Compositional Consequences of Ultrafiltration Treatment of White and Red Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wine Samples
2.2. Ultrafiltration of White and Red Wines
2.3. Ultrafiltration of the Highly Phenolic Wine
2.4. Compositional Analysis of Wine, Retentate and Permeate
2.4.1. Basic Chemistry
2.4.2. Wine Colour, Tannins, and Phenolics
2.4.3. Polysaccharides
2.4.4. Proteins
2.5. Statistical and Mass Balance Analysis
3. Results and Discussion
3.1. Composition of Retentate and Permeate following UF of White Wine
3.2. Composition of Retentate and Permeate following UF of Red Wine
3.3. Remediation of a Highly Phenolic Wine by UF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kammakakam, I.; Lai, Z. Next-generation ultrafiltration membranes: A review of material design, properties, recent progress, and challenges. Chemosphere 2023, 317, 137669. [Google Scholar] [CrossRef] [PubMed]
- Meireles, M.; Aimar, P.; Sanzhez, V. Effects of protein fouling on the apparent pore size distribution of sieving membranes. J. Membr. Sci. 1991, 56, 13–28. [Google Scholar] [CrossRef]
- de Boer, R. From Milk Byproducts to Milk Ingredients: Upgrading the Cycle; John Wiley & Sons, Ltd.: Chichester, UK, 2014. [Google Scholar] [CrossRef]
- Hsu, J.C.; Heatherbell, D.A.; Flores, J.H.; Watson, B.T. Heat-unstable proteins in grape juice and wine. II. Characterisation and removal by ultrafiltration. Am. J. Enol. Vitic. 1987, 38, 17–22. [Google Scholar] [CrossRef]
- Pabby, A.K.; Rizvi, S.S.; Requena, A.M.S. Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- Tahkt Ravanchi, M.; Kaghazchi, T.; Kargari, A. Application of membrane separation processes in petrochemical industry: A review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Govindasamy-Lucey, S.; Jaeggi, J.J.; Johnson, M.E.; Wang, T.; Lucey, J.A. Use of cold ultrafiltered retentates for standardization of mils for pizza cheese: Impact on yield and functionality. Int. Dairy J. 2005, 15, 941–955. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Ng, C.Y.; Lim, Y.P.; Ng, G.K. Ultrafiltration in food processing industry: Review on application, membrane fouling, and fouling control. Food Bioprocess Technol. 2012, 5, 1143–1156. [Google Scholar] [CrossRef]
