Multi-Elemental Analysis of Edible Insects, Scorpions, and Tarantulas from French (Online) Market and Human Health Risk Assessment Due to Their Consumption: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Chemicals and Reagents
2.3. Reference Materials and Samples
2.4. Analytical Procedures
2.4.1. Sample Preparation
2.4.2. Multi-Elemental Analysis by ICP-MS/MS
2.4.3. Data Processing
3. Results and Discussion
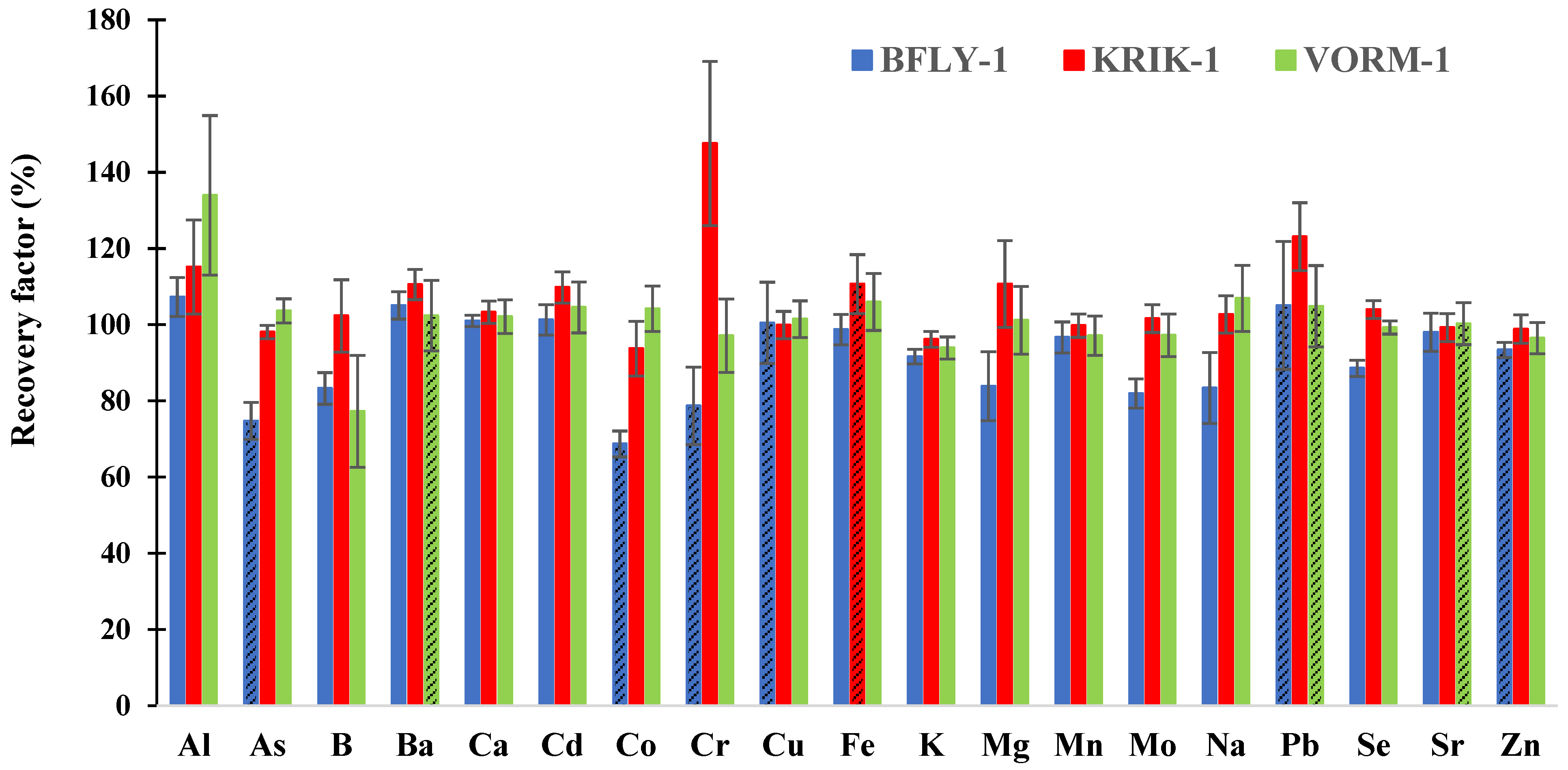
3.1. Method Validation
3.2. Analysis of Commercially Available Edible Insects
| Sample | As | Cd | Pb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Measured | Maximum Recommended Consumption (Number per Day) | Measured | Maximum Recommended Consumption (Number per Day) | Measured | Maximum Recommended Consumption (Number per Day) | ||||
| Adults | Children | Adults | Children | Adults | Children | ||||
| Ants | |||||||||
| 1 | 43.86 ± 0.66 | 401 | 120 | 85.4 ± 1.7 | 80 | 24 | 106.8 ± 5.4 | 280 | 84 |
| 2 | 44.13 ± 0.77 | 429 | 129 | 79.60 ± 0.15 | 93 | 28 | 104.1 ± 1.2 | 309 | 93 |
| Crickets | |||||||||
| 1 | 7.70 ± 0.40 | 145,196 | 43,559 | 36.89 ± 0.39 | 11,834 | 3550 | 18.296 ± 0.018 | 103,882 | 31,165 |
| 2 | 6.97 ± 0.18 | 178,103 | 53,431 | 38.95 ± 0.72 | 12,445 | 3733 | 15.18 ± 0.51 | 139,021 | 41,706 |
| 3 | 6.449 ± 0.084 | 182,939 | 54,882 | 29.35 ± 0.92 | 15,696 | 4709 | 16.7 ± 2.9 | 120,097 | 36,029 |
| 4 | 12.42 ± 0.65 | 41,399 | 12,420 | 13.75 ± 0.11 | 14,602 | 4381 | 36.62 ± 0.81 | 23,870 | 7161 |
| 5 | 3.6 ± 2.7 | 271,389 | 81,417 | 73 ± 10 | 5182 | 1555 | 53.6 ± 2.4 | 30,729 | 9219 |
| 6 | 2.622 ± 0.086 | 423,019 | 126,906 | 65.90 ± 0.49 | 6572 | 1972 | 33.4 ± 3.8 | 56,454 | 16,936 |
| 7 | 19.57 ± 0.73 | 13,757 | 4127 | 76.07 ± 0.92 | 1382 | 415 | 96.9 ± 3.0 | 4723 | 1417 |
| 8 | 21.07 ± 0.67 | 12,726 | 3818 | 76.6 ± 3.8 | 1367 | 410 | 62.0 ± 5.6 | 7352 | 2206 |
| Locusts | |||||||||
| 1 | 35.0 ± 1.0 | 8137 | 2441 | 31.78 ± 0.34 | 3499 | 1050 | 50.194 ± 0.060 | 9645 | 2894 |
| 2 | 47.44 ± 0.12 | 4912 | 1474 | 40.9 ± 2.3 | 2225 | 667 | 73.4 ± 1.9 | 5397 | 1619 |
| Scorpions | |||||||||
| 1 | 725 ± 36 | 62 | 19 | 539 ± 22 | 32 | 10 | 235 ± 14 | 324 | 97 |
| 2 | 383.5 ± 2.4 | 200 | 60 | 2272 ± 19 | 13 | 4 | 123.6 ± 3.1 | 1053 | 316 |
| 3 | 233.1 ± 3.3 | 226 | 68 | 1001 ± 38 | 21 | 6 | 162 ± 17 | 554 | 166 |
| 4 | 192.20 ± 0.23 | 127 | 38 | 771.406 ± 0.046 | 12 | 4 | 64.6 ± 9.7 | 643 | 193 |
| Tarantulas | |||||||||
| 1 | 93.78 ± 0.51 | 65 | 19 | 13,200 ± 97 | 2 | 1 | 633.3 ± 6.3 | 163 | 49 |
