Evaluation of Different Pectic Materials Coming from Citrus Residues in the Production of Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Materials
2.2.1. Water-Soluble Orange Residue Extract (WSE)
2.2.2. Semi-Pure Pectins
2.2.3. Raw Matter Composition and Characteristics
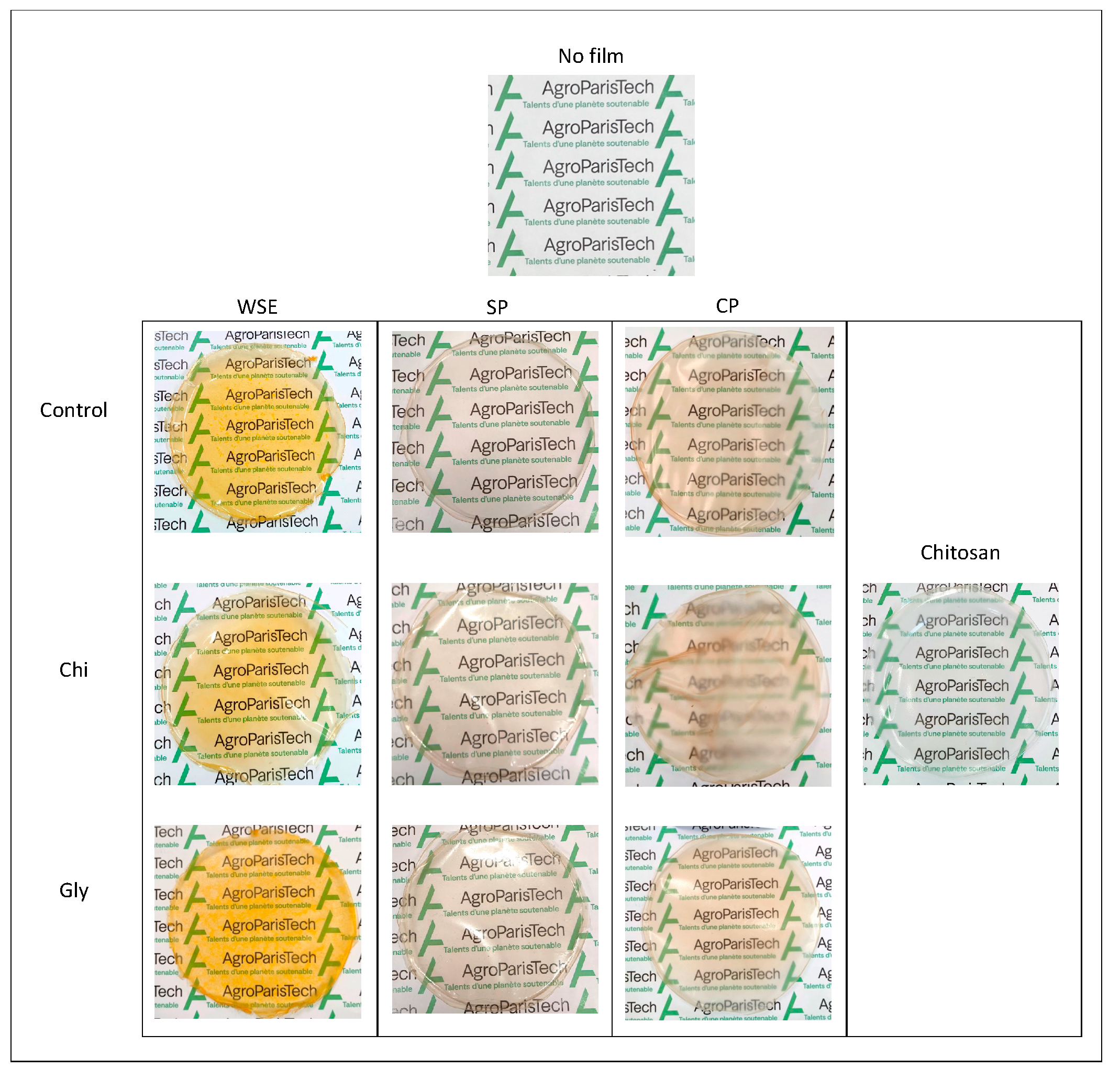
2.3. Films Preparation
2.4. Films Characterization
2.4.1. Chemical Properties
2.4.2. Functional Properties
2.4.3. Aspect and Structure
2.5. Statistical Analysis
3. Results and Discussion
3.1. Raw Matter Characteristics
3.1.1. General Composition
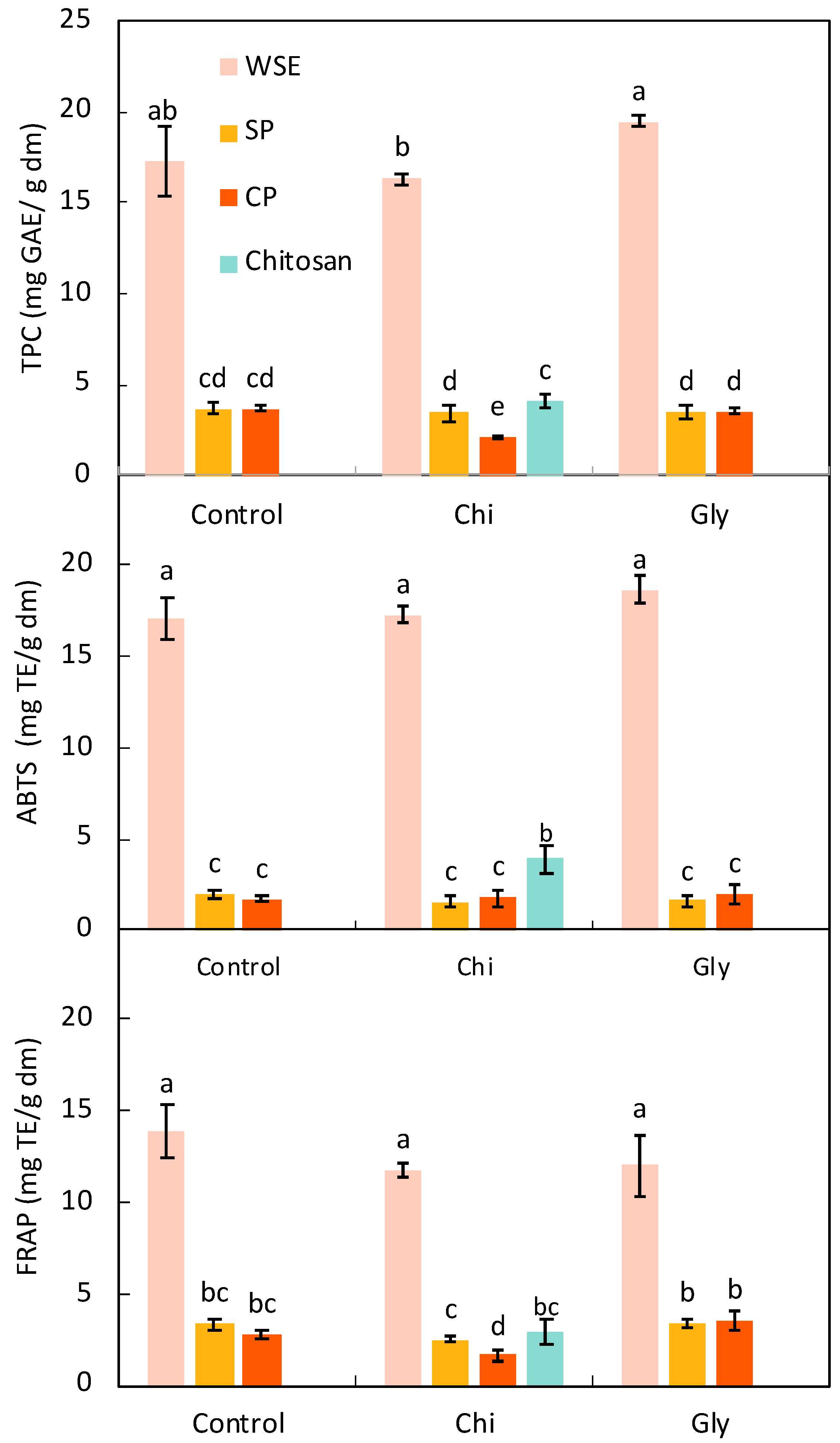
3.1.2. Total Phenolic Content and Antioxidant Activity of the Raw Matter
3.2. Films Characteristics
3.2.1. Chemical Properties
TPC and Antioxidant Activity of the Films
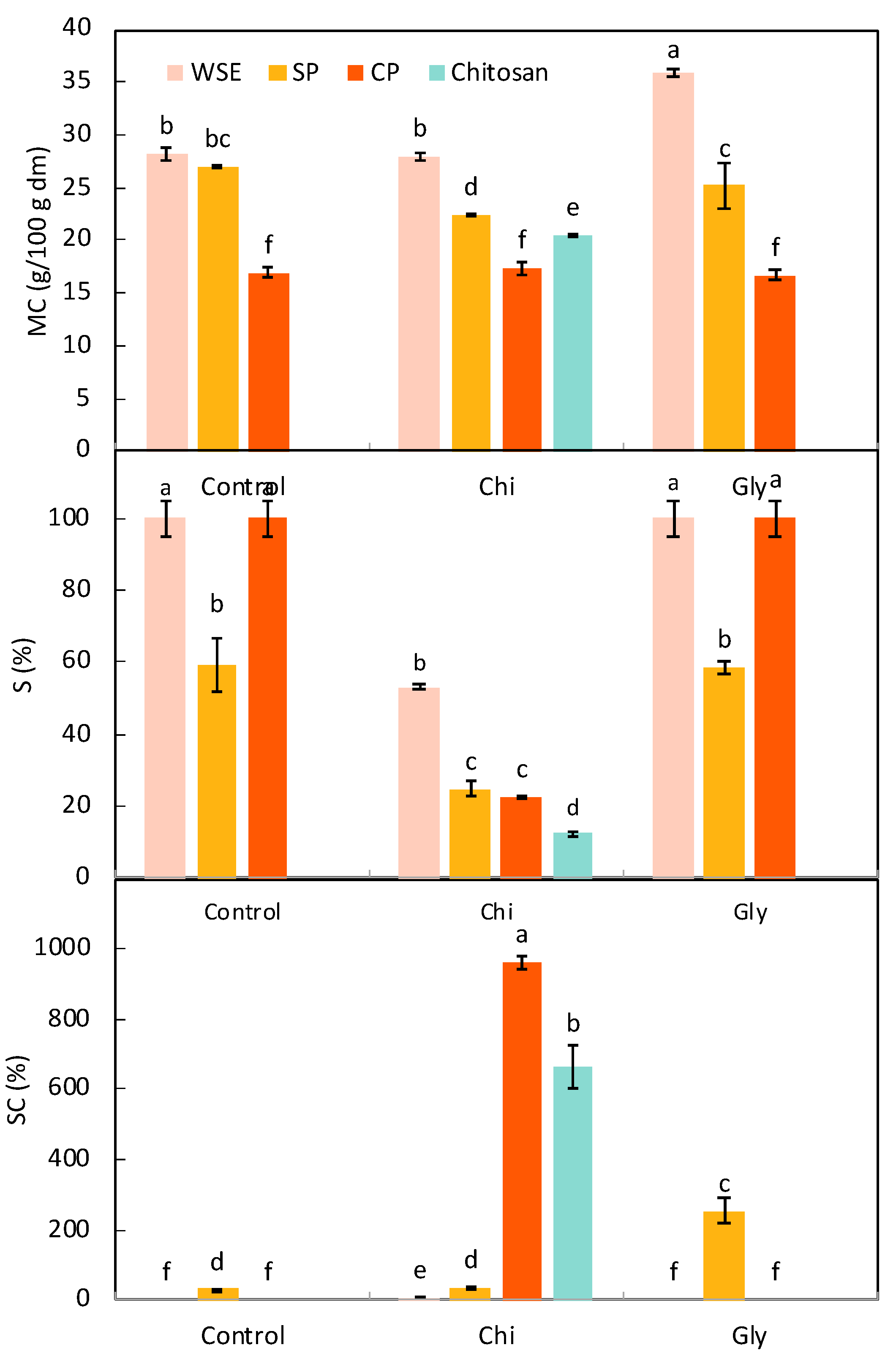
