Effects of High Hydrostatic Pressure on the Distribution of Oligosaccharides, Pinitol, Soysapapogenol A, and Fatty Acids in Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Soybeans
2.3. Determination of Pinitol, Oligosaccharides, and Soyasapogenol A
2.3.1. Pinitol and Oligosaccharides Extract Sample Preparation
2.3.2. Quantification of Pinitol and Oligosaccharides
2.3.3. Soyasapogenol A Extract Sample Preparation
2.3.4. Quantification of Soyasapogenol A
2.4. Determination of α-Linolenic Acid, Linoleic Acid, Oleic Acid, Palmitic Acid, and Stearic Acid
2.4.1. Fatty Acids Extract Sample Preparation
2.4.2. Quantification of α-Linolenic Acid, Linoleic Acid, Oleic Acid, Palmitic Acid, and Stearic Acid
2.5. Statistical Analysis
3. Results
3.1. Pinitol and Oligosaccharide Concentrations in Pressurized Soybean
3.2. Soyasapogenol A Concentration in Pressurized Soybean
3.3. Pinitol, Oligosaccharides, and Soyasapogenol A Concentrations in Boiled Soybean
3.4. Fatty Acid Concentrations in Pressurized Soybean
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamamoto, K. Food processing by high hydrostatic pressure. Biosci. Biotechnol. Biochem. 2017, 81, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Ikezaki, M.; Kataoka, C.; Hori, S.; Aoki, T.; Kuribayashi, T.; Kaneoke, M.; Iguchi, A.; Shigematsu, T. High hydrostatic pressure pasteurization of a draft sake brewed using a Niigata-sake yeast. High Press. Res. 2019, 39, 301–312. [Google Scholar] [CrossRef]
- Shigematsu, T.; Furukawa, N.; Takaoka, R.; Hayashi, M.; Sasao, S.; Ueno, S.; Nakajima, K.; Kido, M.; Nomura, K.; Iguchi, A. Effect of high pressure on the saccharification of starch in the tuberous root of sweet potato (Ipomoea batatas). Biophys. Chem. 2017, 231, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Shigematsu, T.; Karo, M.; Hayashi, M.; Fujii, T. Effects of High Hydrostatic Pressure on Water Absorption of Adzuki Beans. Foods 2015, 4, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, A.; Ikarashi, M.; Maruyama, A.; Hori, S.; Nomura, K.; Shigematsu, T. High-throughput screening of food additives with synergistic effects on high hydrostatic pressure inactivation of budding yeast. High Press. Res. 2019, 39, 280–292. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Rodolfi, M.; Ganino, T.; Morbarigazzi, M.; Chiavaro, E. Effects of high hydrostatic pressure on physico-chemical and structural properties of two pumpkin species. Food Chem. 2019, 274, 281–290. [Google Scholar] [CrossRef]