| 2 | 191.8 ± 4.2 | 326 | 98 | 9650 ± 190 | 3 | 1 | 464.79 ± 0.89 | 229 | 69 |
| 3 | 120.8 ± 1.3 | 476 | 143 | 8582 ± 48 | 3 | 1 | 1064 ± 27 | 92 | 28 |
| Water bug | |||||||||
| 1 | 59.31 ± 0.64 | 681 | 204 | 3.46 ± 0.15 | 4556 | 1367 | 60.96 ± 0.98 | 1126 | 338 |
| 2 | 86.4 ± 2.6 | 416 | 125 | 6.98 ± 0.14 | 2009 | 603 | 104.9 ± 1.4 | 582 | 175 |
| Worms | |||||||||
| 1 | 39.73 ± 0.76 | 75,962 | 22,789 | 82.6 ± 2.1 | 14,267 | 4280 | 10.8 ± 1.7 | 475,050 | 142,515 |
| 2 | 470 ± 15 | 1483 | 445 | 53.9 ± 4.2 | 5049 | 1515 | 87.3 ± 5.2 | 13,571 | 4071 |
| 3 | 21.25 ± 0.62 | 20,567 | 6170 | 16.777 ± 0.061 | 10,172 | 3052 | 7.425 ± 0.011 | 100,064 | 30,019 |
| 4 | 25.65 ± 0.79 | 119,520 | 35,856 | 79.25 ± 0.76 | 15,105 | 4532 | 7.5 ± 2.5 | 694,891 | 208,467 |
| 5 | 26.50 ± 0.53 | 138,018 | 41,405 | 83.5 ± 2.9 | 17,104 | 5131 | 6.03 ± 0.28 | 1,031,129 | 309,339 |
| 6 | 25.66 ± 0.12 | 103,376 | 31,013 | 77.7 ± 2.8 | 13,331 | 3999 | 6.32 ± 0.39 | 713,524 | 214,057 |
| Sample | Ca | K | Mg | Na |
|---|---|---|---|---|
| Ants | ||||
| 1 | 539 ± 62 | 681 ± 2 | 187 ± 2 | 1135 ± 5 |
| 2 | 458 ± 87 | 677 ± 5 | 184 ± 3 | 1117 ± 22 |
| Crickets | ||||
| 1 | 1268 ± 13 | 7567 ± 150 | 1215 ± 11 | 2577 ± 9 |
| 2 | 1399 ± 16 | 8308 ± 120 | 1316 ± 34 | 2835 ± 66 |
| 3 | 1397 ± 21 | 8538 ± 79 | 1314 ± 43 | 2874 ± 33 |
| 4 | 1798 ± 12 | 5263 ± 61 | 727 ± 1 | 1771 ± 12 |
| 5 | 1053 ± 66 | 9116 ± 330 | 746 ± 12 | 2424 ± 130 |
| 6 | 1034 ± 53 | 9078 ± 220 | 801 ± 10 | 2448 ± 15 |
| 7 | 496 ± 2 | 7017 ± 26 | 867 ± 13 | 1259 ± 12 |
| 8 | 411 ± 8 | 6559 ± 1 | 777 ± 2 | 1162 ± 6 |
| Locusts | ||||
| 1 | 559 ± 24 | 6510 ± 150 | 749 ± 34 | 937 ± 57 |
| 2 | 481 ± 21 | 6350 ± 460 | 658 ± 4 | 860 ± 19 |
| Scorpions | ||||
| 1 | 1149 ± 52 | 5946 ± 510 | 430 ± 7 | 10,597 ± 1009 |
| 2 | 1792 ± 14 | 5101 ± 37 | 542 ± 7 | 4152 ± 23 |
| 3 | 2102 ± 42 | 6903 ± 4 | 784 ± 28 | 6150 ± 7 |
| 4 | 1163 ± 4 | 4883 ± 53 | 417 ± 4 | 4495 ± 28 |
| Tarantulas | ||||
| 1 | 5372 ± 14 | 1298 ± 10 | 1944 ± 43 | 32,232 ± 320 |
| 2 | 2518 ± 74 | 1794 ± 38 | 1984 ± 12 | 27,079 ± 620 |
| 3 | 2748 ± 26 | 2220 ± 12 | 1765 ± 40 | 40,454 ± 260 |
| Water bug | ||||
| 1 | 632 ± 2 | 3950 ± 4 | 695 ± 5 | 3150 ± 2 |
| 2 | 865 ± 18 | 4041 ± 18 | 614 ± 10 | 5113 ± 49 |
| Worms | ||||
| 1 | 401 ± 10 | 4474 ± 250 | 1894 ± 69 | 521 ± 8 |
| 2 | 1132 ± 1 | 7462 ± 130 | 2565 ± 11 | 126 ± 1 |
| 3 | 788 ± 67 | 6040 ± 29 | 1343 ± 17 | 670 ± 6 |
| 4 | 499 ± 1 | 8335 ± 300 | 3074 ± 6 | 980 ± 10 |
| 5 | 492 ± 6 | 8533 ± 400 | 2896 ± 70 | 982 ± 38 |
| 6 | 501 ± 15 | 8294 ± 50 | 2959 ± 3 | 976 ± 10 |
