Comparison of the Controlled Atmosphere Treatment for Submerged and Solid-State Fermentation of Inonotus obliquus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Fungal Material
2.3. Strain Activation and Purification
2.4. Scale-Up of Strain
2.5. Submerged Fermentation of I. obliquus
2.6. Solid-State Fermentation of I. obliquus
2.7. Freeze-Dried Powder Preparation
2.8. Polysaccharide Contents
2.9. Betulinic Acid Contents
- Column: LiChrospher 100 RP−18 (250 × 4.6 mm, 5 um, Merck, Rahway, NJ, USA).
- Injection volume: 20 µL.
- Temperature of column: 30 °C.
- Mobile phase: Methanol: 0.2% formic acid = 85:15.
- Flow rate: 1 mL/min.
- Detection wavelength: 210 nm.
2.10. DPPH’s Radical Scavenging Potential
2.11. Statistical Analysis
3. Results
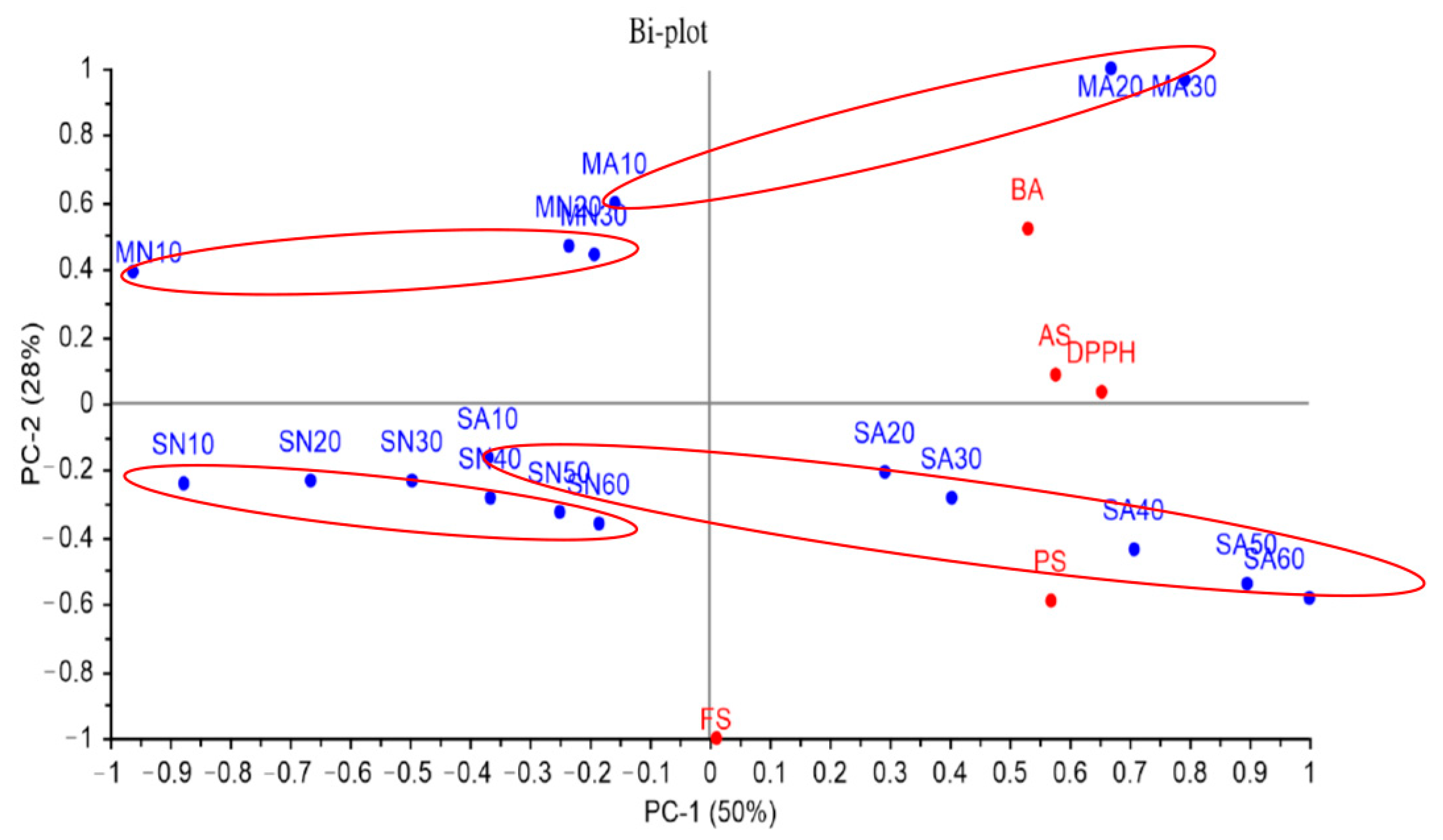
3.1. Significance of Different Treatments
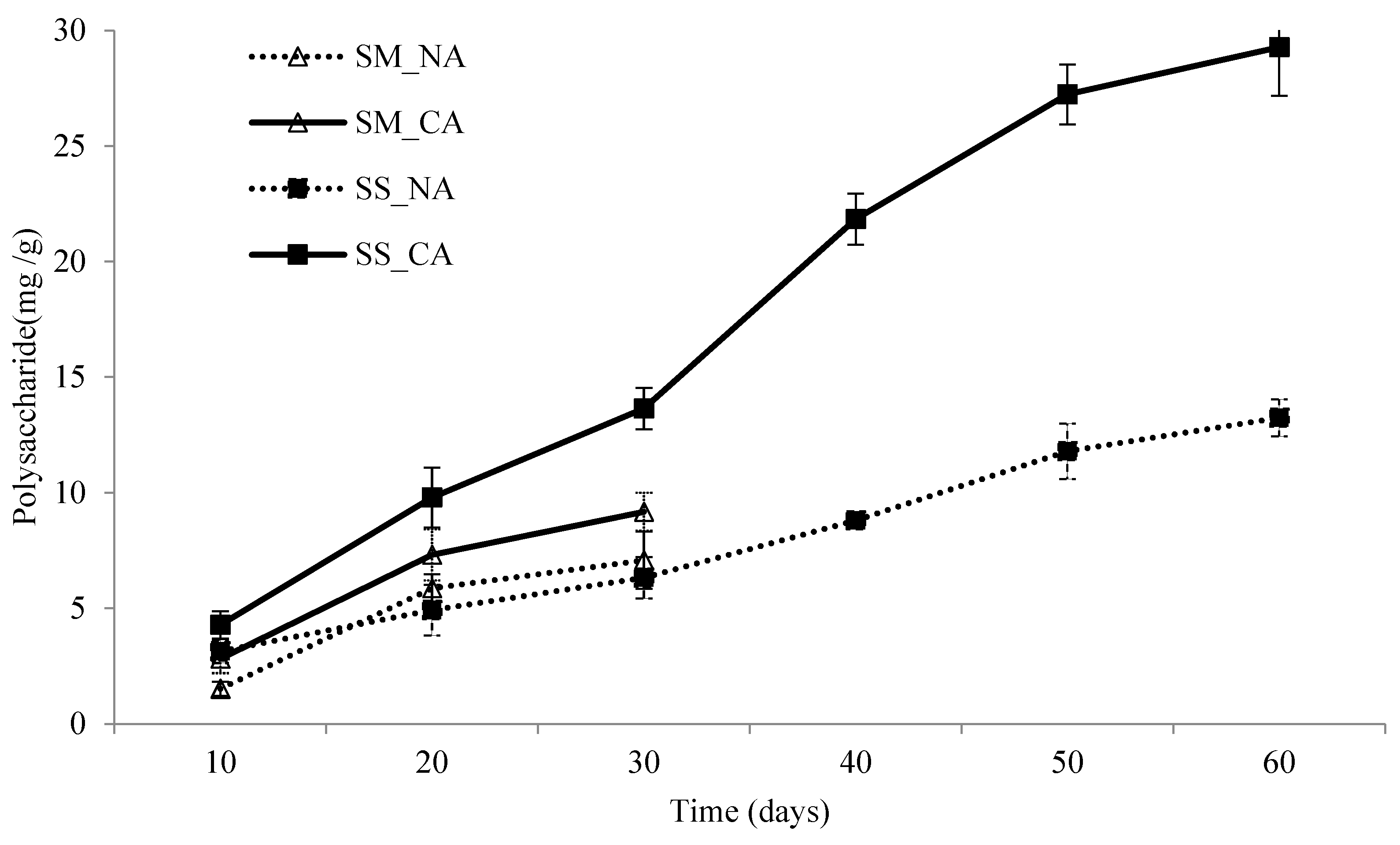
3.2. The Polysaccharide Contents of I. obliquus
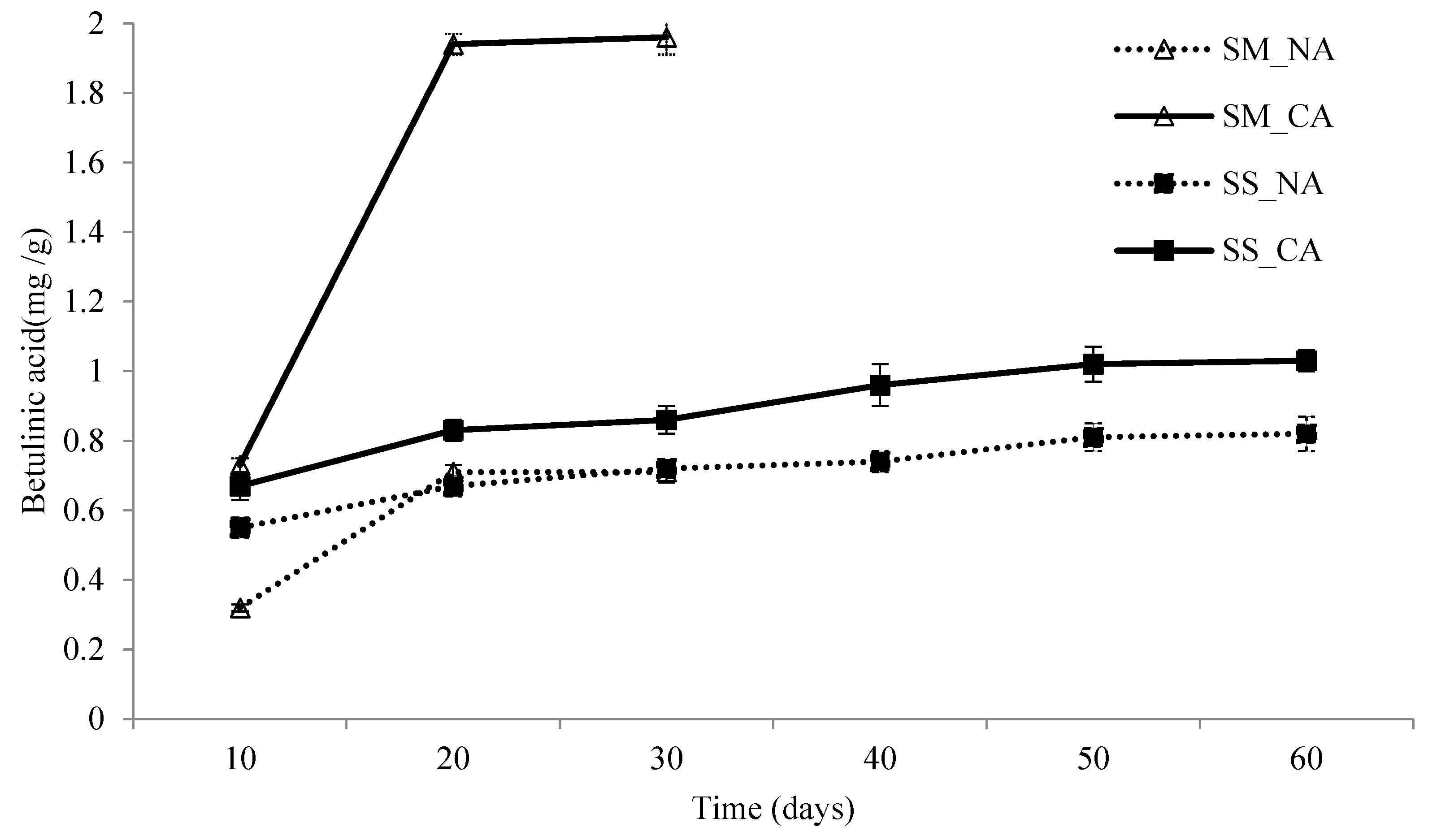
3.3. The Betulinic Acid Contents of I. obliquus
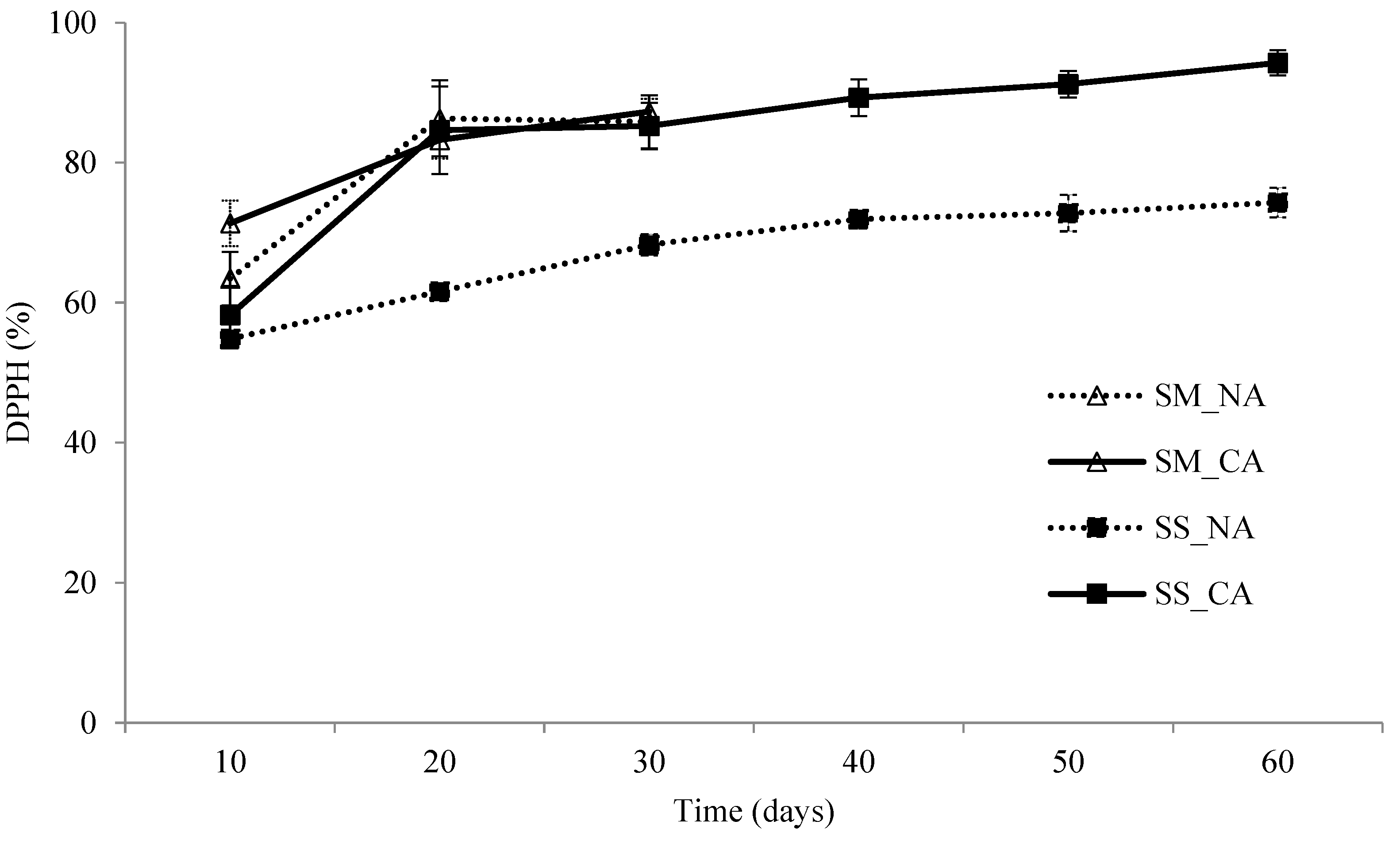
3.4. The DPPH Radical Scavenging Potential of I. obliquus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Bai, Y.; Cui, G.H.; Li, W.L. Study in the extraction of active components and pharmacological abilities of the Inonotus obliquus. J. Jilin Med. Univ. 2017, 38, 460–462. [Google Scholar]
- Koyamaa, T.; Gub, Y.; Takac, A. Fungal medicine Fuscoporia obliqua as a traditional herbal medicine its bioactivities in vivo testing andmedicinal effects. Asian Biomed. 2008, 2, 459–469. [Google Scholar]
- Huang, N.L. Fuscoporia obliqua-a mysterious folk medicinal mushroom in Russia. Edible Fungi China 2002, 21, 7–8. [Google Scholar]
