Changes in Polyphenols and Antioxidant Activity in Fermentation Substrate during Maotai-Flavored Liquor Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Total Phenolic Determination
2.4. Phenolic Compound Analysis
2.5. Determination of the DPPH Free Radical Clearance
2.6. ABTS Measurement
2.7. FRAP Measurement
2.8. Data Statistics and Analysis
3. Results and Discussion
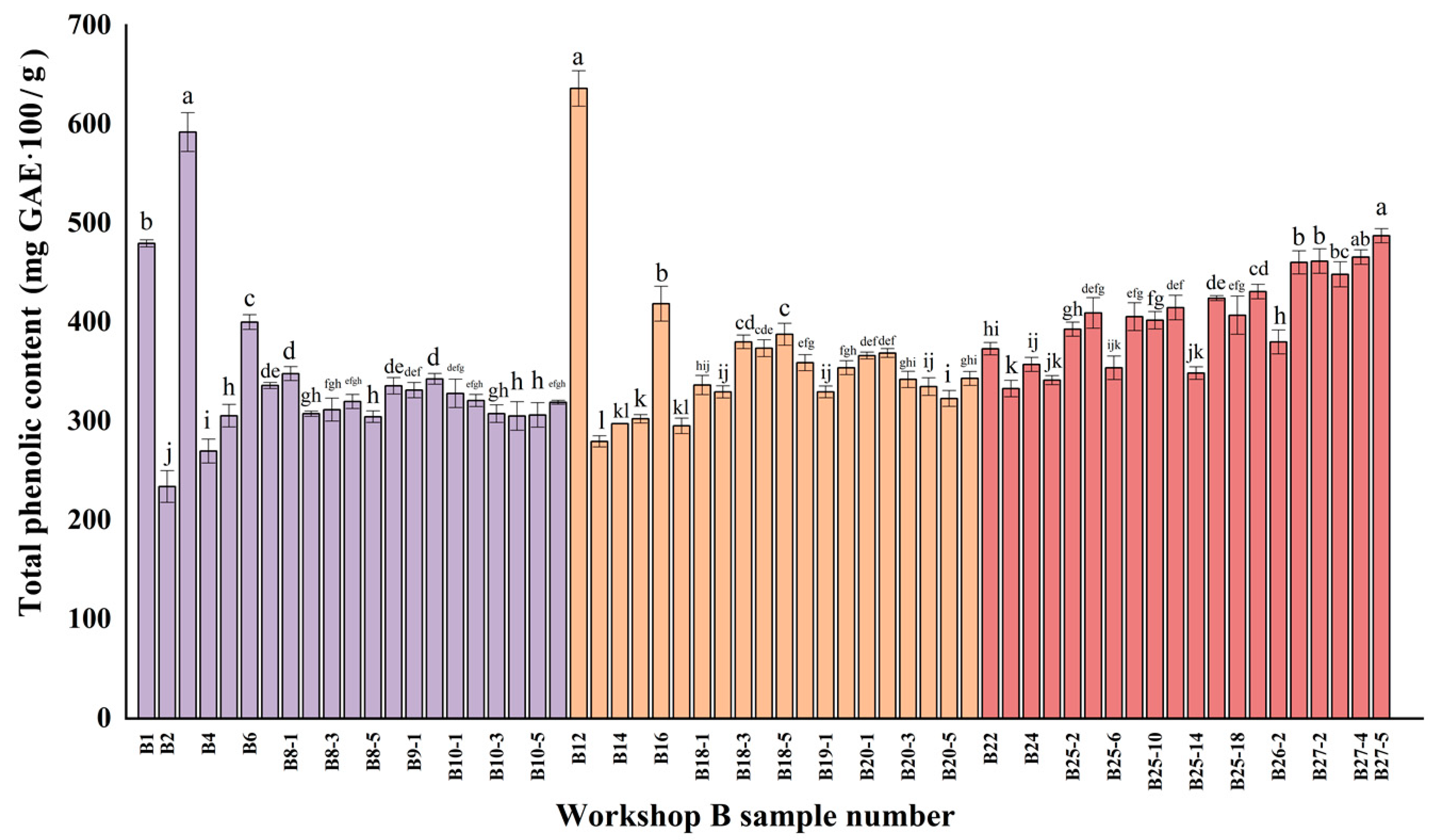
3.1. Total Phenol Analysis
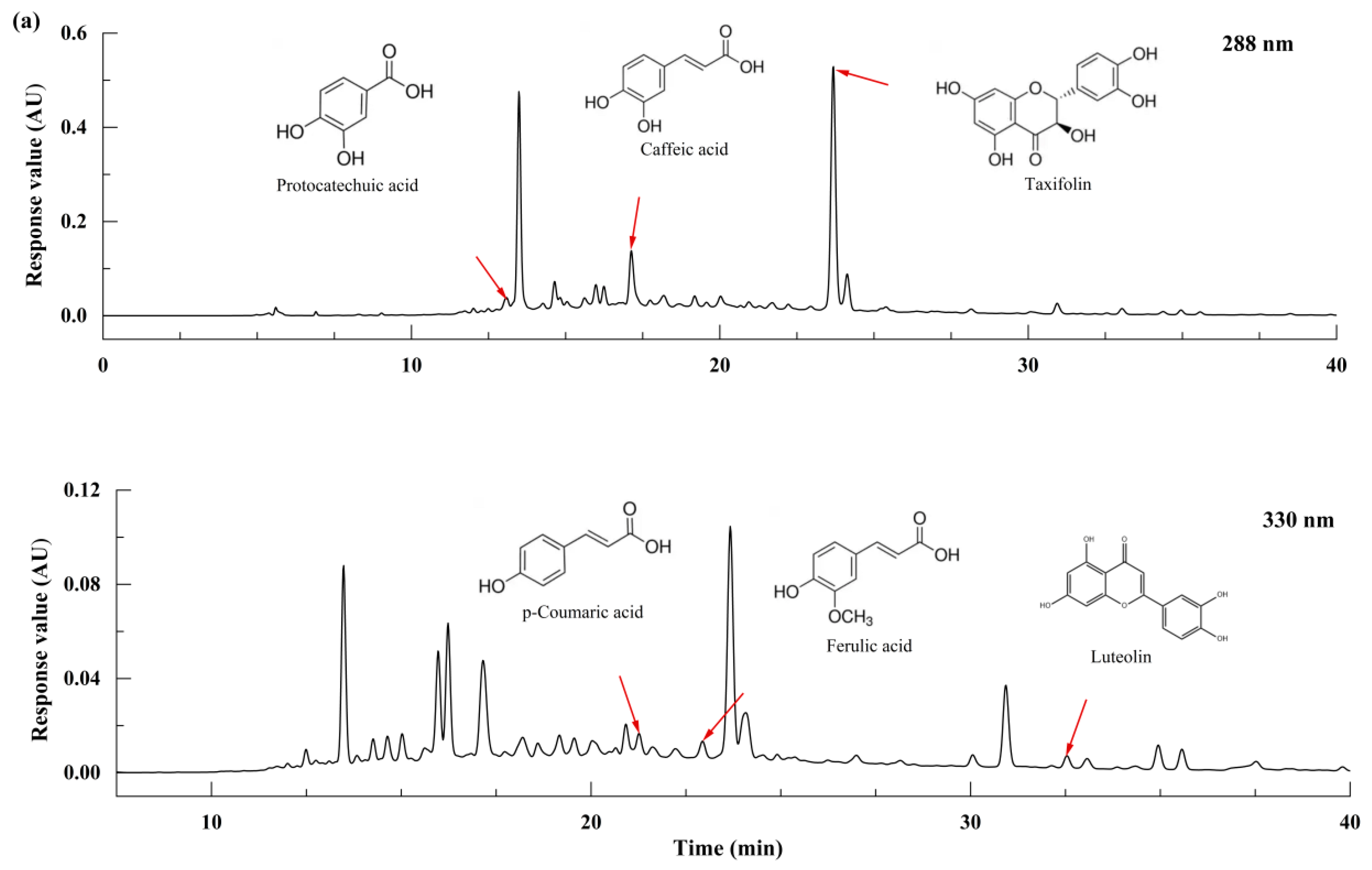
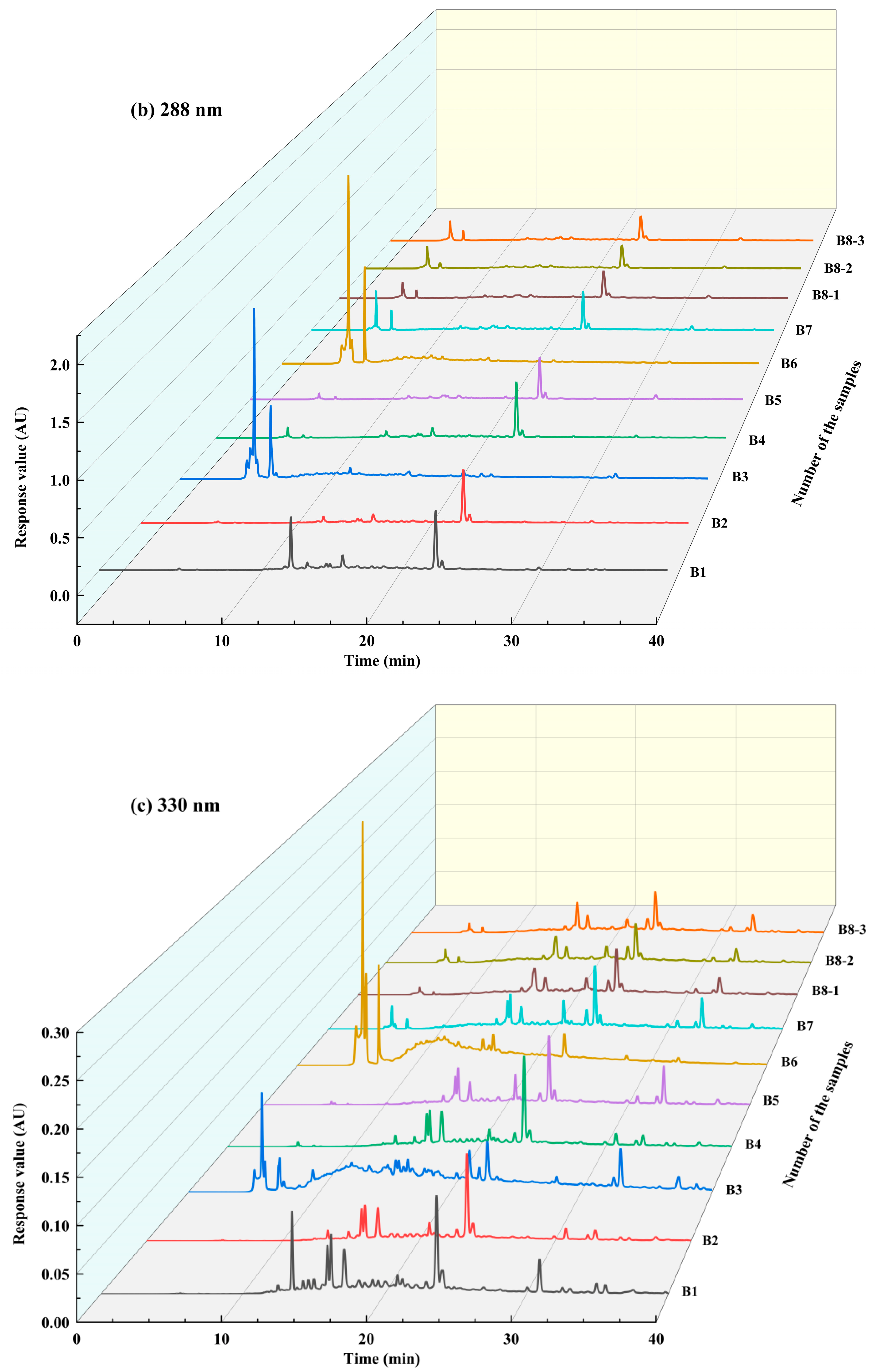
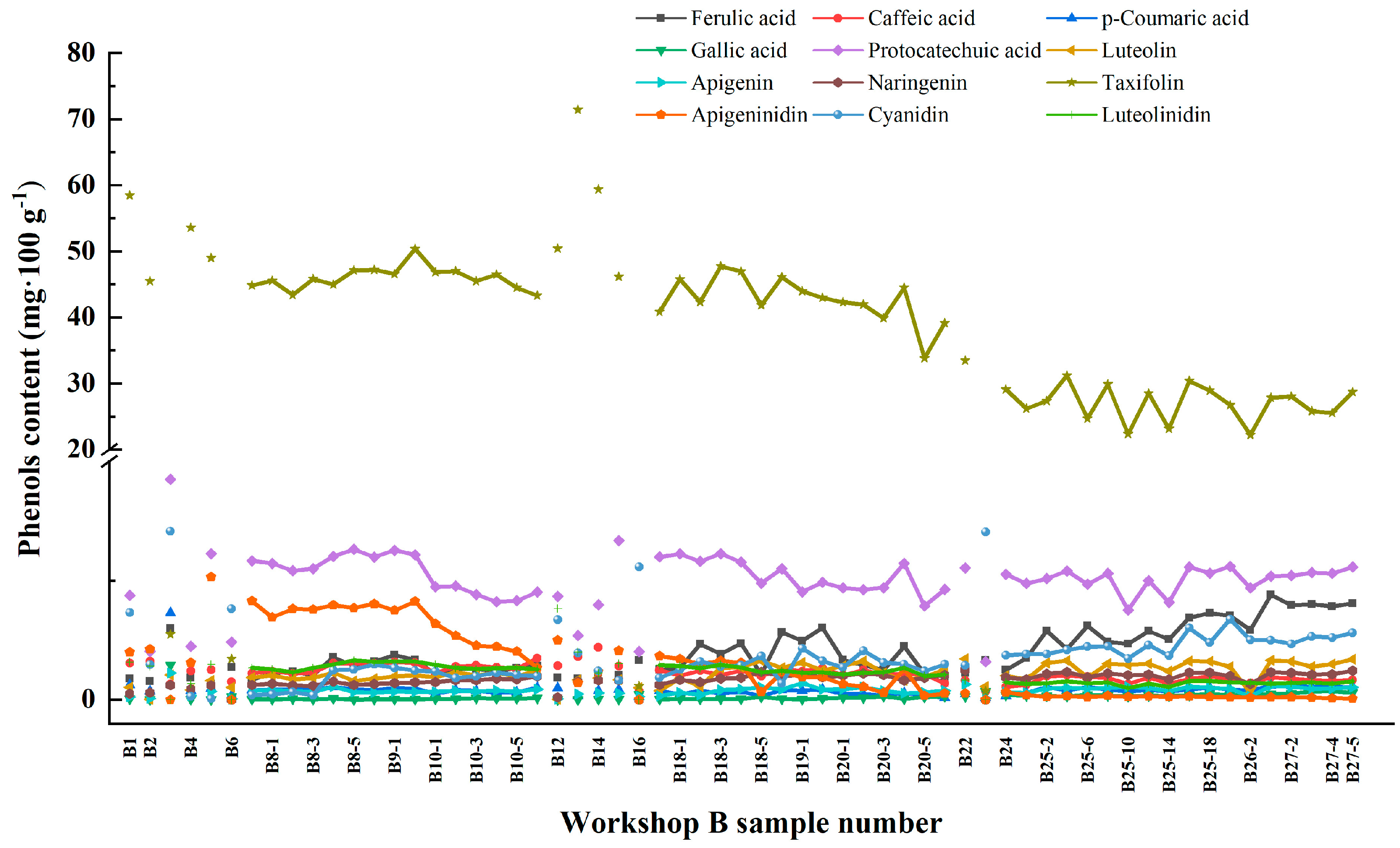
3.2. Identification of Phenolic Substances
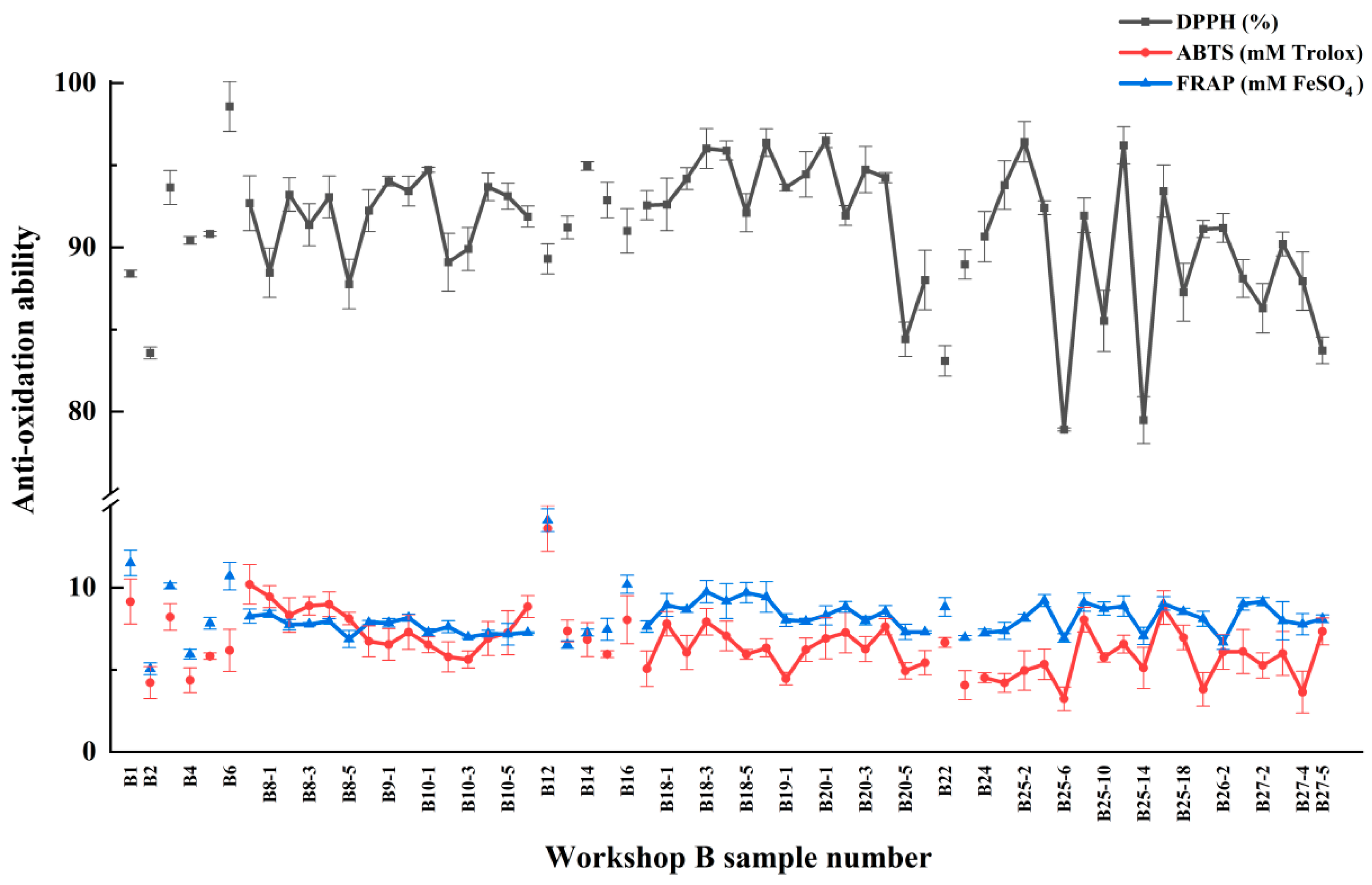
3.3. Antioxidant Activity Analysis
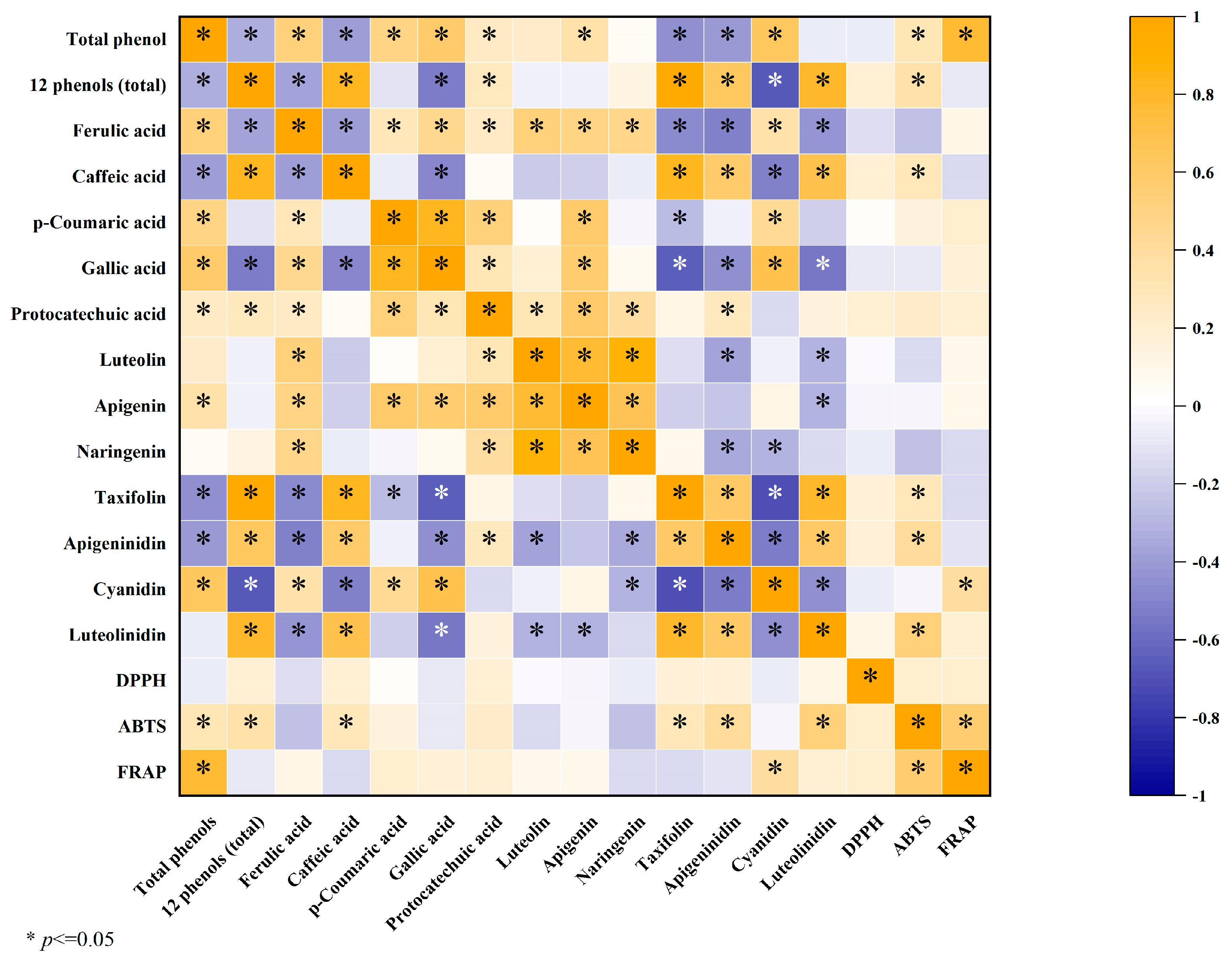
3.4. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bi, R.Y.; Lu, J.; Tang, P.; Shan, Q.; Wang, L.; Wang, F.; Li, C. Study on change law of volatile phenolic compounds in Jiangxiang Baijiu by aqueous two-phase extraction combined with GC-MS/MS. Liquor Mak. Sci. Technol. 2023, 1, 137–144. [Google Scholar]
- Wu, Y.; Sun, Y.; Yin, Y.; Wang, H.; Li, J.; Du, L. Isolation, identification and probiotic characteristics of lactic acid bacteria in fermented grains of sauce-flavor Baijiu. China Brew. 2023, 42, 76–82. [Google Scholar]
- Huang, W.; Cheng, P.Y.; Zhang, J.; Li, L.; Hu, F. Research progress in the factors influencing the flavor formation of Jiangxiang Baijiu and its main flavoring components. Liquor. -Mak. Sci. Technol. 2020, 4, 85–93. [Google Scholar]
- Zhao, G.H.; Zhang, R.F.; Su, D.X.; Lihong, D.; Lei, L.; Zhencheng, W.; Mingwei, Z. Advances in the study of whole grain phenolics and their antioxidant activity. J. Chin. Inst. Food Sci. Technol. 2017, 17, 183–196. [Google Scholar]
- Hao, J.M.; Jin, M.K.; Yang, W.P.; Zhao, M.; Yang, C.; Gao, Z.; Yang, Z.; Fan, W. Purification of flavonoids from wheat grain and their components analysis. Food Sci. Technol. 2019, 44, 203–210. [Google Scholar]
