Abstract
The tomato industry is a relevant socio-economic activity in the European Union, while it generates a large variety of residues. Tomatoes unfit for consumption, tomato peels, seeds, industrial pomace, and plants are examples of residues of this industry. Commonly, some of the residues can be left in the field, composted, used for animal feeding, or valorized through anaerobic digestion. However, more economic value can be attributed to these residues if a biorefinery approach is applied. Indeed, many value-added compounds can be obtained by the integration of different processes while closing the carbon and nutrient loops. The extraction of bioactive compounds followed by anaerobic digestion and composting seems to be a viable proposal for a biorefinery approach. Thus, this study aims to review the biorefinery strategies for valorizing tomato residues, highlighting the main processes proposed. The recovery of lycopene, β-carotene, and phenolic compounds has been widely studied at the lab scale, while energy recovery has already been applied at the industrial scale. Although techno-economic analysis is scarce for tomato residue valorization processes, positive net present values (NPV) and low payback times (PBT) have been reported in the literature. Thus, more work comparing multiple extraction technologies and biorefinery strategies coupled with economic and environmental assessment should be performed to select the most promising management route for tomato residues.
1. Introduction
The increasing pressure from governmental agencies to avoid food waste and the environmental impact of agro-industrial residues have encouraged the scientific community to investigate different strategies for managing and valorizing those waste streams. Biorefinery has been highlighted as a relevant approach for this purpose [1,2]. The integration of well-established processes enhances the recovery of multiple value-added products, focusing on reuse, recycling, and valorization to recover energy. This contributes to the objectives defined in the European Circular Economy Action Plan (CEAP) and other frameworks, such as the European Green Deal (EGD).
The recovery of value-added compounds (proteins, pectin, antioxidants, carotenoids, phenolic compounds, etc.), energy, and nutrients from multiple agro-residues have been widely studied [3,4,5,6]. The use of processes such as extraction, fermentation, anaerobic digestion, pyrolysis, gasification, and composting are the main processes assessed to valorize agro-industrial residues [1,7,8,9,10,11]. However, only recently the integration of those processes has been assessed for some agro-industrial residues applying the biorefinery concept [12,13,14,15]. Although stand-alone processes have proved suitable to valorize the residues with high efficiencies, the use of sequential processes may improve or not the yields of the processes. Almeida et al. [12,13] proved that extracting phenolic compounds from tomato and wine residues did not impair the anaerobic digestion and pyrolysis performance. However, Ninčević Grassino [16] showed that extracting pectin before phenolic compounds drastically reduced the extracting yield. For this reason, the biorefinery viability must be done on a case-by-case basis including techno-economic analysis and life cycle assessment (LCA).
This study will focus on the case study of tomato industries that generate multiple residues including tomatoes unfit for consumption (rotten and green), peels, seeds, pomace, and tomato plants. Thus, the main objective is to summarize the outputs regarding the biorefineries of tomato residues present in the literature. In particular, updated findings will be highlighted regarding specific processes (extraction, anaerobic digestion, and composting) with the greatest potential to be used in biorefinery approaches. An overview of the tomato processing industries, the technological readiness of the processes, and the techno-economic analysis will also be assessed. The review of the tomato residues biorefineries and techno-economic analysis found in the literature for either stand-alone or integrated valorization processes are the main novelty of this work. To the best of the authors’ knowledge, there is no review paper highlighting the biorefinery approaches and economic analysis for tomato residue valorization.
2. Tomato Industry
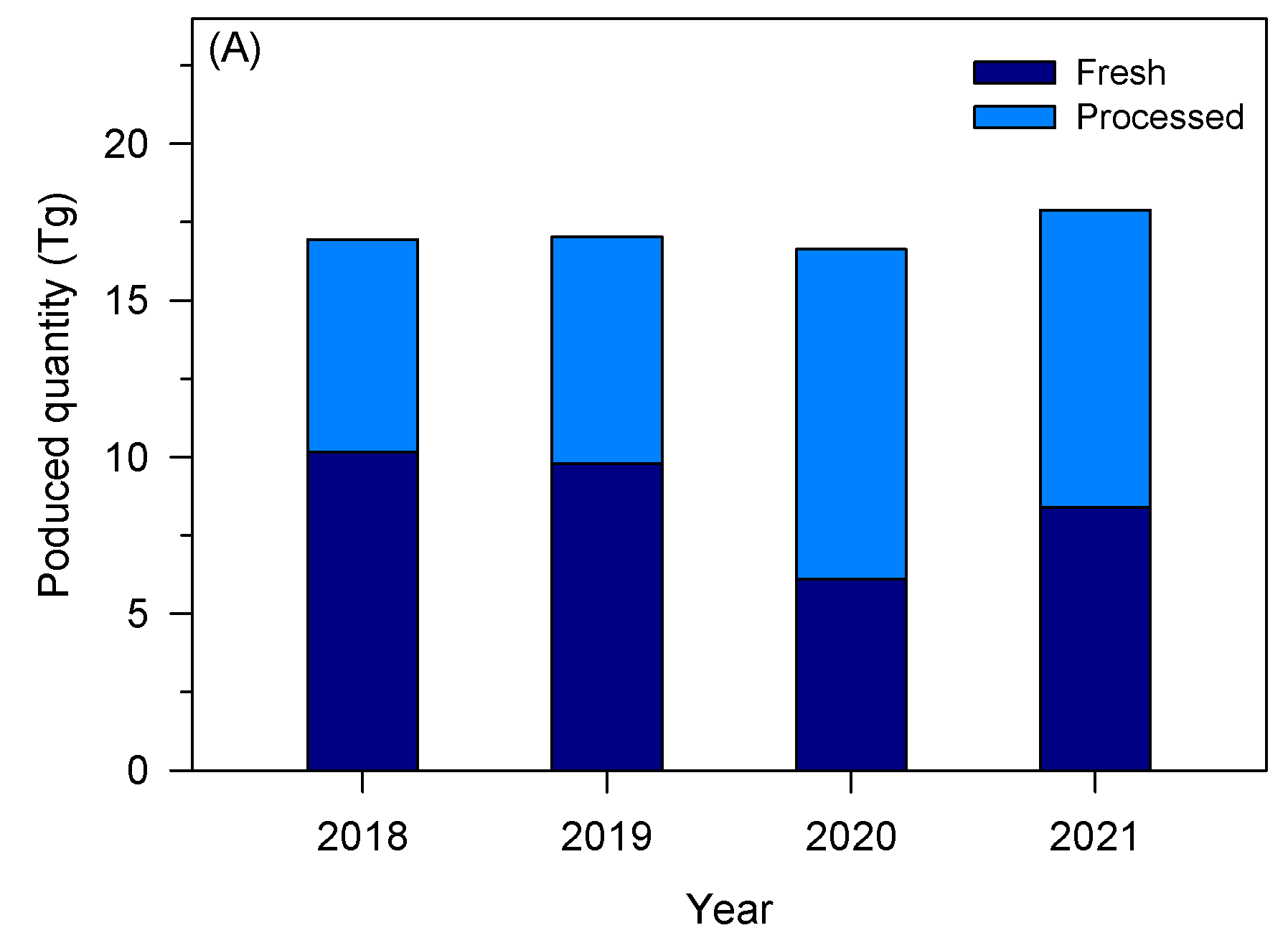
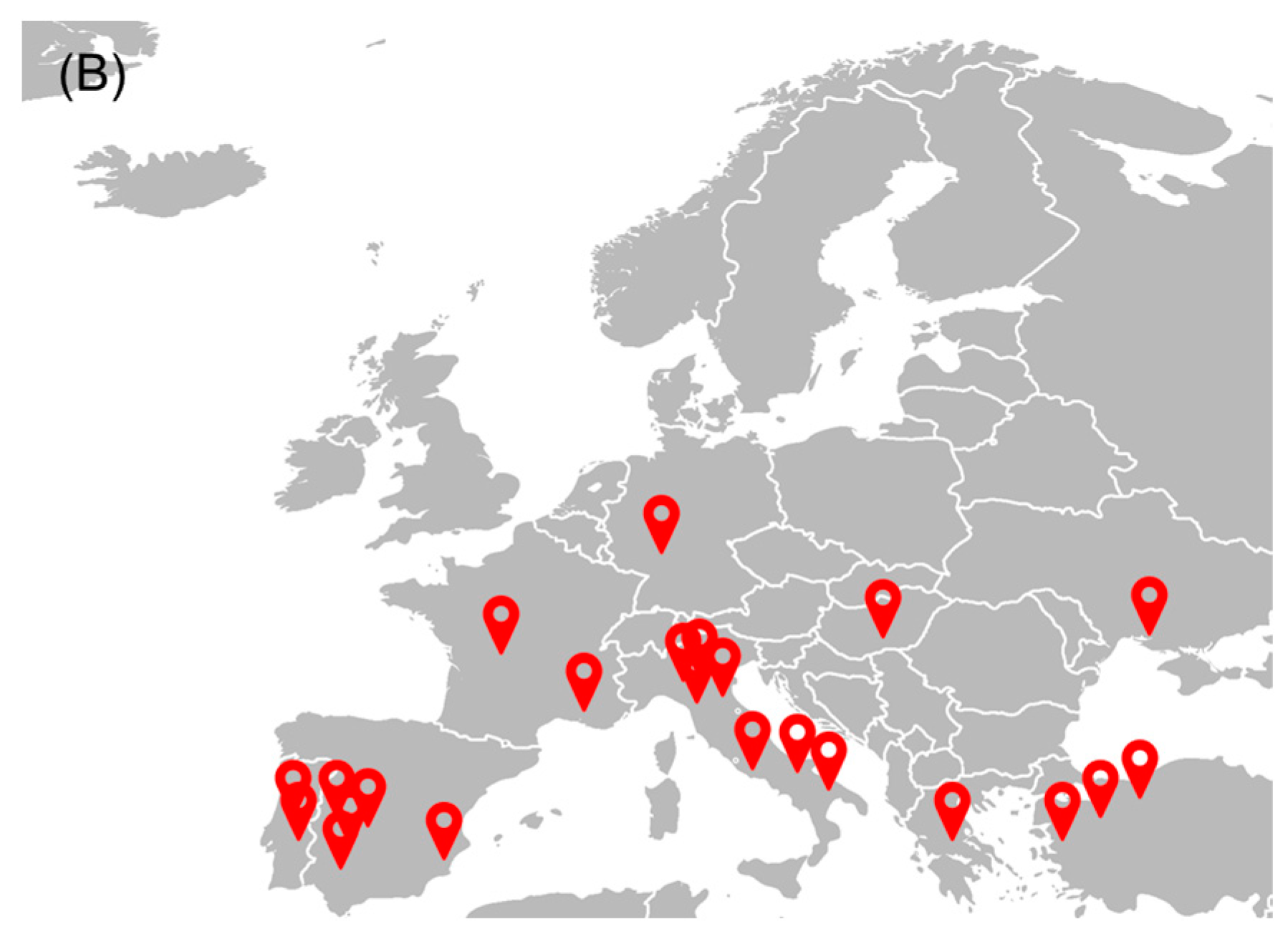
The tomato industry is a relevant socio-economic activity in the European Union (EU), presenting a gross production value of about 91 billion euros [17]. After grapes, tomatoes are among the most produced vegetables and fruits in the EU, representing about 13% in 2022 [18]. Figure 1A shows the quantity of tomatoes produced and processed in the EU over four years. In 2021, about 16.9 Tg of tomatoes were harvested, while Italy, Spain, and Portugal are the top three producers of tomatoes, accounting for 40%, 29%, and 11% of the EU’s total production, respectively [17]. Although tomatoes can be consumed fresh, a large fraction (about 40%) is processed to obtain paste, sauce, puree, and ketchup, making the tomato processing industry also a relevant socio-economic activity. In 2022, the tomato processing industry presented a market value of around 40 billion euros in Western Europe, with an expected annual growth rate ranging between 0.4 and 1.8% [19]. Ready meals, followed by tomato paste, are the most tomato-derivative products produced in Europe [19]. The most relevant groups or companies in Europe are located mainly in Italy, Spain, and Portugal—Figure 1B. Sugal group, Conesa, and Mutti were the main tomato processing industries in Europe in 2020 [20].
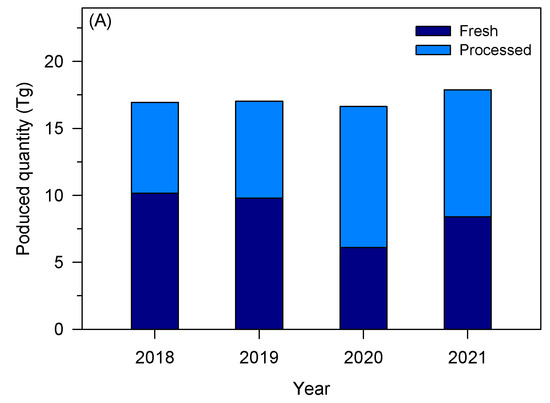
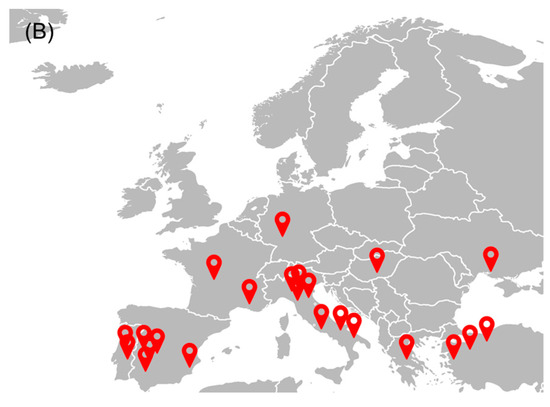
Figure 1.
(A) Quantity of tomato produced to be consumed fresh and after processing in the EU (based on [17]); (B) main tomato processing industries in Europe within the top 50 companies in the sector worldwide (based on [20]).