3.2.2. Functional Properties
Basic Water-Related Properties
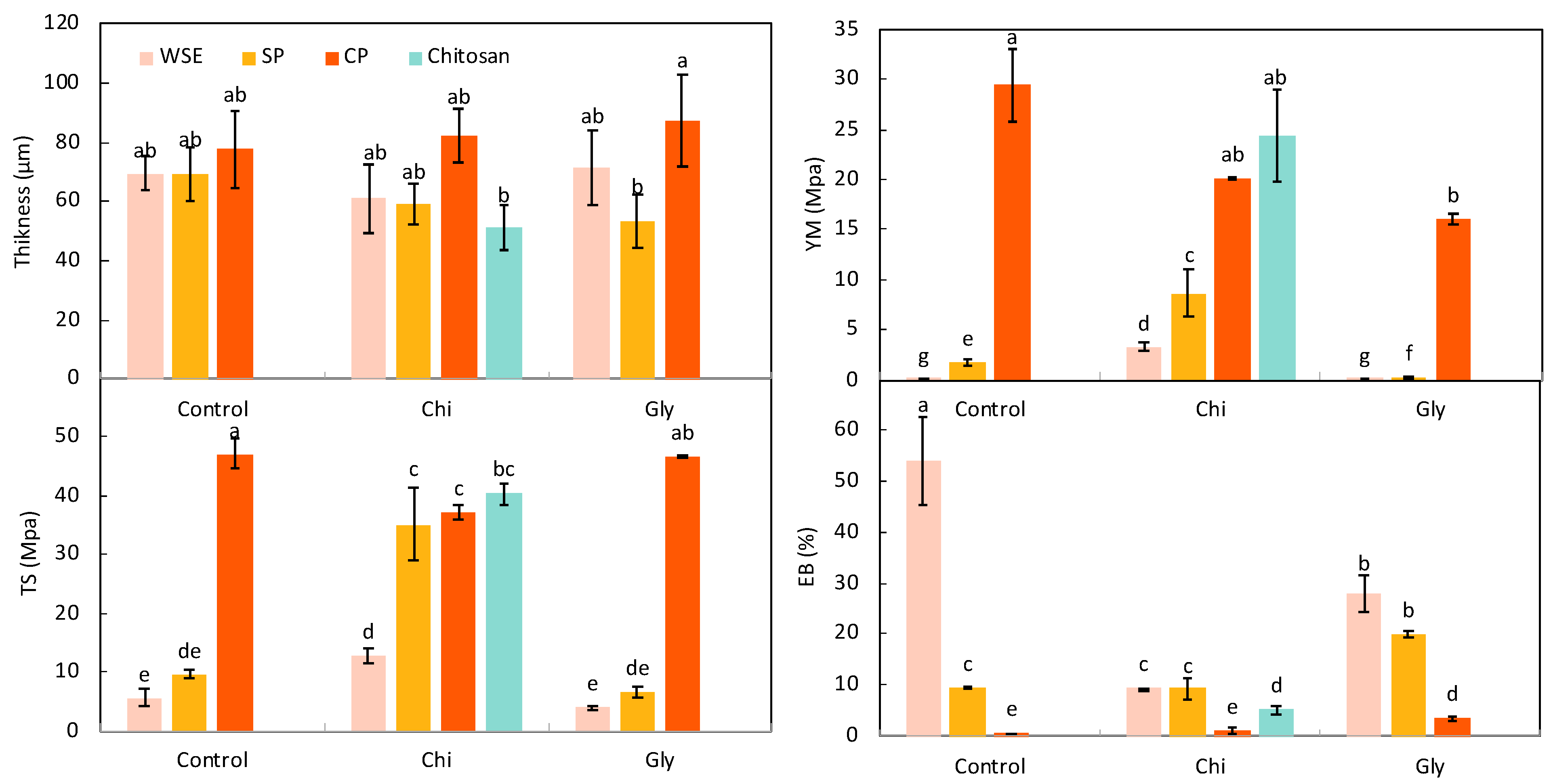
Thickness and Mechanical Properties
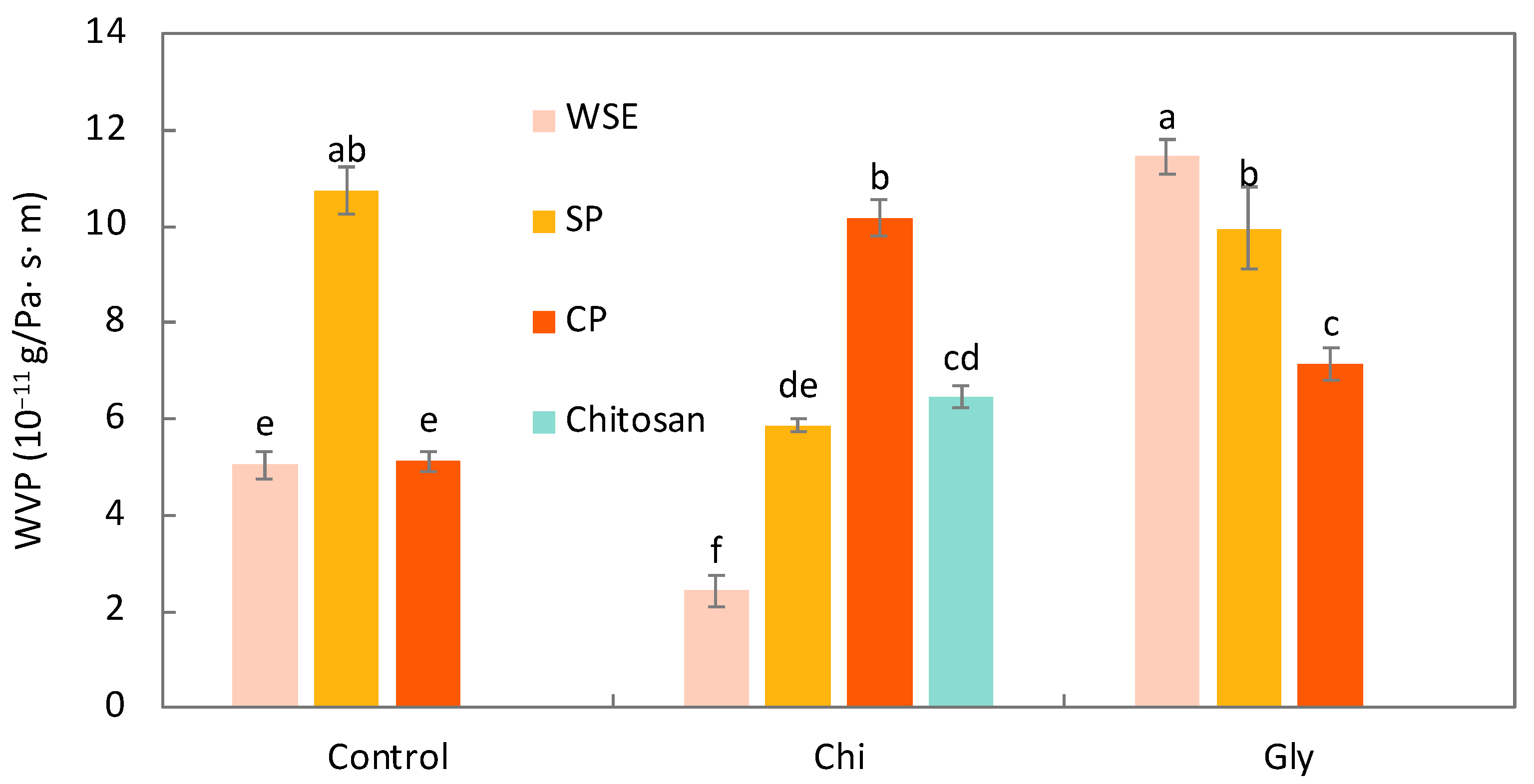
Water Vapor Permeability
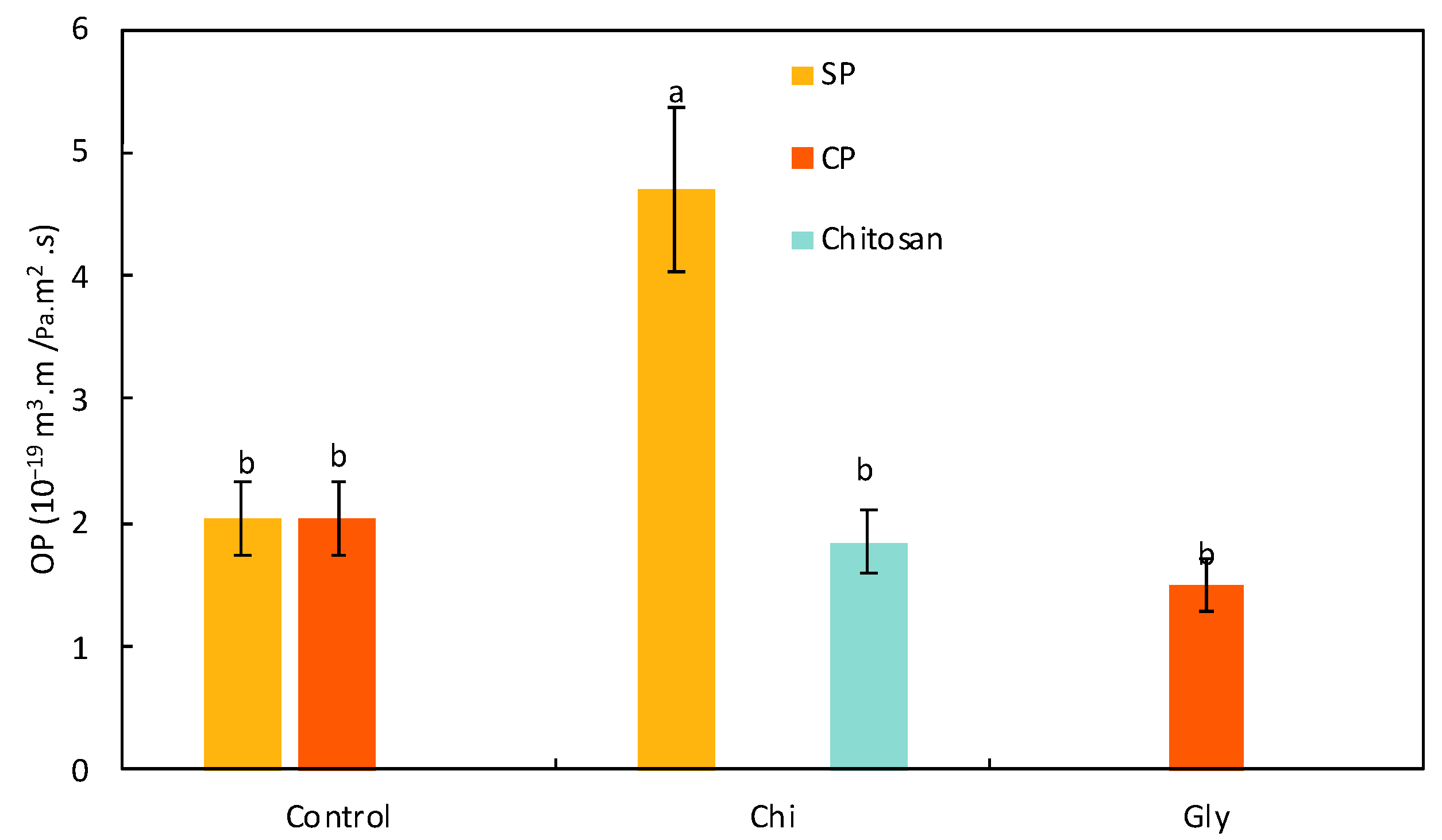
Oxygen Permeability
3.2.3. Aspect and Structure
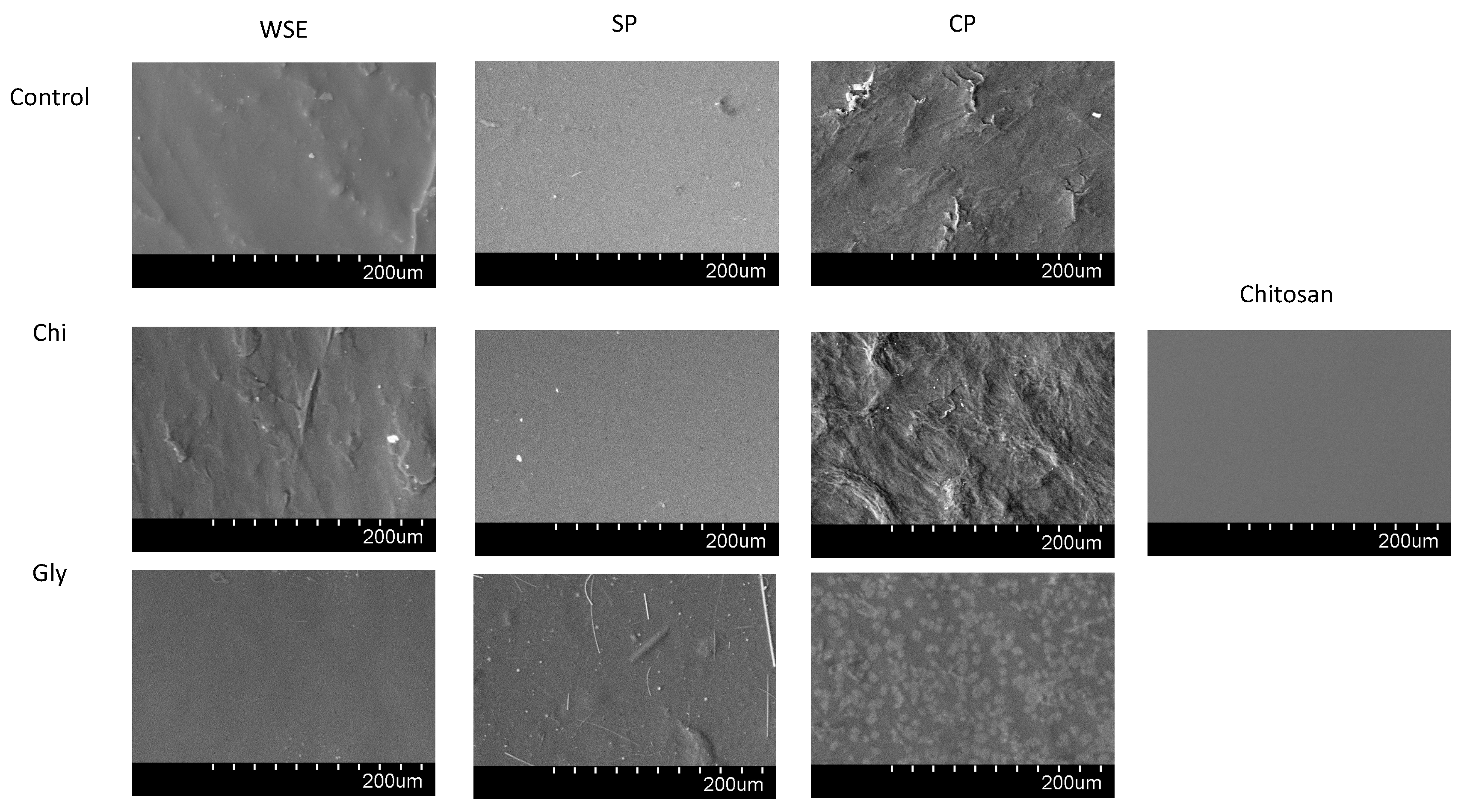
Scanning Electron Microscopy (SEM)
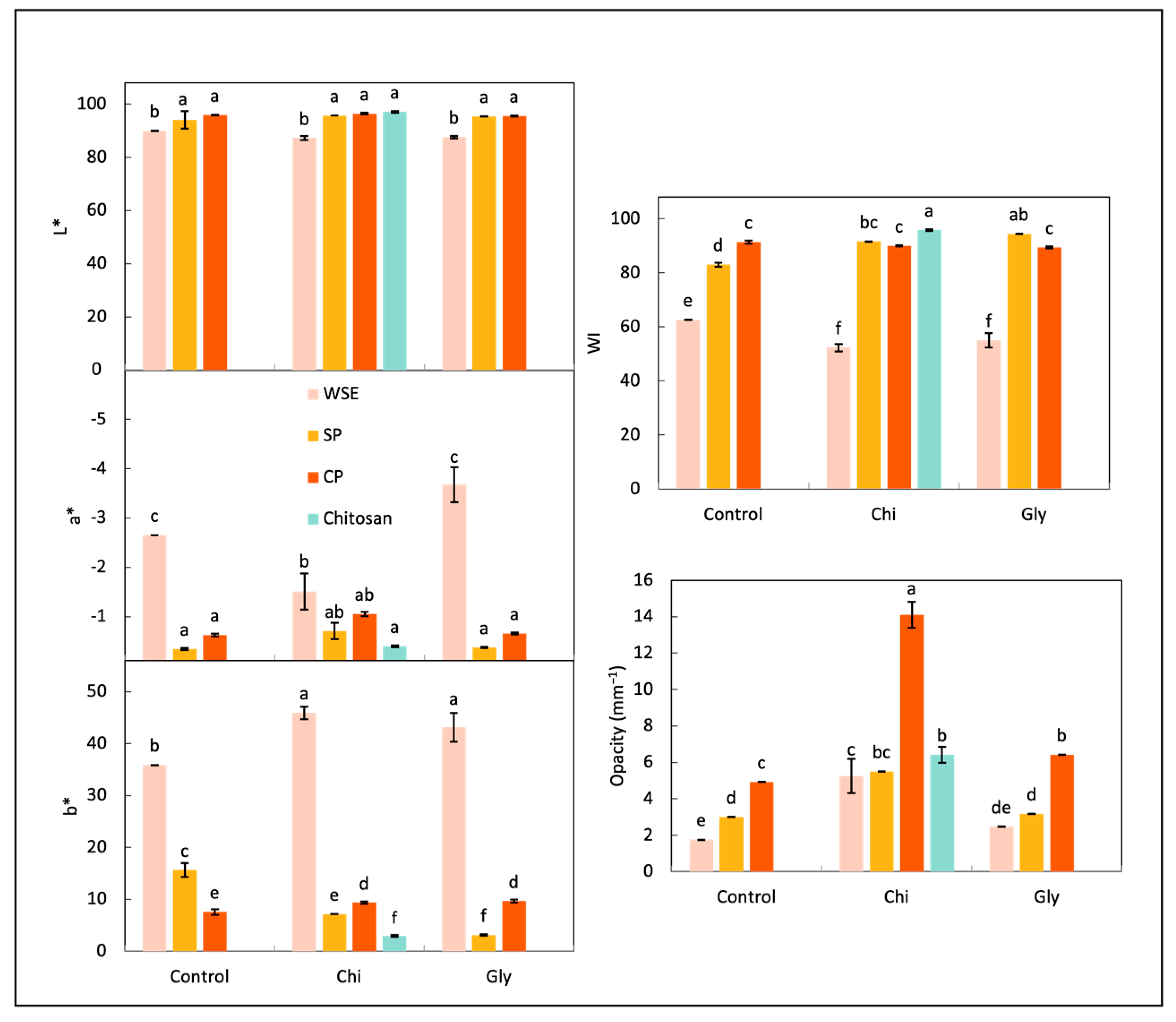
Color and Opacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNEP. Turning off the Tap How the World Can End Plastic Pollution and Create a Circular Economy; UNEP: Nairobi, Kenya, 2023. [Google Scholar]
- UNEP. Chemicals in Plastics: A Technical Report; UNEP: Geneva, Switzerland, 2023. [Google Scholar]