- Li, J.; Du, N.; Yuan, X.; Pang, H.; Guo, X.; Zhang, Y. Determination of volatile phenols in Jiangxiang Baijiu by GC-MS. Liquor. Mak. Sci. Technol. 2017, 1, 98–101. [Google Scholar]
- Li, S.G.; Liu, M.; Liu, F.; Zou, J.Q.; Lu, X.C.; Diao, X.M. Current status and future prospective of sorghum production and seed industry in China. Sci. Agric. Sin. 2021, 54, 471–482. [Google Scholar]
- Jiang, L.L.; Yin, Y.Y.; Yang, J.L.; Tian, D.; Feng, X.; Chen, M.; Cheng, P. Research progress on the effect of sorghum as brewing material on Baijiu quality. China Brew. 2022, 41, 6–11. [Google Scholar]
- Yao, C.; Zhou, H.; Gong, Y.; Liao, Q.; Wu, L.; Li, Y.; Tang, T.; An, M.; Li, G. Phenolic components in Wuliangye Daqu and their antioxidant activity. Food Ferment. Ind. 2019, 45, 55–59. [Google Scholar]
- Sun, B.G.; Huang, M.Q.; Wang, J. Advances in the study of flavor chemistry and health effects of Baijiu. J. Chin. Inst. Food Sci. Technol. 2021, 21, 1–13. [Google Scholar]
- Shi, Y.L.; Fan, W.L.; Xu, Y. Preliminary study on antioxidant activity of Chinese liquor. Sci. Technol. Food Ind. 2015, 36, 95–97. [Google Scholar]
- Chetrariu, A.; Dabija, A. Spent grain from malt whisky: Assessment of the phenolic compounds. Molecules 2021, 26, 3236. [Google Scholar] [CrossRef]
- Hong, J.; Chen, L.; Zhao, D.; Sun, J. Multivariate analysis on the relationship between radical scavenging activities and phenolic compounds of Baijiu and its protective effect against LPS-induced inflammation in THP-1 cells. Int. Food Res. J. 2022, 29, 116–127. [Google Scholar] [CrossRef]
- Deng, W.Y.; Xu, Y.M.; Cheng, W.; Liu, J.; Deng, J.; Wang, H.; Wu, X. Effects of grain soaking process on the production of Jiangxiang Baijiu. Liquor. Mak. Sci. Technol. 2021, 1, 36–41. [Google Scholar]