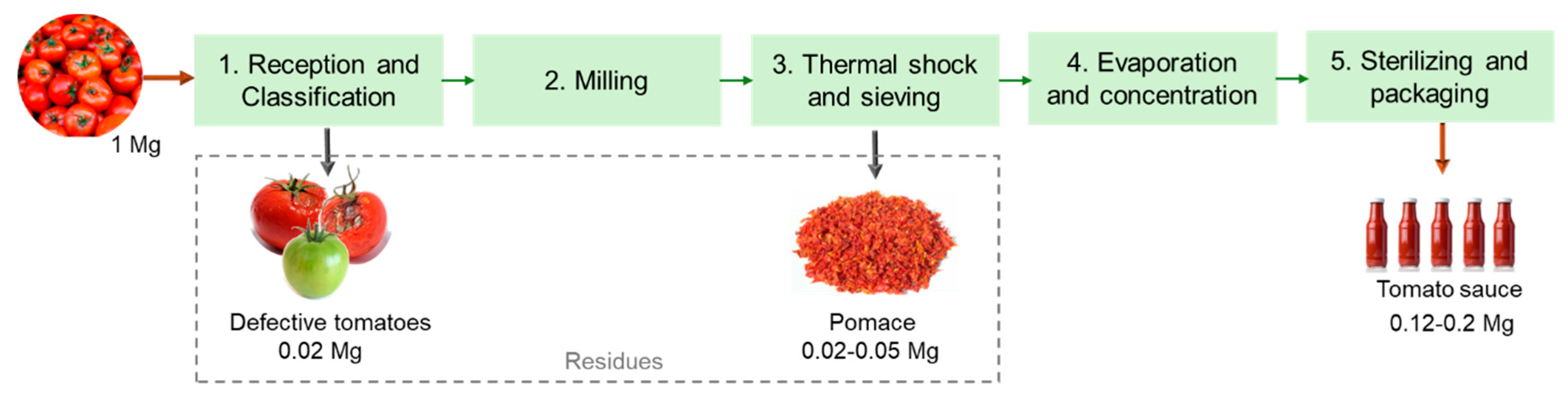
The industrial process for tomato transformation into sauce or paste comprises multiple unit processes, as depicted in Figure 2. Globally, the tomatoes are first evaluated and selected, rejecting the defective tomatoes. Those that meet the quality requirements are driven with water through the production lines facilitating transport and the washing step. Then, the tomatoes are milled and subjected to a thermal shock to secure enzymatic inactivity, preventing product degradation. The peels and seeds are separated from the pulp through sieving with rotating blades. The pulp is then dehydrated using vacuum and temperature to obtain a concentrated product. In the final step, the products are subjected to high temperatures and quickly cooled for sterilization before being bottled [21]. Overall, these industries produce large amounts of residues in the sieving step, which is commonly named tomato pomace and is composed of peels, seeds, and some pulp. According to the literature, about 2–5% of the raw material weight corresponds to tomato pomace [22]. Thus, around 332 Gg of tomato pomace were generated by industries in the EU in 2021. During the selection and washing step, the immature, defective, or damaged tomatoes are also discarded, and the amount of rejection typically corresponds to 2% of the raw material weight [23].
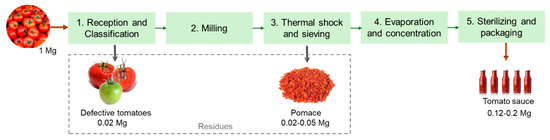
Figure 2.
Schematic representation of a tomato processing industry (based on Lu et al. [22]).
Besides tomato pomace and defective tomatoes, during the harvest season, unripe tomatoes and tomato leaves and stalks are left in the agricultural field, as well as fully ripe tomatoes (rotten). About 15 Mg/ha∙year of residues, including unmarketable tomatoes, leaves, and stalks, may be produced in greenhouse systems [24]. Considering a harvested area of 208,630 ha [18], about 3.1 Tg of residues were generated in the EU during 2022. Moreover, tomatoes can also reach an advanced stage of maturation during commercialization, and all these streams are considered waste in the tomato industry [25].
3. Tomato Residue Composition
The main general physical and chemical characteristics of tomato residues are presented in Table 1. The five residues of tomato presented can be divided into two main categories: the fruit components (tomato rotten, unripe tomato, and tomato pomace) and the more lignocellulosic materials (tomato plant and tomato stalks).
The rotten tomato is mostly composed of water, with a moisture content ranging from 93.8–94.7%. However, 81.1–89.9% of the total solids remaining are organic matter, meaning that both materials have the potential to be biologically processed. The tomato fruit is acidic, with a pH of around 4.5, which must be considered when biological treatments are implemented due to its sensibility to pH. Tomato pomace (peels and seeds) has lower moisture levels (57.6–89.1%) but organic matter content (87.8–97.8%) in the same order as the rotten tomato. The structural carbohydrates (hemicellulose and cellulose), lignin, and protein contents of rotten tomatoes are similar to tomato pomace. According to Del Valle et al. [26], fiber, protein, and lipids are the main components of tomato pomace on a dry matter basis.
Tomato plants and tomato stalks have a similar composition because the whole plant includes the stems. The natural pH of both residues rounds the neutrality (5.4–7.6), and moisture content is between 80.3–92.4%. Similar to tomato fruit and tomato pomace, the plant is rich in lignin (7.6–28.5%), hemicellulose (6.4–16.9%), cellulose (15.5–33.1%), and proteins (13.8–18.6%).

Table 1.
General characterization of tomato rotten, pomace, plant, and stalks.
Table 1.
General characterization of tomato rotten, pomace, plant, and stalks.
| Parameter | Tomato Rotten a | Unripe Tomato b | Tomato Pomace c | Tomato Plant d | Tomato Stalks e |
|---|---|---|---|---|---|
| pH | 4.4–4.6 | 4.0 | 4.6–4.7 | 5.4–7.6 | 7.9 |
| Moisture (%) | 93.8–94.7 | 92.2 | 57.6–89.1 | 80.3–92.4 | 20.77–80.3 |
| Organic matter (%, db) | 81.1–89.9 | 88.0 | 87.8–97.8 | 75.9–79.8 | 63.6–86.3 |
| Ash (%, db) | 15.8–18.9 | - | 3.2–4.1 | 16.0–20.2 | 13.7 |
| Lignin (%, db) | 2.43–19.9 | 4.11 | 13.5–37.3 | 7.6–28.5 | 11.9–17.9 |
| Hemicellulose (%, db) | 3.4–15.2 | 31.02 | 5.4–14.4 | 6.4–16.9 | 7.9–20.4 |
| Cellulose (%, db) | 8.6–16.2 | 23.15 | 7.7–34.0 | 15.5–33.1 | 26.5–43.1 |
| Lipids (%, db) | 2.3–3.4 | 2.75 | 5.0–11.0 | 2.2–11.9 | - |
| Proteins (%, db) | 17.4 | 17.35 | 16.6–30.0 | 13.8–18.6 | - |
| Pectin (%, db) | - | - | 7.5–8.8 | - | - |
| C (%,db) | 42–44 | 38 | 57 | - | 37 |
| N (%, db) | 2.4–3.9 | 2.0 | 0.92 | - | 1.6–2.8 |
| H (%, db) | 5.7–6.5 | 6.0 | 7.5 | - | 5.1 |
| O (%, db) | 27–35 | 42 | 35 | - | 35 |
| Phenolic compounds | |||||
| Caffeic acid (mg/gdb) | - | - | 0.051–1.82 | 0.14–1.5 | 0.25–1.2 |
| p-coumaric acid (mg/gdb) | - | - | 0.0114–2.33 | - | - |
| Chlorogenic acid (mg/gdb) | - | - | 0.04–0.52 | 0.25–3 | 0.25–1.1 |
| Ferulic acid (mg/gdb) | - | - | 0.0023–0.45 | - | - |
| Gallic acid (mg/gdb) | - | - | - | 0.76–4.636 | 0.75–1.0 |
| Naringenin (mg/gdb) | 0.029–25.22 | - | - | - | - |
| Quercetin (mg/gdb) | 0.0373–5.89 | - | - | - | - |
| Rutin (mg/gdb) | 0.0292–1.255 | - | - | 0.15–3.02 | 0.2–1.3 |
| Catechin (mg/gdb) | 0.18–1.23 | - | - | - |
a References [5,12,13,27,28,29,30,31,32,33,34,35,36,37,38,39]; b [5,12,13]; c [26,33,34,35,36,40,41,42,43,44,45,46,47,48,49,50]; d [51,52,53,54,55,56]; e [56,57,58,59,60,61,62,63].
In smaller quantities but with higher value, tomato residues contain carotenoids (β-carotene, lycopene, and lutein) and phenolic compounds. Carotenoids are organic pigments produced by plants and microorganisms and can be divided into two major groups: carotenes and xanthophylls. They are responsible for the yellow, orange, and red colors of many fruits and vegetables. The carotenoid content in tomatoes is affected by agronomic, environmental factors, variety, and ripening stage [64,65]. In addition, the diverse parts of the tomato (peel, pulp, and seeds) can present substantial differences in carotenoid concentration [64], and the extraction process applied significantly affects the result. Those are the main reasons to find a large variability of carotenoid content reported in different studies. This topic will be discussed in depth in Section 4.1.
Also, the phenolic compounds in tomato residues are dependent on the variety of the fruit, the agronomic conditions (light, irrigation, temperature), the maturation stage, the extraction process, and respective conditions (solvent, time), pre-treatments applied, and analytical methodology [12,36,65]. This dependency can explain the wide range of phenolic contents reported. Due to this variability, it is difficult to conclude if tomato residues are richer in flavonoids than phenolic acids or the contrary. Indeed, different authors have reported distinct results—Table 1 [33,36,64,66,67]. Although the varying factors hinder the comparison, an increase in phenolic concentration from tomato seed to tomato peel can be noted [36,67]. Among the phenolic acids, caffeic acid, p-coumaric acid, chlorogenic acid, and ferulic acid are the molecules found in concentrations of potential interest for recovering from tomato pomace [34,35,50,68]. Gallic acid and vanillic acid are also found in tomato residues but in minor amounts. Naringenin, quercetin, rutin, and catechin are the most abundant flavonoids present in tomato fruit residues [32,33,34,35,37,38,39,68]. Catalkaya and Kahveci [50] reported a wide range of rutin concentrations (5–88.1 mg/gdbe—dry basis extract) according to the solvent used, proving the influence of extraction methodology on the phenolic compound profiler.
Phenolic extraction from tomato plants (leaves and stems) has been scarcely studied. Silva-Beltrán et al. [56] analyzed the phenolic profile of different parts of two tomato plants, namely leaves, stems, and their mixture. Gallic acid, chlorogenic acid, caffeic acid, and rutin are the phenolic compounds identified in the tomato plant, in concentrations between 0.76–4.636, 0.25–3, 0.14–1.5, and 0.15–3.02 mg/gdb, respectively. Quercetin and ferulic acid were also identified but in lower concentrations. Considering this study, tomato leaves present the highest phenolic content in the tomato plant.
An increasing demand for these bioactive compounds has been noticed because of the growing awareness of a healthy lifestyle. Indeed, carotenoids and phenolic compounds present biological properties of high interest for the food, cosmetic, and pharmaceutical industries. Because carotenoids are natural pigments, they are used as colorants in foods and cosmetic products [69]. Carotenoids also play an important role in the production of nutraceuticals such as vitamin A (responsible for the protection of the retina from blue and near-ultraviolet light) [69]. It presents high antioxidant activity, enabling its use as a preservative, and some studies revealed that diets rich in carotenoids reduce the risk of prostate cancer [70], benefit osteoporosis [71], and prevent cardiovascular diseases [71]. Phenolic compounds showed antioxidant, anti-inflammatory, and anti-cancer properties [1].
4. Biorefinery Approach for Tomato Residue Valorization
Currently, tomatoes are unfit for consumption, and tomato plants are left in the agriculture field after crop cultivation [12], while at the industrial level, tomato pomace is commonly used for animal feed [72]. More recently, tomato residues have also been used as a substrate in anaerobic digestion to recover energy [73]. Indeed, presently, there are many alternative processes to valorize these residues: extraction of value-added compounds (e.g., pectin, oil, lycopene, β-carotenes, phenolic compounds…), pyrolysis, gasification, anaerobic digestion, and composting. Table 2 summarizes the technology readiness (TRL) of the main technologies used for treating/valorizing tomato residues based on the literature. Many patents were found aiming to extract carotenoids (including lycopene) from tomato residues, considering different strategies. Multiple pre-treatments (WO2003020053A1, US20140316175A1), solvent mixtures (US20100055261A1), and non-conventional extraction processes (CN1799674, CN1334328A, CN101298618A, and CN101121631A) have been claimed in those patents. Proof of their industrial application was not found in the literature. Indeed, 90% of carotenoids are chemically synthesized (US20210115454A1). Although all the technologies mentioned in Table 2 are well-established and may be industrially used for managing tomato residues, valorization may not be maximized with a single process. As mentioned before, the integration of multiple processes aiming at a biorefinery approach may enhance the valorization of tomato residues. Some authors have already studied different biorefinery strategies using tomato residues, and Figure 3 summarizes eight possible approaches.

Table 2.
Technology readiness level (TRL) of the main processes used for treating/valorizing tomato residues considering 3 different scales.
All the biorefineries proposed in the literature using tomato residues include the extraction of valuable compounds, such as carotenoids, phenolics, oil, pectin, and proteins. Ouatmani et al. [82] and Ninčević Grassino et al. [83] proposed a multistage extraction to recover different value-added compounds (oil, phenolic compounds, pectin, and fatty acids). Ouatmani et al. [82] evaluated the extraction of oil (with a yield of 21.69%) from tomato seeds followed by a microwave extraction to recover phenolic and antioxidant compounds (2.31 mg GAE/gdb; 78% of antioxidant activity). Ninčević Grassino [83] studied the effect of removing pectin from tomato peels on phenolic compounds and fatty acids recovery. Indeed, it was proven that tomato peels can be used to extract sequentially pectin (21.7%), phenolic compounds (40.64 mg GAE/gdb), and fatty acids (31.67%, including palmitic acid and linoleic acid). On the contrary, the use of high hydrostatic pressure extraction for pectin recovery counters this conclusion. The recovery efficiency of phenolic compounds from tomato pomace primarily extracted with high hydrostatic pressure for pectin removal was lower than tomato pomace without other pre-processing. The high hydrostatic pressure extraction for tomato pomace affected the recovery yield of phenolic compounds probably because the interest molecules were extracted along with pectin, degraded, or formed complexes with pectin at high pressures [16]. A different strategy for obtaining multiple products was proposed by Bazzarelli et al. [84] The integration of the extraction process with two consecutive membrane systems was used to separate proteins, carbohydrates, and phenolic compounds from tomato leaves. Azabou et al. [85], Baker et al. [49], and Kehili et al. [86] evaluated the valorization of tomato pomace by processing peels and seeds separately. Azabou et al. [85] extracted tomato peels and seeds to obtain bioactive compounds (phenolic compounds: 199.35 mg GAE/gdbe and lycopene: 0.0367 mg/gdb) and oil (17.15%), respectively. The remaining tomato peel and seed extracted were mixed and subjected to a thermal treatment to produce a biosorbent for dye removal. A zero-waste path was evaluated in this work. Although Baker et al. [49] do not assess the biorefinery proposed, a zero-waste path is also presented. Briefly, the tomato peels are extracted to obtain carotenoids, while tomato seeds are treated with grinders and membrane processes to recover proteins. The remaining solids (from tomato seed and peel) are mainly composed of fibers that can be used for animal feed. The sequential extraction of carotenoids and proteins was evaluated by Kehili et al. [86] Indeed, the purity of proteins recovered was higher after the supercritical fluid extraction. The solid spent of the extraction was submitted to a thermal treatment to hydrolyze the cellulose and hemicellulose into smaller molecules that can be used in fermentation processes to produce bioethanol. Kehili et al. [86] concluded that raw tomato seeds and peels lead to higher concentrations of sugars than extracted solids. Even so, an extensive techno-economic analysis should be performed to realize which strategy would be the most profitable. Allison and Simmons [87] and Almeida et al. [12,13] conjugated the extraction process with an energetic valorization process for three different types of tomato residues. The studies concluded that the extraction of bioactive compounds (phenolic compounds, β-carotene, and lycopene) did not negatively affect the energetic recovery compared to a stand-alone process. Thus, bioactive compounds can be recovered without compromising the energetic value either through anaerobic digestion or pyrolysis.
