] [CrossRef]
- Panchev, I.; Delchev, N.; Kovacheva, D.; Slavov, A. Physicochemical characteristics of inulins obtained from Jerusalem artichoke (Helianthus tuberosus L.). Eur. Food Res. Technol. 2011, 233, 889–896. [Google Scholar] [CrossRef]
- Mudannayake, D.C.; Jayasena, D.D.; Wimalasiri, K.M.S.; Ranadheera, C.S.; Ajlouni, S. Inulin fructans–food applications and alternative plant sources: A review. Int. J. Food Sci. Technol. 2022, 57, 5764–5780. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef]
- Qin, Y.-Q.; Wang, L.-Y.; Yang, X.-Y.; Xu, Y.-J.; Fan, G.; Fan, Y.-G.; Ren, J.-N.; An, Q.; Li, X. Inulin: Properties and Health Benefits. Food Funct. 2023, 14, 2948–2968. [Google Scholar] [CrossRef]
- Melilli, M.G.; Buzzanca, C.; Di Stefano, V. Quality characteristics of cereal-based foods enriched with different degree of polymerization inulin: A review. Carbohydr. Polym. 2024, 332, 121918. [Google Scholar] [CrossRef]
- Sissons, M. Development of novel pasta products with evidence based impacts on health—A Review. Foods 2022, 11, 123. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Di Stefano, V.; Bonacci, S.; Sollima, L.; Melilli, M.G. Spaghetti Enriched with Inulin: Effect of Polymerization Degree on Quality Traits and α-Amylase Inhibition. Molecules 2022, 27, 2482. [Google Scholar] [CrossRef]
- An, R.; Zhou, X.; He, P.; Lyu, C.; Wang, D. Inulin mitigated antibiotic-induced intestinal microbiota dysbiosis–a comparison of different supplementation stages. Food Funct. 2024, 15, 5429–5438. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Awasthi, M.K.; Varjani, S.; Bhatia, S.K.; Tsai, M.L.; Hsieh, S.-L.; Chen, C.-W.; Dong, C.D. Emerging prospects of macro-and microalgae as prebiotic. Microb. Cell Fact. 2021, 20, 112. [Google Scholar] [CrossRef]
- Ariaee, A.; Wardill, H.R.; Wignall, A.; Prestidge, C.A.; Joyce, P. The Degree of Inulin Polymerization Is Important for Short-Term Amelioration of High-Fat Diet (HFD)-Induced Metabolic Dysfunction and Gut Microbiota Dysbiosis in Rats. Foods 2024, 13, 1039. [Google Scholar] [CrossRef]
- Difonzo, G.; de Gennaro, G.; Caponio, G.R.; Vacca, M.; dal Poggetto, G.; Allegretta, I.; Immirzi, B.; Pasqualone, A. Inulin from Globe Artichoke Roots: A Promising Ingredient for the Production of Functional Fresh Pasta. Foods 2022, 11, 3032. [Google Scholar] [CrossRef]
- Valerio, F.; Russo, F.; de Candia, S.; Riezzo, G.; Orlando, A.; Lonigro, S.L.; Lavermicocca, P. Effects of probiotic Lactobacillus paracasei-enriched artichokes on constipated patients: A pilot study. J. Clin. Gastroenterol. 2010, 44 (Suppl. S1), S49–S53. [Google Scholar] [CrossRef]
- Valerio, F.; De Bellis, P.; Lonigro, S.L.; Morelli, L.; Visconti, A.; Lavermicocca, P. In vitro and in vivo survival and transit tolerance of potentially probiotic strains carried by artichokes in the gastrointestinal tract. Appl. Environ. Microbiol. 2006, 72, 3042–3045. [Google Scholar] [CrossRef]
- Valerio, F.; De Candia, S.; Lonigro, S.L.; Russo, F.; Riezzo, G.; Orlando, A.; De Bellis, P.; Sisto, A.; Lavermicocca, P. Role of the probiotic strain Lactobacillus paracasei LMGP22043 carried by artichokes in influencing faecal bacteria and biochemical parameters in human subjects. J. Appl. Microbiol. 2011, 111, 155–164. [Google Scholar] [CrossRef]
- Grand View Research, 2022. Prebiotics Market Size, Share & Trends Analysis Report by Ingredients (FOS, Inulin, GOS, MOS), by Application (Food & Beverages, Dietary Supplements, Animal Feed), by Region, and Segment Forecasts, 2022–2030. Report ID: 978-1-68038-089. Available online: https://www.grandviewresearch.com/industry-analysis/prebiotics-market# (accessed on 23 May 2024).