- Rahman, H.; Mu, T.; Zhang, M.; Ma, M.; Sun, H. Comparative study of the effects of high hydrostatic pressure on physicochemical, thermal, and structural properties of maize, potato, and sweet potato starches. J. Food Process. Preserv. 2020, 44, e14852. [Google Scholar] [CrossRef]
- Cao, J.; Li, Y.; Li, F.; Liao, X.; Hu, X.; Zhang, Y. Effect of high hydrostatic pressure on chlorophyll/soybean protein isolate interaction and the mixtures properties. Food Hydrocoll. 2022, 128, 107555. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Q.; Zhang, Q.; Ding, J.; Liu, Y.; Qin, W. An updated review of functional properties, debittering methods, and applications of soybean functional peptides. Crit. Rev. Food Sci. Nutr. 2022, 63, 8823–8838. [Google Scholar] [CrossRef]
- Jelena, M.; Christine, A.; Charles, R.H. Current knowledge in soybean composition. J. Am. Oil Chem. Soc. 2014, 91, 363–384. [Google Scholar]
- Ueno, S.; Katayama, T.; Watanabe, T.; Nakajima, K.; Hayashi, M.; Shigematsu, T.; Fujii, T. Enzymatic production of γ-aminobutyric acid in soybeans using high hydrostatic pressure and precursor feeding. Biosci. Biotechnol. Biochem. 2013, 77, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Peñas, E.; Gomez, R.; Frias, J.; Baeza, M.L. Concepcion Vidal-Valverde, High hydrostatic pressure effects on immunoreactivity and nutritional quality of soybean products. Food Chem. 2011, 125, 423–429. [Google Scholar] [CrossRef]
- Ueno, S.; Shigematsu, T.; Watanabe, T.; Nakajima, K.; Murakami, M.; Hayashi, M.; Fujii, T. Generation of free amino acids and γ-aminobutyric acid in water-soaked soybean by high-hydrostatic pressure processing. J. Agric. Food Chem. 2010, 58, 1208–1213. [Google Scholar] [CrossRef]
- Zou, H.; Xu, Z.; Zhao, L.; Wang, Y.; Liao, X. Effects of high pressure processing on the interaction of α-lactalbumin and pelargonidin-3-glucosides. Food Chem. 2019, 285, 22–30. [Google Scholar] [CrossRef]