- Gavazzi-April, C.; Benoit, S.; Doyen, A.; Britten, M.; Pouliot, Y. Preparation of milk protein concentrates by ultrafiltration and continuous diafiltration: Effect of process design on overall efficiency. J. Dairy Sci. 2018, 101, 9670–9679. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Mecchia, A.; Drioli, E. Analyses of hydrodynamic resistances and operating parameters in the ultrafiltration of grape must. J. Food Eng. 2008, 89, 171–177. [Google Scholar] [CrossRef]
- Echavarría, A.P.; Torras, C.; Pagán, J.; Ibarz, A. Fruit juice processing and membrane technology application. Food Eng. Rev. 2011, 3, 136–158. [Google Scholar] [CrossRef]
- Perreault, V.; Gouin, N.; Bérubé, A.; Villeneuve, W.; Pouliot, Y.; Doyen, A. Effect of pectinolytic enzyme pretreatment on the clarification of cranberry juice by ultrafiltration. Membranes 2021, 11, 55. [Google Scholar] [CrossRef]
- Alfonso, M.D.; Bórquez, R. Review of the treatment of seafood processing wastewaters and recovery of proteins therein by membrane separation processes—Prospects of the ultrafiltration of wastewaters from the fish meal industry. Desalination 2002, 142, 29–45. [Google Scholar] [CrossRef]
- El Rayess, Y.; Castro-Muñoz, R.; Cassano, A. Current advances in membrane processing of wines: A comprehensive review. Trends Food Sci. Technol. 2024, 147, 104453. [Google Scholar] [CrossRef]
- Massot, A.; Mietton-Peuchot, M.; Peuchot, C.; Milisic, V. Nanofiltration and reverse osmosis in winemaking. Desalination 2008, 231, 283–289. [Google Scholar] [CrossRef]
- García-Martín, N.; Perez-Magariño, S.; Ortega-Heras, M.; González-Huerta, C.; Mihnea, M.; González-Sanjosé, M.L.; Palacio, L.; Prádanos, P.; Hernández, A. Sugar reduction in musts with nanofiltration membranes to obtain low alcohol content wines. Sep. Purif. Technol. 2010, 76, 158–170. [Google Scholar] [CrossRef]
- Fudge, A.L.; Ristic, R.; Wollan, D.; Wilkinson, K.L. Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption. Aust. J. Grape Wine Res. 2012, 17, S41–S48. [Google Scholar] [CrossRef]
- Pham, D.-T.; Ristic, R.; Stockdale, V.; Jeffery, D.W.; Tuke, J.; Wilkinson, K. Influence of partial dealcoholization on the composition and sensory properties of Cabernet Sauvignon wines. Food Chem. 2020, 325, 126869. [Google Scholar] [CrossRef]