- Song, Y.; Hui, J.; Kou, W.; Xin, R.; Jia, F.; Wang, N.; Hu, F.; Zhang, H.; Liu, H. Identification of Inonotus obliquus and analysis of antioxidation and antitumor activities of polysaccharides. Curr. Microbiol. 2008, 57, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, M.; Zhao, Y.; Wang, Y.; Miao, K.; Wei, Z. Accumulation of antioxidant phenolic constituents in submerged cultures of Inonotus obliquus. Bioresour. Technol. 2009, 100, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Miao, K.; Liu, Y.; Zhao, Y.; Zhang, M.; Pan, S.; Dai, Y. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl. Microbiol. Biotechnol. 2010, 87, 1237–1254. [Google Scholar] [CrossRef] [PubMed]
- Yusoo, S.; Yutaka, T.; Minoru, T. Triterpenoids, steroids, and a new sesquiterpene from Inonotus obliquus (Pers.: Fr.) Pilat. Int. J. Med. Mushrooms. 2002, 4, 77–84. [Google Scholar] [CrossRef]
- Song, F.Q.; Liu, Y.; Kong, X.S.; Chang, W.; Song, G. Progress on Understanding the Anticancer Mechanisms of Medicinal Mushroom: Inonotus Obliquus. Asian Pac. J. Cancer Prev. 2013, 14, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Yamada, T.; Tanaka, R. An unusual lanostane-type triterpenoid, spiroinonotsuoxodiol, and other triterpenoids from Inonotus obliquus. Phytochemistry 2010, 71, 1774–1779. [Google Scholar] [CrossRef]
- Zan, L.F.; Bao, H.Y. Progress in Inonotus hispidus Research. Acta Edulis Fungi 2011, 18, 78–82. [Google Scholar]
- Lee, I.K.; Kim, Y.S.; Jang, Y.W.; Jung, J.Y.; Yun, B.S. New antioxidant polyphenols from the medicinal mushroom Inonotus obliquus. Bioorg. Med. Chem. Lett. 2007, 17, 6678–6681. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Bao, H.Y. Chemical Composition and Pharmacological Actions of Inonotus obliquus. Edible Fungi China 2008, 27, 34–39. [Google Scholar]