- Liu, H.; Ueno, S.; Araki, T. Effect of high-hydrostatic-pressure processing on catechin content in green tea leaves. High Press. Res. 2023, 43, 97–105. [Google Scholar] [CrossRef]
- Saffarionpour, S. Off-Flavors in Pulses and Grain Legumes and Processing Approaches for Controlling Flavor-Plant Protein Interaction: Application Prospects in Plant-Based Alternative Foods. Food Bioprocess Technol. 2024, 17, 1141–1182. [Google Scholar] [CrossRef]
- Ueno, S.; Kawaguchi, Y.; Oshikiri, Y.; Liu, H.; Shimada, R. Enrichment of free amino acid content and reduction of astringent taste compounds in soybean by high hydrostatic pressure. High Press. Res. 2019, 39, 398–407. [Google Scholar] [CrossRef]
- Hayashi, T.; Sakurada, I.; Honda, K.; Motohashi, S.; Uchikura, K. Electrochemical detection of sugar-related compounds using boron-doped diamond electrodes. Anal. Sci. 2012, 28, 127–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsukamoto, C.; Shimada, S.; Igita, K.; Kudou, S.; Kokubun, M.; Okubo, K.; Kitamura, K. Factors affecting isoflavone content in soybean seeds: Changes in isoflavones, saponins, and composition of fatty acids at different temperatures during seed development. J. Agric. Food Chem. 1995, 43, 1184–1192. [Google Scholar] [CrossRef]
- Ko, D.-Y.; Ku, K.-M. Effect of Anti-Obesity and Antioxidant Activity through the Additional Consumption of Peel from ‘Fuji’ Pre-Washed Apple. Foods 2022, 11, 497. [Google Scholar] [CrossRef]
- Kosina, S.M.; Castillo, A.; Schnebly, S.R.; Obendorf, R.L. Soybean seed coat cup unloading on plants with low-raffinose, low-stachyose seeds. Seed Sci. Res. 2009, 19, 145–153. [Google Scholar] [CrossRef]
- Jiang, G.-L.; Chen, P.; Zhang, J.; Florez-Palacios, L.; Zeng, A.; Wang, X.; Bowen, R.A.; Miller, A.; Berry, H. Genetic analysis of sugar composition and its relationship with protein, oil, fiber in soybean. Crop. Sci. 2018, 58, 2413–2421. [Google Scholar] [CrossRef]
- Slupski, J.; Gebcynski, P. Changes due to cooking and sterilization in low molecular, Carbohydrates in immature seeds of five cultivars of common bean. Int. J. Food Sci. Nutr. 2014, 65, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.H.; Hossain, M.A.; Lee, E.; Kanth, B.K.; Park, P.B. Increased salt and drought tolerance by D-pinitol production by transgenic Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2018, 504, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Phang, H.; Shao, G.; Lam, M. Salt Tolerance in Soybean. J. Integr. Plant Biol. 2008, 50, 1196–1212. [Google Scholar] [CrossRef] [PubMed]