- Mora-Rochin, S.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O.; Sánchez-Peña, P.; Reyes-Moreno, C.; Milán-Carrillo, J. Phenolic content and antioxidant activity of tortillas produced from pigmented maize processed by conventional nixtamalization or extrusion cooking. J. Cereal Sci. 2010, 52, 502–508. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Luo, J.; Johnson, S.; Fang, Z. Effect of processing on the phenolic contents, antioxidant activity and volatile compounds of sorghum grain tea. J. Cereal Sci. 2019, 85, 6–14. [Google Scholar] [CrossRef]
- He, Z.; Tao, Y.; Yurong, Y.; Zhebing, H.; Peng, Y.; Jian, W.; Caihong, L.; Shenggang, L. Optimization of oscillating cooking process of brewing sorghum by orthogonal tests. China Brew. 2019, 38, 91–95. [Google Scholar]
- Dordevic, T.M.; Siler-Marinkovic, S.S.; Dimitrijevic-Brankovic, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Tang, W.C.; Zhao, J.S.; Kong, X.; Yun, L. Brief introduction of Jiangxiang Baijiu production technology and principles of key processes. Liquor. Mak. Sci. Technol. 2021, 5, 86–92. [Google Scholar]
- Liu, M.W.; Pan, X.S.; Yin, F.W.; Ge, R. Research on the mechanism of high-temperature accumulation process and wine production analysis of soy-sauce type Baijiu rounds. Liquor. Mak. 2019, 46, 60–64. [Google Scholar]
- Sun, H.Y.; Wang, H.X.; Zhang, P.Z.; Ajlouni, S.; Fang, Z.X. Changes in phenolic content, antioxidant activity, and volatile compounds during processing of fermented sorghum grain tea. Cereal Chem. 2020, 97, 612–625. [Google Scholar] [CrossRef]
- Ravisankar, S.; Dizlek, H.; Awika, J.M. Changes in extractable phenolic profile during natural fermentation of wheat, sorghum and teff. Food Res. Int. 2021, 145, 110426. [Google Scholar] [CrossRef]
- Shen, S.; Huang, R.; Li, C.; Wu, W.; Chen, H.; Shi, J.; Chen, S.; Ye, X. Phenolic compositions and antioxidant activities differ significantly among sorghum grains with different applications. Molecules 2018, 23, 1203. [Google Scholar] [CrossRef]
