Effect of Optimized UV-LED Technology on Modeling, Inactivation Kinetics and Microbiological Safety in Tomato Juice
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.2. Physicochemical and Optical Properties
2.3. Inoculation of Pathogenic Bacteria
2.4. Evaluated Treatments
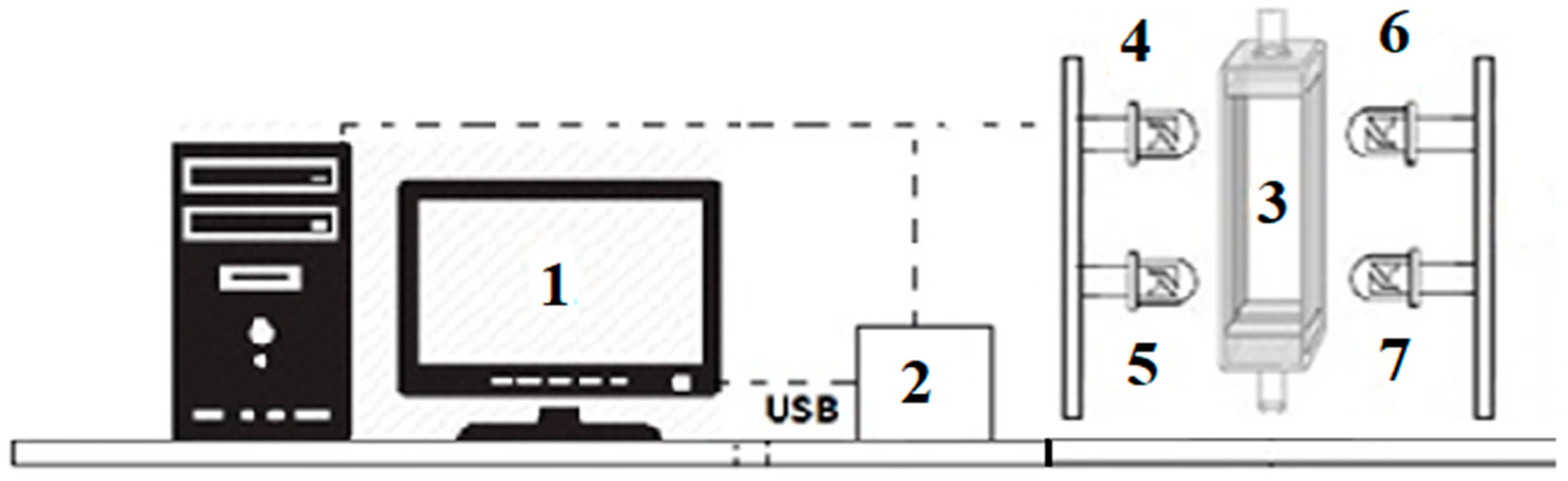
2.4.1. UV-LED Irradiation
2.4.2. Heat Processing
2.5. Experimental Design and Model Validation
2.6. Weibull Distribution Model for Microbial Inactivation
2.7. Microbiological Safety
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters and Optical Properties
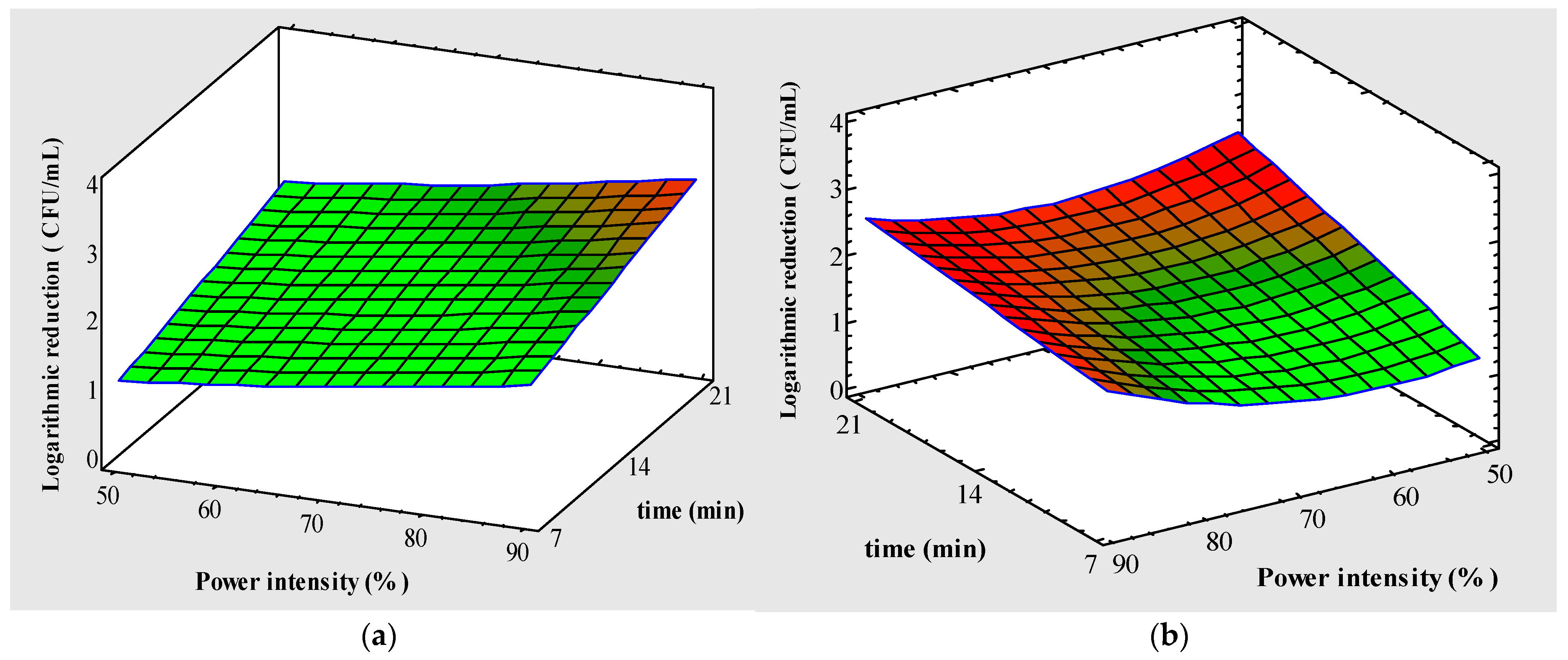
3.2. UV-LED Irradiation Process on the Response Variables
3.2.1. Logarithmic Inactivation of PB1 and PB2
3.2.2. Optimum Model Validation
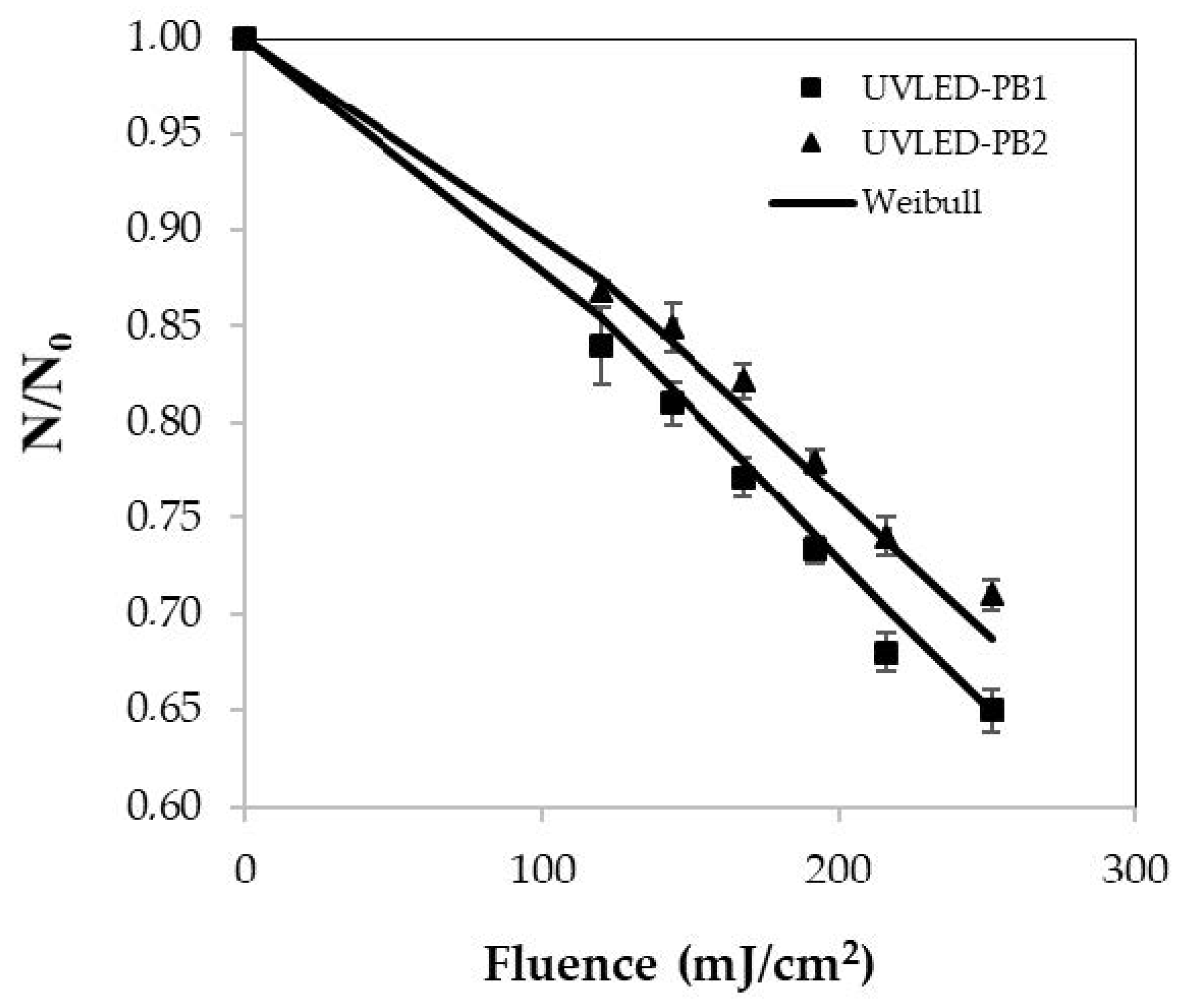
3.3. Microbial Inactivation Kinetics and Weibull Modeling
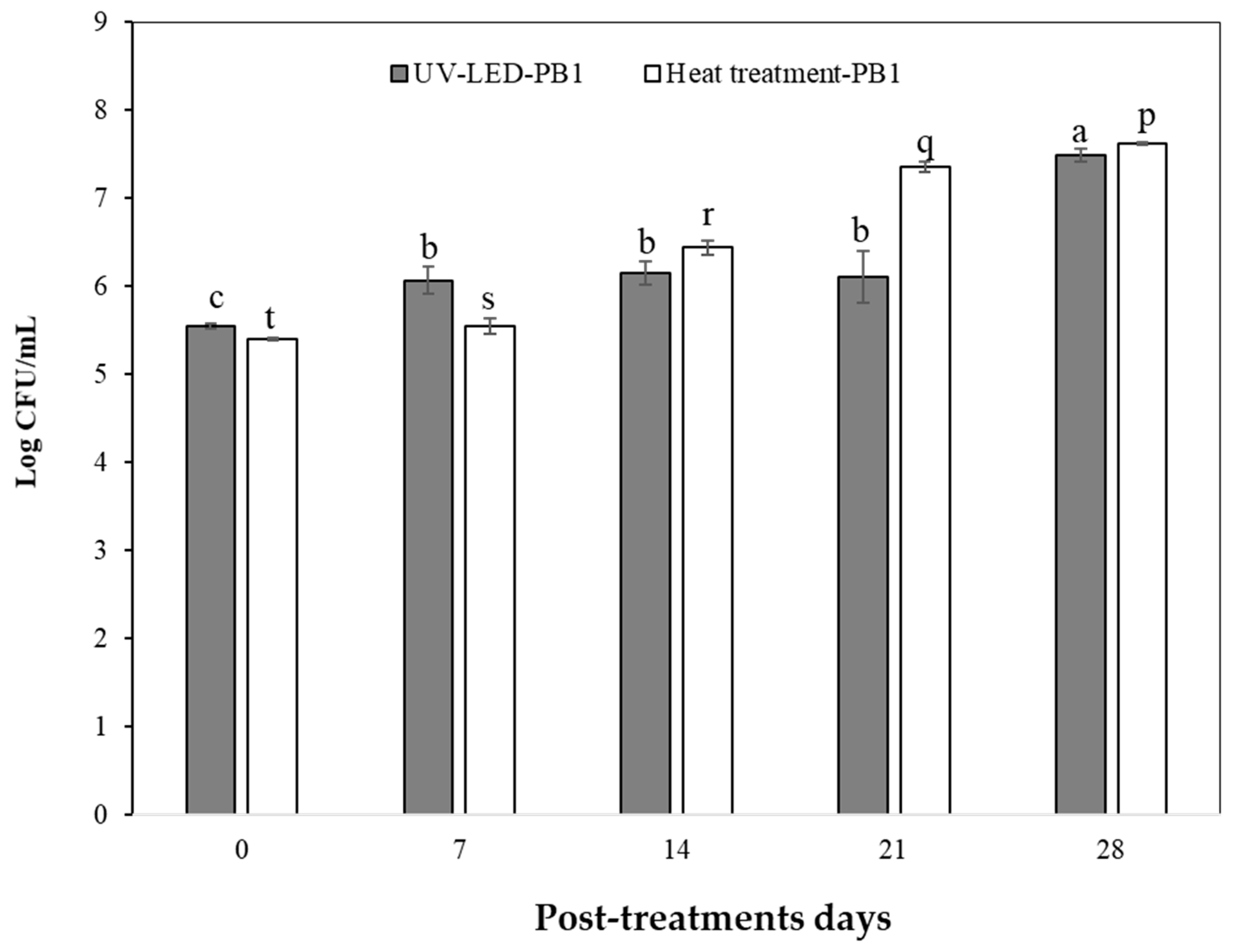
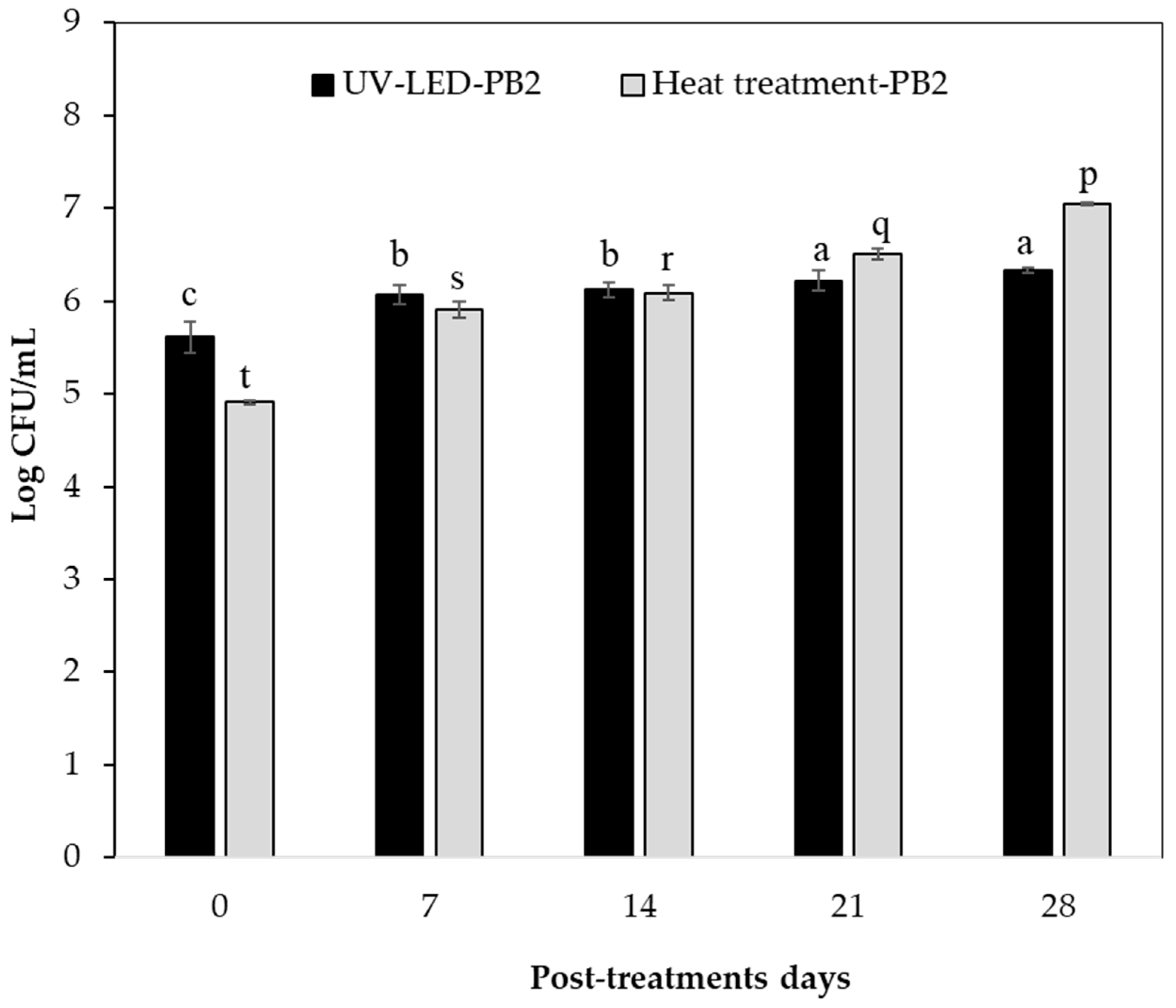
3.4. Microbiological Safety
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruxton, C.H.S.; Myers, M. Fruit Juices: Are They Helpful or Harmful? An Evidence Review. Nutrients 2021, 13, 1815. [Google Scholar] [CrossRef]
- Cui, J.; Lian, Y.; Zhao, C.; Du, H.; Han, Y.; Gao, W.; Xiao, H.; Zheng, J. Dietary Fibers from Fruits and Vegetables and Their Health Benefits via Modulation of Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1514–1532. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Giovagnoli-Vicuña, C.; Cañas-Sarazúa, R. Optimization of Extraction Yield, Flavonoids and Lycopene from Tomato Pulp by High Hydrostatic Pressure-Assisted Extraction. Food Chem. 2019, 278, 751–759. [Google Scholar] [CrossRef]
- Salehi, F. Physico-Chemical Properties of Fruit and Vegetable Juices as Affected by Ultrasound: A Review. Int. J. Food Prop. 2020, 23, 1748–1765. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of Pulsed Electric Fields to Improve Product Yield and Waste Valorization in Industrial Tomato Processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Bhat, R. Impact of Ultraviolet Radiation Treatments on the Quality of Freshly Prepared Tomato (Solanum lycopersicum) Juice. Food Chem. 2016, 213, 635–640. [Google Scholar] [CrossRef]
- Pizarro-Oteíza, S.; Salazar, F. Effect of UV-LED Irradiation Processing on Pectolytic Activity and Quality in Tomato (Solanum lycopersicum) Juice. Innov. Food Sci. Emerg. Technol. 2022, 80, 103097. [Google Scholar] [CrossRef]
- Hinds, L.M.; O’Donnell, C.P.; Akhter, M.; Tiwari, B.K. Principles and Mechanisms of Ultraviolet Light Emitting Diode Technology for Food Industry Applications. Innov. Food Sci. Emerg. Technol. 2019, 56, 102153. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Mandal, R. Non-thermal processing of watermelon and red grape juices in thin-profile continuous-flow pulsed UV light reactors: Effect on microbiological safety and nutritional value. LWT Food Sci. Technol. 2023, 191, 115516. [Google Scholar] [CrossRef]
- Gök, S.B.; Gräf, V.; Stahl, M.R. Engineering Aspects of UV-C. Processing for Liquid Foods. Innov. Food Process. Technol. A Compr. Rev. 2020, 171–181. [Google Scholar] [CrossRef]