- Di Stefano, V.; Pagliaro, A.; Del Nobile, M.A.; Conte, A.; Melilli, M.G. Lentil Fortified Spaghetti: Technological Properties and Nutritional Characterization. Foods 2021, 10, 4. [Google Scholar] [CrossRef]
- Petitot, M.; Boyer, L.; Minier, C.; Micard, V. Fortification of Pasta with Split Pea and Faba Bean Flours: Pasta Processing and Quality Evaluation. Food Res. Int. 2010, 43, 634–641. [Google Scholar] [CrossRef]
- Cleary, L.; Brennan, C. The Influence of a (1 → 3)(1 → 4)-β-D-Glucan Rich Fraction from Barley on the Physico-Chemical Properties and in Vitro Reducing Sugars Release of Durum Wheat Pasta. Int. J. Food Sci. Technol. 2006, 41, 910–918. [Google Scholar] [CrossRef]
- Jung, S.; Rickert, D.A.; Deak, N.A.; Aldin, E.D.; Recknor, J.; Johnson, L.A.; Murphy, P.A. Comparison of Kjeldahl and Dumas methods for determining protein contents of soybean products. J. Am. Oil Chem. Soc. 2003, 80, 1169. [Google Scholar] [CrossRef]
- Bonacci, S.; Di Stefano, V.; Sciacca, F.; Buzzanca, C.; Virzì, N.; Argento, S.; Melilli, M.G. Hemp Flour Particle Size Affects the Quality and Nutritional Profile of the Enriched Functional Pasta. Foods 2023, 12, 774. [Google Scholar] [CrossRef]
- D’Arienzo, R.; Bozzella, G.; Rossi, M.; De Bellis, P.; Lavermicocca, P.; Sisto, A. Distinct immunomodulatory properties of Lactobacillus paracasei strains. J. Appl. Microbiol. 2011, 111, 1482–1491. [Google Scholar] [CrossRef]
- Orlando, A.; Refolo, M.G.; Messa, C.; Amati, L.; Lavermicocca, P.; Guerra, V.; Russo, F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC 2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 2012, 64, 1103–1111. [Google Scholar] [CrossRef]
- Sisto, A.; Luongo, D.; Treppiccione, L.; De Bellis, P.; Di Venere, D.; Lavermicocca, P.; Rossi, M. Effect of Lactobacillus paracasei Culture Filtrates and Artichoke Polyphenols on Cytokine Production by Dendritic Cells. Nutrients 2016, 8, 635. [Google Scholar] [CrossRef]