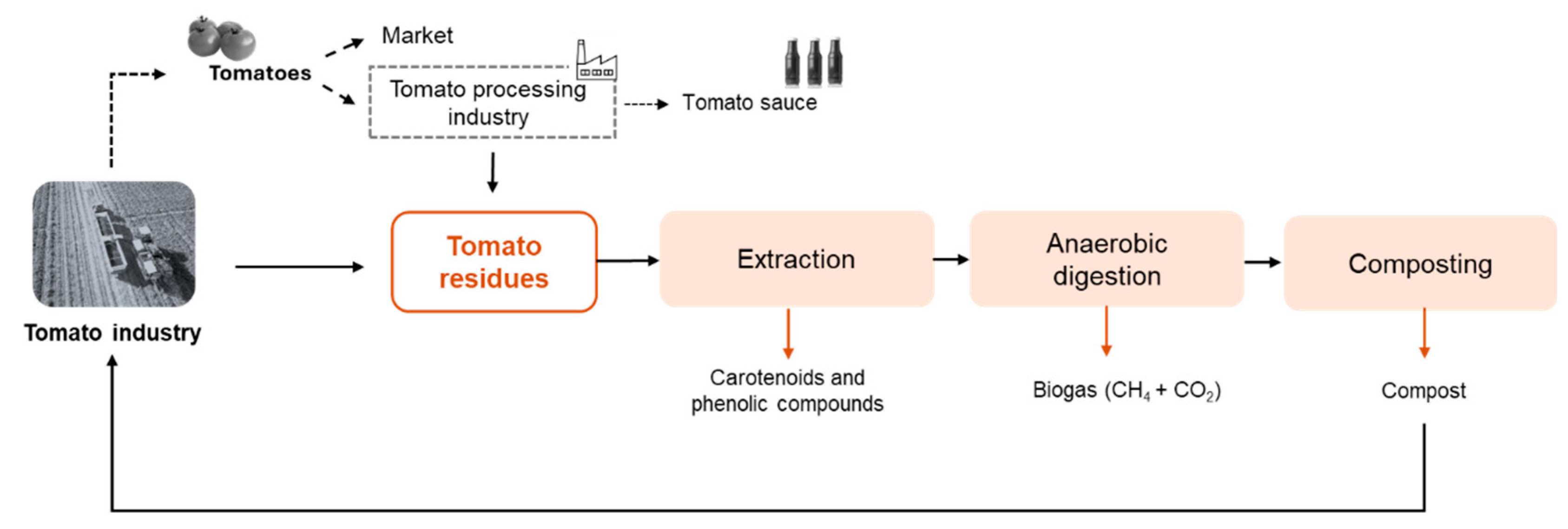
These technological configurations are far from being industrially applied. The comparison between them is hard due to the diverse combinations and results obtained. Moreover, an economic analysis coupled with an LCA should be done to support the selection of the best biorefinery proposition. Even so, this is a recent topic that needs to be investigated in depth. More studies and alternatives can be explored first, aiming to maximize the routes of valorization. The authors of the present study propose a strategy to manage and create value from tomato residues (Figure 4), which allows the carbon cycle (and other nutrients) to be closed. The proposal includes the extraction of bioactive compounds (phenolic compounds, β-carotenes, and lycopene), followed by anaerobic digestion, producing biogas, and composting process to stabilize the organic matter of digestate, generating a stable compost. In addition, many other processes can be added to the biorefinery approach to separate and purify the products. For example, the extract rich in bioactive compounds can be treated by sequential membrane processes to divide the main compounds into single products, as Bazzarelli et al. [84] proposed. The fractionation of extracts obtained from tomato residues has been scarcely studied through membrane processes [84]. However, microfiltration, ultrafiltration, and nanofiltration have been used to separate proteins, carbohydrates, and phenolic compounds from agro-food by-products (olive-mill wastewater, orange press liquor, grape pomace, and others) [88]. Although the separation and purification of value-added compounds is possible, the crude extract presents a mixture of bioactive compounds that may present positive synergistic effects, enhancing the beneficial properties of the molecules. Moreover, separating and purifying increases the operating costs; thus, the application of the crude extract should be prioritized whenever possible [1].
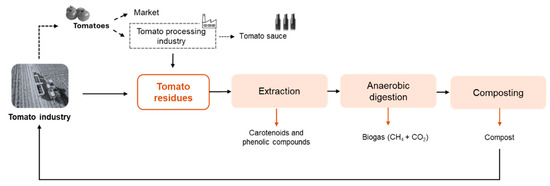
Figure 4.
Biorefinery approach proposed in the present study to manage and create value for tomato residues (tomato rotten, green, peels, seeds, pomace, and tomato plant).
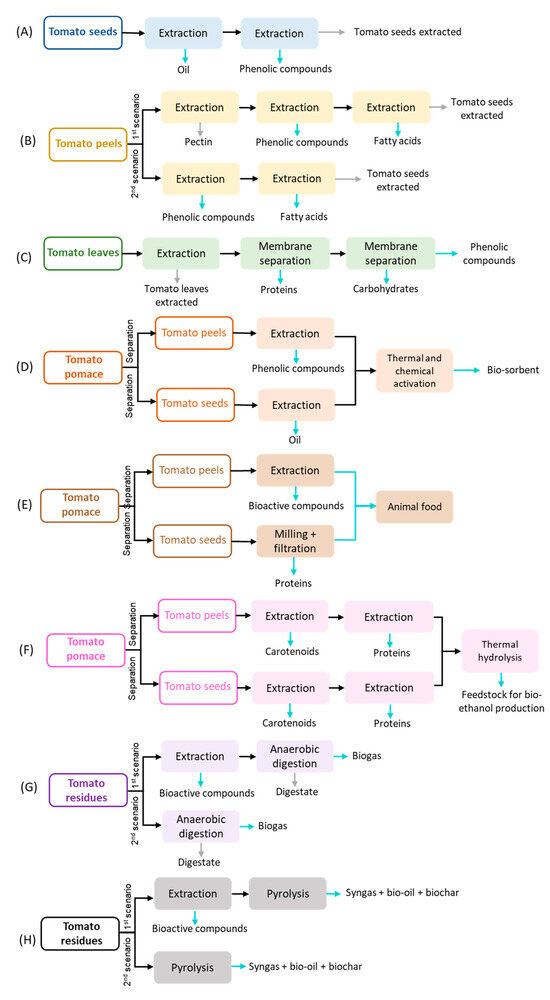
Figure 3.
Biorefinery approach proposed to manage and create value for tomato residues based on Ouatmani et al. [82] (A); Ninčević Grassino [83] (B); Bazzarelli et al. [84] (C); Azabou et al. [85] (D); Baker et al. [49] (E); Kehili et al. [86] (F); Almeida et al. [12]; Allison and Simmons [87] (G); and Almeida et al. [13] (H).
Figure 3.
Biorefinery approach proposed to manage and create value for tomato residues based on Ouatmani et al. [82] (A); Ninčević Grassino [83] (B); Bazzarelli et al. [84] (C); Azabou et al. [85] (D); Baker et al. [49] (E); Kehili et al. [86] (F); Almeida et al. [12]; Allison and Simmons [87] (G); and Almeida et al. [13] (H).
4.1. Recovering Value-Added Compounds through Extraction Processes
Solid-liquid extraction processes (SLE) are commonly used to recover carotenoids and phenolic compounds from tomato residues. These processes separate the soluble molecules present in the solid matrix through solvent contact. The molecules of interest are dissolved in the solvent, while diffusion mechanisms occur until equilibrium is reached. All strategies that improve the solubility of the molecule and diffusion of the solvent enhance extraction efficiency [89]. The operating conditions of SLE for recovering lycopene and β-carotene from tomato residues are summarized in Table 3. Hexane, acetone, methanol, and ethyl lactate are the most used solvents for extracting carotenoids. Mixtures with some of those solvents have enhanced the extraction efficiencies. Indeed, a mixture of 50% hexane and 50% ethyl acetate allowed an extraction efficiency of 16% and 30% higher than individual ethyl acetate and hexane, respectively [90]. A similar trend was observed for other solvents [90].
In addition to the solvent concentration, other operating conditions play an important role in extraction efficiency. Typically, higher temperatures, liquid-solid (L/S) ratios, and time of contact lead to high extraction yields [91]. Even so, room temperature and L/S ratios between 10 and 100 mL/g have been commonly used among conventional extraction processes to recover carotenoids.
Non-conventional extraction processes, such as ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), and supercritical fluid extraction (SFE), have also been studied to recover carotenoids from tomato residues. Design of experiments (DoEs) is one of the most used methodologies to optimize the operating conditions for maximizing the recovery of carotenoids [91,92,93,94]. More recently, the DoE was also applied to compare different SLE processes aiming to select the most suitable [93].
According to Kumcuoglu et al. [91], time is the most important factor in UAE. The lycopene yield increased sharply in the first minutes, whereas after 20 min, the yield remained constant [91]. UAE allows for obtaining similar carotenoid yields while reducing the extraction time by 1.5 to 3.0 times compared with the conventional extraction method [95]. Large sonication times (>40 min) can cause sample deterioration [96].
MAE can also reduce the time of extraction and enhance the lycopene yield. Indeed, Ho et al. [97] obtained about 170 mg/kgdb of lycopene in 1 min with MAE, while 138 mg/kgdb was achieved in 30 min with the conventional process.
SFE was studied as an alternative to extraction processes based on toxic organic solvents. Multiple units have been used to report the results obtained through SFE, hindering its comparison (Table 3). Nevertheless, Popescu et al. [98] obtained extracts richer in lycopene content through conventional extraction (4500 mg/kgdbe) than SFE (2500 mg/kgdbe). Ubeyitogullari and Ciftci [99] reported statistically similar carotenoid content extracted through SFE and hexanic extraction. However, the bioaccessibility of lycopene in the extracts was enhanced through SFE compared with hexanic extraction.
Other strategies have been evaluated to improve the extraction of carotenoids, including sequential extractions and pre-treatments. Strati and Oreopoulou [100] revealed that in the second and third extraction step about 20–30% and 5–15%, respectively, of the total carotenoid is still recovered. Drying and milling are the most common pre-treatments applied. Indeed, most of the authors dry and mill the tomato samples before studying the extraction. Nonetheless, Allison and Simmons [87], Chada et al. [93], and Lazzarini et al. [101] evaluated the effect of different drying methods. According to Allison and Simmons [87], higher carotenoid concentrations were obtained for vacuum-dried pomace (293–476 mg/kgdb). For solar-dried pomace, the lycopene content was below the detection limit (<5 mg/kgdb). Freeze-drying and non-thermal air drying enhanced (about 2.38–6.08 times) the recovery of lycopene compared to non-drying and heat drying (85 °C, 2 h) [101]. Moreover, non-drying revealed higher concentrations (34.11 mg/kgdbe) of lycopene than heat drying (15.87 mg/kgdbe). The selection of the drying process, as well as the temperature, is relevant because the carotenoids are sensitive to high temperatures. Authors have shown that temperatures higher than 70 °C increase the degradation rate of lycopene and β-carotene [102,103,104,105]. Thus, oven drying under 70 °C is highly recommended.
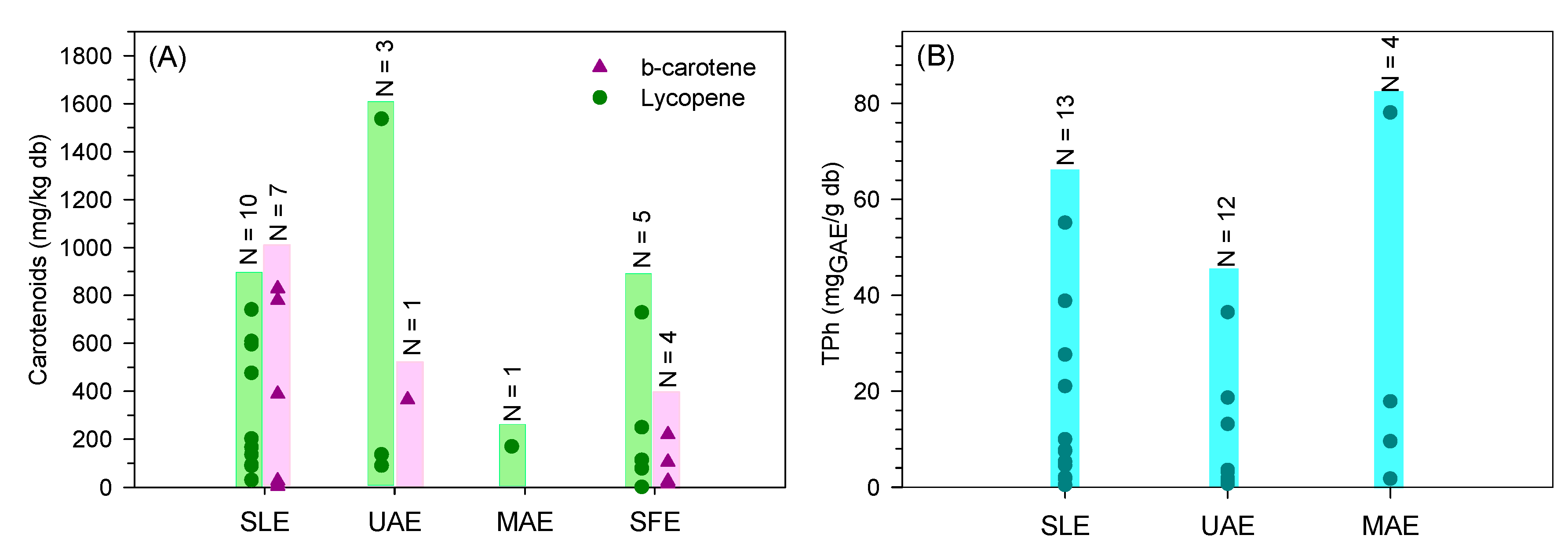
The wide range of β-carotene and lycopene content extracted from tomato fruit residues is well visible in Figure 5A. Indeed, the different operating conditions and units used in the studies are responsible for the variability of results. Furthermore, the lycopene and β-carotene content strongly depend on the tomato variety. Adalid et al. [106] studied the composition of 49 varieties of tomatoes and reported a large variability in lycopene and β-carotene content ranging between 0.4–167 and 2.9–14.6 mg/kgdb, respectively. Even so, no clear evidence is noted among the different solid-liquid extractions (conventional, UAE, MAE, and SFE). A more concise and systematic study comparing the multiple technologies and an economic evaluation should be done to select the most promising extraction process to recover carotenoids.

Table 3.
β-carotene and lycopene content (mg/kgdb) achieved by SLE of tomato residues.
Table 3.