- Kim, M.Y.; Chung, L.M.; Lee, S.J.; Ahn, J.K.; Kim, E.H.; Kim, M.J.; Kim, S.L.; Moon, H.I.; Ro, H.M.; Kang, E.Y.; et al. Comparison of free amino acid, carbohydrates concentrations in Korean edible and medicinal mushrooms. Food Chem. 2009, 113, 386–393. [Google Scholar] [CrossRef]
- Yoshiyuki, K. New anticancer agents: In vitro and in vivo evaluation of the antitumor and antimetastatic actions of various compounds isolated from medicinal plants. In Vivo 2005, 19, 37–60. [Google Scholar]
- Hu, H.; Zhang, Z.; Lei, Z.; Yang, Y.; Sugiura, N. Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. J. Biosci. Bioeng. 2009, 107, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R. Immunomodulatory Activity of the Water Extract from Medicinal Mushroom Inonotus obliquus. Mycobiology 2005, 33, 5. [Google Scholar] [CrossRef]
- Ham, S.S.; Kim, S.H.; Moon, S.Y.; Chung, M.J.; Cui, C.B.; Han, E.K.; Chung, C.K.; Choe, M. Antimutagenic effects of subfractions of Chaga mushroom (Inonotus obliquus) extract. Mutat. Res. 2009, 672, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Hur, H.; Chang, K.C.; Lee, T.S.; Ka, K.H.; Jankovsky, L. Introduction to Distribution and Ecology of Sterile Conks of Inonotus obliquus. Mycobiology 2008, 36, 4. [Google Scholar] [CrossRef]
- Kim, J.H.; Sung, N.Y.; Kwon, S.K.; Srinivasan, P.; Song, B.S.; Choi, J.I.; Yoon, Y.; Kim, K.K.; Byun, M.W.; Kim, M.R.; et al. Gamma-irradiation improves the color and antioxidant properties of Chaga mushroom (Inonotus obliquus) extract. J. Med. Food. 2009, 12, 5. [Google Scholar] [CrossRef]