- Terzioğlu, P.; Güney, F.; Parın, F.N.; Şen, İ.; Tuna, S. Biowaste Orange Peel Incorporated Chitosan/Polyvinyl Alcohol Composite Films for Food Packaging Applications. Food Packag. Shelf Life 2021, 30, 100742. [Google Scholar] [CrossRef]
- Zhu, F. Polysaccharide Based Films and Coatings for Food Packaging: Effect of Added Polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Priyadarshi, R.; Łopusiewicz, L.; Biswas, D.; Chandel, V.; Rhim, J.W. Recent Progress in Pectin Extraction, Characterization, and Pectin-Based Films for Active Food Packaging Applications: A Review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and Characterization of Pectin Films Activated by Nanoemulsion and Pickering Emulsion Stabilized Marjoram (Origanum majorana L.) Essential Oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Yang, L.; Gou, Q.; Han, L.; Yu, Q. li Utilization of Watermelon Peel as a Pectin Source and the Effect of Ultrasound Treatment on Pectin Film Properties. LWT 2021, 147, 111569. [Google Scholar] [CrossRef]
- Machado, B.R.; Facchi, S.P.; de Oliveira, A.C.; Nunes, C.S.; Souza, P.R.; Vilsinski, B.H.; Popat, K.C.; Kipper, M.J.; Muniz, E.C.; Martins, A.F. Bactericidal Pectin/Chitosan/Glycerol Films for Food Pack Coatings: A Critical Viewpoint. Int. J. Mol. Sci. 2020, 21, 8663. [Google Scholar] [CrossRef] [PubMed]
- Robert Nastasi, J.; Kontogiorgos, V.; Dara Daygon, V.; Fitzgerald, M.A. Pectin-Based Films and Coatings with Plant Extracts as Natural Preservatives: A Systematic Review. Trends Food Sci. Technol. 2022, 120, 193–211. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S.; Nagy, K.S.A.; Eweis, M. Surface Modification of Polypropylene Films by Chitosan and Chitosan/Pectin Multilayer. Carbohydr. Polym. 2008, 71, 187–195. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical Properties of the Edible Films from the Blends of High Methoxyl Apple Pectin and Chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Kuo, T.-Y.; Kuo, J.-Y.; Tseng, Y.-P.; Wang, D.-M.; Lai, J.-Y.; Hsieh, H.-J. Novel Chitosan-Pectin Composite Membranes with Enhanced Strength, Hydrophilicity and Controllable Disintegration. Carbohydr. Polym. 2010, 82, 1236–1242. [Google Scholar] [CrossRef]
- Singhal, S.; Swami Hulle, N.R. Citrus Pectins: Structural Properties, Extraction Methods, Modifications and Applications in Food Systems—A Review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. 2021. Available online: https://www.fao.org/faostat/es/#home (accessed on 3 July 2024).