- Iland, P.; Bruer, N.; Ewart, A.; Markides, A.; Sitters, J. Monitoring the Winemaking Process from Grapes to Wine: Techniques and Concepts; Patrick Iland Wine Promotions Ptd Ltd.: Campbelltown, SA, Australia, 2004. [Google Scholar]
- Vernhet, A. Red wine clarification and stabilization. In Red Wine Technology; Morata, A., Ed.; Academic Press: London, UK, 2019; pp. 237–251. [Google Scholar] [CrossRef]
- Gonçlaves, F.; Fernandes, C.; de Pinho, M.N. White wine clarification by micro/ultrafiltration: Effect of removed colloids in tartaric stability. Sep. Purif. Technol. 2001, 22–23, 423–429. [Google Scholar] [CrossRef]
- Sui, Y.; McRae, J.M.; Wollan, D.; Muhlack, R.A.; Godden, P.; Wilkinson, K.L. Use of ultrafiltration and proteolytic enzymes as alternative approaches for protein stabilisation of white wine. Aust. J. Grape Wine Res. 2021, 27, 234–245. [Google Scholar] [CrossRef]
- Sui, Y.; Wollan, D.; McRae, J.M.; Muhlack, R.; Capone, D.L.; Godden, P.; Wilkinson, K.L. Chemical and sensory profiles of Sauvignon Blanc wine following protein stabilization using a combined ultrafiltration/heat/protease treatment. Front. Nutr. 2022, 9, 799809. [Google Scholar] [CrossRef]
- Waters, E.J.; Alexander, G.; Muhlack, R.; Pocock, K.F.; Colby, C.; O’Neill, B.K.; Høj, P.B.; Jones, P. Preventing protein haze in bottled white wine. Aust. J. Grape Wine Res. 2005, 11, 215–225. [Google Scholar] [CrossRef]
- Cottereau, P.; Solanet, D.; Vuchot, P.; Ferment, E.; Noilet, P. Réduction de la teneur en sucre des moûts. In Proceedings of the 29the World Congress of Vine and Wine of the International Organization of Vine and Wine (OIV), Logrono, Spain, 25–30 June 2006. [Google Scholar]
- El Rayess, Y.; Mietton-Peuchot, M. Membrane technologies in wine industry: An overview. Crit. Rev. Food Sci. Nutr. 2016, 56, 2005–2020. [Google Scholar] [CrossRef]
- Sui, Y.; Wollan, D.; McRae, J.; Muhlack, R.; Tuke, J.; Wilkinson, K. Impact of commercial scale ultrafiltration on the composition of white and rosé wine. Sep. Purif. Technol. 2022, 284, 120227. [Google Scholar] [CrossRef]
- The Australian Wine Research Institute. A new validated method for the determination of free and total sulfur dioxide using a discrete analyser. Tech. Rev. 2017, 231, 6–11. [Google Scholar]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics-adaptation and validation of methyl cellulose precipitable tannin assay and modified Somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef]