- Nakajima, Y.; Sato, Y.; Konishi, T. Antioxidant small phenolic ingredients in Inonotus obliquus (persoon) Pilat (Chaga). Chem. Pharm. Bull. 2007, 55, 5. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jackson, M.A.; Kobori, N.N.; Behle, R.W.; Delalibera, Í., Jr. Liquid culture fermentation for rapid production of desiccation tolerant blastospores of Beauveria bassiana and Isaria fumosorosea strains. J. Invertebr. Pathol. 2015, 127, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Q.; Chen, Y.; Hao, Y. Production of Mycelia and Polysaccharides by Liquid Fermentation of Cordyceps sinensis. Food Science. 2009, 30, 171–174. [Google Scholar]
- Lee, W.H.; Kim, S.Y.; Park, Y.J.; Kim, T.W. Favorable Conditions for Mycelial Growth of Phellinus linteus. Korean J. Mycol. 2004, 32, 95–100. [Google Scholar]
- Lin, J.Y.; Wu, T.Z.; Chou, J.C. In vitro induction of fruiting body in Antrodia cinnamomea—A medicinally important fungus. Bot. Stud. 2006, 47, 267–272. [Google Scholar]
- Yang, F.C. Basic Principles of Liquid Culture of Medicinal Mushrooms and Cultivation Strategies to Enhance the Production of Active Ingredients. Agric. Biotechnol. Ind. Q. 2017, 49, 46–52. [Google Scholar]
- Liang, C.H.; Ho, K.J.; Huang, L.Y.; Tsai, C.H.; Lin, S.Y.; Mau, J.L. Antioxidant properties of fruiting bodies, mycelia, and fermented products of the culinary-medicinal king oyster mushroom, Pleurotus eryngii (higher Basidiomycetes), with high ergothioneine content. Int. J. Med. Mushrooms 2013, 15, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G.; Bordewick, S.; Krahe, N.K.; Ersoy, F. Mycelium vs. Fruiting Bodies of Edible Fungi-A Comparison of Metabolites. Microorganisms 2022, 10, 1379. [Google Scholar] [CrossRef]