- Kuş, N.Ş. Biological Properties of Cyclitols and Their Derivatives. Chem. Biodivers. 2023, 21, e202301064. [Google Scholar] [CrossRef]
- Yu, X.; Li, P.; Li, B.; Yu, F.; Zhao, W.; Wang, X.; Wang, Y.; Gao, H.; Cheng, M.; Li, X. D-Pinitol Improves Diabetic Sarcopenia by Regulation of the Gut Microbiome, Metabolome, and Proteome in STZ-Induced SAMP8 Micei. J. Agric. Food Chem. 2024, 72, 14466–14478. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, Y. Effective reduction of antinutritional factors in soybean meal by acetic acid-catalyzed processing. J. Food Process. Preserv. 2018, 42, e13775. [Google Scholar] [CrossRef]
- Vagadia, B.H.; Vanga, S.K.; Raghavan, V. Inactivation methods of soybean trypsin inhibitor. Trends Food Sci. Technol. 2017, 64, 115–125. [Google Scholar] [CrossRef]
- Kamo, S.; Suzuki, S.; Sato, T. The content of soyasaponin and soyasapogenol in soy foods and their estimated intake in the Japanese. Food Sci. Nutr. 2014, 2, 289–297. [Google Scholar] [CrossRef]
- Ueno, S.; Sasao, S.; Liu, H.; Hayashi, M.; Shigematsu, T.; Kaneko, Y.; Araki, T. Effects of high hydrostatic pressure on β-glucan content, swelling power, starch damage, and pasting properties of high-β-glucan barley flour. High Press. Res. 2019, 39, 509–524. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.-H.; He, R.; Xu, R.; Zhang, L.; Gao, X. Improving Soy Sauce Aroma Using High Hydrostatic Pressure and the Preliminary Mechanism. Foods 2022, 11, 2190. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Nomura, K.; Ando, Y.; Nakaura, Y.; Zhang, Z.; Yamamoto, K. Vacuum impregnation of apple assisted by high hydrostatic pressure. High Press. Res. 2021, 41, 414–428. [Google Scholar] [CrossRef]
- Ueno, S.; Iryo, N.; Sasao, S.; Liu, H.; Atsuzawa, K.; Kaneko, Y.; Shimada, R. Freeze–thaw-induced structural destruction and generation of γ-aminobutyric acid in water-soaked soybeans. Jpn. J. Food Eng. 2021, 20, 41–49. [Google Scholar] [CrossRef]