- De Bellis, P.; Valerio, F.; Sisto, A.; Lonigro, S.L.; Lavermicocca, P. Probiotic table olives: Microbial populations adhering on olive surface in fermentation sets inoculated with the probiotic strain Lactobacillus paracasei IMPC2.1 in an industrial plant. Int. J. Food Microbiol. 2010, 140, 6–13. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Teixeira, F.; Silva, S.K.; Madureira, A.R.; Pintado, M.E. Potential prebiotic activity of Tenebrio molitor insect flour using an optimized in vitro gut microbiota model. Food Funct. 2019, 10, 3909–3922. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, M.; Perez-Morales, R.; Goycoolea, F.M.; Mueller, M.; Praznik, W.; Loeppert, R.; Bermudez-Morales, V.; Zavala-Padilla, G.; Ayala, M.; Olvera, C. Self-assembled high molecular weight inulin nanoparticles: Enzymatic synthesis, physicochemical and biological properties. Carbohydr. Polym. 2019, 215, 160–169. [Google Scholar] [CrossRef]
- Kneifel, W. In vitro growth behaviour of probiotic bacteria in culture media with carbohydrates of prebiotic importance. Microb. Ecol. Health Dis. 2000, 12, 27–34. [Google Scholar] [CrossRef]
- Garbetta, A.; D’Antuono, I.; Melilli, M.G.; Sillitti, C.; Linsalata, V.; Scandurra, S.; Cardinali, A. Inulin Enriched Durum Wheat Spaghetti: Effect of Polymerization Degree on Technological and Nutritional Characteristics. J. Funct. Foods 2020, 71, 104004. [Google Scholar] [CrossRef]
- D’Antuono, I.; Bruno, A.; Linsalata, V.; Minervini, F.; Garbetta, A.; Tufariello, M.; Mita, G.; Logrieco, A.F.; Bleve, G.; Cardinali, A. Fermented Apulian table olives: Effect of selected microbial starters on polyphenols composition, antioxidant activities and bioaccessibility. Food Chem. 2018, 248, 137–145. [Google Scholar] [CrossRef]
- de Albuquerque, T.M.R.; Borges, C.W.P.; Cavalcanti, M.T.; Lima, M.; Magnani, M.; de Souza, E.L. Potential prebiotic properties of flours from different varieties of sweet potato (Ipomoea batatas L.) roots cultivated in Northeastern Brazil. Food Biosci. 2020, 36, 100614. [Google Scholar] [CrossRef]
- Steegmans, M.; Iliaens, S.; Hoebregs, H. Enzymatic, spectrophotometric determination of glucose, fructose, sucrose, and inulin/oligofructose in foods. J. AOAC Int. 2004, 87, 1200–1207. [Google Scholar] [CrossRef]
- Madureira, A.R.; Campos, D.; Gullon, B.; Marques, C.; Rodríguez-Alcalá, L.M.; Calhau, C.; Alonso, J.L.; Sarmento, B.; Gomes, A.M.; Pintado, M. Fermentation of Bioactive Solid Lipid Nanoparticles by Human Gut Microflora. Food Funct. 2016, 7, 516–529. [Google Scholar] [CrossRef]