| Sample | Co | Cr | Cu | Fe | Mn | Mo | Se | Zn |
|---|---|---|---|---|---|---|---|---|
| Ants | ||||||||
| 1 | 0.05241 ± 0.00074 | 0.12074 ± 0.00089 | 19.463 ± 0.042 | 147 ± 3 | 36.39 ± 0.39 | 0.5309 ± 0.0012 | 1.01 ± 0.00 | 113 ± 2 |
| 2 | 0.0519 ± 0.0032 | 0.114 ± 0.011 | 19.34 ± 0.35 | 146 ± 5 | 35.32 ± 0.41 | 0.5188 ± 0.0037 | 1.001 ± 0.012 | 113 ± 3 |
| Crickets | ||||||||
| 1 | 0.0615 ± 0.0053 | 0.0561 ± 0.0096 | 21.50 ± 0.60 | 55.80 ± 0.23 | 55.2 ± 1.6 | 0.5318 ± 0.0026 | 0.616 ± 0.023 | 191 ± 2 |
| 2 | 0.0611 ± 0.0017 | 0.0390 ± 0.0088 | 19.54 ± 0.90 | 50.5 ± 2.5 | 59.3 ± 1.2 | 0.5382 ± 0.0015 | 0.631 ± 0.024 | 197 ± 2 |
| 3 | 0.0557 ± 0.0012 | 0.0406 ± 0.0042 | 20.891 ± 0.048 | 55.2 ± 2.5 | 58.2 ± 1.3 | 0.560 ± 0.015 | 0.6384 ± 0.0027 | 202 ± 8 |
| 4 | 0.03363 ± 0.00092 | 0.0915 ± 0.0031 | 19.98 ± 0.33 | 50.9 ± 1.8 | 63.5 ± 1.3 | 0.6071 ± 0.0051 | 0.3087 ± 0.0035 | 155 ± 2 |
| 5 | 0.0108 ± 0.0013 | 0.02712 ± 0.00031 | 24.2 ± 1.5 | 54.69 ± 0.029 | 30.262 ± 0.052 | 0.723 ± 0.025 | 0.1234 ± 0.0064 | 239 ± 24 |
| 6 | 0.0102 ± 0.0021 | 0.0164 ± 0.0026 | 26.0 ± 2.4 | 49.3 ± 3.3 | 30.1 ± 2.9 | 0.755 ± 0.028 | 0.1311 ± 0.0017 | 256 ± 9 |
| 7 | 0.0835 ± 0.0013 | 0.1380 ± 0.0081 | 53.09 ± 0.42 | 83.58 ± 0.17 | 7.69 ± 0.20 | 1.4969 ± 0.0053 | 0.19229 ± 0.00045 | 150 ± 37 |
| 8 | 0.0880 ± 0.0070 | 0.122 ± 0.017 | 49.31 ± 0.40 | 68.7 ± 1.4 | 4.937 ± 0.048 | 1.531 ± 0.030 | 0.200 ± 0.011 | 135 ± 15 |
| Locusts | ||||||||
| 1 | 0.1218 ± 0.0049 | 0.0836 ± 0.0077 | 60.300 ± 0.030 | 90.33 ± 0.55 | 6.77 ± 0.18 | 0.678 ± 0.018 | 0.3341 ± 0.0071 | 177 ± 25 |
| 2 | 0.1246 ± 0.0047 | 0.2066 ± 0.0093 | 92.7 ± 7.7 | 164 ± 23 | 6.46 ± 0.18 | 0.574 ± 0.021 | 0.2160 ± 0.0068 | 173 ± 17 |
| Scorpions | ||||||||
| 1 | 0.683 ± 0.030 | 0.1658 ± 0.0023 | 81.8 ± 7.4 | 139 ± 11 | 166 ± 12 | 0.206 ± 0.014 | 2.136 ± 0.081 | 381 ± 25 |
| 2 | 1.0358 ± 0.0058 | 0.1609 ± 0.0029 | 130.64 ± 0.60 | 199 ± 4 | 203.0 ± 4.0 | 0.3230 ± 0.0040 | 2.62 ± 0.14 | 624 ± 10 |
| 3 | 0.4908 ± 0.0089 | 0.2082 ± 0.0068 | 128.0 ± 1.1 | 107 ± 1 | 269.1 ± 2.0 | 0.28131 ± 0.00035 | 1.512 ± 0.011 | 609 ± 1 |
| 4 | 0.1637 ± 0.0023 | 0.24 ± 0.16 | 102.87 ± 0.40 | 62.07 ± 0.73 | 68.9 ± 2.7 | 0.14280 ± 0.00099 | 1.083 ± 0.012 | 552 ± 2 |
| Tarantulas | ||||||||
| 1 | 0.4964 ± 0.0021 | 0.1479 ± 0.0029 | 112.33 ± 0.28 | 157 ± 1 | 735.9 ± 7.6 | 0.07640 ± 0.00025 | 1.079 ± 0.014 | 1011 ± 10 |
| 2 | 0.2461 ± 0.0042 | 0.3393 ± 0.0016 | 121.0 ± 2.8 | 218 ± 1 | 351.3 ± 1.1 | 0.1178 ± 0.0020 | 1.179 ± 0.020 | 821 ± 2 |