- Amine, B.; Nadir, A.; Nouara, K.O.; Akila, A.T.; Kamelia, K.; Ghania, K.B.; Kenza, B.; Sara, G.; Naima, D.M.; Nedjima, D.I.; et al. Impact of Thermal and Non-Thermal Pasteurization on the Microbial Inactivation of Fruit Juice: Review. J. Food Microbiol. Saf. Hyg. 2023, 8. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, L.H.; Zeng, X.A.; Han, Z.; Brennan, C.S. Non-Thermal Technologies and Its Current and Future Application in the Food Industry: A Review. Int. J. Food Sci. Technol. 2018, 54, 1–13. [Google Scholar] [CrossRef]
- Baysal, A.H.; Molva, C.; Unluturk, S. UV-C Light Inactivation and Modeling Kinetics of Alicyclobacillus Acidoterrestris Spores in White Grape and Apple Juices. Int. J. Food Microbiol. 2013, 166, 494–498. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and Potential Applications of Ultraviolet Light in the Food Industry—A Critical Review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, J.A.; Barbosa-Cánovas, G.V. Review: Advantages and Limitations on Processing Foods by UV Light. Food Sci. Technol. Int. 2004, 10, 137–147. [Google Scholar] [CrossRef]
- Koutchma, T. Advances in Ultraviolet Light Technology for Non-Thermal Processing of Liquid Foods. Food Bioprocess. Technol. 2009, 2, 138–155. [Google Scholar] [CrossRef]
- Caminiti, I.M.; Palgan, I.; Muñoz, A.; Noci, F.; Whyte, P.; Morgan, D.J.; Cronin, D.A.; Lyng, J.G. The Effect of Ultraviolet Light on Microbial Inactivation and Quality Attributes of Apple Juice. Food Bioprocess. Technol. 2012, 5, 680–686. [Google Scholar] [CrossRef]
- Guerrero-Beltran, J.A.; Ochoa-Velasco, C.E. Ultraviolet-C Light Technology and Systems for Preservation of Fruit Juices and Beverages. In Innovative Food Processing Technologies: A Comprehensive Review; Elsevier: Amsterdam, The Netherlands, 2020; pp. 210–226. ISBN 9780128157824. [Google Scholar]
- Kebbi, Y.; Muhammad, A.I.; Sant’Ana, A.S.; do Prado-Silva, L.; Liu, D.; Ding, T. Recent Advances on the Application of UV-LED Technology for Microbial Inactivation: Progress and Mechanism. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3501–3527. [Google Scholar] [CrossRef] [PubMed]
- Graça, A.; Santo, D.; Quintas, C.; Nunes, C. Growth of Escherichia coli, Salmonella enterica and Listeria spp., and Their Inactivation Using Ultraviolet Energy and Electrolyzed Water, on ‘Rocha’ Fresh-Cut Pears. Food Control 2017, 77, 41–49. [Google Scholar] [CrossRef]
- Allahyari, E.; Carraturo, F.; De Risi, A.; Nappo, A.; Morelli, M.; Cajora, A.; Guida, M. A Sequential Utilization of the UV-A (365 Nm) Fluence Rate for Disinfection of Water, Contaminated with Legionella Pneumophila and Legionella Dumoffii. Environ. Pollut. 2022, 304, 119224. [Google Scholar] [CrossRef] [PubMed]
- Taze, B.H.; Unluturk, S.; Buzrul, S.; Alpas, H. The Impact of UV-C Irradiation on Spoilage Microorganisms and Colour of Orange Juice. J. Food Sci. Technol. 2013, 52, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Richard Wagner, J. DNA Base Damage by Reactive Oxygen Species, Oxidizing Agents, and UV Radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Hariram, U.; Srimagal, A.; Sahu, J.K. Applications of Light Emitting Diodes and Their Mechanism for Food Preservation. J. Food Saf. 2023, 43, e13040. [Google Scholar] [CrossRef]