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the Scientific Perspectives of Citrus By-Products Utilization: Progress towards Circular Economy. Trends Food Sci. Technol. 2021, 111, 549–562. [Google Scholar] [CrossRef]
- Mao, Y.; Robinson, J.P.; Binner, E.R. Current Status of Microwave-Assisted Extraction of Pectin. Chem. Eng. J. 2023, 473, 145261. [Google Scholar] [CrossRef]
- Girón-Hernández, J.; Pazmino, M.; Barrios-Rodríguez, Y.F.; Turo, C.T.; Wills, C.; Cucinotta, F.; Benlloch-Tinoco, M.; Gentile, P. Exploring the Effect of Utilising Organic Acid Solutions in Ultrasound-Assisted Extraction of Pectin from Apple Pomace, and Its Potential for Biomedical Purposes. Heliyon 2023, 9, e17736. [Google Scholar] [CrossRef] [PubMed]
- Umaña, M.; Llull, L.; Bon, J.; Eim, V.S.; Simal, S. Artificial Neural Networks to Optimize Oil-in-Water Emulsion Stability with Orange By-Products. Foods 2022, 11, 3750. [Google Scholar] [CrossRef] [PubMed]
- Taghavi Kevij, H.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Mechanical, Physical, and Bio-Functional Properties of Biopolymer Films Based on Gelatin as Affected by Enriching with Orange Peel Powder. Polym. Bull. 2021, 78, 4387–4402. [Google Scholar] [CrossRef]
- Rathinavel, S.; Saravanakumar, S.S. Development and Analysis of Poly Vinyl Alcohol/Orange Peel Powder Biocomposite Films. J. Nat. Fibers 2020, 18, 2045–2054. [Google Scholar] [CrossRef]
- Singha, P.; Rani, R.; Badwaik, L.S. Sweet Lime Peel-, Polyvinyl Alcohol- and Starch-Based Biodegradable Film: Preparation and Characterization. Polym. Bull. 2022, 80, 589–605. [Google Scholar] [CrossRef]
- Leites, C.L.; Julia Menegotto Frick, P.; Isabel Cristina, T. Influence of the Incorporation Form of Waste from the Production of Orange Juice in the Properties of Cassava Starch-Based Films. Food Hydrocoll. 2021, 117, 106730. [Google Scholar] [CrossRef]
- Meydanju, N.; Pirsa, S.; Farzi, J. Biodegradable Film Based on Lemon Peel Powder Containing Xanthan Gum and TiO2–Ag Nanoparticles: Investigation of Physicochemical and Antibacterial Properties. Polym. Test. 2022, 106, 107445. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Guo, Y.; Yan, X.; Liu, W.; Huang, S. Preparation and Characterization of Soluble Dietary Fiber Edible Packaging Films Reinforced by Nanocellulose from Navel Orange Peel Pomace. Polymers 2024, 16, 315. [Google Scholar] [CrossRef]
- Yun, D.; Liu, J. Recent Advances on the Development of Food Packaging Films Based on Citrus Processing Wastes: A Review. J. Agric. Food Res. 2022, 9, 100316. [Google Scholar] [CrossRef]
- Chhatariya, H.F.; Srinivasan, S.; Choudhary, P.M.; Begum, S.S. Corn Starch Biofilm Reinforced with Orange Peel Powder: Characterization of Physicochemical and Mechanical Properties. Mater. Today Proc. 2022, 59, 884–892. [Google Scholar] [CrossRef]
- Pieczykolan, E.; Kurek, M.A. Use of Guar Gum, Gum Arabic, Pectin, Beta-Glucan and Inulin for Microencapsulation of Anthocyanins from Chokeberry. Int. J. Biol. Macromol. 2019, 129, 665–671. [Google Scholar] [CrossRef]
- Umaña, M.; Dalmau, M.E.; Eim, V.S.; Femenia, A.; Rosselló, C. Effects of Acoustic Power and PH on Pectin-enriched Extracts Obtained from Citrus By-products. Modelling of the Extraction Process. J. Sci. Food Agric. 2019, 99, 6893–6902. [Google Scholar] [CrossRef]
- AOAC. AOAC Method 934.06 Moisture in Dried Fruits. AOAC Int. 2000, 215, 1. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; AOAC: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Eim, V.S.; Simal, S.; Rosselló, C.; Femenia, A. Effects of Addition of Carrot Dietary Fibre on the Ripening Process of a Dry Fermented Sausage (Sobrassada). Meat Sci. 2008, 80, 173–182. [Google Scholar] [CrossRef]
- Dalmau, M.E.; Bornhorst, G.M.; Eim, V.; Rosselló, C.; Simal, S. Effects of Freezing, Freeze Drying and Convective Drying on in Vitro Gastric Digestion of Apples. Food Chem. 2017, 215, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.A.; Delgadillo, I.; Waldron, K.W.; Selvendran, R.R. Isolation and Analysis of Cell Wall Polymers from Olive Pulp. Mod. Methods Plant Anal. 1996, 17, 19–44. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New Insights into an Old Polymer Are Starting to Gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Manrique, G.D.; Lajolo, F.M. FT-IR Spectroscopy as a Tool for Measuring Degree of Methyl Esterification in Pectins Isolated from Ripening Papaya Fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Vallespir, F.; Rodríguez, Ó.; Cárcel, J.A.; Rosselló, C.; Simal, S. Ultrasound Assisted Low-Temperature Drying of Kiwifruit: Effects on Drying Kinetics, Bioactive Compounds and Antioxidant Activity. J. Sci. Food Agric. 2019, 99, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Vallespir, F.; Rodríguez, Ó.; Eim, V.S.; Rosselló, C.; Simal, S. Effects of Freezing Treatments before Convective Drying on Quality Parameters: Vegetables with Different Microstructures. J. Food Eng. 2019, 249, 15–24. [Google Scholar] [CrossRef]
- Daniel Daza, L.; Homez-Jara, A.; Fernando Solanilla, J.; Váquiro, H.A. Effects of Temperature, Starch Concentration, and Plasticizer Concentration on the Physical Properties of Ulluco (Ullucus Tuberosus Caldas)-Based Edible Films. Int. J. Biol. Macromol. 2018, 120, 1834–1845. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, I.; Vikman, M.; Harlin, A.; Orelma, H. Enzymatic Degradation and Pilot-Scale Composting of Cellulose-Based Films with Different Chemical Structures. J. Polym. Environ. 2020, 28, 458–470. [Google Scholar] [CrossRef]
- Bueno, J.N.N.; Corradini, E.; De Souza, P.R.; Marques, V.D.S.; Radovanovic, E.; Muniz, E.C. NC-ND License Films Based on Mixtures of Zein, Chitosan, and PVA: Development with Perspectives for Food Packaging Application. Polym. Test. 2021, 101, 107279. [Google Scholar] [CrossRef]
- Cazón, P.; Morales-Sanchez, E.; Velazquez, G.; Vázquez, M. Measurement of the Water Vapor Permeability of Chitosan Films: A Laboratory Experiment on Food Packaging Materials. J. Chem. Educ. 2022, 99, 2403–2408. [Google Scholar] [CrossRef]
- Wexler, A. Vapor Pressure Formulation for Water in Range 0 to 100 Degrees C. A Revision. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1976, 80, 775. [Google Scholar] [CrossRef] [PubMed]
- ASTM D3895; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM: West Conshohocken, PA, USA, 2017.
- Daza, L.D.; Parra, D.O.; Rosselló, C.; Arango, W.M.; Eim, V.S.; Váquiro, H.A. Influence of Ulluco Starch Modified by Annealing on the Physicochemical Properties of Biodegradable Films. Polymers 2022, 14, 4251. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 2; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 3 July 2024).