- Hulle, S.; Kaushik, N.; Rao, S. Effect of high pressure processing on rheological properties, pectinmethylesterase activity and microbiological characteristics of Aloe Vera (Aloe barbadensis Miller) juice. Int. J. Food Prop. 2015, 18, 1597–1612. [Google Scholar] [CrossRef]
- Braspaiboon, S.; Laokuldilok, T. High Hydrostatic Pressure: Influences on Allergenicity, Bioactivities, and Structural and Functional Properties of Proteins from Diverse Food Sources. Foods 2024, 13, 922. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Wang, X.; Li, H.; Liao, X.; Lao, F.; Wu, J.; Li, J. Decoding the Effects of High Hydrostatic Pressure and High-Temperature Short-Time Sterilization on the Volatile Aroma Profile of Red Raspberry Juice. Foods 2023, 13, 1574. [Google Scholar] [CrossRef] [PubMed]
- Olmo-Cunillera, A.; Ribas-Agustí, A.; Lozano-Castellón, J.; Pérez, M.; Ninot, A.; Romero-Aroca, A.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. High hydrostatic pressure enhances the formation of oleocanthal and oleacein in ‘Arbequina’ olive fruit. Food Chem. 2024, 437, 137902. [Google Scholar] [CrossRef] [PubMed]
- Bensaada, S.; Peruzzi, G.; Cubizolles, L.; Denayrolles, M.; Bennetau-Pelissero, C. Traditional and Domestic Cooking Dramatically Reduce Estrogenic Isoflavones in Soy Foods. Foods 2024, 13, 999. [Google Scholar] [CrossRef]
- Yang, H.; Gao, J.; Yang, A.; Chen, H. The ultrasound-treated soybean seeds improve edibility and nutritional quality of soybean sprouts. Food Res. Int. 2015, 77, 704–710. [Google Scholar] [CrossRef]
- Guerrero-Beltran, J.; Estrada-Girón, Y.; Swanson, B.; Barbosa-Cánovas, G. Pressure and temperature combination for inactivation of soymilk trypsin inhibitors. Food Chem. 2009, 116, 676–679. [Google Scholar] [CrossRef]
- Wu, X.; Tan, M.; Zhu, Y.; Duan, H.; Ramaswamy, H.S.; Bai, W.; Wang, C. The influence of high pressure processing and germination on anti-nutrients contents, in vitro amino acid release and mineral digestibility of soybeans. J. Food Compos. Anal. 2023, 115, 104953. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna Saldívar, S.O. Inactivation Methods of Trypsin Inhibitor in Legumes: A Review. J. Food Sci. 2017, 83, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Laguna, L.; Picouet, P.; Guàrdia, M.D.; Renard, C.M.G.C.; Sarkar, A. In vitro gastrointestinal digestion of pea protein isolate as a function of pH, food matrices, autoclaving, high-pressure and re-heat treatments. LWT Food Sci. Technol. 2017, 84, 511–519. [Google Scholar] [CrossRef]