| 3 | 0.5862 ± 0.0049 | 0.2992 ± 0.0031 | 80.3 ± 1.2 | 224.2 ± 0.4 | 275.6 ± 4.9 | 0.1194 ± 0.0076 | 2.013 ± 0.028 | 734 ± 8 |
| Water bug | ||||||||
| 1 | 0.10819 ± 0.00033 | 0.179 ± 0.031 | 12.69 ± 0.36 | 129 ± 1 | 10.03 ± 0.14 | 0.15052 ± 0.00042 | 0.469 ± 0.010 | 147.2 ± 0.4 |
| 2 | 0.2217 ± 0.0054 | 0.211 ± 0.025 | 11.199 ± 0.080 | 474 ± 340 | 14.674 ± 0.053 | 0.2358 ± 0.0086 | 0.5047 ± 0.0076 | 119 ± 1 |
| Worms | ||||||||
| 1 | 0.04598 ± 0.00043 | 0.0231 ± 0.0045 | 16.6 ± 1.3 | 46.6 ± 5.0 | 10.72 ± 0.12 | 0.960 ± 0.010 | 0.819 ± 0.012 | 106 ± 6 |
| 2 | 0.001978 ± 0.000015 | 0.0203 ± 0.0019 | 9.12 ± 0.11 | 32.85 ± 0.97 | 19.390 ± 0.055 | 0.3557 ± 0.0035 | 0.6344 ± 0.0096 | 175 ± 4 |
| 3 | 0.048699 ± 0.000032 | 0.0504 ± 0.0074 | 9.714 ± 0.096 | 51.8 ± 2.4 | 17.7 ± 1.6 | 0.699 ± 0.011 | 0.2437 ± 0.0022 | 73.5 ± 3.0 |
| 4 | 0.01844 ± 0.00023 | 0.0237 ± 0.0043 | 19.08 ± 0.20 | 56.7 ± 9.7 | 11.762 ± 0.024 | 0.55998 ± 0.00063 | 0.10654 ± 0.00047 | 105 ± 1 |
| 5 | 0.01959 ± 0.00073 | 0.0798 ± 0.0096 | 20.02 ± 0.73 | 51.3 ± 1.8 | 11.187 ± 0.057 | 0.573 ± 0.014 | 0.1073 ± 0.0067 | 107 ± 3 |
| 6 | 0.0198 ± 0.0024 | 0.0223 ± 0.0067 | 18.45 ± 0.12 | 49.34 ± 0.49 | 11.10 ± 0.15 | 0.5681 ± 0.0089 | 0.10155 ± 0.00046 | 101 ± 1 |
| Sample | Al | B | Ba | Sr |
|---|---|---|---|---|
| Ants | ||||
| 1 | 99.5 ± 2.5 | 0.742 ± 0.024 | 17.54 ± 0.37 | 4.828 ± 0.091 |
| 2 | 105 ± 10 | 0.715 ± 0.031 | 14.753 ± 0.029 | 4.24 ± 0.23 |
| Crickets | ||||
| 1 | 5.49 ± 0.74 | 0.757 ± 0.073 | 0.5008 ± 0.0019 | 2.794 ± 0.077 |
| 2 | 3.06 ± 0.43 | 0.5543 ± 0.0056 | 0.464 ± 0.013 | 2.75 ± 0.10 |
| 3 | 4.3 ± 1.7 | 0.553 ± 0.011 | 0.489 ± 0.038 | 2.9160 ± 0.0040 |
| 4 | 20.4 ± 1.2 | 0.398 ± 0.012 | 0.786 ± 0.043 | 2.472 ± 0.048 |
| 5 | 12.3 ± 7.6 | 0.690 ± 0.043 | 1.06 ± 0.16 | 2.54 ± 0.13 |
| 6 | 3.79 ± 0.72 | 0.314 ± 0.021 | 0.773 ± 0.067 | 2.40 ± 0.33 |
| 7 | 6.29 ± 0.32 | 0.771 ± 0.018 | 1.4569 ± 0.0065 | 1.861 ± 0.039 |
| 8 | 4.07 ± 0.60 | 0.714 ± 0.041 | 1.171 ± 0.048 | 1.636 ± 0.043 |
| Locusts | ||||
| 1 | 55.3 ± 2.4 | 0.387 ± 0.031 | 0.9767 ± 0.0055 | 1.895 ± 0.028 |
| 2 | 119 ± 15 | 0.400 ± 0.020 | 1.298 ± 0.049 | 1.0888 ± 0.0059 |
| Scorpions | ||||
| 1 | 283 ± 17 | 0.659 ± 0.020 | 8.23 ± 0.64 | 4.80 ± 0.45 |
| 2 | 64.25 ± 0.54 | 0.3993 ± 0.0042 | 11.071 ± 0.018 | 10.58 ± 0.11 |
| 3 | 69.0 ± 2.0 | 0.763 ± 0.034 | 7.078 ± 0.062 | 17.99 ± 0.11 |
| 4 | 103.7 ± 0.5 | 0.429 ± 0.017 | 4.269 ± 0.028 | 5.436 ± 0.055 |
| Tarantulas | ||||
| 1 | 132.8 ± 0.05 | 0.898 ± 0.040 | 28.83 ± 0.17 | 31.22 ± 0.14 |
| 2 | 169 ± 3 | 1.0023 ± 0.0036 | 23.78 ± 0.41 | 31.08 ± 0.93 |