- Kaewarsar, E.; Chaiyasut, C.; Lailerd, N.; Makhamrueang, N.; Peerajan, S.; Sirilun, S. Optimization of mixed inulin, fructooligosaccharides, and galactooligosaccharides as prebiotics for stimulation of probiotics growth and function. Foods 2023, 12, 1591. [Google Scholar] [CrossRef]
- Brennan, C.S.; Tudorica, C.M. Evaluation of potential mechanisms by which dietary fibre additions reduce the predicted glycaemic index of fresh pastas. Int. J. Food Sci. Technol. 2008, 43, 2151–2162. [Google Scholar] [CrossRef]
- Manno, D.; Filippo, E.; Serra, A.; Negro, C.; De Bellis, L.; Miceli, A. The influence of inulin addition on the morphological and structural properties of durum wheat pasta. Int. J. Food Sci. Technol. 2009, 44, 2218–2224. [Google Scholar] [CrossRef]
- Mueller, M.; Reiner, J.; Fleischhacker, L.; Viernstein, H.; Loeppert, R.; Praznik, W. Growth of Selected Probiotic Strains with Fructans from Different Sources Relating to Degree of Polymerization and Structure. J. Funct. Foods 2016, 24, 264–275. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Introducing inulin-type fructans. Br. J. Nutr. 2005, 93, S13–S25. [Google Scholar] [CrossRef]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. Synergistic effect of different dietary fibres in pasta on in vitro starch digestion? Food Chem. 2015, 172, 245–250. [Google Scholar] [CrossRef]
- Morreale, F.; Benavent-Gila, Y.; Rosell, C.M. Inulin enrichment of gluten free breads: Interaction between inulin and yeast. Food Chem. 2019, 278, 545–551. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Logtenberg, M.J.; Akkerman, R.; An, R.; Hermes, G.D.A.; Haan, B.J.; Faas, M.M.; Zoetendal, E.G.; Schols, H.A.; Vos, P. Fermentation of Chicory Fructo-Oligosaccharides and Native Inulin by Infant Fecal Microbiota Attenuates Pro-Inflammatory Responses in Immature Dendritic Cells in an Infant-Age-Dependent and Fructan-Specific Way. Mol. Nutr. Food Res. 2020, 64, 2000068. [Google Scholar] [CrossRef]
- Pham, V.T.; Seifert, N.; Richard, N.; Raederstorff, D.; Steinert, R.; Prudence, K.; Mohajeri, M.H. The effects of fermentation products of prebiotic fibres on gut barrier and immune functions in vitro. PeerJ 2018, 6, e5288. [Google Scholar] [CrossRef]