- Prasad, A.; Du, L.; Zubair, M.; Subedi, S.; Ullah, A.; Roopesh, M.S. Applications of Light-Emitting Diodes (LEDs) in Food Processing and Water Treatment. Food Eng. Rev. 2020, 12, 268–289. [Google Scholar] [CrossRef]
- Popović, V.; Koutchma, T.; Pagan, J. Emerging Applications of Ultraviolet Light-Emitting Diodes for Foods and Beverages. In Innovative Food Processing Technologies: A Comprehensive Review; Elsevier: Amsterdam, The Netherlands, 2021; pp. 335–344. ISBN 9780128157824. [Google Scholar]
- Song, K.; Taghipour, F.; Mohseni, M. Microorganisms Inactivation by Wavelength Combinations of Ultraviolet Light-Emitting Diodes (UV-LEDs). Sci. Total Environ. 2019, 665, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Finardi, S.; Hoffmann, T.G.; Schmitz, F.R.W.; Bertoli, S.L.; Khayrullin, M.; Neverova, O.; Ponomarev, E.; Goncharov, A.; Kulmakova, N.; Dotsenko, E.; et al. Comprehensive Study of Light-Emitting Diodes (LEDs) and Ultraviolet-LED Lights Application in Food Quality and Safety. J. Pure Appl. Microbiol. 2021, 15, 1125–1135. [Google Scholar] [CrossRef]
- Chatterley, C.; Linden, K. Demonstration and Evaluation of Germicidal UV-LEDs for Point-of-Use Water Disinfection. J. Water Health 2010, 8, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, D.; Ferrario, M.; Schenk, M.; Guerrero, S. Effect of pilot-scale UV-C light treatment assisted by mild heat on E. coli, L. plantarum and S. cerevisiae Inactivation in Clear and Turbid Fruit Juices. Storage Study of Surviving Populations. Int. J. Food Microbiol. 2020, 332, 108767. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current Pathogenic Escherichia coli Foodborne Outbreak Cases and Therapy Development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef]
- Mansur, A.R.; Lee, H.S.; Lee, C.J. A Review of the Efficacy of Ultraviolet C Irradiation for Decontamination of Pathogenic and Spoilage Microorganisms in Fruit Juices. J. Microbiol. Biotechnol. 2023, 33, 419–429. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, S.H.; Kim, S.H.; Kang, D.H. Synergistic Effect of Ohmic Heating and UV-C Irradiation for Inactivation of Escherichia coli O157:H7, Salmonella typhimurium and Listeria monocytogenes in Buffered Peptone Water and Tomato Juice. Food Control 2019, 102, 69–75. [Google Scholar] [CrossRef]
- Jaiaue, P.; Piluk, J.; Sawattrakool, K.; Thammakes, J.; Malasuk, C.; Thitiprasert, S.; Thongchul, N.; Siwamogsatham, S. Mathematical Modeling for Evaluating Inherent Parameters Affecting UVC Decontamination of Indicator Bacteria. Appl. Environ. Microbiol. 2022, 88, e0214821. [Google Scholar] [CrossRef]
- Ghate, V.; Kumar, A.; Kim, M.J.; Bang, W.S.; Zhou, W.; Yuk, H.G. Effect of 460 Nm Light Emitting Diode Illumination on Survival of Salmonella Spp. on Fresh-Cut Pineapples at Different Irradiances and Temperatures. J. Food Eng. 2017, 196, 130–138. [Google Scholar] [CrossRef]
- Quintero-Ramos, A.; Churey, J.J.; Hartman, P.; Barnard, J.; Worobo, R.W. Modeling of Escherichia coli Inactivation by UV Irradiation at Different PH Values in Apple Cider. J. Food Prot. 2004, 67, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Rattanakul, S.; Oguma, K. Inactivation Kinetics and Efficiencies of UV-LEDs against Pseudomonas Aeruginosa, Legionella Pneumophila, and Surrogate Microorganisms. Water Res. 2018, 130, 31–37. [Google Scholar] [CrossRef]