- Chen, H.J.; Chen, Y.S.; Liu, S.L.; Liou, B.K.; Chen, C.S. The Influence of Submerged Fermentation of Inonotus obliquus with Control Atmosphere Treatment on Enhancing Bioactive Ingredient Contents. Appl. Biochem. Biotechnol. 2020, 191, 412–425. [Google Scholar] [CrossRef]
- Chen, H.J.; Chen, Y.S.; Liu, S.L.; Liou, B.K.; Chen, C.S. The Increase of Bioactive Ingredients by Solid State Fermentation of Inonotus obliquus with Spent Substrate. Waste Biomass Valorization 2020, 11, 6725–6739. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, M.; Zhao, Y.; Miao, K.; Pan, S.; Cao, F.; Dai, Y. Analysis of antioxidant metabolites by solvent extraction from sclerotia of Inonotus obliquus (Chaga). Phytochem. Anal. 2011, 22, 95–102. [Google Scholar] [CrossRef]
- Zhao, G.; Yan, W.; Cao, D. Simultaneous determination of betulin and betulinic acid in white birch bark using RP-HPLC. J. Pharm. Biomed. Anal. 2007, 43, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.J.; Chen, C. Enzymatic modification by tannase increases the antioxidant activity of green tea. Food Res. Int. 2008, 41, 130–137. [Google Scholar] [CrossRef]
- Chen, Y.S.; Liu, B.L.; Chang, Y.N. Bioactivities and sensory evaluation of Pu-erh teas made from three tea leaves in an improved pile fermentation process. J. Biosci. Bioeng. 2010, 109, 557–563. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, M.; Zhao, Y.; Miao, K.; Jiang, H. NMR-based metabonomic analysis on effect of light on production of antioxidant phenolic compounds in submerged cultures of Inonotus obliquus. Bioresour. Technol. 2009, 100, 4481–4487. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, J. Enhanced phenolic antioxidants production in submerged cultures of Inonotus obliquus in a ground corn stover medium. Biochem. Eng. J. 2011, 58–59, 103–109. [Google Scholar] [CrossRef]
