Catalytic Mode and Product Specificity of an α-Agarase Reveal Its Direct Catalysis for the Production of Agarooligosaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gene Construction, Protein Production, and Purification
2.2. Activity Assay
2.3. SDS-PAGE Analysis
2.4. Hydrolysis Reaction of Polysaccharides
2.5. Hydrolysis Reaction of AGO
2.6. Isolation and Purification of Cm-AGA-Produced AOS
2.7. Product Analysis
2.8. Molecular Mass Determination
2.9. Multiple Amino Acid Sequence Alignments
2.10. Protein Structural Prediction and Molecular Docking
2.11. Construction of Mutants and Production of Cm-AGA Mutant Proteins
2.12. Statistical Analysis
3. Results
3.1. Expression and Purification of Cm-AGA
3.2. Product Analysis Using Agarose as the Substrate
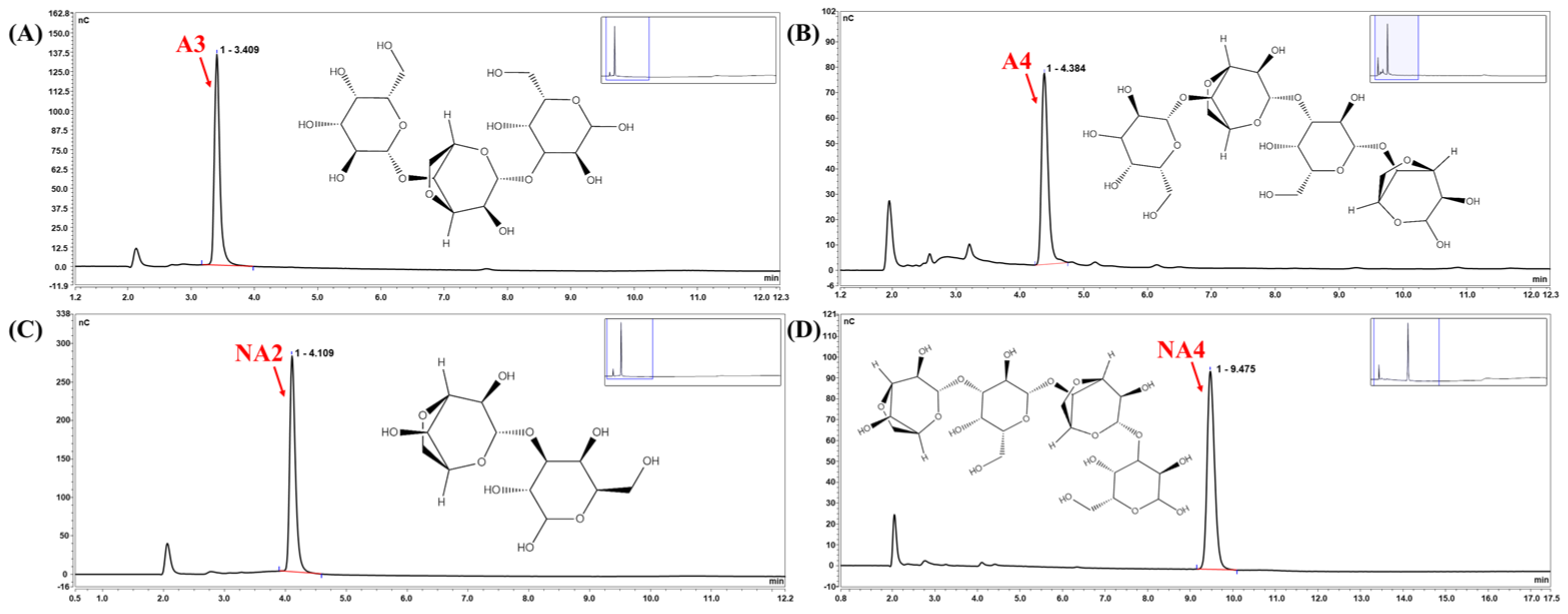
3.3. Identification of the Cm-AGA Hydrolysis Pattern
3.4. Multiple Amino Acid Sequence Alignments and Site-Directed Mutation Verification
3.5. Molecular Docking
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marinho-Soriano, E. Agar polysaccharides from Gracilaria species (Rhodophyta, Gracilariaceae). J. Biotechnol. 2001, 89, 81–84. [Google Scholar] [CrossRef]
- Li, J.; Han, F.; Lu, X.; Fu, X.; Ma, C.; Chu, Y.; Yu, W. A simple method of preparing diverse neoagaro-oligosaccharides with beta-agarase. Carbohydr. Res. 2007, 342, 1030–1033. [Google Scholar] [CrossRef]
- Chi, W.J.; Chang, Y.K.; Hong, S.K. Agar degradation by microorganisms and agar-degrading enzymes. Appl. Microbiol. Biotechnol. 2012, 94, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Qiao, X.; Jiang, T.; Fu, X.; He, Y.; Zhao, X. Agarose oligosaccharide- silver nanoparticle- antimicrobial peptide- composite for wound dressing. Carbohydr. Polym. 2021, 269, 118258. [Google Scholar] [CrossRef]
- Yu, S.; Yun, E.J.; Kim, D.H.; Park, S.Y.; Kim, K.H. Anticariogenic activity of agarobiose and agarooligosaccharides derived from red macroalgae. J. Agric. Food Chem. 2019, 67, 7297–7303. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Gong, Q.; Wang, Y.; Ma, Y.; Li, J.; Yu, W. Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe 2006, 12, 260–266. [Google Scholar] [CrossRef]
- Kang, O.L.; Ghani, M.; Hassan, O.; Rahmati, S.; Ramli, N. Novel agaro-oligosaccharide production through enzymatic hydrolysis: Physicochemical properties and antioxidant activities. Food Hydrocoll. 2014, 42, 304–308. [Google Scholar] [CrossRef]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Mizushima, K.; Hirai, Y.; Harusato, A.; Ohnogi, H.; Yamaji, R.; Inui, H.; Nakano, Y.; et al. Oligosaccharides from agar inhibit murine intestinal inflammation through the induction of heme oxygenase-1 expression. J. Gastroenterol. 2013, 48, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Na, C.S.; Cho, S.S.; Kim, K.M.; Lee, J.H.; Chen, X.Q.; Ku, S.K.; Cho, I.J.; Kim, E.J.; Lee, J.H.; et al. Hepatoprotective effect of neoagarooligosaccharide via activation of Nrf2 and enhanced antioxidant efficacy. Biol. Pharm. Bull. 2020, 43, 619–628. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, E.J.; Yu, S.; Kim, K.H.; Kang, N.J. Different levels of skin whitening activity among 3,6-anhydro-l-galactose, agarooligosaccharides, and neoagarooligosaccharides. Mar. Drugs 2017, 15, 321. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, W.; Hao, C.; Mao, X. Agaropentaose protects SH-SY5Y cells against 6-hydroxydopamine-induced neurotoxicity through modulating NF-κB and p38MAPK signaling pathways. J. Funct. Foods 2019, 57, 222–232. [Google Scholar] [CrossRef]
- Xu, X.-Q.; Su, B.-M.; Xie, J.-S.; Li, R.-K.; Yang, J.; Lin, J.; Ye, X.-Y. Preparation of bioactive neoagaroligosaccharides through hydrolysis of Gracilaria lemaneiformis agar: A comparative study. Food Chem. 2018, 240, 330–337. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Yu, P.; Liang, Q.-Y.; Mu, D.-S.; Du, Z.-J. Inducible expression of agar-degrading genes in a marine bacterium Catenovulum maritimus Q1T and characterization of a β-agarase. Appl. Microbiol. Biotechnol. 2020, 104, 10541–10553. [Google Scholar] [CrossRef]
- Michel, G.; Nyval-Collen, P.; Barbeyron, T.; Czjzek, M.; Helbert, W. Bioconversion of red seaweed galactans: A focus on bacterial agarases and carrageenases. Appl. Microbiol. Biotechnol. 2006, 71, 23–33. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, B.; Jiang, Q.R.; Ke, C.H. Changes in gut-associated flora and bacterial digestive enzymes during the development stages of abalone (Haliotis diversicolor). Aquaculture 2012, 338–341, 147–153. [Google Scholar] [CrossRef]
- Fu, X.T.; Kim, S.M. Agarase: Review of major sources, categories, purification method, enzyme characteristics and applications. Mar. Drugs 2012, 8, 200–218. [Google Scholar] [CrossRef]
- Zhang, C.; Kim, S.K. Research and application of marine microbial enzymes: Status and prospects. Mar. Drugs 2010, 8, 1920–1934. [Google Scholar] [CrossRef]
- Potin, P.; Richard, C.; Rochas, C.; Kloareg, B. Purification and characterization of the alpha-agarase from Alteromonas agarlyticus (Cataldi) comb. nov., strain GJ1B. Eur. J. Biochem. 1993, 214, 599–607. [Google Scholar] [CrossRef]
- Flament, D.; Barbeyron, T.; Jam, M.; Potin, P.; Czjzek, M.; Kloareg, B.; Michel, G. Alpha-agarases define a new family of glycoside hydrolases, distinct from beta-agarase families. Appl. Environ. Microbiol. 2007, 73, 4691–4694. [Google Scholar] [CrossRef]
- You, Y.; Xie, W.; Li, C.; Gu, Z.; Ban, X.; Zhang, F.; Li, Z. Characterization and efficient production of an α-agarase from marine bacterium Catenovulum maritimum STB14. Food Bioeng. 2022, 2, 12037. [Google Scholar] [CrossRef]
- Seok, J.H.; Kim, H.S.; Hatada, Y.; Nam, S.W.; Kim, Y.H. Construction of an expression system for the secretory production of recombinant α-agarase in yeast. Biotechnol. Lett. 2012, 34, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Xie, W.; You, Y.; Ban, X.; Zhang, A.; Li, C.; Gu, Z.; Li, Z. Structural basis for the cold activation and adaptation of an α-agarase from marine bacterium Catenovulum agarivorans STB13. Food Biosci. 2023, 53, 102630. [Google Scholar] [CrossRef]