- Unluturk, S.; Atilgan, M.R.; Baysal, A.H.; Unluturk, M.S. Modeling Inactivation Kinetics of Liquid Egg White Exposed to UV-C Irradiation. Int. J. Food Microbiol. 2010, 142, 341–347. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, H.; Sánchez Basurto, L.A.; Protasenko, V.V.; Bharadwaj, S.; Islam, M.; Moraru, C.I. Inactivation of Listeria and E. Coli by Deep-UV LED: Effect of Substrate Conditions on Inactivation Kinetics. Sci. Rep. 2020, 10, 3411. [Google Scholar] [CrossRef]
- Xiang, Q.; Fan, L.; Zhang, R.; Ma, Y.; Liu, S.; Bai, Y. Effect of UVC Light-Emitting Diodes on Apple Juice: Inactivation of Zygosaccharomyces Rouxii and Determination of Quality. Food Control 2020, 111, 107082. [Google Scholar] [CrossRef]
- Zhai, Y.; Tian, J.; Ping, R.; Xiu, H.; Xiang, Q.; Shen, R.; Wang, Z. Effects of Ultraviolet-C Light-Emitting Diodes at 275 Nm on Inactivation of Alicyclobacillus Acidoterrestris Vegetative Cells and Its Spores as Well as the Quality Attributes of Orange Juice. Food Sci. Technol. Int. 2020, 27, 334–343. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, C.; Yuk, H.G.; Khoo, G.H.; Zhou, W. Application of Light-Emitting Diodes in Food Production, Postharvest Preservation, and Microbiological Food Safety. Compr. Rev. Food Sci. Food Saf. 2015, 14, 719–740. [Google Scholar] [CrossRef]
- Baykuş, G.; Akgün, M.P.; Unluturk, S. Effects of Ultraviolet-Light Emitting Diodes (UV-LEDs) on Microbial Inactivation and Quality Attributes of Mixed Beverage Made from Blend of Carrot, Carob, Ginger, Grape and Lemon Juice. Innov. Food Sci. Emerg. Technol. 2020, 67, 102572. [Google Scholar] [CrossRef]
- Norton, T.; Sun, D.W. Computational Fluid Dynamics (CFD)—An Effective and Efficient Design and Analysis Tool for the Food Industry: A Review. Trends Food Sci. Technol. 2006, 17, 600–620. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.A.; Vega-Gálvez, A.; Zambra, C.E.; Moraga, N.O. Modeling 3D Conjugate Heat and Mass Transfer for Turbulent Air Drying of Chilean Papaya in a Direct Contact Dryer. Heat Mass Transf. 2016, 53, 11–24. [Google Scholar] [CrossRef]
- Keshavarzfathy, M.; Taghipour, F. Computational Modeling of Ultraviolet Light-Emitting Diode (UV-LED) Reactor for Water Treatment. Water Res. 2019, 166, 115022. [Google Scholar] [CrossRef]
- Soro, A.B.; Whyte, P.; Bolton, D.J.; Tiwari, B.K. Modelling the Effect of UV Light at Different Wavelengths and Treatment Combinations on the Inactivation of Campylobacter Jejuni. Innov. Food Sci. Emerg. Technol. 2021, 69, 102626. [Google Scholar] [CrossRef]
- Atilgan, M.R.; Yildiz, S.; Kaya, Z.; Unluturk, S. Kinetic and Process Modeling of UV-C Irradiation of Foods; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128157824. [Google Scholar]
- Nyangaresi, P.O.; Rathnayake, T.; Beck, S.E. Evaluation of Disinfection Efficacy of Single UV-C, and UV-A Followed by UV-C LED Irradiation on Escherichia coli, B. spizizenii and MS2 Bacteriophage, in Water. Sci. Total Environ. 2023, 859, 160256. [Google Scholar] [CrossRef]