- Li, Y.; Meng, S.; Shi, M.; Hu, X.; Yang, Y.; Zhang, Z. Bioactivity Evaluation of Crude Polysaccharide from Rice Bran Fermented by Preussia aemulans and the Changes in its Nutritional Contents. J. Food Biochem. 2016, 40, 664–672. [Google Scholar] [CrossRef]
- Kim, Y.O.; Han, S.B.; Lee, H.W.; Ahn, H.J.; Yoon, Y.D.; Jung, J.K.; Kim, H.M.; Shin, C.S. Immuno-stimulating effect of the endo-polysaccharide produced by submerged culture of Inonotus obliquus. Life Sci. 2005, 77, 2438–2456. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Chen, H. Comparative antioxidative characteristics of polysaccharide-enriched extracts from natural sclerotia and cultured mycelia in submerged fermentation of Inonotus obliquus. Food Chem. 2011, 127, 74–79. [Google Scholar] [CrossRef]
- Zhao, F.; Mai, Q.; Ma, J.; Xu, M.; Wang, X.; Cui, T.; Qiu, F.; Han, G. Triterpenoids from Inonotus obliquus and their antitumor activities. Fitoterapia 2015, 101, 34–40. [Google Scholar] [CrossRef]
- Zhang, D.M.; Xu, H.G.; Wang, L.; Li, Y.J.; Sun, P.H.; Wu, X.M.; Wang, G.J.; Chen, W.M.; Ye, W.C. Betulinic Acid and its Derivatives as Potential Antitumor Agents. Med. Res. Rev. 2015, 35, 1127–1155. [Google Scholar] [CrossRef]
- Bai, Y.H.; Feng, Y.Q.; Mao, D.B.; Xu, C.P. Optimization for betulin production from mycelial culture of Inonotus obliquus by orthogonal design and evaluation of its antioxidant activity. J. Taiwan Inst. Chem. Eng. 2012, 43, 663–669. [Google Scholar] [CrossRef]
- Cui, Y.; Kim, D.S.; Park, K.C. Antioxidant effect of Inonotus obliquus. J. Ethnopharmacol. 2005, 96, 79–85. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, X.; Li, J. Chemical properties and antioxidant activity of exopolysaccharides fractions from mycelial culture of Inonotus obliquus in a ground corn stover medium. Food Chem. 2012, 134, 1899–1905. [Google Scholar] [CrossRef]