- Bindon, K.A.; Kassara, S.; Solomon, M.; Bartel, C.; Smith, P.A.; Barker, A.; Curtin, C. Commercial Saccharomyces cerevisiae yeast strains significantly impact Shiraz tannin and polysaccharide composition with implications for wine colour and astringency. Biomolecules 2019, 9, 466. [Google Scholar] [CrossRef]
- Culbert, J.A.; McRae, J.M.; Condé, B.C.; Schmidtke, L.M.; Nicholson, E.L.; Smith, P.A.; Howell, K.S.; Boss, P.K.; Wilkinson, K.L. Influence of production method on the chemical composition, foaming properties, and quality of Australian carbonated and sparkling white wines. J. Agric. Food Chem. 2017, 65, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-Y.; Zhang, Q.-A.; Li, E.-C.; Zhang, Y.F. Ions in wine and their relation to electrical conductivity under ultrasound irradiation. J. AOAC Int. 2017, 100, 1516–1523. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Peralta, R.M.; Peralta, R.A.; Rodriguez-Castellon, E.; Moreira, R.F.P.M. Adding value to aluminosilicate solid wastes to produce adsorbents, catalysts and filtration membranes for water and wastewater treatment. J. Mater. Sci. 2021, 56, 1039–1063. [Google Scholar] [CrossRef]
- Vernhet, A.; Moutounet, M. Fouling of organic microfiltration membranes by wine constituents: Importance, relative impact of wine polysccharides and polyphenols and incidence of membrane properties. J. Membr Sci. 2002, 201, 103–122. [Google Scholar] [CrossRef]
- El Rayess, Y.; Albasi, C.; Bacchin, P.; Taillandier, P.; Mietton-Peuchot, M.; Devatine, A. Analysis of membrane fouling during cross-flow microfiltration of wine. Innov. Food Sci. Emerg. Technol. 2012, 16, 398–408. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, L.; Wang, J.; Duan, C.; Sun, Y.; Kong, Q.; He, F. Research progress of protein haze in white wines. Food Sci. Hum. Wellness 2023, 12, 1427–1438. [Google Scholar] [CrossRef]
- van Sluyter, S.C.; McRae, J.M.; Falconer, R.J.; Smith, P.A.; Bacic, A.; Waters, E.J.; Marangon, M. Wine protein haze: Mechanisms of formation and advances in prevention. J. Agric. Food Chem. 2015, 63, 4020–4030. [Google Scholar] [CrossRef] [PubMed]