| 3 | 167 ± 1 | 1.068 ± 0.049 | 28.57 ± 0.12 | 21.73 ± 0.21 |
| Water bug | ||||
| 1 | 81.1 ± 1.4 | nd | 1.0085 ± 0.0063 | 1.2820 ± 0.0020 |
| 2 | 144 ± 3 | nd | 1.492 ± 0.016 | 1.424 ± 0.036 |
| Worms | ||||
| 1 | 14.2 ± 2.3 | 0.758 ± 0.012 | 2.238 ± 0.053 | 3.71 ± 0.20 |
| 2 | 1.406 ± 0.029 | nd | 0.857 ± 0.050 | 0.901 ± 0.016 |
| 3 | 14.86 ± 0.96 | 1.184 ± 0.028 | 2.22 ± 0.14 | 3.222 ± 0.069 |
| 4 | 0.490 ± 0.070 | 2.28 ± 0.23 | 3.974 ± 0.071 | 3.619 ± 0.064 |
| 5 | 1.5 ± 1.2 | 2.128 ± 0.070 | 3.83 ± 0.20 | 3.57 ± 0.11 |
| 6 | 0.4421 ± 0.0020 | 2.49 ± 0.36 | 4.003 ± 0.043 | 3.5216 ± 0.0024 |
| Insect | As (µg kg−1) | Cd (µg kg−1) | Pb (µg kg−1) | Ca (mg kg−1) | K (mg kg−1) | Mg (mg kg−1) | Na (mg kg−1) | Reference |
|---|---|---|---|---|---|---|---|---|
| Cricket (n = 3) | 300–400 | 22–28 | n.d.-19 | 612–2630 | 8100–13,500 | 740–1880 | 1130–15,900 | [27] |
| Cricket (n = 8) | 3.6–21.1 | 13.8–76.6 | 16.7–96.9 | 411–1798 | 5263–9116 | 727–1316 | 1162–2874 | This work |
| Locust (n = 1) | 300 | n.d. | 16 | 864 | 6060 | 490 | 2160 | [27] |
| Locust (n = 2) | 35.0–47.4 | 31.8–40.9 | 50.2–73.4 | 481–559 | 6350–6510 | 658–749 | 860–937 | This work |
| Mealworm (n = 3) | 200–620 | 30–200 | n.d.-100,000 | 941–2380 | 7560–10,000 | 870–1450 | 1290–1510 | [27] |
| Mealworm (n = 5) | 25.7–39.7 | 79.3–83.8 | 6.0–10.8 | 401–788 | 4474–8533 | 1343–3074 | 521–982 | This work |
| Silkworm (n = 1) | 200 | n.d. | 9 | 622 | 1090 | 480 | 3650 | [27] |
| Silkworm (n = 1) | 470 | 53.9 | 47.3 | 1132 | 7462 | 2565 | 126 | This work |
| Water bug (n = 5) | 320.9–561.5 | 220–346 | 336–420 | 197.4–286.2 | [23] | |||
| Water bug (n = 2) | 632–855 | 3950–4041 | 614–695 | 3150–5113 | This work |
| Insect | Co | Cr | Cu | Fe | Mn | Mo | Se | Zn | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Cricket (n = 3) | n.d.-0.045 | 0.174–0.267 | 16.3–23.9 | 40.9–80.7 | 9.62–45.8 | 0.327–17.2 | 0.474–0.899 | 125–173 | [27] |
| Cricket (n = 8) | 0.0102–0.0880 | 0.0164–0.1380 | 19.54–53.09 | 49.3–83.6 | 7.7–63.5 | 0.560–1.531 | 0.123–0.638 | 135–256 | This work |
| Locust (n = 1) | n.d. | 0.140 | 35.7 | 42.4 | 4.44 | 0.734 | 0.307 | 140 | [27] |
| Locust (n = 2) | 0.1218–0.1246 | 0.0836–0.2066 | 60.3–92.7 | 90.3–164 | 6.46–6.77 | 0.574–0.678 | 0.216–0.334 | 173–177 | This work |
| Mealworm (n = 3) | 0.018–0.033 | 0.164–0.624 | 11.3–28.2 | 36.2–253 | 5.36–410 | 0.37–0.897 | 0.474–0.547 | 93.8–126 | [27] |
| Mealworm (n = 5) | 0.01844–0.04870 | 0.0223–0.0798 | 9.71–20.02 | 46.6–56.7 | 10.7–17.7 | 0.560–0.960 | 0.102–0.819 | 73.5–107 | This work |