- RStudio Team. RStudio: Integrated Development for R. RStudio; RStudio Inc.: Boston, MA, USA, 2022. [Google Scholar]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Recent Trends on the Valorization Strategies for the Management of Citrus By-Products. Food Rev. Int. 2019, 37, 91–120. [Google Scholar] [CrossRef]
- Torrado, A.M.; Cortés, S.; Salgado, J.M.; Max, B.; Rodríguez, N.; Bibbins, B.P.; Converti, A.; Manuel Domínguez, J. Citric acid production from orange peel wastes by solid-state fermentation. Braz. J. Microbiol. 2011, 42, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.B.; Silva, R.D.; Alonso, J.D.; Brienzo, M.; Silva, N.C.; Perotto, G.; Otoni, C.G.; Azeredo, H.M.C. Bioplastics from Orange Processing Byproducts by an Ecoefficient Hydrothermal Approach. Food Packag. Shelf Life 2023, 38, 101114. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, C.-M.; Li, T.; Liang, R.-H.; Luo, J.; Li, T.I.; Luo, S.-J. Critical Reviews in Food Science and Nutrition Pectin Modifications: A Review Pectin Modifications: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, E.; Osselló, C.; Eim, V.; Ratti, C.; Simal, S. Ultrasound-Assisted Aqueous Extraction of Biocompounds from Orange Byproduct: Experimental Kinetics and Modeling. Antioxidants 2020, 9, 352. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Do Thi, N. Effects of Extraction and Processing Methods on Antioxidant Compound Contents and Radical Scavenging Activities of Laver (Porphyra tenera). Prev. Nutr. Food Sci. 2014, 19, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Enzymatically Extracted Apple Pectin Possesses Antioxidant and Antitumor Activity. Molecules 2021, 26, 1434. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.K. Antioxidant Effects of Chitin, Chitosan, and Their Derivatives. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 73, pp. 15–31. [Google Scholar]
- Monsoor, M.A.; Proctor, A. Preparation and Functional Properties of Soy Hull Pectin. J. Am. Oil Chem. Soc. 2001, 78, 709–713. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Pasini Cabello, S.D.; Takara, E.A.; Marchese, J.; Ochoa, N.A. Influence of Plasticizers in Pectin Films: Microstructural Changes. Mater. Chem. Phys. 2015, 162, 491–497. [Google Scholar] [CrossRef]
- Song, K.; Hao, Y.; Liu, Y.; Cao, R.; Zhang, X.; He, S.; Wen, J.; Zheng, W.; Wang, L.; Zhang, Y. Preparation of Pectin-Chitosan Hydrogels Based on Bioadhesive-Design Micelle to Prompt Bacterial Infection Wound Healing. Carbohydr. Polym. 2023, 300, 120272. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Gui, Q.; Yang, H. Drug-Loaded Chitosan Film Prepared via Facile Solution Casting and Air-Drying of Plain Water-Based Chitosan Solution for Ocular Drug Delivery. Bioact. Mater. 2020, 5, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.A.; Bhatia, S.; Al-Harrasi, A.; Afzaal, M.; Saeed, F.; Anwer, M.K.; Khan, M.R.; Jawad, M.; Akram, N.; Faisal, Z. Mechanical Properties of Protein-Based Food Packaging Materials. Polymers 2023, 15, 1724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, J.H. Plasticization of Pea Starch Films with Monosaccharides and Polyols. J. Food Sci. 2006, 71, E253–E261. [Google Scholar] [CrossRef]
- Martins, J.G.; De Oliveira, A.C.; Garcia, P.S.; Kipper, M.J.; Martins, A.F. Durable Pectin/Chitosan Membranes with Self-Assembling, Water Resistance and Enhanced Mechanical Properties. Carbohydr. Polym. 2018, 188, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.L.; Coffin, D.R.; Konstance, R.P.; Onwulata, C.I. Extrusion of Pectin/Starch Blends Plasticized with Glycerol. Carbohydr. Polym. 2000, 41, 317–325. [Google Scholar] [CrossRef]
- Abera Asfaw, W.; Dekeba Tafa, K.; Satheesh, N. Optimization of Citron Peel Pectin and Glycerol Concentration in the Production of Edible Film Using Response Surface Methodology. Heliyon 2023, 8, 13724. [Google Scholar] [CrossRef]
- Han, H.S.; Song, K. Bin Antioxidant Activities of Mandarin (Citrus unshiu) Peel Pectin Films Containing Sage (Salvia officinalis) Leaf Extract. Int. J. Food Sci. Technol. 2020, 55, 3173–3181. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the Gap for Bioplastic Use in Food Packaging: An Update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef] [PubMed]
- Carolina, A.; Ribeiro, B.; Pacheco Cunha, A.; Morais, L.; Da Silva, R.; Lincoln, A.; Mattos, A.; Sousa De Brito, E.; Pontes, M.; Ricardo, S. From Mango By-Product to Food Packaging: Pectin-Phenolic Antioxidant Films from Mango Peels. Int. J. Biol. Macromol. 2021, 193, 1138–1150. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Morrugares-Carmona, R.; Wellner, N.; Cross, K.; Bajka, B.; Waldron, K.W. Development of Pectin Films with Pomegranate Juice and Citric Acid. Food Chem. 2016, 198, 101–106. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Alim, A.; Iqbal, M.; Yang, X.; Sun, L.; Guo, Y. Citrus Pectin Films Enriched with Thinned Young Apple Polyphenols for Potential Use as Bio-Based Active Packaging. CyTA-J. Food 2019, 17, 695–705. [Google Scholar] [CrossRef]
- Yun, D.; Wang, Z.; Li, C.; Chen, D.; Liu, J. Antioxidant and Antimicrobial Packaging Films Developed Based on the Peel Powder of Different Citrus Fruits: A Comparative Study. Food Biosci. 2022, 51, 102319. [Google Scholar] [CrossRef]
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; Friedman, M.; McHugh, T.H. Physical and Antibacterial Properties of Edible Films Formulated with Apple Skin Polyphenols. J. Food Sci. 2011, 76, M149–M155. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and Characterization of Active Films Based on Chitosan Incorporated Tea Polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Ureña, M.; Phùng, T.T.T.; Gerometta, M.; de Siqueira Oliveira, L.; Chanut, J.; Domenek, S.; Dole, P.; Roudaut, G.; Lagorce, A.; Karbowiak, T. Potential of Polysaccharides for Food Packaging Applications. Part 1/2: An Experimental Review of the Functional Properties of Polysaccharide Coatings. Food Hydrocoll. 2023, 144, 108955. [Google Scholar] [CrossRef]