- Marangon, M.; van Sluyter, S.C.; Haynes, P.A.; Waters, E.J. Grape and wine proteins: Their fractionation by hydrophobic interaction chromatography and identification by chromatographic and proteomic analysis. J. Agric. Food Chem. 2009, 57, 4415–4425. [Google Scholar] [CrossRef] [PubMed]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular properties of red wine compounds and cardiometabolic benefits. Nutr. Metab. Insights 2016, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [PubMed]
- Iland, P.; Bruer, N.; Edwards, G.; Weeks, S.; Wilkes, E. Chemical Analysis of Grapes and Wine: Techniques and Concepts; Patrick Iland Wine Promotions Ptd Ltd.: Campbelltown, SA, Australia, 2004. [Google Scholar]
- Somers, T.C. The polymeric nature of wine pigments. Phytochem 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Gawel, R.; Smith, P.A.; Waters, E.J. Influence of polysaccharides on the taste and mouthfeel of white wine. Aust. J. Grape Wine Res. 2016, 22, 350–357. [Google Scholar] [CrossRef]
- Nel, A.P.; du Toit, W.J.; van Jaarsveld, F.P. Pinking in white wines—A review. S. Afr. J. Enol. Vitic. 2020, 41, 151–157. [Google Scholar] [CrossRef]

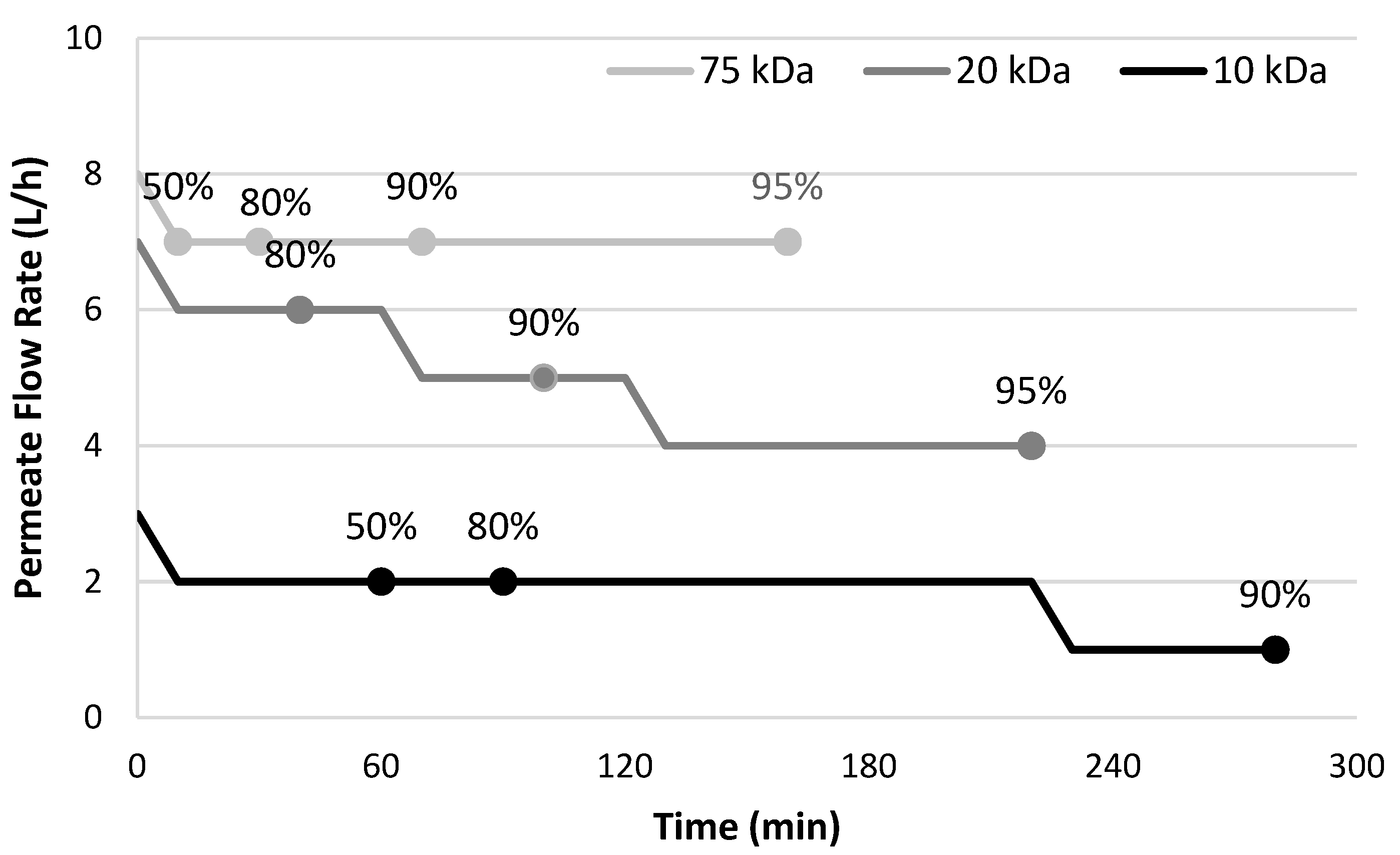
| Degree of Permeation | Feed Volume (mL) | Permeate Volume (mL) | Retentate Volume (mL) |
|---|---|---|---|
| 50% | 5 | 2.5 | 2.5 |
| 80% | 5 | 4 | 1 |
| 90% | 10 | 9 | 1 |
| 95% | 20 | 19 | 1 |
| Membrane MWCO (kDa) | Permeation Degree (%) | pH * | TA (g/L) * | Conductivity (ms/cm) * | Alcohol (abv) * | Protein (mg/L) * | Total Phenolics (a.u.) * | Polysaccharides (g/L) * | |
|---|---|---|---|---|---|---|---|---|---|
| Wine | – | – | 3.03 ± 0.01 e | 6.1 ± 0.01 e | 1.62 ± 0.001 f | 11.9 ± 0.01 ab | 17.0 ± 0.1 f | 2.10 ± 0.01 e | 0.29 ± 0.001 e |
| Retentate | 20 | 50 | 3.23 ± 0.01 ab | 6.2 ± 0.01 e | 1.72 ± 0.003 e | 11.7 ± 0.01 c | 13.5 ± 0.2 h | 1.54 ± 0.05 f | 0.29 ± 0.010 e |
| 80 | 3.23 ± 0.01 ab | 6.5 ± 0.03 d | 1.81 ± 0.001 cd | 11.9 ± 0.01 b | 15.3 ± 0.1 g | 2.59 ± 0.01 d | 0.57 ± 0.012 d | ||
| 90 | 3.24 ± 0.01 a | 6.7 ± 0.01 c | 1.80 ± 0.003 d | 12.0 ± 0.01 ab | 27.7 ± 0.3 e | 3.28 ± 0.04 c | 0.89 ± 0.027 c | ||
| 95 | 3.21 ± 0.01 c | 7.3 ± 0.01 b | 1.88 ± 0.001 b | 12.1 ± 0.12 a | 61.4 ± 0.5 b | 6.09 ± 0.13 b | 1.82 ± 0.204 b | ||
| 10 | 50 | 3.23 ± 0.01 abc | 5.7 ± 0.03 f | 1.70 ± 0.020 e | 11.0 ± 0.01 e | 15.2 ± 0.1 g | 1.89 ± 0.01 e | 0.30 ± 0.009 e | |