- Chan, H.L.; Hee, T.K.; Eun, J.Y.; Ah, R.L.; Sa, R.K.; Kim, J.-H.; Choi, I.-G.; Kyoung, H.K. A novel agarolytic β-galactosidase acts on agarooligosaccharides for complete hydrolysis of agarose into monomers. Appl. Environ. Microbiol. 2014, 80, 5965–5973. [Google Scholar]
- Vera, C.; Guerrero, C.; Conejeros, R.; Illanes, A. Synthesis of galacto-oligosaccharides by β-galactosidase from Aspergillus oryzae using partially dissolved and supersaturated solution of lactose. Enzym. Microb. Technol. 2012, 50, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Tiangpook, S.; Nhim, S.; Prangthip, P.; Pason, P.; Tachaapaikoon, C.; Ratanakhanokchai, K.; Waeonukul, R. Production of a sseries of long-chain isomaltooligosaccharides from maltose by Bacillus subtilis AP-1 and Associated Prebiotic Properties. Foods 2023, 12, 1499. [Google Scholar] [CrossRef] [PubMed]
- Borromei, C.; Cavazza, A.; Merusi, C.; Corradini, C. Characterization and Quantitation of Short-Chain Fructooligosaccharides and Inulooligosaccharides in Fermented Milks by High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Odjo, S.; Béra, F.; Jacquet, N.; Richel, A.; Malumba, P. Characterization of saccharides released during an in vitro pepsin-pancreatin digestion of corn flour using HPAEC-PAD. Starch Strke 2016, 68, 691–699. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Gu, Z.; Ban, X.; Hong, Y.; Cheng, L.; Li, C. Cyclodextrin glycosyltransferase-catalyzed products from starch enhance the stability of microencapsulated cinnamaldehyde emulsion. Food Hydrocoll. 2023, 144, 108991. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Su, X.; Xu, J.; Chen, H.; Chen, J.; Yan, X. Analysis of agaro-oligosaccharide by liquid chromatography coupled with electrospray ionization-quadrupole-time of flight-mass spectrometry. Anal. Chem. 2011, 39, 7. [Google Scholar]
- Li, W.; Wang, Y.; Han, J.; Zhang, J.; Li, B.; Qi, X.; Zhang, Y.; Hu, F.; Liu, H. UPLC-Q-TOF-MS and UPLC-QQQ-MS were used for the qualitative and quantitative analysis of oligosaccharides in Fufang Ejiao Syrup. J. Pharm. Biomed. Anal. 2023, 224, 115193. [Google Scholar] [CrossRef]
- Ali, A.H.; Zou, X.; Lu, J.; Abed, S.M.; Yao, Y.; Tao, G.; Jin, Q.; Wang, X. Identification of phospholipids classes and molecular species in different types of egg yolk by using UPLC-Q-TOF-MS. Food Chem. 2017, 221, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ladunga, I. Finding similar nucleotide sequences using network BLAST searches. Curr. Protoc. Bioinform. 2017, 58, 3.3.1–3.3.25. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Hatada, Y.; Miyazaki, M.; Nogi, Y.; Ito, S.; Horikoshi, K. Purification and characterization of a novel alpha-agarase from a Thalassomonas sp. Curr. Microbiol. 