- Menezes, N.M.C.; Longhi, D.A.; Ortiz, B.O.; Junior, A.F.; de Aragão, G.M.F. Modeling the inactivation of Aspergillus fischeri and Paecilomyces niveus ascospores in apple juice by different ultraviolet light irradiances. Int. J. Food Microbiol. 2020, 333, 108773. [Google Scholar] [CrossRef] [PubMed]
- Biral, D.; Maria, M.; Augusto, O.; Guilherme, N.; Astrath, C.; Caroline, N.; Terezinha, I.; Previdelli, S.; Vataru, C.; Martha, J.; et al. Effect of Ultraviolet (UV-C) Radiation on Spores and Biofilms of Alicyclobacillus spp. in Industrialized Orange Juice. Int. J. Food Microbiol. 2019, 305, 108238. [Google Scholar] [CrossRef]
- Menezes, N.M.C.; Tremarin, A.; Junior, A.F.; de Aragão, G.M.F. Effect of Soluble Solids Concentration on Neosartorya fischeri Inactivation Using UV-C Light. Int. J. Food Microbiol. 2019, 296, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, R.R.; Sanches, M.A.R.; de Castilhos, M.B.M.; Cantú-Lozano, D.; Telis-Romero, J. Determination of the Rheological Behavior and Thermophysical Properties of Malbec Grape Juice Concentrates (Vitis vinifera). Food Res. Int. 2020, 137, 109431. [Google Scholar] [CrossRef] [PubMed]
- Mirondo, R.; Barringer, S. Improvement of Flavor and Viscosity in Hot and Cold Break Tomato Juice and Sauce by Peel Removal. J. Food Sci. 2015, 80, S171–S179. [Google Scholar] [CrossRef]
- Akgün, M.P.; Ünlütürk, S. Effects of Ultraviolet Light Emitting Diodes (LEDs) on Microbial and Enzyme Inactivation of Apple Juice. Int. J. Food Microbiol. 2017, 260, 65–74. [Google Scholar] [CrossRef]
- Fredericks, I.N.; du Toit, M.; Krügel, M. Efficacy of Ultraviolet Radiation as an Alternative Technology to Inactivate Microorganisms in Grape Juices and Wines. Food Microbiol. 2011, 28, 510–517. [Google Scholar] [CrossRef]
- Kim, D.K.; Kang, D.H. Inactivation Efficacy of a Sixteen UVC LED Module to Control Foodborne Pathogens on Selective Media and Sliced Deli Meat and Spinach Surfaces. LWT 2020, 130, 109422. [Google Scholar] [CrossRef]
- Yu, H.C.; Tan, F.J. Optimization of Ultrasonic-Assisted Enzymatic Hydrolysis Conditions for the Production of Antioxidant Hydrolysates from Porcine Liver by Using Response Surface Methodology. Asian-Australas. J. Anim. Sci. 2017, 30, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Brugnini, G.; Rodríguez, S.; Rodríguez, J.; Rufo, C. Effect of UV-C Irradiation and Lactic Acid Application on the Inactivation of Listeria monocytogenes and Lactic Acid Bacteria in Vacuum-Packaged Beef. Foods 2021, 10, 1217. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.; Mohamed Ahmed, I.A.; Ahmed, K.A.; Yehia, H.M.; Abdelkarim, D.O.; El-Abedein, A.I.Z.; Alhamdan, A. Response Surface Methodology (RSM) Optimization of the Physicochemical Quality Attributes of Ultraviolet (UV-C)-Treated Barhi Dates. Plants 2022, 11, 2322. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.R.; Linden, K.G.; Asce, M. Standardization of Methods for Fluence "UV Dose… Determination in Bench-Scale UV Experiments. J. Environ. Eng. 2003, 129, 209–215. [Google Scholar] [CrossRef]