- Yan, R.; Hao, H.; Miao, Z.; Wang, B.; Sun, J.; Sun, B. Analysis of metabolism of 4-methyl/ethyl guaiacol during strong flavor Baijiu fermentation. Food Ferment. Ind. 2022, 49, 41–46. [Google Scholar]
- Jin, S.P.; Hui, M.; Tian, Q. Research progress on flavor substances and influencing factors of Maotai-flavor liquor. Bulletin of Fermentation and Sci. Technol. 2022, 51, 164–168. [Google Scholar]
- Wang, L.; Hu, X.X.; Huang, Y.G. Characteristics of bacterial microflora structure in environmental samples from different rounds of Maotai-flavor liquor fermentation in Maotai Town. Food Sci. 2021, 42, 185–192. [Google Scholar]
- Zheng, L.; Qin, P.; Lu, Y.; Wu, Z.; Zhang, C.; Li, X.; Liang, H. Research progress of microbial diversity and metabolic process during the fermentation of sauce-flavor Baijiu. China Brew. 2022, 41, 6–11. [Google Scholar]
- Lu, Z.; Wang, H.Z.; Li, E.Z.; Yang, K.; Zhang, J.S.; Gao, S.Q. Screening of a mold strain from high-temperature Daqu & Identification of its volatile fermentation products. Liquor. Mak. Sci. Technol. 2019, 7, 50–55. [Google Scholar]
- Yang, X.X.; Huang, Y.Y.; Yi, C.Y.; Li, Y.; Guo, R.Q.; Zhu, X.Q. Relationship between polyphenol content and antioxidant activity of different soybean varieties. J. Chin. Cereals Oils Assoc. 2022, 37, 86–91. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects-a review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar]
- Wang, X.; Liu, W.W.; Cao, X.L. Enzymatic extraction of phenolic components from distiller’s grains. Food Sci. 2011, 32, 114–119. [Google Scholar]
- Yang, J. Extraction and Separation of Total Flavonoids from Distiller’s Grains and Their Biological Activities. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2022. [Google Scholar]
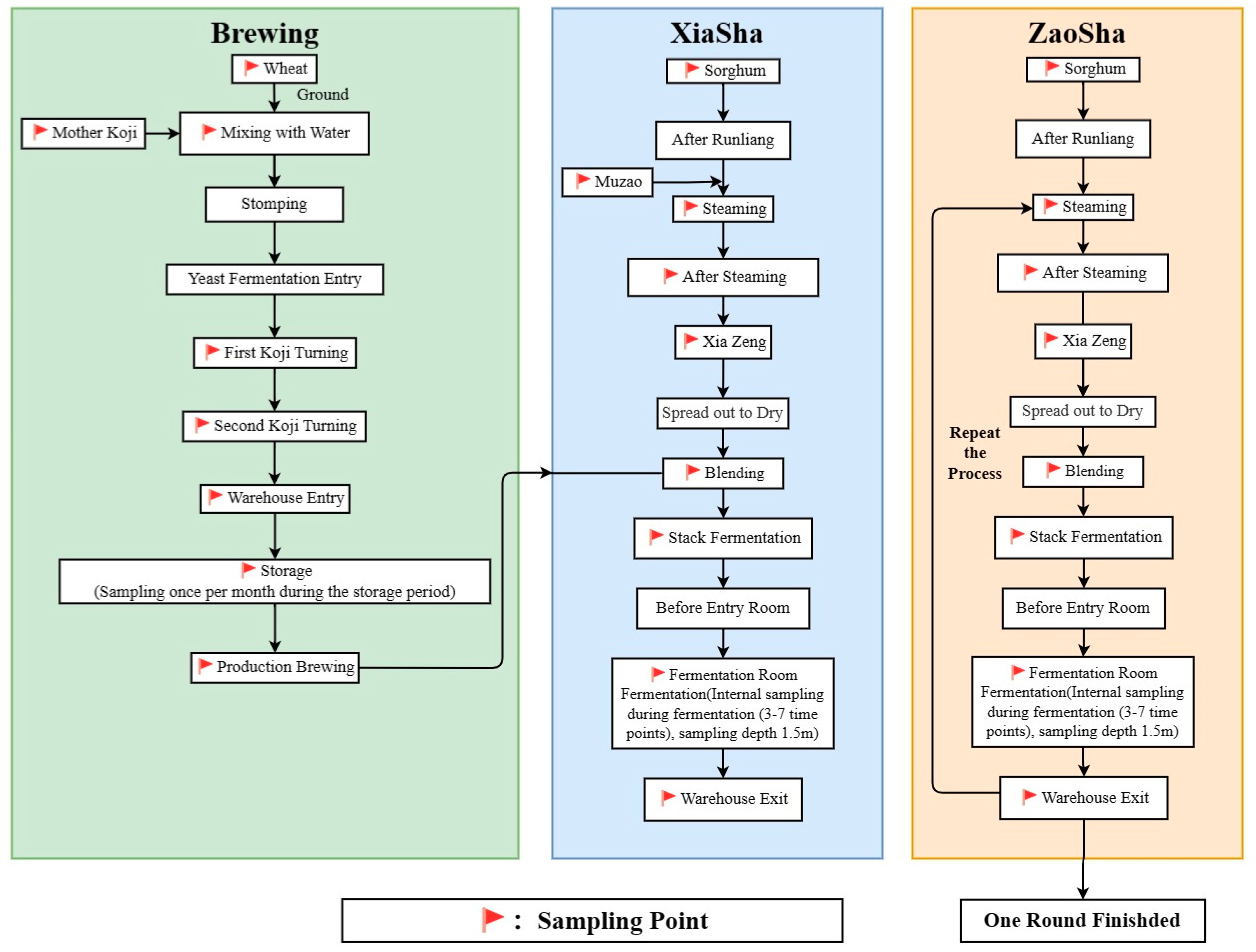
| Sample Name of Xiasha Stage | Number | Sample Name of Zaosha Stage | Number | Sample Name of Single-Round Stage | Number | |||
|---|---|---|---|---|---|---|---|---|