β-carotene and lycopene content (mg/kgdb) achieved by SLE of tomato residues.
| Ref. | Operation Conditions | Residue | β-Carotene | Lycopene | Total Carotenoids |
|---|---|---|---|---|---|
| Conventional extraction | |||||
| [87] | Solvent: hexane:acetone:ethanol (50:25:25%); T: 100 °C; t: 90 min; L/S: 100; dp: <1 mm | Pomace | - | 476 | - |
| [90] | Solvent: hexane:ethyl acetate (50:50%); T: 25 °C; t: 30 min; L/S: 10; dp: <1 mm | Pomace | 4.75 | 30.3 | 36.5 |
| [91] | Solvent: hexane:methanol:acetone (50:25:25%); T: 20–60 °C; t: 10–40 min; L/S: 20–50; dp: 0.286 mm | Pomace | - | 93.9 | - |
| [100] | Solvent: ethyl lactate; T: 25 °C; t: 40 min; L/S: 10; dp: <1 mm | Pomace | - | 203 | - |
| [107] | Solvent: hexane; t: 12 h; L/S: 30; dp: <0.3 mm | Peels | 27.9 | 609 | - |
| [95] | Solvent: acetone; T: 20 °C; t: 20 min; L/S: 30; dp: <1 mm | Peels | 389 | 136 | 568 |
| [98] | Solvent: ethyl acetate; L/S: 12.5 | Tomato | ~9500 b/828 | ~8500 b/741 | - |
| Pomace | ~5900 b/780 | ~4500 b/595 | - | ||
| [106] | Solvent: ethanol: hexane (57:43%); t: 60 min; L/S: 70; dp: <0.4 mm | Tomato | 14.6 | 167 | - |
| [108] | Solvent: Capric acid/lauric acid (33:67%); t: 62 min; L/S: 61 | Tomato | - | 79.9 a | - |
| [108] | Solvent: acetone; L/S: 61 | Tomato | 3.7 a/4.0 | 81.5 a/89 | 88.6 a/96.3 |
| [12] | Solvent: hexane; t: 6 h; L/S: 67 | Rotten | 53.2 b | 6.08 b | - |
| Green | 8.6 b | 2.57 b | - | ||
| Plant | 53.2 b | 13.8 b | - | ||
| [109] | Solvent: ethanol:acetic acid (95:5%); T: 25 °C; t: 72 h | Stem | - | 1772 b | |
| Leaves | - | 5354 b | |||
| Root | - | 493 b | |||
| Plant | - | 2845 b | |||
| Ultrassoud-assisted extraction | |||||
| [91] | Solvent: hexane:methanol:acetone (50:25:25%); t: 1–30 min; L/S: 20–50; power: 50–90 W; dp: 0.286 mm | Pomace | - | 90.1 | - |
| [92] | Solvent: ethanol; t: 20 min; T: 65 °C; L/S: 72; amplitude: 65; dp: <0.732 mm | Pomace | - | 1536 | 1408 |
| [95] | Solvent: acetone; T: 20 °C; t: 5–20 min; L/S: 30; Power: 45 kHz; dp: <1 mm | Peels | 365 | 136 | 539 |
| [101] | Solvent: acetone:n-hexane; t: 20 min; T: 63 °C; S/L: 0.1 | Pomace | 5717.46 b | 96.55 b | - |
| [96] | Solvent: acetonitrile:methanol (80:20%); T: 20–30 °C; t: 45 min; L/S: 180; power: 100 W | Green | 30 a | 45 a | - |
| Red-ripe | 80 a | 700 a | - | ||
| Microwave-assisted extraction | |||||
| [97] | Solvent: ethyl acetate; t: 1 min; L/S: 20; power: 400 W; energy: 24 kJ; dp: <5 mm | Pomace | - | 170 | - |
| Supercritical fluid extraction | |||||
| [98] | Solvent: CO2; P: 400 bar; T: 70 °C; solvent flow rate: 11 kg/h; mass sample: 180 g | Tomato | ~2000 b/105 | ~1500 b/79 | - |
| Pomace | ~2200 b/220 | ~2500 b/250 | - | ||
| [99] | Solvent: CO2; T: 80 °C; t: 20 min; mass sample: 12 g; P: 300 bar; solvent flow rate: 1 L/min | Pomace | 220 b/25.3 | 990 b/114 | - |
| [110] | Solvent: CO2; T: 80 °C; t: 20 min; P: 500 bar; solvent flow rate: 30.4 mg/s; dp: <1 mm | Pomace | - | 1.32 a/1.4 | |
| [107] | Solvent: CO2; t: 180 min; mass sample: 10 g; P: 400 bar; solvent flow rate: 4 g/min; dp: <0.3 mm | Peels | 16.4 | 729 | - |
| Enzymatic-assisted extraction | |||||
| [94] | Enzyme: 1.4%; time: 92 min; T: 52 °C | Pomace | - | 3996 | - |
a mg/kgfw—fresh weight; b mg/kgdbe—dry basis extract; S/L—solid-liquid ratio in mL/g; dp—avarage particle diameter.
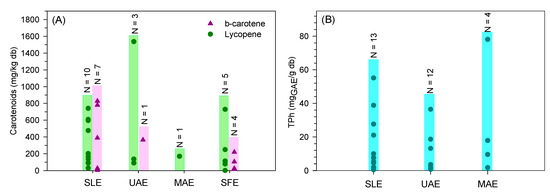
Figure 5.
Distribution of (A)—carotenoids and total phenolic content, (B)—obtained through conventional extraction (SLE), ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), and supercritical fluid extraction (SFE) found in the literature (N represents the number of results from the literature).
Figure 5.
Distribution of (A)—carotenoids and total phenolic content, (B)—obtained through conventional extraction (SLE), ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), and supercritical fluid extraction (SFE) found in the literature (N represents the number of results from the literature).
The recovery of phenolic compounds from tomato residues through SLE and non-conventional SLE processes are summarized in Table 4 and Table 5, respectively. Similar to the carotenoids, many authors have also used DoEs to evaluate the operating conditions that maximize the extraction of phenolic compounds [50,111,112]. To synthesize the information, only the best results are presented in Table 4 and Table 5.
Total phenolic content (TPh) has been reported in a range of 0.72–78.06 mgGAE/gdb for tomato peel, 0.67–2.03 mgGAE/gdb for seed, 1.72–9.55 mgGAE/gdb for whole fruit and 0.43–55.1 mgGAE/gdb for tomato pomace. A reduced number of studies evaluating the phenolic extraction from tomato plants was found. Nevertheless, 1.71 mgGAE/gdb was reported [109]. Arab et al. [113] measured higher contents of phenolics in tomato leaves (162–240 mgGAE/gdb).
Water, ethanol, methanol, and acetone are the main solvents used for phenolic compound extraction. The diffusion of phenolics from the solid to the liquid matrix is promoted by diluted organic solvents [16,114]. Indeed, total phenolic content recovery increased from 14.56 to 36.44 mgGAE/gdb when the solvent used was 96% and 70% ethanol, respectively [16].
Besides the solvent, temperature and time are also important operational conditions because, typically, high extraction times and temperatures enhance the traditional SLE efficiency. Ninčević Grassino et al. [83] evaluated the influence of time and revealed an increase in the total phenolic and total flavonoid content from 27.62 to 38.79 mgGAE/gdb and 2.21 to 4.80 mgQE/gdb when time rises from 3 to 12 h, respectively. Temperatures between 20 and 60 °C are commonly applied to extract phenolic compounds from tomato residues. Also, the L/S ratio, as reported before, is an important factor affecting the extraction yield, and 5–60 mL/g is the range commonly reported.

Table 4.
Total phenolic compounds (TPh—mgGAE/gdb) and total flavonoid content (TFC—mgQE/gdb) achieved by a solid-liquid extraction of tomato residues.
Table 4.
Total phenolic compounds (TPh—mgGAE/gdb) and total flavonoid content (TFC—mgQE/gdb) achieved by a solid-liquid extraction of tomato residues.
| Ref. | Operation Conditions | Residue | TPh | TFC |
|---|---|---|---|---|
| [50] | Solvent: ethyl acetate:ethanol (50:50) t: 30 min; T: 25 °C L/S: 5 | Pomace | 27.6 b | |
| [115] | Solvent: methanol (95%); t: 30 min; T: 50 °C; L/S: 50 | Pomace | 5.3 | 2.21 |
| [116] | Solvent: ethanol:water (99.5:0.05%) t: 6 h; T: 25 °C; L/S: 60 | Pomace | 55.1/199 b | 102 b |
| [117] | Solvent: methanol:water (80:20%) t: overnight; T: 25 °C; L/S: 10; dp: <0.5 mm | Pomace | 0.43 | - |
| Solvent: ethanol:water (50:50%); t: 20 min; T: 20 °C; L/S: 10 | Pomace | 0.028 a | - | |
| [118] | Solvent: ethanol:water (50:50%); t: 20 min; T: 20 °C; L/S: 10Pre-treated with a pulsed electric field (200 Pulses, 2.0 kV/cm) | Pomace | ||
| 0.056 a | - | |||
| [83] | Solvent: ethanol:water (70:30%) t: 12 h | Peel | 38.79 | 4.80 |
| [119] | Solvent: methanol (80%); T: 60 °C | Peel | 21 | - |
| [120] | Solvent: ethanol:water (70:30%) | Seed | 2.03 | - |
| [35] | Solvent: ethanol (80%); t: 1 h; T: 25 °C; L/S: 5 | Tomato | 115 b/7.7 | - |
| Pomace | 104 b/9.96 | - | ||
| [121] | Solvent: ethanol:water (70:30); t: 24 h; T: 25 °CPre-treated with microwaves during 30 sPre-treated with microwaves during 300 s | Tomato | 1.92 a | 2.34 a |
| 1.97 a | 2.58 a | |||
| 6.95 a | 10.68 a | |||
| [122] | Solvent: acetone; t: 1 h; T: 60 °C; L/S: 20 | Tomato | 0.278 a | - |
| [123] | Solvent: water; t: 2 h; T: 90 °C | Tomato | 7.56 b | |
| [12] | Solvent: ethanol; t: 4 h; T: 50 °C; L/S: 20 | Tomato | 27.5 b/4.5 | - |
| Green | 9.90 b/1.72 | - | ||
| Plant | 27.1 b/1.71 | - | ||
| [27] | Solvent: water; t: 6 h; T: 0 °C; L/S: 20; dp: <1 mm | Rotten | 14.2 b/7.5 | - |
| [124] | Solvent: methanol; t: 24 h; L/S: 5 | Green | 0.225 a | 0.184 a |
| Tomato | 0.205 a | 0.210 a | ||
| [109] | Solvent: ethanol:acetic acid (95:5%); t: 72 h; T: 25 °C | Stem | 45 b | 28 b |
| Leaves | 125.5 b | 61.96 b | ||
| Root | 24.5 b | 19 b | ||
| Plant | 75 b | 52 b |
a Phenolic concentration in mg/gfw; b phenolic concentration in mg/gdbe; L/S—liquid-solid ratio in mL/g; dp—avarage particle diameter.
UAE and MAE have been widely studied as an alternative to traditional SLE. Ethanol concentration and time of sonication were considered the most important factors in UAE [16,112]. A non-linear relationship between total phenolic compounds and ethanol concentration was reported by Solaberrieta et al. [112] Ethanol concentrations of around 60–70% improved the extraction of phenolic compounds [16,112]. Time of sonication between 5 to 15 min was studied [16,112] and a linear correlation with phenolics was reported. A rise of about 16% in TPh was noted when the time of sonication increased from 5 to 15 min. Longer extraction times (about 1 h) in UAE were also used in other works [34,66], but a wide range of results for tomato pomace was reported probably because other operating conditions and the tomato variety are different.
Bakić et al. [114] used MAE with a power range of 0 to 500 W to maintain the temperature in the defined setpoint (25, 55, or 90 °C) and short periods (5 and 10 min). Higher extraction temperatures lead to richer extracts in phenolic and flavonoid content. A rise of 55 °C to 90 °C induced an increase in bioactive concentration from 55.44 to 78.06 mgGAE/gdb [114]. Using the DoE methodology, Solaberrieta et al. [112] evaluated and optimized time, temperature, ethanol concentration, and L/S ratio in the MAE of total phenolic content. Ethanol concentration and temperature were the most important operating conditions. Diluted ethanol (63%) and high temperatures (80 °C) maximized the TPh (1.72 mg/gdb) obtained through MAE from seeds [112]. Although for different tomato residues, Solaberrieta et al. [112] and Plaza et al. [111] compared the optimization of UAE with MAE and both reported similar phenolic compounds recovery. Besides operating conditions, the ripening stage and pre-treatments also explain the wide range of results reported (Figure 5B).
The variety of tomato fruit and tomato plants led to different phenolic recoveries even when the same extraction conditions were used [37,67,109,113,125]. For example, the total phenolic content for different species of tomato leaves ranged from 83.35–125.5 mgGAE/gdbe [109].
Another important aspect to be highlighted is the fact that the total phenolic content does not follow a well-defined trend regarding the tomato maturation stages [65,124]. Among the four stages (green, green-orange, orange-red, and red-ripe), the total phenolic content from the HLY 18 tomato variety increases from green to orange-red and then decreases until the red-ripe stage [124]. In contrast, for the Lyco 2 tomato, the total phenolic concentration remains constant during the ripening stages, a pattern also reported by Helyes and Lugasi [126]. As the same recovery process was used, the tomato variety determines the phenolic evolution in the different ripening stages. Raffo et al. [127] reported a decrease in phenolic concentration during the maturation process. Overall, no conclusions can be drawn about phenolic evolution during the ripening process.
Drying is the pre-treatment common to all the extraction studies of TPh from tomato residues. Similar to the recovery of carotenoids, the yield of phenolics extraction is also affected by the drying process. Segoviano-León et al. [66] reported a TPh of 242.3 mgGAE/gdb for extracting oven-dried tomato pomace, while freeze-dried tomato pomace reached a TPh of 508.3 mgGAE/gdb.
In summary, the wide range of values reported (Figure 5B) shows that similar results can be achieved by any of the processes (conventional—SLE, UAE, and MAE). More concise studies optimizing the operating conditions while comparing the different extraction processes should be done to select the most promising one at an industrial scale. Moreover, an economic analysis should be performed, including the extraction of tomato residues without pre-drying.

Table 5.
Total phenolic compounds (TPh—mgGAE/gdb) and total flavonoid content (TFC—mgQE/gdb) achieved by an ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), accelerated solvent extraction (ASE), and enzymatic-assisted extraction (EAE) of tomato residues.
Table 5.