- Bolton, J.R.; Mayor-Smith, I.; Linden, K.G. Rethinking the Concepts of Fluence (UV Dose) and Fluence Rate: The Importance of Photon-Based Units—A Systemic Review. Photochem. Photobiol. 2015, 91, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Unluturk, S.; Atilgan, M.R. Microbial Safety and Shelf Life of UV-C Treated Freshly Squeezed White Grape Juice. J. Food Sci. 2015, 80, M1831–M1841. [Google Scholar] [CrossRef] [PubMed]
- Briones, L.S.; Reyes, J.E.; Tabilo-Munizaga, G.E.; Pérez-Won, M.O. Microbial Shelf-Life Extension of Chilled Coho Salmon (Oncorhynchus kisutch) and Abalone (Haliotis rufescens) by High Hydrostatic Pressure Treatment. Food Control 2010, 21, 1530–1535. [Google Scholar] [CrossRef]
- Begum, M.; Hocking, A.D.; Miskelly, D. Inactivation of Food Spoilage Fungi by Ultra Violet (UVC) Irradiation. Int. J. Food Microbiol. 2009, 129, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Koutchma, T.; Keller, S.; Chirtel, S.; Parisi, B. Ultraviolet Disinfection of Juice Products in Laminar and Turbulent Flow Reactors. Innov. Food Sci. Emerg. Technol. 2004, 5, 179–189. [Google Scholar] [CrossRef]
- Adubofuor, J.; Amankwah, E.A.; Arthur, B.S.; Appiah, F. Comparative Study Related to Physico-Chemical Properties and Sensory Qualities of Tomato Juice and Cocktail Juice Produced from Oranges, Tomatoes and Carrots. Afr. J. Food Sci. 2010, 4, 427–433. [Google Scholar]
- Hsu, K.C.; Tan, F.J.; Chi, H.Y. Evaluation of Microbial Inactivation and Physicochemical Properties of Pressurized Tomato Juice during Refrigerated Storage. LWT 2008, 41, 367–375. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.; Kang, D. Bactericidal Effect of 266 to 279 nm Wavelength UVC-LEDs for Inactivation of Gram Positive and Gram Negative Foodborne Pathogenic Bacteria and Yeasts. Food Res. Int. 2017, 97, 280–287. [Google Scholar] [CrossRef]
- Rezaee, R.; Maleki, A.; Jafari, A.; Mazloomi, S.; Zandsalimi, Y.; Mahvi, A.H. Application of Response Surface Methodology for Optimization of Natural Organic Matter Degradation by UV/H2O2 Advanced Oxidation Process. J. Environ. Health Sci. Eng. 2014, 12, 67. [Google Scholar] [CrossRef]
- Jafari, N.; Jafarizadeh-Malmiri, H.; Hamzeh-Mivehroud, M.; Adibpour, M. Optimization of UV Irradiation Mutation Conditions for Cellulase Production by Mutant Fungal Strains of Aspergillus Niger through Solid State Fermentation. Green Process. Synth. 2017, 6, 333–340. [Google Scholar] [CrossRef]
- Wu, W.J.; Ahn, B.Y. Statistical Optimization of Ultraviolet Irradiate Conditions for Vitamin D2 Synthesis in Oyster Mushrooms (Pleurotus ostreatus) Using Response Surface Methodology. PLoS ONE 2014, 9, e95359. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jo, D.; Kang, M.; Khan, F.; Doo, S.; Youl, C.; Kim, Y.; Ryu, U. Bactericidal Effect of Ultraviolet C Light-Emitting Diodes: Optimization of Efficacy toward Foodborne Pathogens in Water. J. Photochem. Photobiol. B Biol. 2021, 222, 112277. [Google Scholar] [CrossRef] [PubMed]
- Anjaly, M.G.; Prince, M.V.; Warrier, A.S.; Lal, A.M.N.; Mahanti, N.K.; Pandiselvam, R.; Thirumdas, R.; Sreeja, R.; Rusu, A.V.; Trif, M.; et al. Design Consideration and Modelling Studies of Ultrasound and Ultraviolet Combined Approach for Shelf-Life Enhancement of Pine Apple Juice. Ultrason. Sonochem. 2022, 90, 106166. [Google Scholar] [CrossRef]