- Xu, X.; Quan, L.; Shen, M. Effect of chemicals on production, composition and antioxidant activity of polysaccharides of Inonotus obliquus. Int. J. Biol. Macromol. 2015, 77, 143–150. [Google Scholar] [CrossRef]
- Liou, B.K.; Chuang, G.C.C. Principles and Practices of Sensory Evaluation of Food; New Wun Ching Developmental Publishing Co., Ltd.: New Taipei City, Taiwan, 2020. [Google Scholar]
| Source | DF | p-Value | ||
|---|---|---|---|---|
| Polysaccharide | Betulinic Acid | DPPH | ||
| FS a | 1 | 0.04 | 0.05 | 0.03 |
| AS | 1 | <0.01 | 0.02 | <0.01 |
| FT | 2 | <0.01 | <0.01 | <0.01 |
| FS × AS | 1 | 0.04 | 0.03 | 0.01 |
| AS × FT | 2 | 0.02 | 0.08 | 0.07 |
| FS × FT | 2 | 0.47 | 0.02 | 0.07 |
| FS × AS × FT | 2 | 0.05 | 0.10 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-J.; Chen, Y.-S.; Lin, K.-M.; Tsai, S.-W.; Liao, M.-J.; Yeh, C.-S.; Liu, S.-L. Comparison of the Controlled Atmosphere Treatment for Submerged and Solid-State Fermentation of Inonotus obliquus. Foods 2024, 13, 2275. https://doi.org/10.3390/foods13142275
Chen H-J, Chen Y-S, Lin K-M, Tsai S-W, Liao M-J, Yeh C-S, Liu S-L. Comparison of the Controlled Atmosphere Treatment for Submerged and Solid-State Fermentation of Inonotus obliquus. Foods. 2024; 13(14):2275. https://doi.org/10.3390/foods13142275
Chicago/Turabian StyleChen, Hsin-Jung, Yuh-Shuen Chen, Kuo-Min Lin, Shuo-Wen Tsai, Mei-Jine Liao, Chia-Sheng Yeh, and Shih-Lun Liu. 2024. "Comparison of the Controlled Atmosphere Treatment for Submerged and Solid-State Fermentation of Inonotus obliquus" Foods 13, no. 14: 2275. https://doi.org/10.3390/foods13142275