| Silkworm (n = 1) | n.d. | 0.054 | 3.90 | 11.7 | 5.71 | 0.118 | 0.357 | 38.6 | [27] |
| Silkworm (n = 1) | 0.001978 | 0.0203 | 9.12 | 32.85 | 19.390 | 0.3557 | 0.6344 | 175 | This work |
| Water bug (n = 5) | 22.2–420 | 253–1121 | 19.8–42.2 | 49.8–72.2 | [23] | ||||
| Water bug (n = 2) | 11.2–12.7 | 129–474 | 10.03–14.67 | 119–147 | This work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Van Huis, A. Edible insects contributing to food security? Agric. Food Secur. 2015, 4, 20. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs. World Population Prospects 2022: Summary of Results; Report No. 3; United Nations: New York, NY, USA, 2022. [Google Scholar]
- Wood, P.; Tavan, M. A review of the alternative protein industry. Curr. Opin. Food Sci. 2022, 47, 100869. [Google Scholar] [CrossRef]
- Cappellozza, S.; Leonardi, M.G.; Savoldelli, S.; Carminati, D.; Rizzolo, A.; Cortellino, G.; Terova, G.; Moretto, E.; Badaile, A.; Concheri, G.; et al. A first attempt to produce proteins from insects by means of a circular economy. Animals 2019, 9, 278. [Google Scholar] [CrossRef]
- Kusch, S.; Fiebelkorn, F. Environmental impact judgments of meat, vegetarian, and insect burgers: Unifying the negative footprint illusion and quantity insensitivity. Food Qual. Prefer. 2019, 78, 103731. [Google Scholar] [CrossRef]
- de Koning, W.; Dean, D.; Vriesekoop, F.; Aguiar, L.K.; Anderson, M.; Mongondry, P.; Oppong-Gyamfi, M.; Urbano, B.; Luciano, C.A.G.; Jiang, B.; et al. Drivers and inhibitors in the acceptance of meat alternatives: The case of plant and insect-based proteins. Foods 2020, 9, 1292. [Google Scholar] [CrossRef]
- McClements, D.J.; Barrangou, R.; Hill, C.; Kokini, J.L.; Lila, M.A.; Meyer, A.S.; Yu, L. Building a resilient, sustainable, and healthier food supply through innovation and technology. Annu. Rev. Food Sci. Technol. 2021, 12, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Hadi, J.; Brightwell, G. Safety of alternative proteins: Technological, environmental and regulatory aspects of cultured meat, plant-based meat, insect protein and single-cell protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Diet and Health. Diet and Health; National Academies Press: Washington, DC, USA, 1989. [Google Scholar]
- World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Mehri, A. Trace elements in human nutrition (ii)—An update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef]