| Raw sorghum | B1 | Raw sorghum | B12 | Wine spirits before mixing with Daqu | B22 | |||
| Pre-mixing grain spirits | B2 | Grain spirits before mixing with cellar spirits | B13 | Daqu | B23 | |||
| Muzao | B3 | Grain spirits after mixing with cellar spirits | B14 | Wine spirits after mixing with Daqu | B24 | |||
| Grain spirits after mixing Muzao | B4 | Wine spirits before mixing with Daqu | B15 | Stacking 1 day of wine spirits | Depth 0.3 m | B25-1 | ||
| Wine spirits before mixing with Daqu | B5 | Daqu | B16 | Depth 1.5 m | B25-2 | |||
| Daqu | B6 | Wine spirits after mixing with Daqu | B17 | Stacking 3 day of wine spirits | Depth 0.3 m | B25-5 | ||
| Wine spirits after mixing with Daqu | B7 | Stacking 1 day of wine spirits | Depth 0.3 m | B18-1 | Depth 1.5 m | B25-6 | ||
| Stacking 1 day of wine spirits | Depth 0.3 m | B8-1 | Depth 1.5 m | B18-2 | Stacking 5 day of wine spirits | Depth 0.3 m | B25-9 | |
| Depth 1.5 m | B8-2 | Stacking 2 day of wine spirits | Depth 0.3 m | B18-3 | Depth 1.5 m | B25-10 | ||
| Stacking 2 day of wine spirits | Depth 0.3 m | B8-3 | Depth 1.5 m | B18-4 | Stacking 7 day of wine spirits | Depth 0.3 m | B25-13 | |
| Depth 1.5 m | B8-4 | Stacking 3 day of wine spirits | Depth 0.3 m | B18-5 | Depth 1.5 m | B25-14 | ||
| Stacking 3 day of wine spirits | Depth 0.3 m | B8-5 | Depth 1.5 m | B18-6 | Stacking 9 day of wine spirits | Depth 0.3 m | B25-17 | |
| Depth 1.5 m | B8-6 | Pre-cellar spirits | Depth 0.3 m | B19-1 | Depth 1.5 m | B25-18 | ||
| Pre-cellar spirits | Depth 0.3 m | B9-1 | Depth 1.5 m | B19-2 | Pre-cellar spirits | Depth 0.3 m | B26-1 | |
| Depth 1.5 m | B9-2 | Fermentation of wine spirits in the cellar for 3 days | B20-1 | Depth 1.5 m | B26-2 | |||
| Fermentation of wine spirits in the cellar for 3 days | B10-1 | Fermentation of wine spirits in the cellar for 7 days | B20-2 | Fermentation of wine spirits in the cellar for 3 days | B27-1 | |||