- Despond, S.; Espuche, E.; Cartier, N.; Domard, A. Barrier Properties of Paper–Chitosan and Paper–Chitosan–Carnauba Wax Films. J. Appl. Polym. Sci. 2005, 98, 704–710. [Google Scholar] [CrossRef]
- Mulla, Z.M.; Ahmed, J.; Vahora, A.; Pathania, S. Effect of Pectin Incorporation on Characteristics of Chitosan Based Edible Films. J. Food Meas. Charact. 2023, 17, 5569–5581. [Google Scholar] [CrossRef]
- Vonnie, J.M.; Rovina, K.; ‘Aqilah, N.M.N.; Felicia, X.W.L. Development and Characterization of Biosorbent Film from Eggshell/Orange Waste Enriched with Banana Starch. Polymers 2023, 15, 2414. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.I.; Balandrán-Quintana, R.; López-Franco, Y.I.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Yamakita, E.; Nakashima, S. Water Retention of Calcium-Containing Pectin Studied by Quartz Crystal Microbalance and Infrared Spectroscopy with a Humidity Control System. J. Agric. Food Chem. 2018, 66, 34. [Google Scholar] [CrossRef]
- dos Santos, V.S.; Lorevice, M.V.; Baccarin, G.S.; da Costa, F.M.; da Silva Fernandes, R.; Aouada, F.A.; de Moura, M.R. Combining Chitosan Nanoparticles and Garlic Essential Oil as Additive Fillers to Produce Pectin-Based Nanocomposite Edible Films. Polymers 2023, 15, 2244. [Google Scholar] [CrossRef]
- Hosseini-Parvar, S.H.; Keramat, J.; Kadivar, M.; Khanipour, E.; Motamedzadegan, A. Optimising Conditions for Enzymatic Extraction of Edible Gelatin from the Cattle Bones Using Response Surface Methodology. Int. J. Food. Sci. Technol. 2009, 44, 467–475. [Google Scholar] [CrossRef]
- Moreira, R.B.; Teixeira, J.A.; Furuyama-Lima, A.M.; Souza, N.C.D.; Siqueira, A.B. Preparation, Characterization and Evaluation of Drug-Delivery Systems: Pectin and Mefenamic Acid Films. Thermochim. Acta 2014, 590, 100–106. [Google Scholar] [CrossRef]
- Abid, M.; Cheikhrouhou, S.; Renard, C.M.G.C.; Bureau, S.; Cuvelier, G.; Attia, H.; Ayadi, M.A. Characterization of Pectins Extracted from Pomegranate Peel and Their Gelling Properties. Food Chem. 2017, 215, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Onwudiwe, D.C.; Mbonu, J.I. Green Synthesis, Structural Characterization and Photocatalytic Activities of Chitosan-ZnO Nano-Composite. J. Inorg. Organomet. Polym. Mater. 1988, 31, 3356–3367. [Google Scholar] [CrossRef]
- Dhanikula, A.B.; Panchagnula, R. Development and Characterization of Biodegradable Chitosan Films for Local Delivery of Paclitaxel. AAPS J. 2004, 6, 1–12. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głab, M.; Kedzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef]
- Wathoni, N.; Yuan Shan, C.; Yi Shan, W.; Rostinawati, T.; Indradi, R.B.; Pratiwi, R.; Muchtaridi, M. Characterization and Antioxidant Activity of Pectin from Indonesian Mangosteen (Garcinia mangostana L.) Rind. Heliyon 2019, 5, e02299. [Google Scholar] [CrossRef]
- de Oliveira AC, S.; Ferreira, L.F.; de Oliveira Begali, D.; Ugucioni, J.C.; de Sena Neto, A.R.; Yoshida, M.I.; Borges, S.V. Thermoplasticized Pectin by Extrusion/Thermo-Compression for Film Industrial Application. J. Polym. Environ. 2021, 29, 2546–2556. [Google Scholar] [CrossRef]
| * Compound/ Characteristic | Orange Residue | WSE 1 | SP | CP |
|---|---|---|---|---|
| Moisture (g/100 g dm) | 6.10 ± 0.16 a | 3.13 ± 0.31 b | 3.16 ± 0.32 b | 2.57 ± 0.55 b |
| Lipid (g/100 g dm) | 0.96 ± 0.03 a | nd | nd | nd |
| Proteins (g/100 g dm) | 3.58 ± 0.13 b | 4.36 ± 0.26 b | 5.54 ± 0.32 a | nd |
| Ashes (g/100 g dm) | 3.13 ± 0.03 c | 3.43 ± 0.04 b | 0.67 ± 0.01 d | 3.84 ± 0.04 a |
| Total carbohydrates (g/100 g dm) | 92.15 ± 0.13 c | 92.21 ± 0.13 c | 93.79 ± 0.13 b | 96.15 ± 0.14 a |
| Alcohol-insoluble residues (g/100 g dm) | 46.45 ± 0.90 b | 6.79 ± 0.19 c | 50.83 ± 2.13 b | 99.28 ± 2.97 a |
| Carbohydrate composition of the fiber (g/100 g dm) | ||||
| Uronic acid | 19.61 ± 1.24 c | 4.14 ± 0.03 d | 39.83 ± 1.89 b | 89.37 ± 5.11 a |
| Arabinose | 5.28 ± 0.24 a | 0.47 ± 0.05 c | 2.73 ± 0.21 b | 0.96 ± 0.07 c |
| Galactose | 2.78 ± 0.14 b | 0.33 ± 0.04 c | 2.49 ± 0.22 b | 5.27 ± 0.05 a |
| Rhamnose | 0.22 ± 0.05 b | 0.05 ± 0.01 c | 0.21 ± 0.04 b | 0.40 ± 0.08 a |
| Glucose | 5.85 ± 0.22 a | 0.04 ± 0.01 c | 0.44 ± 0.01 b | 0.54 ± 0.01 b |
| Xylose | 1.32 ± 0.07 a | 0.05 ± 0.01 c | 0.15 ± 0.04 bc | 0.25 ± 0.07 b |
| Mannose | 0.83 ± 0.06 a | 0.02 ± 0.01 c | 0.23 ± 0.05 b | 0.37 ± 0.08 b |
| Fucose | 0.15 ± 0.01 a | nd | 0.07 ± 0.01 b | nd |
| Pectins | 29.37 ± 0.17 c | 5.0 ± 0.13 d | 46.51 ± 1.16 b | 100.13 ± 5.31 a |
| Degree of methylation (%) | 39.46 ± 0.01 d | 41.74 ± 0.72 c | 98.53 ± 0.11 a | 58.92 ± 0.67 b |
| Total phenolic compounds (mg GAE/g dm) | 12.78 ± 0.34 b | 30.11 ± 0.70 a | 3.22 ± 0.25 c | 1.71 ± 0.07 d |
| Antioxidant activity (mg TE/g dm) | ||||
| ABTS method | 12.90 ± 0.54 b | 21.06 ± 0.65 a | 3.82 ± 0.39 c | 1.66 ± 0.36 d |
| FRAP method | 10.08 ± 0.61 b | 23.65 ± 0.65 a | 2.33 ± 0.41 c | 1.47 ± 0.23 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umaña, M.; Simal, S.; Dalmau, E.; Turchiuli, C.; Chevigny, C. Evaluation of Different Pectic Materials Coming from Citrus Residues in the Production of Films. Foods 2024, 13, 2138. https://doi.org/10.3390/foods13132138
Umaña M, Simal S, Dalmau E, Turchiuli C, Chevigny C. Evaluation of Different Pectic Materials Coming from Citrus Residues in the Production of Films. Foods. 2024; 13(13):2138. https://doi.org/10.3390/foods13132138
Chicago/Turabian StyleUmaña, Mónica, Susana Simal, Esperanza Dalmau, Christelle Turchiuli, and Chloé Chevigny. 2024. "Evaluation of Different Pectic Materials Coming from Citrus Residues in the Production of Films" Foods 13, no. 13: 2138. https://doi.org/10.3390/foods13132138
APA StyleUmaña, M., Simal, S., Dalmau, E., Turchiuli, C., & Chevigny, C. (2024). Evaluation of Different Pectic Materials Coming from Citrus Residues in the Production of Films. Foods, 13(13), 2138. https://doi.org/10.3390/foods13132138