Total phenolic compounds (TPh—mgGAE/gdb) and total flavonoid content (TFC—mgQE/gdb) achieved by an ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), accelerated solvent extraction (ASE), and enzymatic-assisted extraction (EAE) of tomato residues.
| Ref. | Operation Conditions | Tomato | TPh | TFC |
|---|---|---|---|---|
| Ultrasound-assisted extraction (UAE) | ||||
| [16] | Solvent: ethanol: water:(70:30); t: 15 min; L/S: 50 | Pomace | 36.44 | 3.58 |
| [33] | Solvent: methanol; L/S: 10; | Pomace | 3.2 | - |
| [34] | Solvent: methanol; t: 50 min; T: 25 °C L/S: 16.7; dp: <1 mm | Pomace | 1.23 | 0.42 |
| [111] | Solvent: water; t: 60 min; T: 25 °C; L/S: 10; amplitude: 50% | Pomace | 18.65 | - |
| [128] | Solvent: n-hexane; t: 2 min; T: 25; re-extracted with ethanol during 24 h | Pomace | 13.15 | - |
| [67] | Solvent: methanol; t: 1 h; L/S: 10; dp: <0.2 mm | Peel | 0.72–3.52 | 0.84–5.72 |
| Seed | 0.67–1.22 | 0.5–0.69 | ||
| [112] | Solvent: ethanol:water (61:39%); t: 15 min; L/S: 0.05; amplitude: 85%; dp: <1 mm | Seeds | 1.61 | - |
| [37] | Solvent: methanol; t: 1 h; T: 25 °C; L/S: 10 | Tomato | 1.83–3.43 | 0.12–0.35 |
| [36] | Solvent: methanol; t: 5 min; L/S: 1 | Red-ripe peel | 0.369 b | - |
| Green peel | 0.299 b | - | ||
| Red-ripe seed | 0.176 b | - | ||
| Green seed | 0.133 b | - | ||
| Pomace | 0.146 b | - | ||
| [113] | Solvent: methanol; t: 1 h; T: 40 °C; L/S: 100; dp: <1 mm | Leaves | 240 | 184 |
| Microwave-assisted extraction (MAE) | ||||
| [111] | Solvent: water; L/S: 0.005; t: 60 min; power: 120 W | Pomace | 17.87 | |
| [114] | Solvent: methanol:water (50:50) t: 10 min; T: 90; L/S: 50; power: 0–500 W | Peel | 78.06 | 65.85 |
| [112] | Solvent: ethanol:water (63:37%); t: 15 min; T: 80 °C; L/S: 0.08; Frequency: 2.45 GHz; dp: <1 mm | Seeds | 1.72 | - |
| [38] | Solvent: ethanol:water (66.2:33.8) t: 2 min; T: 96.5 °C L/S: 50 | Tomato | 9.55 | - |
| [25] | Solvent: ethanol (100%); t: 20 min; T: 180 °C; L/S: 45; dp: <0.85 mm | Rotten | 66.8 b | 3.89 b |
| [25] | Solvent: ethanol:water (47.4:52.6); t: 20 min; T: 180 °C; L/S: 22; dp: <0.85 mm | Rotten | 43.9 b | 3.50 b |
| Accelerated solvent extraction | ||||
| [16] | Solvent: water; t: 10 min; T: 45 °C; pressure: 600 MPa | Peels | 49.92 | - |
| Enzymatic-assisted extraction | ||||
| [50] | Solvent: ethyl acetate/enzyme; t: 1 h; T: 50 °C L/S: 5; enzyme: Celluclast:Viscozyme | Pomace | 28.9 b | - |
a Phenolic concentration in mg/gfw; b phenolic concentration in mg/gdbe; L/S—liquid-solid ratio in mL/g; dp—avarage particle diameter.
4.2. Recovering Energy through Anaerobic Digestion
Anaerobic digestion (AD) is a biological process in which microorganisms can break down biodegradable organic material in the absence of oxygen, producing biogas (CH4 and CO2) and remaining digestate (sludge with microorganisms and undegraded substrate and water). As can be seen in Table 1, tomato fruit residues (tomato rotten, green, fruit, and pomace) are mainly composed of organic matter (>80%). Thus, high biochemical methane potential (BMP) values are expected. Indeed, tomato residues have been studied for energy recovery. Some of the literature results are summarized in Table 6. BMP values range between 84.7–330, 124–369, and 28.8–365 mLCH4/gVS for tomato pomace (peels and seeds), tomato fruit, and tomato plant (leaves and stems), respectively. The process complexity and the variations of the operational conditions are the main reasons for the large range of values reported. Indeed, the substrate-to-inoculum ratio (SIR) significantly affects the AD performance [62,129,130]. A SIR of 1, 2, 3, and 4 led to a methane yield of 56, 32, 48, and 29 mLCH4/gVS for tomato pomace, respectively [129]. In contrast, lower SIR, such as 0.25 and 0.5, allowed it to reach a BMP value of 211 and 261 mLCH4/gVS, respectively [129]. High SIR causes an organic matter overload to the microbial population, and the anaerobic medium acidifies, inhibiting methanogenesis because methanogenic microorganisms are sensitive to pH. For SIR ratios higher than 1, acidic pH and VFA/TA (volatile fatty acid/total alkalinity) ratios in the unstable range (0.4–0.8) were reported at the final stage of the anaerobic digestion process [129]. So, the SIR ratio between 0.25 and 0.5 enhances the methane yield and also allows the maintenance of pH in the optimum range for organic matter degradation [62,129]. Moreover, higher SIR causes a delay in biogas production because more time is necessary to achieve enough methanogenic microorganisms in the inoculum that degrade the organic matter [62].

Table 6.
Biochemical methane potential (BMP—mLCH4/gVS) of tomato residues.
Lignocellulosic materials typically have a lower methane yield because lignin is difficult to biodegrade. Tomato plants (leaves and stems) are rich in lignin, as can be seen in Table 1; thus, lower BMP values are expected. However, Li et al. [146] reported a BMP value of 28.8 mLCH4/gVS, which is extremely lower when compared with other studies. These discrepant results are probably due to the SIR ratio (4) used by the authors.
Total volatile solids (tVS) concentration in the reaction mixture is also an important factor for AD. Camarena-Martínez et al. [62] evaluated the effect of tVS and stated that an increase from 13.0 gVS/L to 19.5 gVS/L leads to a methane production increase from 181 to 208 mLCH4/gVS. It is important to note that BMP rises as the tVS concentration increases until an optimal point; thus, an optimization is commonly required.
Anaerobic co-digestion (AcoD) is an alternative to single anaerobic digestion, allowing the management of multiple substrates simultaneously while producing biogas. Tomato residues have been tested with cattle manure, including cow manure, pig slurry [53,131,132,142,148] and corn stover [53,131,148], food waste [142], cucumber waste [29], and watermelon [131]. From these multiple combinations tested, a methane yield of about 300 mL/gVS can be achieved [28,131,146]. Almeida et al. [5] performed a DoE to determine the best substrate combination for digesting rotten tomatoes, green tomatoes, and tomato plants. Only combinations with a high amount of tomato plants impaired the BMP results. Nonetheless, a maximum of 324 NmLCH4/gVS was achieved with a mixture of 63% of rotten tomato, 20% of green tomato, and 17% of tomato plant [5]. Thus, a tomato industry can treat and valorize simultaneously the multiple residues generated.
Overall, tomato residues have shown a high potential for methane production through AD or AcoD. However, authors should invest more time in selecting and testing more combinations for AcoD, mostly thinking about the regional context and aiming to manage multiple regional residues, as proposed by Aravani et al. [131]
4.3. Closing the Loop through Composting Processes
Composting is a biological process in which microorganisms can break down biodegradable organic material in the presence of oxygen, releasing CO2, H2O, and heat, resulting in a stabilized material known as compost. Composting of tomato residue combined with dairy manure, wheat straw, and sewage sludge has been studied [61,63,80,81,149,150].
Tomato plant residues have been used as a bulk agent to maintain proper FAS in the composting mixture [63,81,149,150]. According to Kulcu [150], 60% of tomato stalks, 30% of separated dairy manure, and 10% of wheat straw are the optimum mixture ratio for composing, attaining suitable values of FAS (30%). The composting of two-phase olive-mill pomace was improved when higher contents of tomato stalks and poultry manure [149]. Şevik et al. [63] revealed high decomposition levels from composting mixtures composed of 5.0%, 67.1%, and 27.9% of olive pomace, dairy manure, and tomato stalks, respectively. The aerobic degradation of tomato stalks with sheep manure extended the thermophilic phase and enhanced the quality of compost regarding nutrients (P, K, N) and humic substances [80].
In another study, tomato green, rotten tomato, and tomato stalks were mixed with chicken manure (20%), and the composting of this mixture led to an organic matter loss of 36.4% [151]. The maturity and stability of the final compost were evaluated by CIELAB color space parameters (validated by physicochemical parameters) and it was concluded that the former method could be used to analyze the composting progress [151].
The combination of compost amendments (such as biochar, peat bog, and zeolite) with tomato stalks and chicken manure was studied [61]. Biochar was the best compost amendment due to its high surface area, improving the regulation of moisture content and free air space and, consequently, enhancing the environmental conditions for the growth of aerobic microorganisms.
Li et al. [152] evaluated the influence of the digestion time in an integrated anaerobic digestion and composting system. Briefly, tomato stalks and leaves were anaerobically degraded during 15, 30, and 45 days generating three different digestates. Those digestates were subjected to composting with corn stalks. The use of digestates formed within 30 and 45 days improved the germination index (a parameter used as a measure of compost maturation). Moreover, a reduction of 18.9-29.0% of greenhouse gas emissions was attained when digestates were composted. This study concluded that composting is an efficient and economical method to properly manage the digestate from AD of tomato residues.
5. Economic Analysis
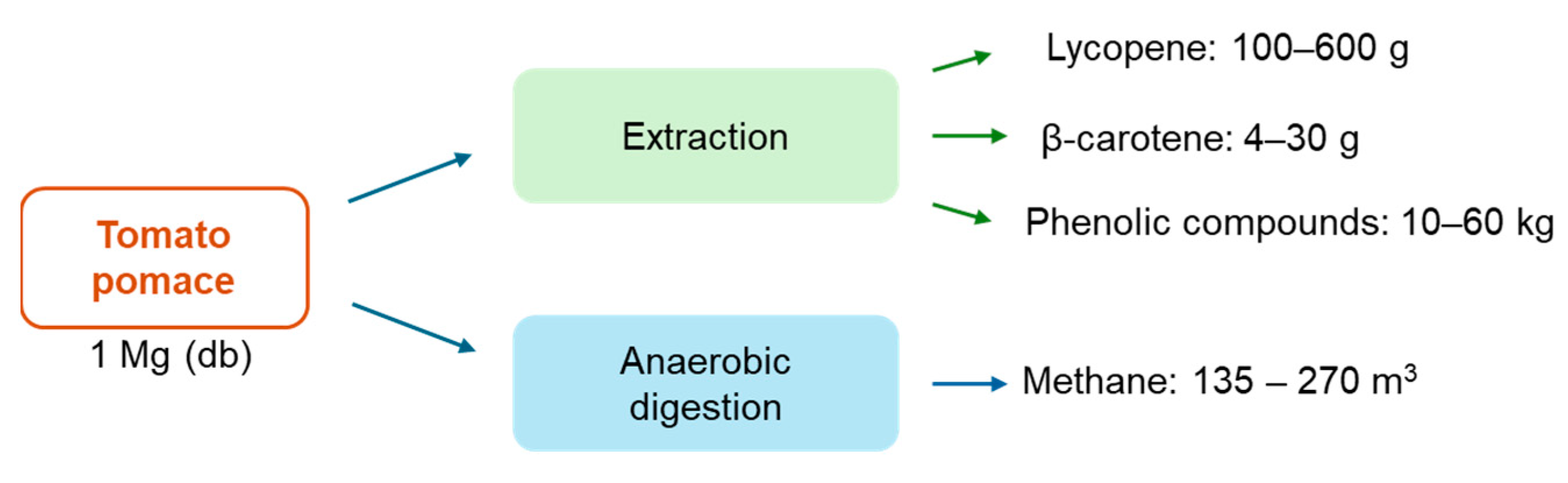
As mentioned before, many alternatives for managing and valorizing tomato residues have been studied. Multiple process yields have been reported according to the operating conditions. Nevertheless, Figure 6 summarizes the ranges of bioactive compounds and methane obtained through extraction and anaerobic digestion of tomato pomace. Indeed, the quantity of bioactive compounds corresponds to a small fraction of the tomato residues. However, the market of lycopene and β-carotene tends to increase with a compound annual growth rate (CAGR) of 5% and 4.3% from 2023 and 2030, respectively [153,154]. In addition, the demand for natural lycopene and β-carotene has increased due to the awareness of a healthy lifestyle. Although there is no established market value for natural extracts with bioactive compounds (lycopene and carotenoids), the commercialized lycopene and β-carotene present high market values. Thus, the studies with economic evaluation assumed high selling prices for extract rich in those compounds (30–50 USD/kg of extract) [73,98]. Overall, the high value of the compounds may compensate for the small amount recovered. A techno-economic analysis must be performed to evaluate the most profitable valorization strategy.
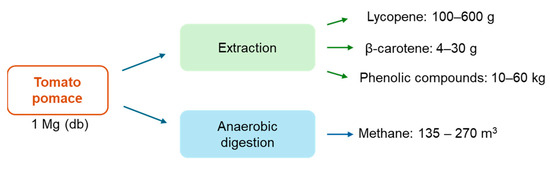
Figure 6.
Range of recoverable products from 1 Mg of tomato pomace.
The profitability of each process and scenario proposed is commonly assessed by three main economic indicators: net present value (NPV), internal return rate (IRR), and payback time (PBT). NPV is a measure of the project profitability including the cash inflows and outflows associated with an investment at an established discount (interest) rate. IRR corresponds to the discount rate, which pays the investment cost at the end of the project (the discount rate at which NPV is zero). If the IRR is higher than the discount rate defined by the market, the project is economically viable. PBT represents the time needed to pay the project investment. Some authors have already evaluated the economic viability of applying supercritical fluid extraction for recovering extracts rich in lycopene and carotenoids from tomato and tomato pomace [98,155] and AcoD for obtaining biogas and digestate [53,156]. Different concentration of solids in the anaerobic reactor was also economically evaluated by some authors (wet if TS < 10% and dry if TS > 15%). Table 7 summarizes the economic data related to the valorization processes of tomato residues. As implied before, the economic viability of a project depends on many assumptions, including the lifetime and interest rate. The projects with extraction processes defined a lifetime of 10 years, while 20 years were specified for projects with anaerobic digestion processes. The interest rate ranged from 5.5 to 25% [98,155,157]. Except for the wet anaerobic co-digestion, all the processes were revealed to be economically viable. Thus, tomato residues can be managed using environmentally friendly technologies while producing profit through the recovery of natural and functional compounds (lycopene and carotenoids), energy (biogas), and digestate (used as fertilizer).

Table 7.
Capital and manufacturing costs, revenues, and economic indicators of different valorization processes for tomato residues.
The extraction of lycopene and carotenoids leads to high revenues (111–10,300 kUSD/y) due to the high value of those compounds. Hatami and Ciftci [155] and Yadav et al. [157] reported very high prices for tomato extracts (423 USD/kg and 13,050 USD/kg, respectively), explaining the revenues reported (Table 7). A sensitivity analysis for the supercritical fluid extraction proposed by Hatami and Ciftci [155] revealed that extract price had the most significant impact on the NPV [158]. Nonetheless, NPV remains positive in the worst scenario [158].