| Fermentation of wine spirits in the cellar for 7 days | B10-2 | Fermentation of wine spirits in the cellar for 14 days | B20-3 | Fermentation of wine spirits in the cellar for 7 days | B27-2 | |||
| Fermentation of wine spirits in the cellar for 14 days | B10-3 | Fermentation of wine spirits in the cellar for 21 days | B20-4 | Fermentation of wine spirits in the cellar for 14 days | B27-3 | |||
| Fermentation of wine spirits in the cellar for 21 days | B10-4 | Fermentation of wine spirits in the cellar for 28 days | B20-5 | Fermentation of wine spirits in the cellar for 21 days | B27-4 | |||
| Fermentation of wine spirits in the cellar for 28 days | B10-5 | Unstrained spirits from the cellar | B21 | Fermentation of wine spirits in the cellar for 28 days | B27-5 | |||
| Unstrained spirits from the cellar | B11 | |||||||
| Phenolic Substances | Chemical Formula | Content (mg/100 g) |
|---|---|---|
| Gallic acid | C7H6O5 | 0.025 ± 0.003 |
| Protocatechuic acid | C7H6O4 | 4.369 ± 0.152 |
| Caffeic acid | C9H8O4 | 1.527 ± 0.340 |
| Luteolinidin | C15H11O5+ | 1.571 ± 0.220 |
| Cyanidin | C15H11O6+ | 3.650 ± 0.053 |
| Apigeninidin | C15H11O4+ | 1.985 ± 0.352 |
| p-Coumaric acid | C9H8O3 | 0.606 ± 0.012 |
| Ferulic acid | C10H10O4 | 0.882 ± 0.105 |
| Taxifolin | C15H12O7 | 58.433 ± 3.266 |
| Luteolin | C15H10O6 | 0.512 ± 0.113 |
| Apigenin | C15H10O5 | 0.118 ± 0.011 |
| Naringenin | C15H12O5 | 0.246 ± 0.037 |
| Sample Number | DPPH (%) | ABTS (mM Trolox) | FRAP (mM FeSO4) |
|---|---|---|---|
| B1 | 88.40 ± 0.21 | 9.15 ± 1.37 | 11.52 ± 0.77 |
| B2 | 83.57 ± 3.36 | 4.22 ± 0.97 | 5.06 ± 0.36 |
| B3 | 93.64 ± 1.03 | 8.21 ± 0.81 | 10.16 ± 0.18 |
| B4 | 90.42 ± 0.23 | 4.36 ± 0.76 | 5.95 ± 0.30 |
| B5 | 90.82 ± 2.14 | 5.83 ± 0.20 | 7.83 ± 0.36 |
| B6 | 98.57 ± 1.52 | 6.18 ± 1.28 | 10.70 ± 0.84 |
| B7 | 92.68 ± 1.67 | 10.24 ± 1.2 | 8.26 ± 0.43 |
| B8-1 | 88.45 ± 3.50 | 9.44 ± 0.67 | 8.40 ± 0.21 |