- Yin, S.-W.; Tang, C.-H.; Wen, Q.-B.; Yang, X.-Q.; Li, L. Functional properties and in vitro trypsin digestibility of red kidney bean (Phaseolus vulgaris L.) protein isolate: Effect of high-pressure treatment. Food Chem. 2008, 110, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Nomura, K.; Ando, Y.; Nakaura, Y.; Zhang, Z.; Yamamoto, K. Vacuum impregnation of liquid into carrot assisted by high hydrostatic pressure. High Press. Res. 2023, 43, 142–155. [Google Scholar] [CrossRef]
- Nakaura, Y.; Sok, C.; Morimatsu, K.; Yamamoto, K. Effect of incubation temperature after high hydrostatic pressure treatment of Escherichia coli on the detection of injured populations. High Press. Res. 2024, 44, 105–115. [Google Scholar] [CrossRef]
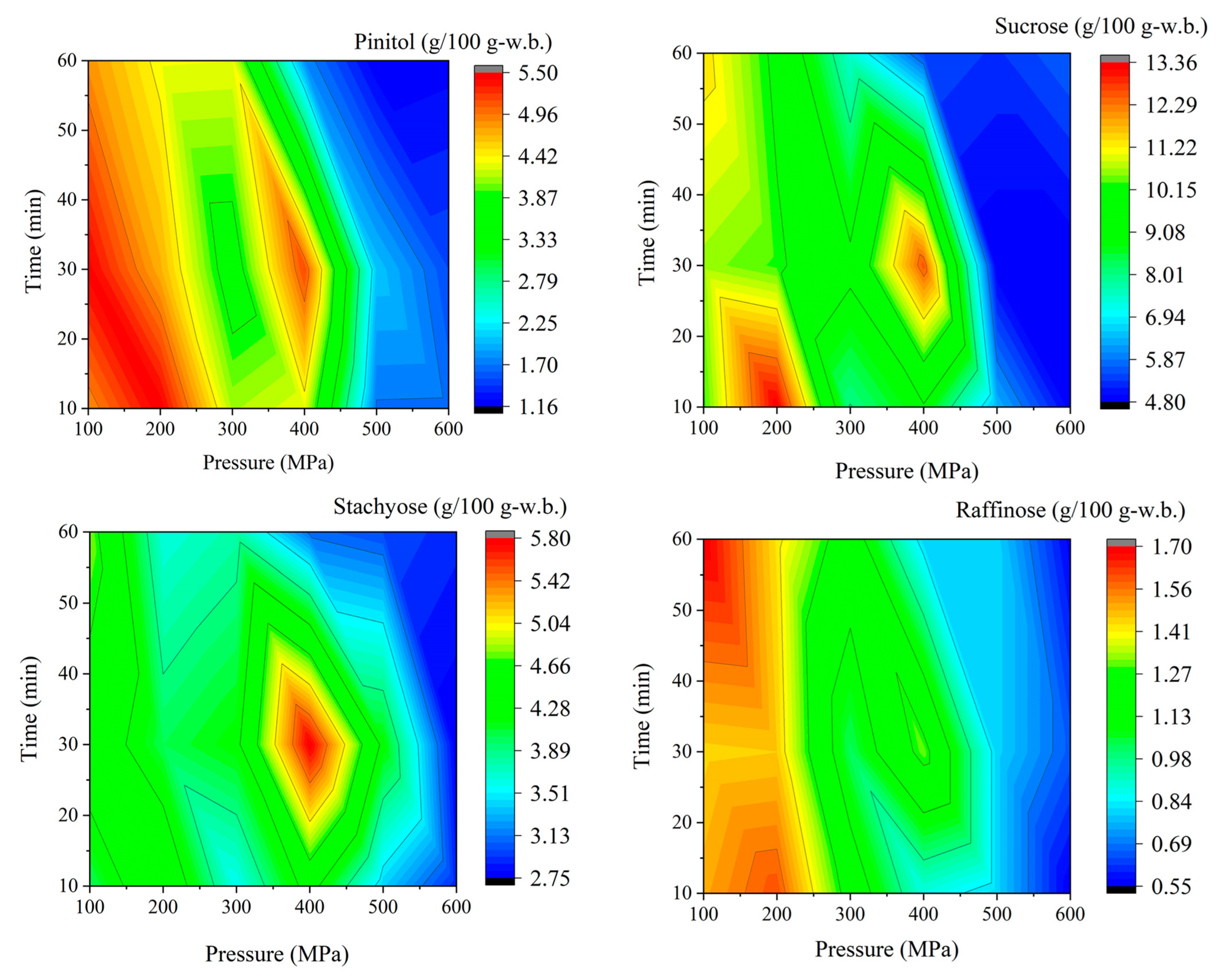
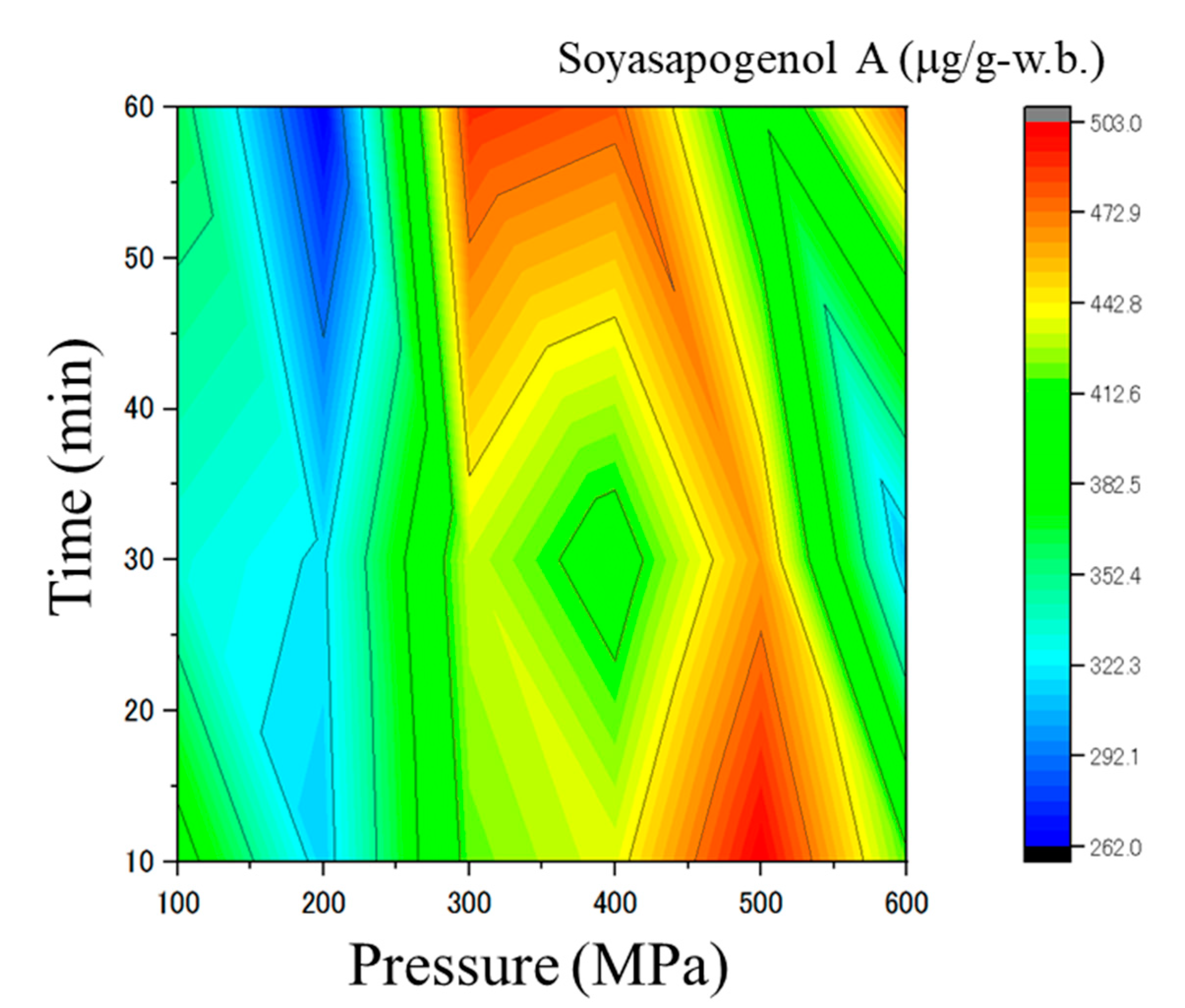
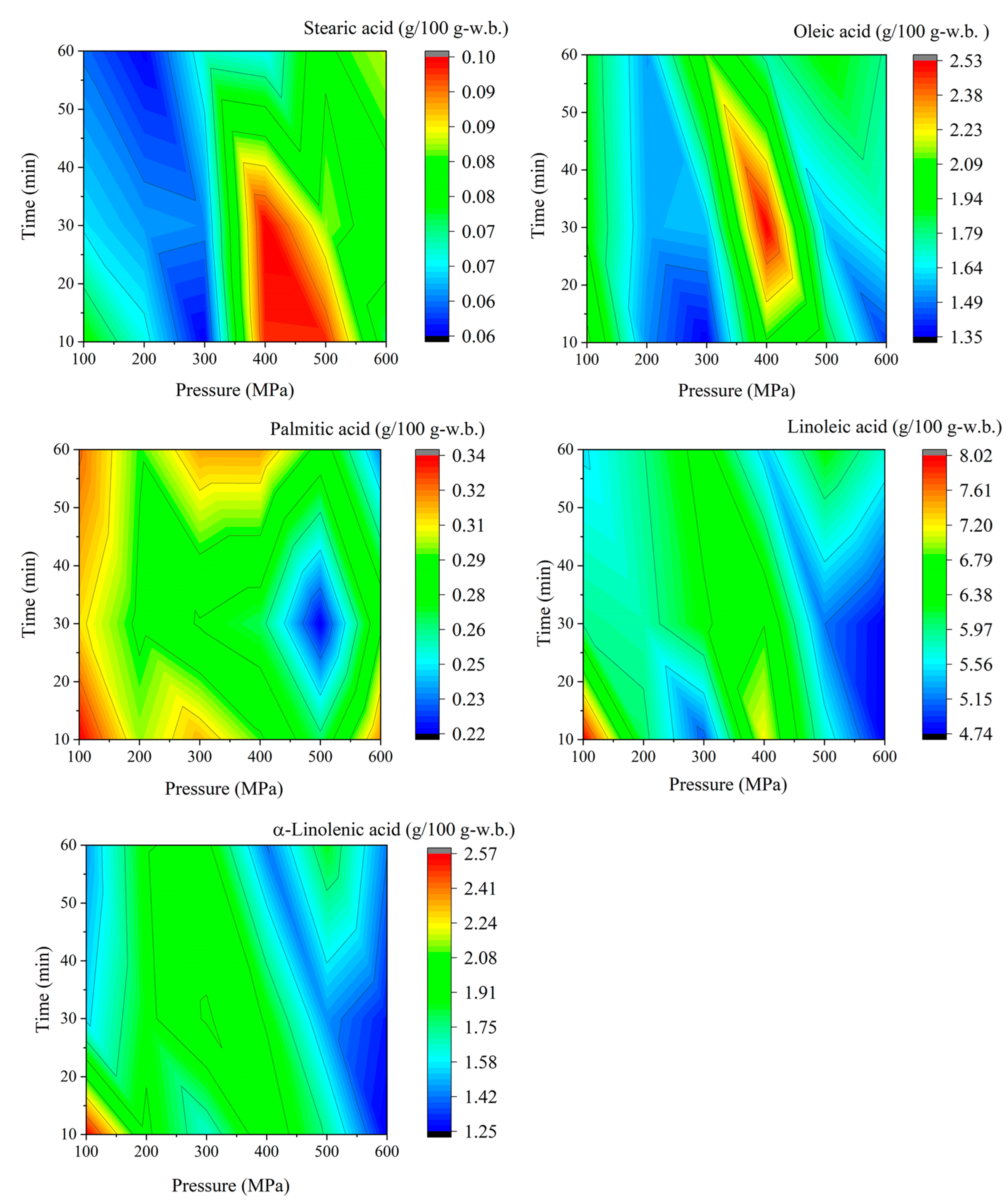
| Pressure (MPa) | Time (min) | Pinitol (g/100 g) | Sucrose (g/100 g) | Stachyose (g/100 g) | Raffinose (g/100 g) | Soyasapogenol A (µg/g) |
|---|---|---|---|---|---|---|
| Untreated | Untreated | 2.52 ± 0.53 | 14.69 ± 2.43 | 5.88 ± 1.27 | 1.82 ± 0.49 | 429.53 ± 13.07 |
| 100 | 10 | 4.86 ± 0.12 | 10.24 ± 1.01 | 3.99 ± 0.12 | 1.53 ± 0.32 | 394.48 ± 3.51 |
| 100 | 30 | 5.46 ± 0.37 | 10.61 ± 0.47 | 4.52 ± 0.19 | 1.45 ± 0.33 | 333.63 ± 0.57 |
| 100 | 60 | 4.84 ± 0.07 | 11.40 ± 0.26 | 4.79 ± 0.08 | 1.70 ± 0.02 | 362.55 ± 4.34 |
| 200 | 10 | 5.48 ± 0.07 | 13.35 ± 0.74 | 4.61 ± 0.69 | 1.60 ± 0.15 | 313.70 ± 8.51 |
| 200 | 30 | 4.71 ± 0.14 | 10.28 ± 0.60 | 4.02 ± 0.31 | 1.46 ± 0.05 | 320.32 ± 1.72 |
| 200 | 60 | 4.34 ± 0.08 | 9.97 ± 0.22 | 3.64 ± 0.18 | 1.43 ± 0.05 | 262.73 ± 9.88 |
| 300 | 10 | 4.13 ± 0.05 | 7.98 ± 0.10 | 3.58 ± 0.09 | 1.22 ± 0.17 | 419.61 ± 5.85 |
| 300 | 30 | 3.65 ± 0.42 | 9.29 ± 0.24 | 4.22 ± 0.21 | 1.02 ± 0.23 | 431.98 ± 3.09 |
| 300 | 60 | 4.33 ± 0.34 | 7.39 ± 0.20 | 3.75 ± 0.25 | 1.20 ± 0.05 | 490.37 ± 6.29 |
| 400 | 10 | 4.31 ± 0.71 | 9.0 ± 1.99 | 4.40 ± 0.78 | 0.88 ± 0.12 | 436.66 ± 4.02 |
| 400 | 30 | 5.15 ± 0.31 | 12.57 ± 0.04 | 5.78 ± 0.56 | 1.31 ± 0.10 | 400.52 ± 9.94 |