Regarding AcoD, dry systems lead to profitable projects with NPV higher than 197 kUSD and IRR higher than the established discount rate (8%) [53,156]. Moreover, the conversion of methane in heat and electricity, including a combined heat and power (CHP) system, increased the NPV by 50% [156]. The revenues from the digestate accounted for about 50% of the total revenues [53,156]. Thus, all the products from anaerobic digestion have a high value. Indeed, digestate presents a high content of nutrients (N, P, and K) that can be used to replace a fraction of commercial fertilizers. The authors assumed prices for bio-methane, heat, electricity, and digestate of 0.54 USD/m3, 0.16 USD/kWh, 0.04 USD/kWh, and 30 USD/t, respectively [53,156]. A sensibility analysis revealed that the efficiency of the CHP, methane yield, and price of the products are the main variables affecting the NPV [53,156].
Only Scaglia et al. [73] evaluated the economic viability of a biorefinery for tomato pomace. SFE, followed by AD, allowed to obtain revenues higher than the manufacturing costs. Thus, applying biorefinery approaches may generate profits of 1450 kUSD/y [73]. Biorefineries with different feedstock also showed to be economically profitable [1]. This is a very good indicator for exploring biorefineries to manage and valorize tomato residues while the carbon loop (and nutrients) is closed. If the tomato processing industries adopt the valorization of tomato pomace, a positive contribution to the circular economy is expected by promoting the efficient use of resources, minimizing waste, and encouraging the recycling and reuse of by-products. By transforming waste into valuable secondary products, companies can lower disposal costs, reduce their environmental footprint, and create additional revenue. However, these processes might offer limited improvements in economic return in the short term due to initial investment costs. Long-term gains are expected as the industry adapts and scales these innovations, potentially leading to more substantial economic benefits and a more sustainable production model. Besides the economic analysis, in the literature, some recent studies have highlighted the need for a rigorous evaluation before using food residues, namely in terms of safety and risk assessment [159].
6. Conclusions and Future Perspectives
The tomato industry is a relevant socio-economic activity in the EU that generates diverse residues in large amounts. Tomatoes unfit for consumption (rotten and green), tomato plants, tomato seeds, tomato peels, and tomato pomace are among the most common residues widely studied, aiming at proper management and valorization. Extraction of β-carotene, lycopene, and phenolic compounds and anaerobic digestion for biogas production are the processes most widely studied in the literature. Although many studies have extracted carotenoids and phenolic compounds from tomato residues, there are many variables affecting the results hindering the comparison between different works. For this reason, comparative studies should be performed using the same residue, pretreatments, and analytical procedures to understand which extraction process maximizes the recovery of β-carotenes and phenolic compounds. Moreover, the results should be normalized by the initial biomass weight used as well as by the extract obtained to uniformize the units and allow the comparison between works. Both forms inform about the extraction performance, but in different relevant ways. The biorefinery approach has been gradually studied for tomato residues to maximize revenues and safeguard the environment. The integration of multiple processes, mainly to extract bioactive compounds, has been reported in the literature. Higher or similar efficiencies were reported by sequential processes when compared to stand-alone processes. Considering that good results were reported for extracting bioactive compounds, anaerobic digestion (or co-digestion), and composting, a more complex biorefinery, including those processes, must be investigated aiming to close the carbon (and nutrients) loop as suggested by the authors. The valorization of multiple residues simultaneously in co-digestion strategies using common agro-residues from a specific region should also be investigated, aiming to treat and valorize the waste streams in industrial plants. The techno-economic analysis shows good perspectives about recovering carotenoids from tomato residues because high profits were reported in the literature. However, more economic evaluations comparing different valorization processes and using the biorefinery approach should be performed to select the most profitable way of managing tomato residues. Even the drying effect on bioactive compound extraction should be economically assessed, assuring that higher efficiencies cover the drying operating costs. Before any recovery, safety assessment studies are necessary, particularly involving microbiological assessments or the determination of toxic contaminants in the residues. In addition, the life cycle assessment regarding tomato residue management is still lacking in the literature. However, these holistic data are of high relevance for understanding and selecting the best methods to valorize the waste streams. To sum up, more comparative studies, economic evaluations, and life cycle assessments of different strategies to valorize tomato residues will ensure a well-planned solution to reduce the cost of tomato industries with the management of these residues and boost the circular economy in the future.
Funding
This work was financially supported by Fundação para a Ciência e Tecnologia (FCT, Portugal), which provided a Ph.D grant (2020.08445.BD) to Patrícia V. Almeida. The authors thank FCT/MCTES for the financial support to CERES (https://doi.org/10.54499/UIDB/00102/2020) through national funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Almeida, P.V.; Gando-Ferreira, L.M.; Quina, M.J. Biorefinery perspective for industrial potato peel management: Technology readiness level and economic assessment. J. Environ. Chem. Eng. 2023, 11, 110049. [Google Scholar] [CrossRef]
- Caldeira, C.; Vlysidis, A.; Fiore, G.; De Laurentiis, V.; Vignali, G.; Sala, S. Sustainability of food waste biorefinery: A review on valorisation pathways, techno-economic constraints, and environmental assessment. Bioresour. Technol. 2020, 312, 123575. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Mu, T.H.; Ma, M.M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.P.; Sousa, A.M.; Gando-Ferreira, L.M.; Quina, M.J. Grape Pomace as a Natural Source of Phenolic Compounds: Solvent Screening and Extraction Optimization. Molecules 2023, 28, 2715. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.V.; Rodrigues, R.P.; Teixeira, L.M.; Santos, A.F.; Martins, R.C.; Quina, M.J. Bioenergy production through mono and co-digestion of tomato residues. Energies 2021, 14, 5563. [Google Scholar] [CrossRef]
- Almeida, P.V.; Gando-Ferreira, L.M.; Quina, M.J.; Slezak, R.; Klepacz-Smolka, A. Evaluation of gas-phase pyrolysis from agro-industrial residues. In WASTES: Solutions, Treatments and Opportunities IV; Vilarinho, C., Castro, F., Quina, M.J., Eds.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Rodrigues, R.P.; Gando-ferreira, L.M.; Quina, M.J. Increasing Value of Winery Residues through Integrated Biorefinery Processes: A Review. Molecules 2022, 27, 4709. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Sanchez, M.; Inocencio-García, P.J.; Alzate-Ramírez, A.F.; Alzate, C.A.C. Potential and Restrictions of Food-Waste Valorization through Fermentation Processes. Fermentation 2023, 9, 274. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Andrade, C.; Pereira, J.A.M.; Perestrelo, R.; Câmara, J.S. Current Challenges in the Sustainable Valorisation of Agri-Food Wastes: A Review. Processes 2023, 11, 20. [Google Scholar] [CrossRef]
- Murugesan, P.; Raja, V.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Food waste valorisation via gasification—A review on emerging concepts, prospects and challenges. Sci. Total Environ. 2022, 851, 157955. [Google Scholar] [CrossRef]
- Assis, T.I.; Gonçalves, R.F. Valorization of food waste by anaerobic digestion: A bibliometric and systematic review focusing on optimization. J. Environ. Manag. 2022, 320, 115763. [Google Scholar] [CrossRef]
- Almeida, P.V.; Rodrigues, R.P.; Gaspar, M.C.; Braga, M.E.M.; Quina, M.J. Integrated management of residues from tomato production: Recovery of value-added compounds and biogas production in the biorefinery context. J. Environ. Manag. 2021, 299, 113505. [Google Scholar] [CrossRef]
- Almeida, P.V.; Rodrigues, R.P.; Slezak, R.; Quina, M.J. Effect of phenolic compound recovery from agro-industrial residues on the performance of pyrolysis process. Biomass Convers. Biorefinery 2022, 12, 4257–4269. [Google Scholar] [CrossRef]
- Bühlmann, C.H.; Mickan, B.S.; Tait, S.; Bahri, P.A. Developing a food waste biorefinery: Lactic acid extraction using anionic resin and impacts on downstream biogas production. Chem. Eng. J. 2022, 431, 133243. [Google Scholar] [CrossRef]
- Cristóbal, J.; Caldeira, C.; Corrado, S.; Sala, S. Techno-economic and profitability analysis of food waste biorefineries at European level. Bioresour. Technol. 2018, 259, 244–252. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Ostojić, J.; Miletić, V.; Djaković, S.; Bosiljkov, T.; Zorić, Z.; Ježek, D.; Rimac Brnčić, S.; Brnčić, M. Application of high hydrostatic pressure and ultrasound-assisted extractions as a novel approach for pectin and polyphenols recovery from tomato peel waste. Innov. Food Sci. Emerg. Technol. 2020, 64, 102424. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agricultural Organization of the United Nations. 2021. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 5 October 2023).
- FAOSTAT. Food and Agricultural Organization of the United Nations. 2022. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 6 October 2023).
- International Euromonitor, Global Market Report: Processed Tomatoes. 2023. Available online: https://www.tomatonews.com/en/consumption_67.html (accessed on 7 October 2023).
- Colvine, S.; Branthôme, F.-X. TOP50 Tomato Processing Companies Worldwide in 2020. 2021. Available online: https://www.tomatonews.com/en/top50-tomato-processing-companies-worldwide-in-2020_2_1295.html (accessed on 7 October 2023).
- Sugal Group. 2023. Available online: https://sugal-group.com (accessed on 8 October 2023).
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Fritsch, C.; Staebler, A.; Happel, A.; Márquez, M.A.C.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, valorization and application of bio-waste derived compounds from potato, tomato, olive and cereals: A review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Pane, C.; Celano, G.; Piccolo, A.; Villecco, D.; Spaccini, R.; Palese, A.M.; Zaccardelli, M. Effects of on-farm composted tomato residues on soil biological activity and yields in a tomato cropping system. Chem. Biol. Technol. Agric. 2015, 2, 4. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Barreira, M.F.; Carvalho, A.M.; Oliveira, M.B.P.P.; Curran, T.P.; Ferreira, I.C.F.R. Valorisation of tomato wastes for development of nutrient-rich antioxidant ingredients: A sustainable approach towards the needs of the today’s society. Innov. Food Sci. Emerg. Technol. 2017, 41, 160–171. [Google Scholar] [CrossRef]
- Del Valle, M.; Cámara, M.; Torija, M. Chemical characterization of tomato pomace. J. Sci. Food Agric. 2006, 86, 1232–1236. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Mendes, C.V.T.; Pinela, S.R.; Moreira, R.; Carvalho, M.G.V.S.; Quina, M.J.; Braga, M.E.M.; Portugal, A.T. Assessment of Agroforestry Residues: Their Potential within the Biorefinery Context. ACS Sustain. Chem. Eng. 2019, 7, 17154–17165. [Google Scholar] [CrossRef]
- Ferrer, P.; Cambra-lópez, M.; Cerisuelo, A.; Peñaranda, D.S.; Moset, V. The use of agricultural substrates to improve methane yield in anaerobic co-digestion with pig slurry: Effect of substrate type and inclusion level. Waste Manag. 2014, 34, 196–203. [Google Scholar] [CrossRef]
- Gil, A.; Siles, J.Á.; Serrano, A.; Martín, M.Á. Mixture optimization of anaerobic co-digestion of tomato and cucumber waste. Environ. Technol. 2015, 36, 2628–2636. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-pejó, E.; Gónzalez-Fernández, C. Agroindustrial waste as a resource for volatile fatty acids production via anaerobic fermentation. Bioresour. Technol. 2020, 297, 122486. [Google Scholar] [CrossRef]
- Ćetković, G.; Savatović, S.; Čanadanović-Brunet, J.; Djilas, S.; Vulić, J.; Mandić, A.; Četojević-Simin, D. Valorisation of phenolic composition, antioxidant and cell growth activities of tomato waste. Food Chem. 2012, 133, 938–945. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Adani, F.; Sciarria, T.P.; Squillace, P.; Scarafoni, A.; Iametti, S.; Scaglia, B. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Raw and Fermented Tomato Pomace and Their Correlations with Aglycate-Polyphenols. Antioxidants 2020, 9, 179. [Google Scholar] [CrossRef]
- Nour, V.; Panaite, T.D.; Ropota, M.; Turcu, R.; Corbu, A.R.; Nour, V.; Panaite, T.D.; Ropota, M.; Turcu, R.; Trandafir, I. Nutritional and bioactive compounds in dried tomato processing waste. CyTA-J. Food 2018, 16, 222–229. [Google Scholar] [CrossRef]
- Perea-Domínguez, X.P.; Hernández-Gastelum, L.Z.; Olivas-Olguin, H.R.; Espinosa-Alonso, L.G.; Valdez-Morales, M.; Medina-Godoy, S. Phenolic composition of tomato varieties and an industrial tomato by-product: Free, conjugated and bound phenolics and antioxidant activity. J. Food Sci. Technol. 2018, 55, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Carle, R.; Astudillo, L.; Guzmán, L.; Gutiérrez, M.; Carrasco, G.; Palomo, I. Antioxidant and Antiplatelet Activities in Extracts from Green and Fully Ripe Tomato Fruits (Solanum lycopersicum) and Pomace from Industrial Tomato Processing. Evidence-Based Complement. Altern. Med. 2013, 2013, 867578. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit. Phytochemistry 2010, 71, 1848–1864. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Alarcón-Flores, M.I.; Romero-González, R.; Martínez Vidal, J.L.; Garrido Frenich, A. Multiclass Determination of Phenolic Compounds in Different Varieties of Tomato and Lettuce by Ultra High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Int. J. Food Prop. 2016, 19, 494–507. [Google Scholar] [CrossRef]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef]