| B8-2 | 93.21 ± 1.02 | 8.33 ± 1.05 | 7.75 ± 0.31 |
| B8-3 | 91.37 ± 1.28 | 8.89 ± 0.56 | 7.77 ± 0.10 |
| B8-4 | 93.06 ± 1.28 | 8.98 ± 0.75 | 7.95 ± 0.16 |
| B8-5 | 87.76 ± 4.51 | 8.12 ± 0.38 | 6.87 ± 0.52 |
| B8-6 | 92.23 ± 1.28 | 6.73 ± 0.94 | 7.87 ± 0.13 |
| B9-1 | 94.03 ± 0.30 | 6.54 ± 0.96 | 7.86 ± 0.26 |
| B9-2 | 93.42 ± 0.91 | 7.29 ± 1.06 | 8.17 ± 0.21 |
| B10-1 | 94.72 ± 0.15 | 6.52 ± 0.48 | 7.27 ± 0.22 |
| B10-2 | 89.09 ± 4.76 | 5.78 ± 0.92 | 7.62 ± 0.37 |
| B10-3 | 89.90 ± 3.31 | 5.62 ± 0.52 | 6.98 ± 0.08 |
| B10-4 | 93.68 ± 1.84 | 6.90 ± 1.03 | 7.19 ± 0.21 |
| B10-5 | 93.11 ± 1.79 | 7.25 ± 1.34 | 7.16 ± 0.66 |
| B11 | 91.87 ± 0.64 | 8.84 ± 0.67 | 7.26 ± 0.05 |
| B12 | 89.30 ± 0.92 | 13.69 ± 1.4 | 14.1 ± 0.69 |
| B13 | 91.21 ± 0.69 | 7.36 ± 0.66 | 6.53 ± 0.22 |
| B14 | 94.94 ± 0.26 | 6.83 ± 1.03 | 7.26 ± 0.21 |
| B15 | 92.87 ± 1.09 | 5.95 ± 0.21 | 7.46 ± 0.67 |
| B16 | 91.00 ± 1.35 | 8.04 ± 1.46 | 10.21 ± 0.55 |
| B17 | 92.56 ± 1.89 | 5.06 ± 1.08 | 7.63 ± 0.35 |
| B18-1 | 92.61 ± 1.60 | 7.79 ± 0.74 | 8.94 ± 0.70 |
| B18-2 | 94.18 ± 1.66 | 6.05 ± 1.04 | 8.66 ± 0.17 |
| B18-3 | 96.01 ± 1.21 | 7.92 ± 0.81 | 9.75 ± 0.68 |
| B18-4 | 95.89 ± 0.58 | 7.06 ± 0.90 | 9.18 ± 1.06 |
| B18-5 | 92.10 ± 4.16 | 5.93 ± 0.30 | 9.69 ± 0.62 |
| B18-6 | 96.37 ± 2.84 | 6.33 ± 0.55 | 9.43 ± 0.93 |
| B19-1 | 93.64 ± 2.20 | 4.46 ± 0.38 | 8.02 ± 0.39 |
| B19-2 | 94.44 ± 2.38 | 6.22 ± 0.71 | 7.95 ± 0.18 |
| B20-1 | 96.50 ± 0.43 | 6.91 ± 1.25 | 8.31 ± 0.58 |
| B20-2 | 91.94 ± 2.60 | 7.26 ± 1.23 | 8.83 ± 0.33 |
| B20-3 | 94.73 ± 2.41 | 6.26 ± 0.76 | 7.99 ± 0.26 |
| B20-4 | 94.23 ± 0.31 | 7.62 ± 0.50 | 8.57 ± 0.33 |
| B20-5 | 84.40 ± 4.04 | 4.93 ± 0.50 | 7.30 ± 0.47 |
| B21 | 88.01 ± 1.81 | 5.43 ± 0.74 | 7.30 ± 0.07 |
| B22 | 83.09 ± 0.92 | 6.66 ± 0.30 | 8.82 ± 0.57 |
| B23 | 88.96 ± 3.89 | 4.06 ± 0.88 | 6.96 ± 0.12 |
| B24 | 90.65 ± 1.53 | 4.52 ± 0.30 | 7.26 ± 0.21 |
| B25-1 | 93.78 ± 1.48 | 4.20 ± 0.57 | 7.37 ± 0.52 |