- Pham, V.T.; Mohajeri, M.H. The application of in vitro human intestinal models on the screening and development of pre-and probiotics. Benef. Microbes 2018, 9, 725–742. [Google Scholar] [CrossRef]
- Uerlings, J.; Schroyen, M.; Willems, E.; Tanghe, S.; Bruggeman, G.; Bindelle, J.; Everaert, N. Differential effects of inulin or its fermentation metabolites on gut barrier and immune function of porcine intestinal epithelial cells. J. Funct. Foods 2020, 67, 103855. [Google Scholar] [CrossRef]
- Akbari, P.; Fink-Gremmels, J.; Willems, R.H.A.M.; Difilippo, E.; Schols, H.A.; Schoterman, M.H.C.; Garssen, J.; Braber, S. Characterizing Microbiota-Independent Effects of Oligosaccharides on Intestinal Epithelial Cells: Insight into the Role of Structure and Size: Structure–Activity Relationships of Non-Digestible Oligosaccharides. Eur. J. Nutr. 2017, 56, 1919–1930. [Google Scholar] [CrossRef]
- Vogt, L.M.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Ramasamy, U.; Schols, H.A.; de Vos, P. Toll-like receptor 2 activation by beta2-->1-fructans protects barrier function of T84 human intestinal epithelial cells in a chain length-dependent manner. J. Nutr. 2014, 144, 1002–1008. [Google Scholar] [CrossRef]
- Chen, X.; de Vos, P. Structure-function relationship and impact on the gut-immune barrier function of non-digestible carbohydrates and human milk oligosaccharides applicable for infant formula. Crit. Rev. Food Sci. 2023, 2023, 2199072. [Google Scholar] [CrossRef]
- Ragavan, M.L.; Hemalatha, S. The functional roles of short chain fatty acids as postbiotics in human gut: Future perspectives. Food Sci. Biotechnol. 2024, 33, 275–285. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the intestinal epithelium: Modulation of proliferation and inflammation in homeostasis and disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- van Trijp, M.P.; Rios-Morales, M.; Witteman, B.; Abegaz, F.; Gerding, A.; An, R.; Koehorst, M.; Evers, B.; van Dongen, K.C.V.; Zoetendal, E.G.; et al. Intraintestinal fermentation of fructo-and galacto-oligosaccharides and the fate of short-chain fatty acids in humans. Iscience 2024, 27, 109208. [Google Scholar] [CrossRef]

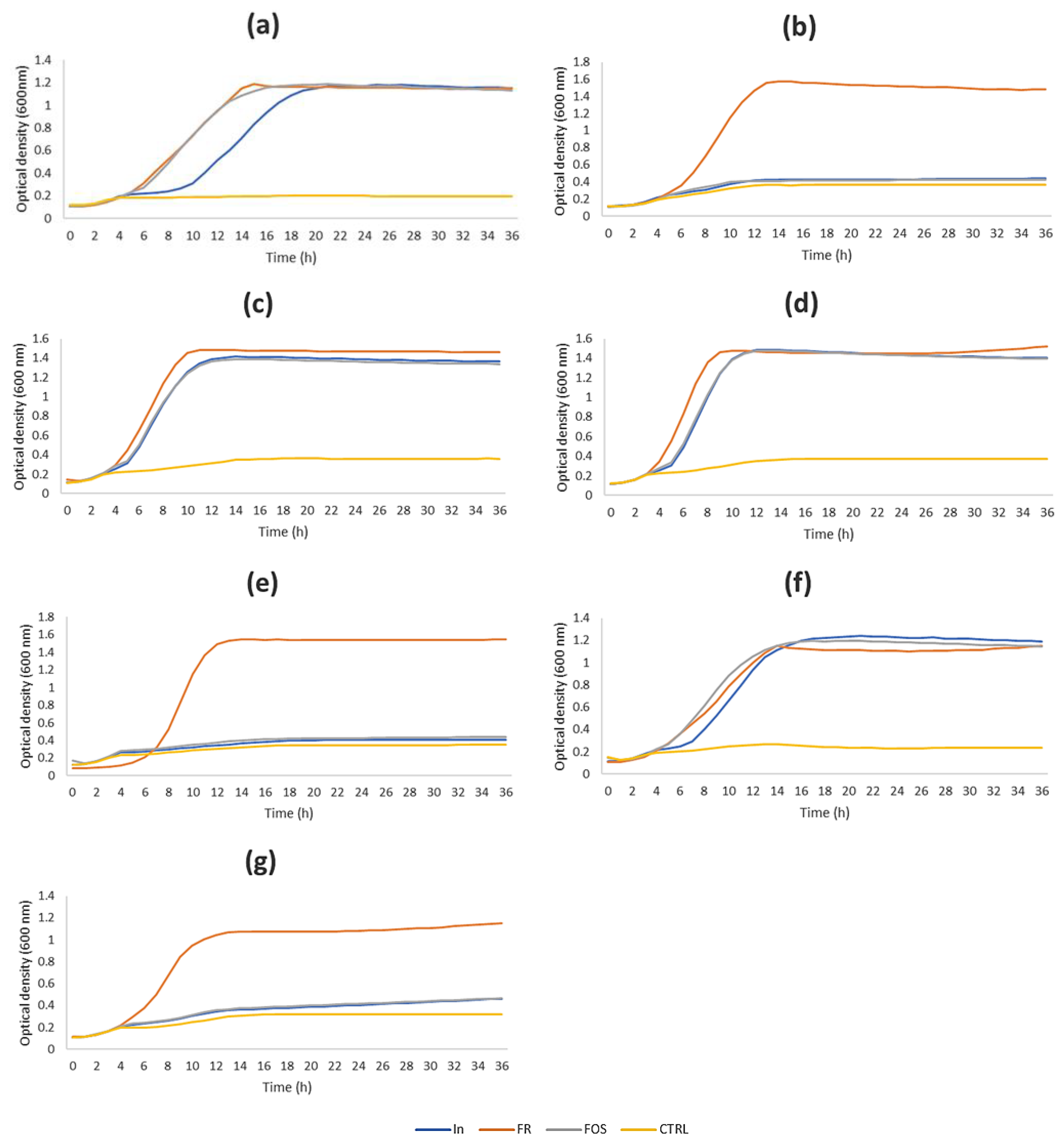
| Microorganism | Origin | Media Incubation Conditions |