- Türkdoğan, M.K.; Kilicel, F.; Kara, K.; Tuncer, I.; Uygan, I. Heavy metals in soil, vegetables and fruits in the endemic upper gastrointestinal cancer region of Turkey. Environ. Toxicol. Pharmacol. 2003, 13, 17–179. [Google Scholar] [CrossRef]
- Islam, M.S.; Islam, A.R.M.T.; Phoungthong, K.; Ustaoğlu, F.; Tokatli, C.; Ahmed, R.; Ibrahim, K.A.; Idris, A.M. Potentially toxic elements in vegetable and rice species in Bangladesh and their exposure assessment. J. Food Compos. Anal. 2022, 106, 104350. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Idris, A.M.; Phoungthong, K.; Habib, M.A.; Mustafa, R.A. Geochemical speciation and bioaccumulation of trace elements in different tissues of pumpkin in the abandoned soils: Health hazard perspective in a developing country. Toxin Rev. 2022, 41, 1124–1138. [Google Scholar] [CrossRef]
- Proc, K.; Bulak, P.; Wiącek, D.; Bieganowski, A. Hermetia illucens exhibits bioaccumulative potential for 15 different elements—Implications for feed and food production. Sci. Total Environ. 2020, 723, 138125. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Dhanani, K.; Hoffman, L.C. Food safety of consuming black soldier fly (Hermetia illucens) larvae: Microbial, heavy metal and cross-reactive allergen risks. Foods 2021, 10, 101934. [Google Scholar] [CrossRef]
- Migula, P.; Przybyłowicz, W.J.; Mesjasz-Przybyłowicz, J.; Augustyniak, M.; Nakonieczny, M.; Głowacka, E.; Tarnawska, M. Micro-PIXE Studies of Elemental Distribution in Sap-Feeding Insects Associated with Ni Hyperaccumulator, Berkheya coddii. Plant Soil 2007, 293, 197–207. [Google Scholar] [CrossRef]
- Orłowski, G.; Mróz, L.; Kadej, M.; Smolis, A.; Tarnawski, D.; Karg, J.; Campanaro, A.; Bardiani, M.; Harvey, D.J.; Méndez, M.; et al. Breaking down Insect Stoichiometry into Chitin-Based and Internal Elemental Traits: Patterns and Correlates of Continent-Wide Intraspecific Variation in the Largest European Saproxylic Beetle. Environ. Pollut. 2020, 262, 114064. [Google Scholar] [CrossRef]
- Momoshima, N.; Sugihara, S.; Hibino, K.; Nakamura, Y. Elemental Concentrations of Aquatic Insect Larvae and Attached Algae on Stone Surfaces in an Uncontaminated Stream. J. Radioanal. Nucl. Chem. 2009, 280, 107–111. [Google Scholar] [CrossRef]
- Köksal Erman, Ö. Elemental Composition in Two Water Beetles (Dytiscus thianschanicus, Dytiscus persicus) (Dytiscidae: Coleoptera) as Revealed by WDXRF Spectroscopy. Biol. Trace Elem. Res. 2011, 143, 1541–1563. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, M.; Bhattacharyya, B.; Bhagawati, S.; Sarmah, K. Nutritional Composition of Some Commonly Available Aquatic Edible Insects of Assam, India. Insects 2022, 13, 976. [Google Scholar] [CrossRef]