| B25-2 | 96.42 ± 1.23 | 4.95 ± 1.20 | 8.17 ± 0.21 |
| B25-5 | 92.41 ± 0.42 | 5.33 ± 0.93 | 9.21 ± 0.36 |
| B25-6 | 78.91 ± 4.09 | 3.23 ± 0.72 | 6.93 ± 0.26 |
| B25-9 | 91.94 ± 1.06 | 8.05 ± 0.75 | 9.11 ± 0.55 |
| B25-10 | 85.52 ± 4.87 | 5.75 ± 0.29 | 8.72 ± 0.40 |
| B25-13 | 96.21 ± 1.13 | 6.56 ± 0.54 | 8.87 ± 0.62 |
| B25-14 | 79.48 ± 5.43 | 5.11 ± 1.25 | 7.06 ± 0.53 |
| B25-17 | 93.42 ± 2.59 | 8.78 ± 1.02 | 9.03 ± 0.41 |
| B25-18 | 87.26 ± 3.76 | 6.96 ± 0.75 | 8.55 ± 0.19 |
| B26-1 | 91.11 ± 3.52 | 3.81 ± 1.02 | 8.10 ± 0.46 |
| B26-2 | 91.17 ± 3.88 | 6.08 ± 1.06 | 6.67 ± 0.43 |
| B27-1 | 88.10 ± 2.15 | 6.11 ± 1.34 | 9.01 ± 0.38 |
| B27-2 | 86.29 ± 2.50 | 5.26 ± 0.77 | 9.14 ± 0.25 |
| B27-3 | 90.20 ± 0.72 | 5.99 ± 1.34 | 7.98 ± 1.17 |
| B27-4 | 87.94 ± 1.78 | 3.64 ± 1.27 | 7.77 ± 0.65 |
| B27-5 | 83.72 ± 1.81 | 7.33 ± 0.82 | 8.10 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, D.; Chen, C.; Yang, Y.; Tian, J.; Tu, H.; Yang, F.; Ye, X. Changes in Polyphenols and Antioxidant Activity in Fermentation Substrate during Maotai-Flavored Liquor Processing. Foods 2024, 13, 1928. https://doi.org/10.3390/foods13121928
Ni D, Chen C, Yang Y, Tian J, Tu H, Yang F, Ye X. Changes in Polyphenols and Antioxidant Activity in Fermentation Substrate during Maotai-Flavored Liquor Processing. Foods. 2024; 13(12):1928. https://doi.org/10.3390/foods13121928
Chicago/Turabian StyleNi, Derang, Chao Chen, Yubo Yang, Jinhu Tian, Huabin Tu, Fan Yang, and Xingqian Ye. 2024. "Changes in Polyphenols and Antioxidant Activity in Fermentation Substrate during Maotai-Flavored Liquor Processing" Foods 13, no. 12: 1928. https://doi.org/10.3390/foods13121928
APA StyleNi, D., Chen, C., Yang, Y., Tian, J., Tu, H., Yang, F., & Ye, X. (2024). Changes in Polyphenols and Antioxidant Activity in Fermentation Substrate during Maotai-Flavored Liquor Processing. Foods, 13(12), 1928. https://doi.org/10.3390/foods13121928