| 80 | 3.22 ± 0.01 bc | 6.3 ± 0.15 e | 1.79 ± 0.003 d | 11.3 ± 0.02 d | 37.0 ± 0.1 d | 2.79 ± 0.01 d | 0.57 ± 0.028 d | ||
| 90 | 3.22 ± 0.01 abc | 6.3 ± 0.05 e | 1.84 ± 0.005 c | 11.2 ± 0.01 d | 56.2 ± 0.8 c | 3.48 ± 0.04 c | 1.60 ± 0.078 b | ||
| 95 | 3.19 ± 0.01 d | 7.7 ± 0.03 a | 2.00 ± 0.001 a | 12.0 ± 0.01 ab | 170.4 ± 0.1 a | 8.59 ± 0.02 a | 2.20 ± 0.082 a | ||
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.001 | ||
| Wine | – | – | 3.03 ± 0.01 e | 6.1 ± 0.01 a | 1.62 ± 0.001 cd | 11.9 ± 0.01 a | 17.0 ± 0.1 a | 2.10 ± 0.01 a | 0.29 ± 0.001 a |
| Permeate | 20 | 50 | 3.25 ± 0.01 b | 5.6 ± 0.02 cd | 1.59 ± 0.001 ab | 11.3 ± 0.02 e | 6.4 ± 0.3 b | 1.49 ± 0.01 d | 0.13 ± 0.015 bc |
| 80 | 3.26 ± 0.01 a | 5.7 ± 0.04 bc | 1.63 ± 0.002 bc | 11.6 ± 0.01 d | 6.4 ± 0.4 b | 1.61 ± 0.01 c | 0.12 ± 0.018 bcd | ||
| 90 | 3.26 ± 0.01 a | 5.8 ± 0.05 bc | 1.64 ± 0.001 ab | 11.6 ± 0.01 c | 5.1 ± 0.1 b | 1.62 ± 0.01 c | 0.12 ± 0.010 bcd | ||
| 95 | 3.26 ± 0.01 a | 5.8 ± 0.11 bc | 1.65 ± 0.002 a | 11.8 ± 0.02 b | 5.9 ± 0.1 b | 1.89 ± 0.01 b | 0.16 ± 0.030 b | ||
| 10 | 50 | 3.22 ± 0.01 d | 5.0 ± 0.09 e | 1.56 ± 0.004 f | 10.0 ± 0.01 h | 5.2 ± 1.1 b | 0.20 ± 0.01 g | 0.08 ± 0.005 de | |
| 80 | 3.25 ± 0.01 b | 5.5 ± 0.06 d | 1.60 ± 0.0105 e | 10.2 ± 0.02 g | 5.4 ± 0.1 b | 0.61 ± 0.01 f | 0.08 ± 0.020 de | ||
| 90 | 3.24 ± 0.01 c | 5.5 ± 0.04 d | 1.62 ± 0.006 d | 11.0 ± 0.01 f | 5.1 ± 0.2 b | 0.79 ± 0.01 e | 0.07 ± 0.004 e | ||
| 95 | 3.25 ± 0.01 b | 5.9 ± 0.01 ab | 1.64 ± 0.001 a | 11.7 ± 0.04 c | 6.0 ± 0.3 b | 1.63 ± 0.04 c | 0.10 ± 0.018 cde | ||
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.001 |
| Membrane MWCO (kDa) | Permeation Degree (%) | pH | TA (g/L) * | Conductivity (ms/cm) | Alcohol (abv) | Total Anthocyanins (mg/L) * | Colour Density (a.u.) * | Total Phenolics (a.u.) * | Tannins/ Epicatechin (g/L) * | Polysaccharides (g/L) * | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wine | – | – | 3.52 ± 0.01 ab | 6.7 ± 0.07 f | 2.64 ± 0.01 f | 14.7 ± 0.01 a | 453 ± 2.9 g | 13.7 ± 0.03 g | 56 ± 0.71 f | 1.09 ± 0.01 e | 1.11 ± 0.03 f |
| Retentate | 75 | 50 | 3.56 ± 0.01 a | 7.1 ± 0.01 ef | 2.65 ± 0.01 f | 14.2 ± 0.35 bcd | 633 ± 25 fg | 19.0 ± 0.01 f g | 57 ± 7.4 f | 1.27 ± 0.01 e | 1.00 ± 0.08 f |