- Sharma, A.; Mahmoud, H.; Pendyala, B.; Balamurugan, S.; Patras, A. UV-C Inactivation of Microorganisms in Droplets on Food Contact Surfaces Using UV-C Light-Emitting Diode Devices. Front. Food Sci. Technol. 2023, 3, 1–10. [Google Scholar] [CrossRef]
- Pierscianowski, J.; Popović, V.; Biancaniello, M.; Bissonnette, S.; Zhu, Y.; Koutchma, T. Continuous-Flow UV-C Processing of Kale Juice for the Inactivation of E. Coli and Assessment of Quality Parameters. Food Res. Int. 2021, 140, 110085. [Google Scholar] [CrossRef]
- Kaya, Z.; Yildiz, S.; Ünlütürk, S. Effect of UV-C Irradiation and Heat Treatment on the Shelf Life Stability of a Lemon-Melon Juice Blend: Multivariate Statistical Approach. Innov. Food Sci. Emerg. Technol. 2015, 29, 230–239. [Google Scholar] [CrossRef]
- Ngadi, M.; Smith, J.P.; Cayouette, B. Kinetics of Ultraviolet Light Inactivation of Escherichia coli O157:H7 in Liquid Foods. J. Sci. Food Agric. 2003, 83, 1551–1555. [Google Scholar] [CrossRef]
- Green, A.; Popović, V.; Pierscianowski, J.; Biancaniello, M.; Warriner, K.; Koutchma, T. Inactivation of Escherichia coli, Listeria and Salmonella by Single and Multiple Wavelength Ultraviolet-Light Emitting Diodes. Innov. Food Sci. Emerg. Technol. 2018, 47, 353–361. [Google Scholar] [CrossRef]
- Nur Hossain, M.; Fakruddin, M.; Nurul Islam, M. Effect of Chemical Additives on the Shelf Life of Tomato Juice. Am. J. Food Technol. 2011, 6, 914–923. [Google Scholar] [CrossRef][Green Version]
- Van Boekel, M.A.J.S. On the Use of the Weibull Model to Describe Thermal Inactivation of Microbial Vegetative Cells. Int. J. Food Microbiol. 2002, 74, 139–159. [Google Scholar] [CrossRef]
- Tran, M.T.T.; Farid, M. Ultraviolet Treatment of Orange Juice. Innov. Food Sci. Emerg. Technol. 2004, 5, 495–502. [Google Scholar] [CrossRef]
- Torkamani, A.E.; Niakousari, M. Impact of UV-C Light on Orange Juice Quality and Shelf Life. Int. Food Res. J. 2011, 18, 1265–1268. [Google Scholar]
- García Carrillo, M.; Ferrario, M.; Guerrero, S. Effectiveness of UV-C Light Assisted by Mild Heat on Saccharomyces Cerevisiae KE 162 Inactivation in Carrot-Orange Juice Blend Studied by Flow Cytometry and Transmission Electron Microscopy. Food Microbiol. 2018, 73, 1–10. [Google Scholar] [CrossRef]
- Fan, L.; Liu, X.; Dong, X.; Dong, S.; Xiang, Q.; Bai, Y. Effects of UVC Light-Emitting Diodes on Microbial Safety and Quality Attributes of Raw Tuna Fillets. LWT-Food Sci. Technol. 2020, 139, 110553. [Google Scholar] [CrossRef]
- Chun, H.H.; Kim, J.Y.; Song, K. Bin Inactivation of Foodborne Pathogens in Ready-to-Eat Salad Using UV-C Irradiation. Food Sci. Biotechnol. 2010, 19, 547–551. [Google Scholar] [CrossRef]
- Lim, W.; Harrison, M.A. Effectiveness of UV Light as a Means to Reduce Salmonella Contamination on Tomatoes and Food Contact Surfaces. Food Control 2016, 66, 166–173. [Google Scholar] [CrossRef]

| Run | UV-LED Irradiation Conditions | Inactivation PB1 (Log CFU/mL) | Inactivation PB2 (Log CFU/mL) | ||
|---|---|---|---|---|---|
| A | B | C | Observed | Observed | |
| 1 | 90 | 7 | 272 | 1.86 | 2.19 |
| 2 | 50 | 14 | 278 | 0.75 | 2.39 |