|---|---|---|
| Bifidobacterium animalis ssp. lactis Bb12® | Chr. Hansen (Hørsholm, Denmark) | MRS Supplemented with 0.05% (w/v) L-cysteine-HCl Anaerobic, 37 °C |
| Lacticaseibacillus casei 01 | Chr. Hansen (Hørsholm, Denmark) | MRS, aerobic 37 °C |
| Lacticaseibacillus rhamnosus GG | Chr. Hansen (Hørsholm, Denmark) | MRS, aerobic 37 °C |
| Lacticaseibacillus casei 431 | Chr. Hansen (Hørsholm, Denmark) | MRS, aerobic 37 °C |
| Lacticaseibacillus paracasei IMPC2.1 | CNR-ISPA | MRS, aerobic 37 °C |
| Lacticaseibacillus casei IMPC4.1 | CNR-ISPA | MRS, aerobic 37 °C |
| Lacticaseibacillus paracasei/casei P1 | CNR-ISPA | MRS, aerobic 37 °C |
| Trait | CTRL | Inulin Fortified Pasta | p 1 (0.05) |
|---|---|---|---|
| Sensory properties | |||
| Elasticity | 6.5 ± 0.2 | 6.5 ± 0.3 | ns |
| Firmness | 7.3 ± 0.4 | 6.3 ± 0.4 | *** |
| Fibrous | 6.3 ± 0.3 | 6.2 ± 0.3 | ns |
| Bulkiness | 7.8 ± 0.2 | 6.0 ± 0.2 | *** |
| Adhesiveness | 6.5 ± 0.3 | 6.0 ± 0.1 | * |
| Color | 8.0 ± 0.1 | 7.5 ± 0.1 | * |
| Odor | 8.0 ± 0.1 | 7.0 ± 0.1 | ** |
| Taste | 7.8 ± 0.2 | 7.0 ± 0.1 | * |
| Overall Quality Score | 7.3 ± 0.3 | 6.6 ± 0.1 | * |
| Color | |||
| L* | 55 ± 3.0 | 66 ± 2.0 | *** |
| a* | 5.5 ± 1.2 | 3.1 ± 0.8 | *** |
| b* | 17 ± 1.8 | 10 ± 2.1 | *** |
| Cooking quality | |||
| OCT (min) | 11.5 | 7.5 | *** |
| Swelling index | 2.20 ± 0.06 | 1.79 ± 0.04 | * |
| Water Absorption (%) | 89 ± 1.5 | 96 ± 0.7 | *** |
| Amino Acids | CTRL | Inulin-Fortified Pasta | p 1 Value |
|---|---|---|---|
| g/100 g | |||
| Alanine | 2.369 ± 0.005 | 2.153 ± 0.011 | *** |
| Leucine | 1.446 ± 0.008 | 1.530 ± 0.002 | *** |
| Methionine | 0.247 ± 0.004 | 0.259 ± 0.003 | ns |
| Phenylalanine | 0.016 ± 0.002 | 0.029 ± 0.004 | ns |
| Lysine | 1.013 ± 0.002 | 0.971 ± 0.004 | *** |
| Cystine | 1.517 ± 0.023 | 1.526 ± 0.004 | ns |
| Hystidine | 1.831 ± 0.004 | 1.813 ± 0.003 | * |
| TAA | 8.4365 ± 0.012 | 8.2795 ± 0.013 | *** |
| Maximum OD in 36 h | |||||||
|---|---|---|---|---|---|---|---|
| Prebiotic Compound | L. casei 431 | L. casei 01 | L. paracasei IMPC2.1 | L. paracasei IMPC4.1 | L. rhamnosus GG | B. animalis subsp. lactis Bb12 | L. paracasei/casei P1 |
| In | 1.18 ± 0.008 Ab | 0.44 ± 0.036 Aa | 1.41 ± 0.010 Ac | 1.48 ± 0.009 Ad | 0.41 ± 0.035 Aa | 1.23 ± 0.040 Ab | 0.46 ± 0.008 Aa |