| 80 | 3.49 ± 0.02 b | 8.0 ± 0.14 de | 2.82 ± 0.06 def | 14.2 ± 0.07 bcd | 73 ± 88 de | 37.0 ± 0.59 e | 86 ± 4.4 e | 2.34 ± 0.21 d | 1.80 ± 0.29 def | ||
| 90 | 3.50 ± 0.01 b | 8.7 ± 0.49 cd | 2.91 ± 0.08 cde | 14.3 ± 0.28 bcd | 1175 ± 19 d | 43.7 ± 0.11 de | 122 ± 9.7 cd | 2.58 ± 0.13 cd | 2.17 ± 0.37 de | ||
| 95 | 3.49 ± 0.01 b | 10.8 ± 0.21 c | 3.08 ± 0.01 bc | 14.2 ± 0.01 bcd | 2006 ± 1.0 ab | 62.5 ± 0.77 b | 190 ± 5.6 b | 3.27 ± 0.09 b | 2.75 ± 0.10 d | ||
| 20 | 50 | 3.52 ± 0.01 ab | 7.2 ± 0.01 ef | 2.62 ± 0.04 f | 14.2 ± 0.01 bcd | 698 ± 18 fg | 20.4 ± 0.06 fg | 63 ± 5.9 f | 1.38 ± 0.02 e | 1.17 ± 0.11 ef | |
| 80 | 3.50 ± 0.01 b | 8.5 ± 0.07 d | 2.72 ± 0.23 ef | 14.3 ± 0.01 bcd | 1194 ± 136 d | 40.0 ± 0.65 de | 113 ± 14.4 d | 2.68 ± 0.03 c | 2.27 ± 0.15 d | ||
| 90 | 3.50 ± 0.03 b | 8.8 ± 0.28 cd | 2.83 ± 0.02 def | 14.5 ± 0.01 abc | 1868 ± 179 bc | 49.4 ± 0.59 cd | 135 ± 9.6 c | 3.09 ± 0.11 b | 5.60 ± 0.06 c | ||
| 95 | 3.49 ± 0.01 b | 9.6 ± 1.3 c | 2.97 ± 0.08 bcd | 14.6 ± 0.07 ab | 2209 ± 233 a | 74.3 ± 0.80 a | 212 ± 8.5 a | 3.97 ± 0.01 a | 13.28 ± 0.34 b | ||
| 10 | 50 | 3.50 ± 0.03 d | 9.1 ± 0.14 c | 3.16 ± 0.03 b | 14.0 ± 0.07 d | 818 ± 35 ef | 26.2 ± 0.41 f | 73 ± 9.3 ef | 1.37 ± 0.02 e | 1.29 ± 0.06 ef | |
| 80 | 3.42 ± 0.04 c * | 12.5 ± 0.14 b | 3.98 ± 0.21 a | 13.5 ± 0.14 e | 1700 ± 87 ac | 50.3 ± 0.03 cd | 166 ± 10.0 b | 3.25 ± 0.09 b | 2.87 ± 0.48 d | ||
| 90 | 3.42 ± 0.01 c * | 13.5 ± 0.42 a | 3.91 ± 0.03 a | 14.1 ± 0.01 cd | 1952 ± 201 bc | 55.4 ± 0.09 bc | 166 ± 10.7 b | 3.72 ± 0.06 a | 15.65 ± 0.71 a | ||
| p | 0.012 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Wine | – | – | 3.52 ± 0.01 d | 6.7 ± 0.07 a | 2.64 ± 0.01 a | 14.7 ± 0.01 a | 453 ± 2.9 a | 13.7 ± 0.03 a | 56 ± 0.71 a | 1.09 ± 0.01 | 1.11 ± 0.03 a |
| Permeate | 75 | 50 | 3.53 ± 0.01 d | 5.8 ± 0.14 abc | 2.34 ± 0.08 b | 14.1 ± 0.14 c | 152 ± 43 b | 3.8 ± 0.13 b | 19.4 ± 4.60 b | nd | 0.08 ± 0.01 b |
| 80 | 3.53 ± 0.01 d | 5.9 ± 0.14 abc | 2.39 ± 0.06 b | 14.1 ± 0.07 c | 150 ± 23 b | 3.6 ± 0.04 b | 19.5 ± 2.69 b | nd | 0.03 ± 0.01 b | ||
| 90 | 3.53 ± 0.01 d | 6.2 ± 0.07 abc | 2.42 ± 0.01 b | 14.5 ± 0.07 b | 167 ± 22 b | 4.2 ± 0.08 b | 20.9 ± 2.47 b | nd | 0.08 ± 0.01 b | ||
| 95 | 3.53 ± 0.01 d | 6.2 ± 0.07 abc | 2.40 ± 0.01 b | 14.6 ± 0.07 ab | 139 ± 9 b | 3.3 ± 0.02 b | 18.0 ± 0.71 b | nd | 0.03 ± 0.01 b | ||