| 400 | 60 | 1.79 ± 0.13 | 5.54 ± 0.20 | 3.07 ± 0.25 | 0.8 ± 0.10 | 479.21 ± 5.21 |
| 500 | 10 | 1.68 ± 0.09 | 6.38 ± 0.16 | 3.37 ± 0.09 | 0.79 ± 0.02 | 502.89 ± 7.69 |
| 500 | 30 | 2.04 ± 0.18 | 4.81 ± 0.10 | 4.22 ± 0.21 | 0.81 ± 0.12 | 463.63 ± 3.13 |
| 500 | 60 | 1.16 ± 0.1 | 5.27 ± 0.00 | 2.97 ± 0.16 | 0.79 ± 0.02 | 386.49 ± 33.58 |
| 600 | 10 | 1.67 ± 0.30 | 4.85 ± 0.34 | 2.89 ± 0.33 | 0.55 ± 0.05 | 417.60 ± 51.59 |
| 600 | 30 | 1.52 ± 0.05 | 4.83 ± 0.17 | 2.75 ± 0.13 | 0.66 ± 0.04 | 308.32 ± 42.37 |
| 600 | 60 | 1.21 ± 0.02 | 5.72 ± 0.07 | 2.90 ± 0.05 | 0.56 ± 0.02 | 475.39 ± 19.47 |
| Boiling Time (min) | Pinitol (g/100 g) | Sucrose (g/100 g) | Stachyose (g/100 g) | Raffinose (g/100 g) | Soyasapogenol A (µg/g) |
|---|---|---|---|---|---|
| 0 | 2.52 ± 0.53 a | 14.69 ± 2.43 a | 5.88 ± 1.27 a | 1.82 ± 0.49 a | 429.53 ± 13.07 a |
| 10 | 3.43 ± 0.27 a | 11.83 ± 0.25 a | 4.62 ± 0.23 a | 1.47 ± 0.08 a | 363.17 ± 1.09 a |
| 20 | 3.28 ± 0.31 a | 12.03 ± 0.60 a | 4.77 ± 0.12 a | 1.36 ± 0.04 a | 290.23 ± 0.94 a |
| 30 | 3.47 ± 0.21 a | 11.49 ± 0.53 a | 4.24 ± 0.10 a | 1.48 ± 0.18 a | 313.19 ± 0.46 ac |
| 40 | 4.24 ± 0.44 b | 13.07 ± 0.41 a | 5.18 ± 0.26 a | 1.74 ± 0.09 a | 321.26 ± 1.84 a |
| 50 | 3.91 ± 0.21 b | 13.52 ± 0.28 a | 5.76 ± 0.45 a | 1.72 ± 0.10 a | 290.07 ± 3.09 b |
| 60 | 3.82 ± 0.20 b | 13.67 ± 0.59 a | 5.01 ± 0.32 a | 1.76 ± 0.05 a | 279.15 ± 1.17 a |
| Pressure (MPa) | Time (min) | a-Linolenic Acid (g/100 g-w.b.) | Linoleic Acid (g/100 g-w.b.) | Oleic Acid (g/100 g-w.b.) | Palmitic Acid (g/100 g-w.b.) | Stearic Acid (g/100 g-w.b.) |
|---|---|---|---|---|---|---|
| Untreated | Untreated | 1.73 ± 0.35 | 5.62 ± 0.43 | 1.44 ± 0.15 | 0.31 ± 0.03 | 0.06 ± 0.01 |
| 100 | 10 | 2.57 ± 1.11 | 8.02 ± 2.08 | 2.12 ± 0.58 | 0.34 ± 0.04 | 0.08 ± 0.01 |
| 100 | 30 | 1.55 ± 0.25 | 5.95 ± 0.89 | 1.91 ± 0.11 | 0.31 ± 0.03 | 0.07 ± 0.01 |
| 100 | 60 | 1.47 ± 0.27 | 5.52 ± 0.82 | 1.87 ± 0.26 | 0.32 ± 0.01 | 0.06 ± 0.01 |
| 200 | 10 | 1.94 ± 0.87 | 6.07 ± 2.06 | 1.52 ± 0.11 | 0.30 ± 0.02 | 0.07 ± 0.00 |
| 200 | 30 | 1.87 ± 0.78 | 5.85 ± 1.59 | 1.56 ± 0.26 | 0.29 ± 0.05 | 0.07 ± 0.01 |
| 200 | 60 | 1.90 ± 0.60 | 5.96 ± 1.19 | 1.54 ± 0.08 | 0.29 ± 0.02 | 0.06 ± 0.01 |