| FR | 1.20 ± 0.022 Ac | 1.57 ± 0.010 Ba | 1.49 ± 0.014 Bb | 1.47 ± 0.007 Ab | 1.55 ± 0.006 Ba | 1.15 ± 0.003 Bd | 1.15 ± 0.025 Bd |
| FOS | 1.19 ± 0.06 Ac | 0.43 ± 0.012 Aa | 1.39 ± 0.012 Ad | 1.48 ± 0.012 Ae | 0.44 ± 0.003 Aa | 1.19 ± 0.004 ABc | 0.47 ± 0.009 Ab |
| Control | 0.19 ± 0.003 Ba | 0.36 ± 0.003 Cde | 0.35 ± 0.001 Cd | 0.37 ± 0.001 Be | 0.35 ± 0.005 Cd | 0.24 ± 0.005 Cb | 0.30 ± 0.001 Cc |
| Induction period (h) | |||||||
| In | 7 | – 1 | 3.5 | 3.5 | – | 4 | – |
| FR | 3 | 4 | 3 | 3 | 6 | 1.5 | 3 |
| FOS | 4 | – | 3.5 | 3.5 | – | 2 | – |
| Control | – | – | – | – | – | – | – |
| Sample | T0 | T48 | T0 | T48 | |
|---|---|---|---|---|---|
| Mono-Cultures | |||||
| L. paracasei IMPC2.1 | E. coli ATCC35401 | Prebiotic Activity Score_Monoculture | |||
| Inulin-enriched pasta | 6.81 ± 0.68 a | 9.00 ± 0.79 c | 5.75 ± 0.50 a | 7.63 ± 1.37 c | 0.66 ± 0.17 a |
| FOS | 7.07 ± 0.18 a | 8.51 ± 0.16 bc | 5.88 ± 0.39 a | 7.16 ± 0.28 bc | 0.35 ± 0.21 a |
| Glucose | 7.00 ± 0.14 a | 8.63 ± 1.01 bc | 5.92 ± 0.71 ab | 7.81 ± 0.31 c | - |
| CTRL pasta | 7.07 ± 0.06 a | 7.81 ± 0.53 ab | 5.55 ± 0.23 a | 8.08 ± 1.21 c | −0.88 ± 0.39 b |
| Co-Cultures | |||||
| L. paracasei IMPC2.1 | E. coli ATCC35401 | Prebiotic Activity Score_Co-Culture | |||
| Inulin-enriched pasta | 6.86 ± 0.89 ab | 7.82 ± 1.27 abc | 5.86 ± 0.84 a | 6.06 ± 0.08 a | 0.08 ± 0.03 ab |
| FOS | 6.61 ± 0.61 a | 8.21 ± 0.25 bc | 6.00 ± 0.26 a | 5.37 ± 0.17 a | 0.32 ± 0.16 b |
| Glucose | 6.59 ± 0.58 a | 9.09 ± 0.13 c | 5.86 ± 0.60 a | 5.37 ± 0.31 a | 0.39 ± 0.25 b |
| CTRL pasta | 6.81 ± 0.43 a | 7.31 ± 0.16 ab | 5.50 ± 0.29 a | 7.36 ± 0.19 b | −0.22 ± 0.38 a |
| Sample | Lactate mMol/L | Acetate mMol/L | Butyrate mMol/L | Valerate mMol/L | Propionate mMol/L |
|---|---|---|---|---|---|
| L. paracasei IMPC2.1 | |||||
| Inulin-enriched pasta | 26.25 ± 2.09 ab | 4.78 ± 0.38 de | - | 3.05 ± 0.58 e | 4.92 ± 1.83 bcd |
| FOS | 51.16 ± 9.44 cd | 3.12 ± 0.91 c | 10.75 ± 1.81 b | 2.92 ± 0.40 e | 5.40 ± 0.41 d |
| Glucose | 65.51 ± 18.39 d | 4.10 ± 1.18 cd | 6.00 ± 0.68 a | 2.59 ± 0.69 de | 4.95 ± 1.64 cd |
| CTRL pasta | 9.18 ± 0.37 a | - | - | 0.98 ± 0.09 bc | 2.30 ± 0.12 a |
| E. coli ATCC35401 | |||||