- Aghajanzadeh-Golshani, A.; Maheri-Sis, N.; Mirzaei-Aghsaghali, A.; Baradaran-Hasanzadeh, A. Comparison of Nutrional Value of Tomato Pomace and Brewer’s Grain for Ruminants Using in vitro Gas Production. Asian J. Anim. Vet. Adv. 2010, 5, 43–51. [Google Scholar]
- Assi, J.A.; King, A.J. Production, Modeling, and Education—Manganese Amendment and Pleurotus ostreatus Treatment to Convert Tomato Pomace for Inclusion in Poultry Feed. Poult. Sci. 2008, 87, 1889–1896. [Google Scholar] [CrossRef]
- Abdollahzadeh, F.; Pirmohammadi, R.; Farhoomand, P.; Fatehi, F.; Pazhoh, F.F. The Effect of Ensiled Mixed Tomato and Apple Pomace on Holstein Dairy Cow. Ital. J. Anim. Sci. 2016, 9, 212–216. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Greco, R.; Evangelou, A.; Komilis, D. Anaerobic digestion of tomato processing waste: Effect of alkaline pretreatment. J. Environ. Manag. 2015, 163, 49–52. [Google Scholar] [CrossRef]
- Allison, B.J.; Cádiz, J.C.; Karuna, N.; Jeoh, T.; Simmons, C.W. The Effect of Ionic Liquid Pretreatment on the Bioconversion of Tomato Processing Waste to Fermentable Sugars and Biogas. Appl. Biochem. Biotechnol. 2016, 179, 1227–1247. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, U.; Mushtaq, Z.; Ahmad, R.S.; Asghar, N. Characterization, oxidative perspectives and consumer acceptability of tomato waste powder supplemented cookies. J. Anim. Plant Sci. 2017, 27, 2045–2055. [Google Scholar]
- Dziedzic, K.; Górecka, D.; Szwengiel, A.; Michniewicz, J.; Drożdżyńska, A.; Walkowiak, J. Interactions between fecal bacteria, bile acids and components of tomato pomace. Food Sci. Biotechnol. 2019, 28, 649–655. [Google Scholar] [CrossRef]
- Vidal, M.; Bastos, D.; Silva, L.; Gaspar, D.; Paulo, I.; Matos, S.; Vieira, S.; Bordado, J.M.; Galhano dos Santos, R. Up-cycling tomato pomace by thermochemical liquefaction—A response surface methodology assessment. Biomass Bioenergy 2022, 156, 106324. [Google Scholar] [CrossRef]
- Baker, P.W.; Preskett, D.; Krienke, D.; Runager, K.S.; Hastrup, A.C.S.; Charlton, A. Pre-processing Waste Tomatoes into Separated Streams with the Intention of Recovering Protein: Towards an Integrated Fruit and Vegetable Biorefinery Approach to Waste Minimization. Waste Biomass Valorization 2022, 13, 3463–3473. [Google Scholar] [CrossRef]
- Catalkaya, G.; Kahveci, D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep. Purif. Technol. 2019, 219, 55–63. [Google Scholar] [CrossRef]
- Monterumici, C.M.; Rosso, D.; Montoneri, E.; Ginepro, M.; Baglieri, A.; Novotny, E.H.; Kwapinski, W.; Negre, M. Processed vs. Non-Processed Biowastes for Agriculture: Effects of Post-Harvest Tomato Plants and Biochar on Radish Growth, Chlorophyll Content and Protein Production. Int. J. Mol. Sci. 2015, 16, 8826–8843. [Google Scholar] [CrossRef]
- Nisticò, R.; Evon, P.; Labonne, L.; Vaca-Medina, G.; Montoneri, E.; Vaca-Garcia, C.; Negre, M. Post-harvest tomato plants and urban food wastes for manufacturing plastic films. J. Clean. Prod. 2017, 167, 68–74. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Xu, F.; Li, Y.; Li, D.; Wang, G.; Li, S.; Zhang, H.; Wu, Y.; Shah, A.; et al. Reactor performance and economic evaluation of anaerobic co-digestion of dairy manure with corn stover and tomato residues under liquid, hemi-solid, and solid state conditions. Bioresour. Technol. 2018, 270, 103–112. [Google Scholar] [CrossRef]
- Akao, S. Nitrogen, Phosphorus, and Antioxidant Contents in Crop Residues for Potential Cascade Utilization. Waste and Biomass Valorization 2018, 9, 1535–1542. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2019, 12, 26. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Chaidez, C.; Ornelas-Paz, J. de J.; López-Mata, M.A.; Márquez-Ríos, E.; Estrada, M.I. Chemical constitution and effect of extracts of tomato plants byproducts on the enteric viral surrogates. Int. J. Environ. Health Res. 2014, 25, 299–311. [Google Scholar] [CrossRef]
- Taha, I.; Elkafafy, M.S.; Mously, H. El Potential of utilizing tomato stalk as raw material for particleboards. Ain Shams Eng. J. 2018, 9, 1457–1464. [Google Scholar] [CrossRef]
- Üner, B.; Kömbeci, K.; Akgül, M. The utilization of tomato stalk in fiber production: NAOH and cao pulping process. Wood Res. 2016, 61, 927–936. [Google Scholar]
- Çöpür, Y.; Özyürek, Ö.; Tozluoglu, A.; Kütük, S. Enzymatic digestibility of tomato, pepper, and eggplant stalks mixture. BioResources 2012, 7, 3252–3261. [Google Scholar] [CrossRef]
- Covino, C.; Sorrentino, A.; Roscigno, G.; Vece, A.P.; Masi, P.; Di Pierro, P. Enzymatic study for valorization of plant wastes. Ital. J. Food Sci. 2019, 31, 204–209. [Google Scholar] [CrossRef]
- Wei, L.; Shutao, W.; Jin, Z.; Tong, X. Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour. Technol. 2014, 154, 148–154. [Google Scholar] [CrossRef]
- Camarena-Martínez, S.; Martínez-Martínez, J.H.; Saldaña-Robles, A.; Nuñez-Palenius, H.G.; Costilla-Salazar, R.; Valdez-Vazquez, I.; Lovanh, N.; Ruiz-Aguilar, G.M.L. Effects of Experimental Parameters on Methane Production and Volatile Solids Removal from Tomato and Pepper Plant Wastes. BioResources 2020, 15, 4763–4780. [Google Scholar] [CrossRef]
- Şevik, F.; Tosun, İ.; Ekinci, K. Composting of olive processing wastes and tomato stalks together with sewage sludge or dairy manure. Int. J. Environ. Sci. Technol. 2016, 13, 1207–1218. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Siddiqui, M.W.; Hdider, C.; Lenucci, M.S. Inside and Beyond Color: Comparative Overview of Functional Quality of Tomato and Watermelon Fruits. Front. Plant Sci. 2019, 10, 1–26. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Segoviano-León, J.P.; Ávila-Torres, G.A.; Espinosa-Alonso, L.G.; Valdez-Morales, M.; Medina-Godoy, S. Nutraceutical potential of flours from tomato by-product and tomato field waste. J. Food Sci. Technol. 2020, 57, 3525–3531. [Google Scholar] [CrossRef]
- Valdez-Morales, M.; Espinosa-Alonso, L.G.; Espinoza-Torres, L.C.; Delgado-Vargas, F.; Medina-Godoy, S. Phenolic Content and Antioxidant and Antimutagenic Activities in Tomato Peel, Seeds, and Byproducts. J. Agric. Food Chem. 2014, 62, 5281–5289. [Google Scholar] [CrossRef]
- Fuentes, E.; Forero-Doria, O.; Carrasco, G.; Maricá, A.; Santos, L.S.; Alarcó, M.; Palomo, I. Effect of tomato industrial processing on phenolic profile and antiplatelet activity. Molecules 2013, 18, 11526–11536. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Khoo, H.E.; Kong, K.W.; Prasad, K.N.; Galanakis, C.M. Extraction of carotenoids and applications. In Carotenoids: Properties, Processing and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 259–288. ISBN 9780128170670. [Google Scholar]
- Silva, P.B.V.d.; Brenelli, L.B.; Mariutti, L.R.B. Waste and by-products as sources of lycopene, phytoene, and phytofluene—Integrative review with bibliometric analysis. Food Res. Int. 2023, 169, 112838. [Google Scholar] [CrossRef]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as potential source of natural additives for meat industry. A review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef]
- Boccia, F.; Di Donato, P.; Covino, D.; Poli, A. Food waste and bio-economy: A scenario for the Italian tomato market. J. Clean. Prod. 2019, 227, 424–433. [Google Scholar] [CrossRef]
- Scaglia, B.; D’Incecco, P.; Squillace, P.; Dell’Orto, M.; De Nisi, P.; Pellegrino, L.; Botto, A.; Cavicchi, C.; Adani, F. Development of a tomato pomace biorefinery based on a CO2-supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. J. Clean. Prod. 2020, 243, 118650. [Google Scholar] [CrossRef]
- Mendez-Carmona, J.Y.; Ramírez-Guzman, K.N.; Ascacio-Valdes, J.A.; Sepulveda, L.; Aguilar, C.N. Solid-state fermentation for recovery of carotenoids from tomato waste. Innov. Food Sci. Emerg. Technol. 2022, 80, 103108. [Google Scholar] [CrossRef]
- Hu, Y.; Kang, K.; Alvarez, I.Z.; Mia, N.; Rakhra, A. Simulation of O2-Blown Co-Gasification of Wood Chip and Potato Peel for Producing Syngas. Front. Agric. Sci. Eng. 2023, 10, 448–457. [Google Scholar] [CrossRef]
- Memici, M.; Ekinci, K. Pyrolysis of tomato harvest waste as a function of temperature and duration: Characteristics, production energy, and carbon dioxide emission in field conditions. Soil Tillage Res. 2020, 202, 104652. [Google Scholar] [CrossRef]
- Hossain, M.M.; Scott, I.M.; Berruti, F.; Briens, C. Application of Novel Pyrolysis Reactor Technology to Concentrate Bio-oil Components with Antioxidant Activity from Tobacco, Tomato and Coffee Ground Biomass. Waste Biomass Valorization 2018, 9, 1607–1617. [Google Scholar] [CrossRef]
- Midhun Prasad, K.; Murugavelh, S. Experimental investigation and kinetics of tomato peel pyrolysis: Performance, combustion and emission characteristics of bio-oil blends in diesel engine. J. Clean. Prod. 2020, 254, 120115. [Google Scholar] [CrossRef]
- Cáceres, L.A.; McGarvey, B.D.; Briens, C.; Berruti, F.; Yeung, K.K.C.; Scott, I.M. Insecticidal properties of pyrolysis bio-oil from greenhouse tomato residue biomass. J. Anal. Appl. Pyrolysis 2015, 112, 333–340. [Google Scholar] [CrossRef]
- Tabrika, I.; Azim, K.; Mayad, E.H.; Zaafrani, M. Composting of tomato plant residues: Improvement of composting process and compost quality by integration of sheep manure. Org. Agric. 2020, 10, 229–242. [Google Scholar] [CrossRef]
- Şevik, F.; Tosun, I.; Ekinci, K. The effect of FAS and C/N ratios on co-composting of sewage sludge, dairy manure and tomato stalks. Waste Manag. 2018, 80, 450–456. [Google Scholar] [CrossRef]
- Ouatmani, T.; Haddadi-Guemghar, H.; Boulekbache-Makhlouf, L.; Mehidi-Terki, D.; Maouche, A.; Madani, K. A sustainable valorization of industrial tomato seeds (cv Rio Grande): Sequential recovery of a valuable oil and optimized extraction of antioxidants by microwaves. J. Food Process. Preserv. 2022, 46, e16123. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Djaković, S.; Bosiljkov, T.; Halambek, J.; Zorić, Z.; Dragović-Uzelac, V.; Petrović, M.; Brnčić, S.R. Valorisation of Tomato Peel Waste as a Sustainable Source for Pectin, Polyphenols and Fatty Acids Recovery Using Sequential Extraction. Waste Biomass Valorization 2020, 11, 4593–4611. [Google Scholar] [CrossRef]
- Bazzarelli, F.; Mazzei, R.; Papaioannou, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Biorefinery of Tomato Leaves by Integrated Extraction and Membrane Processes to Obtain Fractions That Enhance Induced Resistance against Pseudomonas syringae Infection. Membranes 2022, 12, 585. [Google Scholar] [CrossRef]
- Azabou, S.; Louati, I.; Ben Taheur, F.; Nasri, M.; Mechichi, T. Towards sustainable management of tomato pomace through the recovery of valuable compounds and sequential production of low-cost biosorbent. Environ. Sci. Pollut. Res. Int. 2020, 27, 39402–39412. [Google Scholar] [CrossRef]
- Kehili, M.; Schmidt, L.M.; Reynolds, W.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Biorefinery cascade processing for creating added value on tomato industrial by-products from Tunisia. Biotechnol. Biofuels 2016, 9, 261. [Google Scholar] [CrossRef]
- Allison, B.J.; Simmons, C.W. Valorization of tomato pomace by sequential lycopene extraction and anaerobic digestion. Biomass Bioenergy 2017, 105, 331–341. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic compounds recovered from agro-food by-products using membrane technologies: An overview. Food Chem. 2016; 213, 753–762. [Google Scholar]
- Raynie, D.E. Extraction. In Encyclopedia of Separation Science; Cooke, M., Poole, C.F., Wilson, I.D., Adlaard, E.R., Eds.; Academic Press: Cambridge, MA, USA, 2000; pp. 118–128. ISBN 978-0-12-226770-3. [Google Scholar]
- Strati, I.F.; Oreopoulou, V. Process optimisation for recovery of carotenoids from tomato waste. Food Chem. 2011, 129, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Kumcuoglu, S.; Yilmaz, T.; Tavman, S. Ultrasound assisted extraction of lycopene from tomato processing wastes. J. Food Sci. Technol. 2014, 51, 4102–4107. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pettinato, M.; Casazza, A.A.; Perego, P. A Comprehensive Optimization of Ultrasound-Assisted Extraction for Lycopene Recovery from Tomato Waste and Encapsulation by Spray Drying. Processes 2022, 10, 308. [Google Scholar] [CrossRef]
- Chada, P.S.N.; Santos, P.H.; Rodrigues, L.G.G.; Goulart, G.A.S.; Azevedo dos Santos, J.D.; Maraschin, M.; Lanza, M. Non-conventional techniques for the extraction of antioxidant compounds and lycopene from industrial tomato pomace (Solanum lycopersicum L.) using spouted bed drying as a pre-treatment. Food Chem. X 2022, 13, 100237. [Google Scholar] [CrossRef]
- Tran, Q.T.N.; Nguyen, H.V.H. Optimization of enzyme-assisted lycopene extraction from tomato (Lycopersicon esculentum) peel using rice bran oil. J. Food Meas. Charact. 2023, 17, 5154–5162. [Google Scholar] [CrossRef]
- Prokopov, T.; Nikolova, M.; Taneva, D. Improved carotenoid extraction from Bulgarian tomato peels using ultrasonication. Ann. Univ. Dunarea Jos Galati Fascicle VI–Food Technol. 2017, 41, 41–49. [Google Scholar]
- Figueira, J.A.; Pereira, J.A.M.; Porto-Figueira, P.; Câmara, J.S. Ultrasound-assisted liquid-liquid extraction followed by ultrahigh pressure liquid chromatography for the quanti fi cation of major carotenoids in tomato. J. Food Compos. Anal. 2017, 57, 87–93. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Liceaga, A.M.; San Martín-González, M.F. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT-Food Sci. Technol. 2015, 62, 160–168. [Google Scholar] [CrossRef]
- Popescu, M.; Iancu, P.; Plesu, V.; Todasca, M.C.; Isopencu, G.O.; Bildea, C.S. Valuable Natural Antioxidant Products Recovered from Tomatoes by Green Extraction. Molecules 2022, 27, 4191. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. Enhancing the bioaccessibility of lycopene from tomato processing byproducts via supercritical carbon dioxide extraction. Curr. Res. Food Sci. 2022, 5, 553–563. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. Technol. 2011, 46, 23–29. [Google Scholar] [CrossRef]