2005, 50, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, J.; Liu, D.; Liu, H.; Lu, X.; Yu, W. Characterization of an α-agarase from Thalassomonas sp. LD5 and its hydrolysate. Appl. Microbiol. Biotechnol. 2018, 102, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cui, Z.; Zhang, W.; Lu, J.; Lu, X.; Yu, W. Characterizing of a new α-agarase AgaE from Thalassomonas sp. LD5 and probing its catalytically essential residues. Int. J. Biol. Macromol. 2022, 194, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Jiang, C.; Mao, X. Biochemical characterization and substrate degradation mode of a novel α-agarase from Catenovulum agarivorans. J. Agric. Food Chem. 2019, 67, 10373–10379. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, C.R.; Hong, S.K. Biochemical characterization of a novel cold-adapted agarotetraose-producing α-agarase, AgaWS5, from Catenovulum sediminis WS1-A. Appl. Microbiol. Biotechnol. 2019, 103, 8403–8411. [Google Scholar] [CrossRef] [PubMed]
- Pathiraja, D.; Christiansen, L.; Park, B.; Schultz-Johansen, M.; Bang, G.; Stougaard, P.; Choi, I.-G. A novel auxiliary agarolytic pathway expands metabolic versatility in the agar-degrading marine bacterium Colwellia echini A3T. Appl. Environ. Microbiol. 2021, 87, e00230-21. [Google Scholar] [CrossRef]
- Sakamoto, K.; Asano, S.; Ago, Y.; Hirokawa, T. AlphaFold version 2.0 elucidates the binding mechanism between VIPR2 and KS-133, and reveals an S-S bond (Cys(25)-Cys(192)) formation of functional significance for VIPR2. Biochem. Biophys. Res. Commun. 2022, 636 Pt 1, 10–16. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput.-Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.T.; Chen, C.; Chen, R.X.; Li, R.; Chen, W.L.; Jiang, G.H.; Du, L.L. Michael acceptor molecules in natural products and their mechanism of action. Front. Pharmacol. 2022, 13, 1033003. [Google Scholar] [CrossRef]
- Gani, M.A.; Nurhan, A.D.; Budiatin, A.S.; Siswodihardjo, S.; Khotib, J. Predicting the molecular mechanism of glucosamine in accelerating bone defect repair by stimulating osteogenic proteins. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, 4792. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Z.; Jiang, C.; Sun, J.; Xue, C.; Mao, X. A novel agaro-oligosaccharide-lytic β-galactosidase from Agarivorans gilvus WH0801. Appl. Microbiol. Biotechnol. 2018, 102, 5165–5172. [Google Scholar] [CrossRef] [PubMed]
- Henshaw, J.; Horne-Bitschy, A.; van Bueren, A.L.; Money, V.A.; Bolam, D.N.; Czjzek, M.; Ekborg, N.A.; Weiner, R.M.; Hutcheson, S.W.; Davies, G.J.; et al. Family 6 carbohydrate binding modules in beta-agarases display exquisite selectivity for the non-reducing termini of agarose chains. J. Biol. Chem. 2006, 281, 17099–17107. [Google Scholar] [CrossRef] [PubMed]