| 3 | 70 | 7 | 265 | 0.02 | 0.14 |
| 4 | 70 | 7 | 278 | 1.18 | 1.45 |
| 5 | 50 | 7 | 272 | 0.84 | 1.07 |
| 6 | 90 | 21 | 272 | 3.14 | 2.73 |
| 7 | 50 | 14 | 265 | 0.60 | 0.72 |
| 8 | 50 | 21 | 272 | 2.00 | 2.42 |
| 9 | 70 | 14 | 272 | 1.90 | 1.71 |
| 10 | 70 | 21 | 265 | 1.05 | 0.95 |
| 11 | 90 | 14 | 278 | 1.96 | 2.32 |
| 12 | 90 | 14 | 265 | 0.51 | 1.19 |
| 13 | 70 | 14 | 272 | 1.89 | 1.71 |
| 14 | 70 | 21 | 278 | 1.30 | 2.31 |
| 15 | 70 | 14 | 272 | 1.91 | 1.70 |
| 16 | 90 | 7 | 272 | 1.85 | 2.19 |
| 17 | 50 | 14 | 278 | 0.68 | 2.35 |
| 18 | 70 | 7 | 265 | 0.03 | 0.15 |
| 19 | 70 | 7 | 278 | 1.19 | 1.46 |
| 20 | 50 | 7 | 272 | 0.83 | 1.10 |
| 21 | 90 | 21 | 272 | 3.12 | 2.71 |
| 22 | 50 | 14 | 265 | 0.58 | 0.71 |
| 23 | 50 | 21 | 272 | 2.00 | 2.42 |
| 24 | 70 | 14 | 272 | 1.90 | 1.69 |
| 25 | 70 | 21 | 265 | 1.02 | 0.96 |
| 26 | 90 | 14 | 278 | 1.93 | 2.31 |
| 27 | 90 | 14 | 265 | 0.49 | 1.20 |
| 28 | 70 | 14 | 272 | 1.89 | 1.69 |
| 29 | 70 | 21 | 278 | 1.24 | 2.32 |
| 30 | 70 | 14 | 272 | 1.88 | 1.68 |
| Response | Inactivation PB1 (Log CFU/mL) | Inactivation PB2 (Log CFU/mL) | ||
|---|---|---|---|---|
| Factor | Regression Coefficient (β) | p-Value | Regression Coefficient (β) | p-Value |
| A: Power intensity (%) | −0.6921 | 0.000 * | 0.1642 | 0.000 * |
| B: Time (min) | 1.4288 | 0.000 * | 0.0961 | 0.000 * |
| C: Wavelength (nm) | 13.0194 | 0.000 * | 6.1759 | 0.000 * |
| A2 | 0.00015 | 0.4171 | 0.0010 | 0.000 * |
| AB | 0.00019 | 0.6937 | −0.0014 | 0.000 * |
| AC | 0.00250 | 0.000 * | −0.00103 | 0.000 * |
| B2 | 0.00001 | 0.9931 | −0.00024 | 0.7798 |
| BC | −0.00508 | 0.000 * | 0.00027 | 0.7634 |
| C2 | −0.02407 | 0.000 * | −0.01115 | 0.000 * |
| R2 | 0.9597 | 0.9833 | ||
| R2adj | 0.9386 | 0.9745 | ||
| MAPE | 0.1249 | 0.070 | ||
| DW | 2.070 | 1.710 | ||
| Pathogenic Bacteria (PB) | Parameters | Statistical Indices | |||
|---|---|---|---|---|---|
| δ (mJ/cm2) | p | r2 | SSE | RMSE | |
| PB1: Escherichia coli O157:H7 (ATCC 2592) | 465.2 ± 37.5 | 1.37 ± 0.1 | 0.9 | 0.0001 | 0.0115 |
| PB2: Listeria monocytogenes (ATCC 19115) | 511.3 ± 47.6 | 1.40 ± 0.1 | 0.9 | 0.0001 | 0.0110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, F.; Pizarro-Oteíza, S.; Molinett, S.; Labbé, M. Effect of Optimized UV-LED Technology on Modeling, Inactivation Kinetics and Microbiological Safety in Tomato Juice. Foods 2024, 13, 430. https://doi.org/10.3390/foods13030430
Salazar F, Pizarro-Oteíza S, Molinett S, Labbé M. Effect of Optimized UV-LED Technology on Modeling, Inactivation Kinetics and Microbiological Safety in Tomato Juice. Foods. 2024; 13(3):430. https://doi.org/10.3390/foods13030430
Chicago/Turabian StyleSalazar, Fernando, Sebastián Pizarro-Oteíza, Sebastián Molinett, and Mariela Labbé. 2024. "Effect of Optimized UV-LED Technology on Modeling, Inactivation Kinetics and Microbiological Safety in Tomato Juice" Foods 13, no. 3: 430. https://doi.org/10.3390/foods13030430
APA StyleSalazar, F., Pizarro-Oteíza, S., Molinett, S., & Labbé, M. (2024). Effect of Optimized UV-LED Technology on Modeling, Inactivation Kinetics and Microbiological Safety in Tomato Juice. Foods, 13(3), 430. https://doi.org/10.3390/foods13030430