| Inulin-enriched pasta | 6.25 ± 0.35 a | 0.86 ± 0.23 ab | 17.69 ± 4.70 d | 2.93 ± 0.64 e | 1.53 ± 1.25 a |
| FOS | 30.79 ± 7.87 abc | 1.63 ± 0.54 ab | 8.84 ± 0.10 ab | 2.37 ± 0.14 de | 2.69 ± 1.69 ab |
| Glucose | 13.79 ± 8.53 a | 0.69 ± 0.15 a | 8.75 ± 1.37 ab | 2.69 ± 0.28 de | 1.81 ± 0.60 a |
| CTRL pasta | 5.50 ± 0.70 a | 0.84 ± 0.21 ab | 12.18 ± 3.09 bc | 2.24 ± 0.33 de | 1.07 ± 0.60 a |
| Co-cultures | |||||
| Inulin-enriched pasta | 10.84 ± 7.98 a | 0.89 ± 0.31 ab | 15.87 ± 0.81 cd | 2.40 ± 0.59 de | 2.38 ± 0.13 a |
| FOS | 46.77 ± 33.69 bcd | 1.93 ± 0.41 b | 8.18 ± 1.02 ab | 1.86 ± 0.51 cd | 3.45 ± 0.42 abcd |
| Glucose | 137.31 ± 0.20 e | 0.92 ± 0.09 ab | 9.99 ± 1.37 ab | - | 3.01 ± 2.01 abc |
| CTRL pasta | 8.08 ± 0.40 a | - | - | 0.50 ± 0.14 ab | 2.36 ± 0.47 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bavaro, A.R.; Di Biase, M.; Linsalata, V.; D’Antuono, I.; Di Stefano, V.; Lonigro, S.L.; Garbetta, A.; Valerio, F.; Melilli, M.G.; Cardinali, A. Potential Prebiotic Effect of Inulin-Enriched Pasta after In Vitro Gastrointestinal Digestion and Simulated Gut Fermentation. Foods 2024, 13, 1815. https://doi.org/10.3390/foods13121815
Bavaro AR, Di Biase M, Linsalata V, D’Antuono I, Di Stefano V, Lonigro SL, Garbetta A, Valerio F, Melilli MG, Cardinali A. Potential Prebiotic Effect of Inulin-Enriched Pasta after In Vitro Gastrointestinal Digestion and Simulated Gut Fermentation. Foods. 2024; 13(12):1815. https://doi.org/10.3390/foods13121815
Chicago/Turabian StyleBavaro, Anna Rita, Mariaelena Di Biase, Vito Linsalata, Isabella D’Antuono, Vita Di Stefano, Stella Lisa Lonigro, Antonella Garbetta, Francesca Valerio, Maria Grazia Melilli, and Angela Cardinali. 2024. "Potential Prebiotic Effect of Inulin-Enriched Pasta after In Vitro Gastrointestinal Digestion and Simulated Gut Fermentation" Foods 13, no. 12: 1815. https://doi.org/10.3390/foods13121815
APA StyleBavaro, A. R., Di Biase, M., Linsalata, V., D’Antuono, I., Di Stefano, V., Lonigro, S. L., Garbetta, A., Valerio, F., Melilli, M. G., & Cardinali, A. (2024). Potential Prebiotic Effect of Inulin-Enriched Pasta after In Vitro Gastrointestinal Digestion and Simulated Gut Fermentation. Foods, 13(12), 1815. https://doi.org/10.3390/foods13121815