- Wangpaiboon, K.; Laohawuttichai, P.; Kim, S.Y.; Mori, T.; Nakapong, S.; Pichyangkura, R.; Pongsawasdi, P.; Hakoshima, T.; Krusong, K. A GH13 α-glucosidase from Weissella cibaria uncommonly acts on short-chain maltooligosaccharides. Acta Crystallogr. Sect. D Struct. Biol. 2021, 77 Pt 8, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Lu, X.; Shi, C.; Li, J.; Gu, Y.; Ma, Y.; Chu, Y.; Han, F.; Gong, Q.; Yu, W. Molecular cloning and characterization of a novel beta-agarase, AgaB, from marine Pseudoalteromonas sp. CY24. J. Biol. Chem. 2007, 282, 3747–3754. [Google Scholar] [CrossRef]
- Gloster, T.M.; Turkenburg, J.P.; Potts, J.R.; Henrissat, B.; Davies, G.J. Divergence of catalytic mechanism within a glycosidase family provides insight into evolution of carbohydrate metabolism by human gut flora. Chem. Biol. 2008, 15, 1058–1067. [Google Scholar] [CrossRef]
- Allouch, J.; Jam, M.; Helbert, W.; Barbeyron, T.; Kloareg, B.; Henrissat, B.; Czjzek, M. The three-dimensional structures of two beta-agarases. J. Biol. Chem. 2003, 278, 47171–47180. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, C.; Tiwari, M.K.; Webb, H.; Jam, M.; Czjzek, M.; Svensson, B. Asp271 is critical for substrate interaction with the surface binding site in β-agarase a from Zobellia galactanivorans. Proteins 2019, 87, 34–40. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, L.P.; Hand, G.; Johnson, P.E.; Joshi, M.D.; Körner, M.; Plesniak, L.A.; Ziser, L.; Wakarchuk, W.W.; Withers, S.G. The pKa of the general acid/base carboxyl group of a glycosidase cycles during catalysis: A 13C-NMR study of Bacillus circulans xylanase. Biochemistry 1996, 35, 9958–9966. [Google Scholar] [CrossRef]












| Mutant | Primer Sequence (5′-3′) 1 |
|---|---|
| CM_D994A_F1 | ATAAACTGATGTTTGCCACGCAGACAAATAGCACGAGAAG |
| CM_D994A_F2 | GCGCGTAATCTGCTGCTTG |
| CM_D994A_F3 | CCCACGCCGAAACAAGCG |
| CM_D994A_R3 | CAAACATCAGTTTATCCGGGTTCGC |
| CM_D994E_F1 | ATAAACTGATGTTTGAAACGCAGACAAATAGCACGAGAAG |
| CM_D994E_F2 | GCGCGTAATCTGCTGCTTG |
| CM_D994E_F3 | CCCACGCCGAAACAAGCG |
| CM_D994E_R3 | CAAACATCAGTTTATCCGGGTTCGC |
| CM_D994K_F1 | GATAAACTGATGTTTAAAACGCAGACAAATAGCACGAGAAG |
| CM_D994K_F2 | GCGCGTAATCTGCTGCTTG |
| CM_D994K_F3 | CCCACGCCGAAACAAGCG |
| CM_D994K_R3 | AAACATCAGTTTATCCGGGTTCGCC |
| CM_D994R_F1 | GATAAACTGATGTTTCGCACGCAGACAAATAGCACGAGAAG |
| CM_D994R_F2 | GCGCGTAATCTGCTGCTTG |
| CM_D994R_F3 | CCCACGCCGAAACAAGCG |
| CM_D994R_R3 | AAACATCAGTTTATCCGGGTTCGCC |
| CM_E1129A_F1 | CATTCCTGAGCGGCGCACTGAATCTGGGCGCAAGAACATC |
| CM_E1129A_F2 | GCGCGTAATCTGCTGCTTG |
| CM_E1129A_F3 | CCCACGCCGAAACAAGCG |
| CM_E1129A_R3 | CGCCGCTCAGGAATGTAATGTTC |
| CM_E1129D_F1 | CATTCCTGAGCGGCGACCTGAATCTGGGCGCAAGAACATC |
| CM_E1129D_F2 | GCGCGTAATCTGCTGCTTG |
| CM_E1129D_F3 | CCCACGCCGAAACAAGCG |
| CM_E1129D_R3 | CGCCGCTCAGGAATGTAATGTTC |
| CM_E1129K_F1 | ACATTCCTGAGCGGCAAGCTGAATCTGGGCGCAAG |
| CM_E1129K_F2 | GCGCGTAATCTGCTGCTTG |
| CM_E1129K_F3 | CCCACGCCGAAACAAGCG |
| CM_E1129K_R3 | GCCGCTCAGGAATGTAATGTTCTC |
| CM_E1129R_F1 | ACATTCCTGAGCGGCCGGCTGAATCTGGGCGCAAG |
| CM_E1129R_F2 | GCGCGTAATCTGCTGCTTG |
| CM_E1129R_F3 | CCCACGCCGAAACAAGCG |
| CM_E1129R_R3 | GCCGCTCAGGAATGTAATGTTCTC |