| 300 | 10 | 1.65 ± 0.59 | 5.06 ± 1.20 | 1.35 ± 0.10 | 0.31 ± 0.03 | 0.06 ± 0.01 |
| 300 | 30 | 2.10 ± 0.88 | 6.31 ± 1.96 | 1.58 ± 0.19 | 0.28 ± 0.05 | 0.07 ± 0.01 |
| 300 | 60 | 1.95 ± 0.56 | 6.54 ± 1.20 | 2.12 ± 0.57 | 0.32 ± 0.05 | 0.07 ± 0.01 |
| 400 | 10 | 2.02 ± 0.89 | 7.17 ± 2.39 | 2.07 ± 0.17 | 0.29 ± 0.02 | 0.10 ± 0.04 |
| 400 | 30 | 1.89 ± 0.72 | 6.78 ± 2.18 | 2.53 ± 0.84 | 0.27 ± 0.01 | 0.10 ± 0.06 |
| 400 | 60 | 1.41 ± 0. 37 | 5.43 ± 1.18 | 1.76 ± 0.05 | 0.32 ± 0.02 | 0.07 ± 0.03 |
| 500 | 10 | 1.73 ± 0.92 | 5.73 ± 2.37 | 1.86 ± 0.45 | 0.26 ± 0.04 | 0.10 ± 0.05 |
| 500 | 30 | 1.45 ± 0.99 | 5.15 ± 2.83 | 1.58 ± 0.34 | 0.22 ± 0.03 | 0.09 ± 0.04 |
| 500 | 60 | 1.85 ± 0.51 | 6.29 ± 1.31 | 1.92 ± 0.31 | 0.29 ± 0.02 | 0.08 ± 0.03 |
| 600 | 10 | 1.25 ± 0.46 | 4.75 ± 1.07 | 1.42 ± 0.20 | 0.32 ± 0.04 | 0.08 ± 0.01 |
| 600 | 30 | 1.29 ± 0.33 | 4.76 ± 0.74 | 1.70 ± 0.42 | 0.29 ± 0.03 | 0.08 ± 0.05 |
| 600 | 60 | 1.42 ± 0.34 | 5.74 ± 1.28 | 1.78 ± 0.36 | 0.23 ± 0.01 | 0.09 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, S.; Liu, H.; Kishino, R.; Oshikiri, Y.; Kawaguchi, Y.; Watanabe, A.; Kobayashi, W.; Shimada, R. Effects of High Hydrostatic Pressure on the Distribution of Oligosaccharides, Pinitol, Soysapapogenol A, and Fatty Acids in Soybean. Foods 2024, 13, 2214. https://doi.org/10.3390/foods13142214
Ueno S, Liu H, Kishino R, Oshikiri Y, Kawaguchi Y, Watanabe A, Kobayashi W, Shimada R. Effects of High Hydrostatic Pressure on the Distribution of Oligosaccharides, Pinitol, Soysapapogenol A, and Fatty Acids in Soybean. Foods. 2024; 13(14):2214. https://doi.org/10.3390/foods13142214
Chicago/Turabian StyleUeno, Shigeaki, Hsiuming Liu, Risa Kishino, Yuka Oshikiri, Yuki Kawaguchi, Akio Watanabe, Wataru Kobayashi, and Reiko Shimada. 2024. "Effects of High Hydrostatic Pressure on the Distribution of Oligosaccharides, Pinitol, Soysapapogenol A, and Fatty Acids in Soybean" Foods 13, no. 14: 2214. https://doi.org/10.3390/foods13142214
APA StyleUeno, S., Liu, H., Kishino, R., Oshikiri, Y., Kawaguchi, Y., Watanabe, A., Kobayashi, W., & Shimada, R. (2024). Effects of High Hydrostatic Pressure on the Distribution of Oligosaccharides, Pinitol, Soysapapogenol A, and Fatty Acids in Soybean. Foods, 13(14), 2214. https://doi.org/10.3390/foods13142214