| 20 | 50 | 3.53 ± 0.01 d | 5.4 ± 0.07 cd | 2.30 ± 0.03 b | 13.8 ± 0.01 d | 124 ± 52 b | 2.9 ± 0.13 b | 16.2 ± 5.0 b | nd | 0.04 ± 0.05 b | |
| 80 | 3.62 ± 0.13 a | 5.5 ± 0.21 bcd | 2.36 ± 0.03 b | 14.1 ± 0.01 c | 112 ± 14 c | 2.4 ± 0.02 b | 15.0 ± 0.9 b | nd | 0.04 ± 0.01 b | ||
| 90 | 3.52 ± 0.02 d | 6.0 ± 0.07 abc | 2.36 ± 0.06 b | 14.4 ± 0.01 b | 122 ± 25 b | 2.6 ± 0.04 b | 16.5 ± 2.6 b | nd | 0.05 ± 0.01 b | ||
| 95 | 3.52 ± 0.04 d | 6.3 ± 0.57 ab | 2.48 ± 0.09 ab | 14.5 ± 0.01 b | 161 ± 41 b | 3.4 ± 0.09 b | 20.6 ± 4.4 b | nd | 0.05 ± 0.01 b | ||
| 10 | 50 | 3.61 ± 0.01 ab | 4.6 ± 0.42 d | 1.75 ± 0.01 c | 13.9 ± 0.01 d | 11.4 ± 2.4 c | 0.3 ± 0.01 c | 2.50 ± 0.14 c | nd | 0.05 ± 0.07 b | |
| 80 | 3.58 ± 0.01 c * | 4.7 ± 0.42 d | 1.82 ± 0.01 c | 14.1 ± 0.07 c | 10.3 ± 1.4 c | 0.3 ± 0.01 c | 2.70 ± 0.57 c | nd | 0.04 ± 0.06 b | ||
| 90 | 3.59 ± 0.01 bc * | 4.7 ± 0.07 d | 1.75 ± 0.01 c | 14.4 ± 0.14 b | 7.4 ± 0.9 c | 0.2 ± 0.01 c | 2.60 ± 0.57 c | nd | n.d. | ||
| p | <0.001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | na | <0.0001 |
| pH | TA (g/L) | Alcohol (abv) | Total Phenolics (a.u.) | Polysaccharides (g/L) | Brown Pigments (a.u.) | |
|---|---|---|---|---|---|---|
| Base Wine | 3.03 ± 0.01 | 6.1 ± 0.01 | 11.9 ± 0.01 | 2.10 ± 0.01 | 0.29 ± 0.001 | – |
| Phenolic Wine | 4.0 ± 0.01 | 5.0 ± 0.1 b | 11.8 ± 0.02 a | 13.1 ± 0.6 b | 1.64 ± 0.05 b | 0.17 ± 0.01 b |
| Retentate | 4.0 ± 0.01 | 8.0 ± 0.2 a | 11.7 ± 0.02 a | 71.9 ± 2.0 a | 8.93 ± 0.72 a | 1.20 ± 0.01 a |
| Permeate | 4.0 ± 0.01 | 4.3 ± 0.5 b | 11.6 ± 0.11 b | 6.6 ± 0.2 c | nd | 0.05 ± 0.01 c |
| p | ns | <0.001 | 0.008 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angela, S.; Wollan, D.; Muhlack, R.; Bindon, K.; Wilkinson, K. Compositional Consequences of Ultrafiltration Treatment of White and Red Wines. Foods 2024, 13, 1850. https://doi.org/10.3390/foods13121850
Angela S, Wollan D, Muhlack R, Bindon K, Wilkinson K. Compositional Consequences of Ultrafiltration Treatment of White and Red Wines. Foods. 2024; 13(12):1850. https://doi.org/10.3390/foods13121850
Chicago/Turabian StyleAngela, Stephanie, David Wollan, Richard Muhlack, Keren Bindon, and Kerry Wilkinson. 2024. "Compositional Consequences of Ultrafiltration Treatment of White and Red Wines" Foods 13, no. 12: 1850. https://doi.org/10.3390/foods13121850