- Lazzarini, C.; Casadei, E.; Valli, E.; Tura, M.; Ragni, L.; Bendini, A.; Toschi, T.G. Sustainable Drying and Green Deep Eutectic Extraction of Carotenoids from Tomato Pomace. Foods 2022, 11, 405. [Google Scholar] [CrossRef]
- Tan, S.; Ke, Z.; Chai, D.; Miao, Y.; Luo, K.; Li, W. Lycopene, polyphenols and antioxidant activities of three characteristic tomato cultivars subjected to two drying methods. Food Chem. 2021, 338, 128062. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. Stability of lycopene during spray drying of tomato pulp. LWT 2005, 38, 479–487. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G.; Chatzitakis, P.C.; Nikas, V.A. Prediction of lycopene degradation during a drying process of tomato pulp. J. Food Eng. 2006, 74, 37–46. [Google Scholar] [CrossRef]
- Regier, M.; Mayer-Miebach, E.; Behsnilian, D.; Neff, E.; Schuchmann, H.P. Influences of drying and storage of lycopene-rich carrots on the carotenoid content. Dry. Technol. 2005, 23, 989–998. [Google Scholar] [CrossRef]
- Adalid, A.M.; Roselló, S.; Nuez, F. Evaluation and selection of tomato accessions (Solanum section Lycopersicon) for content of lycopene, β-carotene and ascorbic acid. J. Food Compos. Anal. 2010, 23, 613–618. [Google Scholar] [CrossRef]
- Kehilia, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 extraction and antioxidant activity of lycopene and β-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) peels by-product of a Tunisian industry. Food Bioprod. Process. 2017, 2, 340–349. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsiouras, A.; Mourtzinos, I. Extraction of Lycopene from Tomato Using Hydrophobic Natural Deep Eutectic Solvents Based on Terpenes and Fatty Acids. Foods 2022, 11, 2645. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.D.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total Phenolic, Flavonoid, Tomatine, and Tomatidine Contents and Antioxidant and Antimicrobial Activities of Extracts of Tomato Plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef] [PubMed]
- Hatami, T.; Meireles, M.A.A.; Ciftci, O.N. Supercritical carbon dioxide extraction of lycopene from tomato processing by-products: Mathematical modeling and optimization. J. Food Eng. 2019, 241, 18–25. [Google Scholar] [CrossRef]
- Plaza, A.; Rodríguez, L.; Concha-Meyer, A.A.; Cabezas, R.; Zurob, E.; Merlet, G.; Palomo, I.; Fuentes, E. Effects of Extraction Methods on Phenolic Content, Antioxidant and Antiplatelet Activities of Tomato Pomace Extracts. Plants 2023, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Solaberrieta, I.; Mellinas, C.; Jiménez, A.; Garrigós, M.C. Recovery of Antioxidants from Tomato Seed Industrial Wastes by Microwave-Assisted and Ultrasound-Assisted Extraction. Foods 2022, 11, 3068. [Google Scholar] [CrossRef] [PubMed]
- Arab, M.; Bahramian, B.; Schindeler, A.; Valtchev, P.; Dehghani, F.; McConchie, R. Extraction of phytochemicals from tomato leaf waste using subcritical carbon dioxide. Innov. Food Sci. Emerg. Technol. 2019, 57, 102204. [Google Scholar] [CrossRef]
- Bakić, M.T.; Pedisić, S.; Zorić, Z.; Dragović-Uzelac, V.; Grassino, A.N. Effect of Microwave-Assisted Extraction on Polyphenols Recovery from Tomato Peel Waste. Acta Chim. Slov. 2019, 66, 367–377. [Google Scholar] [CrossRef]
- Costa, C.; Lucera, A.; Marinelli, V.; Del Nobile, M.A.; Conte, A. Influence of different by-products addition on sensory and physicochemical aspects of Primosale cheese. J. Food Sci. Technol. 2018, 55, 4174–4183. [Google Scholar] [CrossRef] [PubMed]
- Azabou, S.; Sebii, H.; Ben, F.; Abid, Y.; Jridi, M.; Nasri, M. Food Bioscience Phytochemical profile and antioxidant properties of tomato by-products as affected by extraction solvents and potential application in refined olive oils. Food Biosci. 2020, 36, 100664. [Google Scholar] [CrossRef]
- Robles-Ramírez, M.C.; Monterrubio-López, R.; Mora-Escobedo, R.; Beltrán-Orozco, M.d.C. Evaluation of extracts from potato and tomato wastes as natural antioxidant additives. Arch. Latinoam. Nutr. 2016, 66, 66–73. [Google Scholar]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of pulsed electric fields to improve product yield and waste valorization in industrial tomato processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K.; Bhargav, V.K. Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J. Food Sci. Technol. 2014, 51, 2568–2575. [Google Scholar] [CrossRef]
- Westphal, A.; Bauerfeind, J.; Rohrer, C.; Ernawita; Böhm, V. Analytical characterisation of the seeds of two tomato varieties as a basis for recycling of waste materials in the food industry. Eur. Food Res. Technol. 2014, 239, 613–620. [Google Scholar] [CrossRef]
- Mahieddine, B.; Amina, B.; Faouzi, S.M.; Sana, B.; Wided, D. Effects of microwave heating on the antioxidant activities of tomato (Solanum lycopersicum). Ann. Agric. Sci. 2018, 63, 135–139. [Google Scholar] [CrossRef]
- Atanasova, A.H.; Denev, P.N.; Tringovska, I.I.; Grozeva, S.Y.; Ganeva, D.G.; Kratchanova, M.G.; Panchev, I.N. Optimization of the key parameters for extraction of polyphenol compounds from tomato fruits (Solanum lycopersicum L.). Kinetics of the process. Bulg. Chem. Commun. 2014, 46, 65–70. [Google Scholar]
- Khatib, M.; Cecchi, L.; Bellumori, M.; Zonfrillo, B.; Mulinacci, N. Polysaccharides and Phenolic Compounds Recovered from Red Bell Pepper, Tomato and Basil By-Products Using a Green Extraction by Extractor Timatic®. Int. J. Mol. Sci. 2023, 24, 16653. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Antioxidant activity and bioactive compound changes during fruit ripening of high-lycopene tomato cultivars. J. Food Compos. Anal. 2011, 24, 588–595. [Google Scholar] [CrossRef]
- Szabo, K.; Diaconeaza, Z.; Catoi, A.; Vodnar, D.C. Screening of Ten Tomato Varieties Processing Waste for Bioactive Components and Their Related Antioxidant and Antimicrobial Activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef]
- Helyes, L.; Lugasi, A. Formation of certain compounds having technological and nutritional importance in tomato fruits during maturation. Acta Aliment. 2006, 35, 183–193. [Google Scholar] [CrossRef]
- Raffo, A.; Leonardi, C.; Fogliano, V.; Ambrosino, P.; Salucci, M.; Gennaro, L.; Bugianesi, R.; Guiofrida, F.; Quaglia, G. Nutritional Value of Cherry Tomatoes (Lycopersicon esculentum Cv. Naomi F1) Harvested at Different Ripening Stages. J. Agric. Food Chem. 2002, 50, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Belović, M.M.; Gironés-Vilaplana, A.; Moreno, D.A.; Milovanović, I.L.; Novaković, A.R.; Karaman, M.A.; Ilić, N.M. Tomato (Solanum lycopersicum L.) Processing main product (juice) and by-product (pomace) bioactivity potential measured as antioxidant activity and angiotensin-converting enzyme inhibition. J. Food Process. Preserv. 2016, 40, 1229–1237. [Google Scholar] [CrossRef]
- Kaidi, K.; Moghrani, H.; Mohammed, D.; Youcef, S.; Slimane, K.; Taleb Ahmed, M. Valorization study of the organic waste resulting from the tomato canning by methanisation. UPB Sci. Bull. Ser. B 2020, 82, 95–108. [Google Scholar]
- Achinas, S.; Li, Y.; Achinas, V.; Euverink, G.J.W. Biogas potential from the anaerobic digestion of potato peels: Process performance and kinetics evaluation. Energies 2019, 12, 2311. [Google Scholar] [CrossRef]
- Aravani, V.P.; Tsigkou, K.; Papadakis, V.G.; Kornaros, M. Biochemical Μethane potential of most promising agricultural residues in Northern and Southern Greece. Chemosphere 2022, 296, 133985. [Google Scholar] [CrossRef] [PubMed]
- Andriamanohiarisoamanana, F.J.; Yasui, S.; Yamashiro, T.; Ramanoelina, V.; Ihara, I.; Umetsu, K. Anaerobic co-digestion: A sustainable approach to food processing organic waste management. J. Mater. Cycles Waste Manag. 2020, 22, 1501–1508. [Google Scholar] [CrossRef]
- Girotto, F.; Cristina Lavagnolo, M.; Gulgun, A.; Piazza, L. Bio-methane production from tomato pomace: Preliminary evaluation of process intensification through ultrasound pre-treatment. J. Mater. Cycles Waste Manag. 2020, 23, 416–422. [Google Scholar] [CrossRef]
- Saghouri, M.; Mansoori, Y.; Rohani, A.; Khodaparast, M.H.H.; Sheikhdavoodi, M.J. Modelling and evaluation of anaerobic digestion process of tomato processing wastes for biogas generation. J. Mater. Cycles Waste Manag. 2018, 20, 561–567. [Google Scholar] [CrossRef]
- Alnakeeb, A.N.; Najim, K.; Ahmed, A. Anaerobic Digestion of Tomato Wastes from Groceries Leftovers: Effect of Moisture Content. Int. J. Curr. Eng. Technol. 2017, 7, 1468–1470. [Google Scholar]
- Aybek, A.; Üçok, S. Determination and evaluation of biogas and methane productions of vegetable and fruit wastes with Hohenheim Batch Test method. Int. J. Agric. Biol. Eng. 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Gunaseelan, V.N. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Rodrigues, D.P.; Klepacz-smolka, A.; Martins, R.C.; Quina, M.J. Comparative analysis of methods and models for predicting biochemical methane potential of various organic substrates. Sci. Total Environ. 2019, 649, 1599–1608. [Google Scholar] [CrossRef]
- Sánchez, F.; Sánchez, J.; San Miguel, G. Biomass resources to hybridize CSP with biomethane: Potential of horticultural residues and drought-tolerant crops. Procedia Comput. Sci. 2016, 83, 1102–1109. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Almeida, P.V.; Martinho, C.M.O.; Gando-Ferreira, L.M.; Quina, M.J. Biochemical Methane Potential Enhancement Through Biomass Fly Ash Addition. In Proceedings of the 9th International Conference on Energy and Environment Research—ICEER 2022—Environmental Science and Engineering, Porto, Portugal, 12–16 September 2022; Caetano, N.S., Felgueiras, M.C., Eds.; Springer: Cham, Switzerland, 2023; pp. 655–663. [Google Scholar]
- Ruiz-Aguilar, G.M.L.; Martínez-Martínez, J.H.; Costilla-Salazar, R.; Camarena-Martínez, S. Using Central Composite Design to Improve Methane Production from Anaerobic Digestion of Tomato Plant Waste. Energies 2023, 16, 5412. [Google Scholar] [CrossRef]
- Xue, X.; Li, X.; Shimizu, N. Synthesis evaluation on thermophilic anaerobic co-digestion of tomato plant residue with cattle manure and food waste. Resour. Environ. Sustain. 2023, 13, 100119. [Google Scholar] [CrossRef]
- Ruiz-Aguilar, G.M.L.; Nuñez-Palenius, H.G.; Lovanh, N.; Camarena-Martínez, S. Comparative Study of Methane Production in a One-Stage vs. Two-Stage Anaerobic Digestion Process from Raw Tomato Plant Waste. Energies 2022, 15, 9137. [Google Scholar] [CrossRef]
- Gil, A.; Siles, J.A.; Carmen Gutiérrez, M.; Martín, M.Á. Evaluation of physicochemical pretreatment of tomato plant for aerobic and anaerobic biodegradation. Biomass Convers. Biorefinery 2019, 9, 489–497. [Google Scholar] [CrossRef]
- Jagadabhi, P.S.; Kaparaju, P.; Rintala, J. Two-stage anaerobic digestion of tomato, cucumber, common reed and grass silage in leach-bed reactors and upflow anaerobic sludge blanket reactors. Bioresour. Technol. 2011, 102, 4726–4733. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, F.; Li, Y.; Lu, J.; Li, S.; Shah, A.; Zhang, X.; Zhang, H.; Gong, X.; Li, G. Reactor performance and energy analysis of solid state anaerobic co-digestion of dairy manure with corn stover and tomato residues. Waste Manag. 2018, 73, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Driller, L.; Chamy, R.; Schuch, W.; Nogueira, M.; Fraser, P. Biogas potential of residues generated by the tomato processing industry under different substrate and inoculum conditions. Acta Hortic. 2017, 1159, 151–158. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, D.; Li, G.; Lu, J.; Li, S. Solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover for biogas production. Bioresour. Technol. 2016, 217, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Sülük, K.; Tosun, İ.; Ekinci, K. Co-composting of two-phase olive-mill pomace and poultry manure with tomato harvest stalks. Environ. Technol. 2017, 38, 923–932. [Google Scholar] [CrossRef]
- Kulcu, R. Composting of Greenhouse Tomato Plant Residues, Wheat Straw, and Separated Dairy Manure, and the Effect of Free Air Space on the Process. Polish J. Environ. Stud. 2014, 23, 1341–1346. [Google Scholar]
- Rashwan, M.; Alkoaik, F.; Abdel-Razzak, H.; Ibrahim, M.; Fulleros, R.; Shady, M.; Abdel-Ghany, A. Evaluation of tomato waste compost stability and maturity using CIELAB color indicator. J. Plant Nutr. 2020, 43, 1427–1437. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Lu, J.; Zhang, X.; Li, S.; Wu, Y.; Li, G. Effects of digestion time in anaerobic digestion on subsequent digestate composting. Bioresour. Technol. 2018, 267, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Vig, H.; Deshmukh, R. Lycopene Market Size, Share, Competitive Landscape and Trend Analysis Report by Form, Nature and Application: Global Opportunity Analysis and Industry Forecast, 2021–2030. 2021. Available online: https://www.alliedmarketresearch.com/lycopene-market-A06684 (accessed on 6 June 2024).
- Beta-Carotene Market Size, Share & Growth Report, 2030. 2018. Available online: https://www.grandviewresearch.com/industry-analysis/beta-carotene-market# (accessed on 6 June 2024).
- Hatami, T.; Ciftci, O.N. A step-by-step technoeconomic analysis of supercritical carbon dioxide extraction of lycopene from tomato processing waste. J. Food Eng. 2023, 357, 111639. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Zhang, Y.; Luo, W.; Li, G. Anaerobic digestion of different agricultural wastes: A techno-economic assessment. Bioresour. Technol. 2020, 315, 123836. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.D.; Khare, T.; Dhamole, P.B. Process development and techno-economic assessment of lycopene extraction from tomatoes using surfactant. Biomass Conv. Biorefinery 2023. [Google Scholar] [CrossRef]
- Hatami, T.; Ciftci, O.N. Techno-economic sensitivity assessment for supercritical CO2 extraction of lycopene from tomato processing waste. J. Supercrit. Fluids 2024, 204, 106109. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).