| CM_R1 2 | CAAGCAGCAGATTACGCGC |
| CM_R2 3 | CGCTTGTTTCGGCGTGGG |
| Oligosaccharide Types | Molecular Weight (Da) |
|---|---|
| 3,6-anhydro-L-galactose (L-AHG) | 162.14 |
| D-Galactose (D-Gal) | 180.16 |
| Agarobiose (A2), Neoagarobiose (NA2) | 324.28 |
| Agarotriose (A3), Neoagarotriose (NA3) | 486.42 |
| Agarotetraose (A4), Neoagarotetraose (NA4) | 630.00 |
| Agaropentose (A5), Neoagaropentose (NA5) | 792.69 |
| Agarohexaose (A6), Neoagarohexaose (NA6) | 936.00 |
| Agaroheptose (A7), Neoagaroheptose (NA7) | 1098.95 |
| Agarooctaose (A8), Neoagarooctaose (NA8) | 1281.00 |
| Agaronanose (A9), Neoagaronanose (NA9) | 1405.22 |
| Ion Mode | Ions | Degree of Polymerization for Oligosaccharides | |||
|---|---|---|---|---|---|
| 3 | 5 | 7 | 9 | ||
| ESI+ | [M + H]+ | 487.2 | 793.3 | 1099.4 | 1405.4 |
| ESI− | [M − H]− | 485.1 * | 791.3 * | 1097.4 * | 1403.6 * |
| Protein | Bacterial Species | GH Family | Substrates Used in the Study | Main Products | Remarks |
|---|---|---|---|---|---|
| AgaA | Alteromonas agarlyticus Strain GJlB | GH96 | Agarose | A4, A6 | Does not act on A4 and A6, possess agarase and β-galactosidase activities |
| Aga33 | Thalassomonas sp. Strain JAMB-A33 | GH96 | Agarose, A4, A6, NA4, NA6 | A4 | Acts on A6 and NA6, but cannot hydrolyze tetrasaccharides |
| AgaD | Thalassomonas sp. LD5 | GH96 | Agarose | A4 | Under alkaline conditions, A4 degrade into A3 with a G-A-G arrangement |
| A4, A6, A8, A10 | Does not act on A2 and A4, and the minimum-length substrate is A6 | ||||
| CaLJ96 | Catenovulum agarivorans | GH96 | Agarose, A3, A5 | A4 | Does not act on A2, A3, or A4, and the minimum-length substrate is A5 |
| AgaWS5 | Catenovulum sediminis WS1-A | GH96 | Agarose | A4 | Under alkaline circumstances, L-AHG residues at the reducing end of A4 (G-A-G-A) were cleaved to form A3 (G-A-G) |
| AgaE | Thalassomonas sp. LD5 | GH96 | Agarose | A4, A6 | None |
| Ce2834, Ce2835 | Colwellia echini A3T | GH96 | Agarose, NA4, NA6 | A4, A6 | None |
| Cm-AGA | Catenovulum maritimum STB14 | GH96 | Agarose | A2, A4 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Tian, Y.; Kong, H.; Li, Z.; Gu, Z.; Li, C.; Hong, Y.; Cheng, L.; Ban, X. Catalytic Mode and Product Specificity of an α-Agarase Reveal Its Direct Catalysis for the Production of Agarooligosaccharides. Foods 2024, 13, 2351. https://doi.org/10.3390/foods13152351
Zeng X, Tian Y, Kong H, Li Z, Gu Z, Li C, Hong Y, Cheng L, Ban X. Catalytic Mode and Product Specificity of an α-Agarase Reveal Its Direct Catalysis for the Production of Agarooligosaccharides. Foods. 2024; 13(15):2351. https://doi.org/10.3390/foods13152351
Chicago/Turabian StyleZeng, Xiaofeng, Yixiong Tian, Haocun Kong, Zhaofeng Li, Zhengbiao Gu, Caiming Li, Yan Hong, Li Cheng, and Xiaofeng Ban. 2024. "Catalytic Mode and Product Specificity of an α-Agarase Reveal Its Direct Catalysis for the Production of Agarooligosaccharides" Foods 13, no. 15: 2351. https://doi.org/10.3390/foods13152351