- Momoshima, N.; Toyoshima, T.; Matsushita, R.; Fukuda, A.; Hibino, K. Metal Concentrations in Japanese Medaka, Mosquitofish and Insect Larvae Living in Uncontaminated Rivers in Kumamoto, Japan. J. Radioanal. Nucl. Chem. 2007, 272, 495–499. [Google Scholar] [CrossRef]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Hoffman, L.C. Why for feed and not for human consumption? The black soldier fly larvae. Compr. Rev. Food Sci. Food. Saf. 2020, 19, 2747–2763. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Simon, E.; Fábián, I.; Tóthmérész, B. The Effects of Ethylene Glycol and Ethanol on the Body Mass and Elemental Composition of Insects Collected with Pitfall Traps. Chemosphere 2009, 77, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Sikora, D.; Proch, J.; Niedzielski, P.; Rzymski, P. Elemental Content of the Commercial Insect-Based Products Available in the European Union. J. Food Compos. Anal. 2023, 121, 105367. [Google Scholar] [CrossRef]
- Chen, Y.A.; Forschler, B.T. Elemental Concentrations in the Frass of Saproxylic Insects Suggest a Role in Micronutrient Cycling. Ecosphere 2016, 7, e01300. [Google Scholar] [CrossRef]
- Simon, E.; Baranyai, E.; Braun, M.; Fábián, I.; Tóthmérész, B. Elemental Concentration in Mealworm Beetle (Tenebrio molitor L.) during Metamorphosis. Biol. Trace Elem. Res. 2013, 154, 81–87. [Google Scholar] [CrossRef]
- Kohlmeyer, U.; Jantzen, E.; Kuballa, J.; Jakubik, S. Benefits of high resolution IC-ICP-MS for the routine analysis of inorganic and organic arsenic species in food products of marine and terrestrial origin. Anal. Bioanal. Chem. 2003, 377, 6–13. [Google Scholar] [CrossRef]
- Beauchemin, D. Sample Introduction Systems in ICPMS and ICPOES; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Linsinger, T. Comparison of a Measurement Result with the Certified Value; Application Note 1; European Reference Materials: Geel, Belgium, 2010. [Google Scholar]
- Sánchez, R.; Todolí, J.L. Assessment of total sample consumption infrared-heated system for minimization of matrix effects in ICP-tandem mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2023, 208, 106779. [Google Scholar] [CrossRef]
- Approval of Fourth Insect as a Novel Food. Available online: https://food.ec.europa.eu/safety/novel-food/authorisations/approval-insect-novel-food_en (accessed on 24 June 2023).
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants; World Health Organization: Geneva, Switzerland, 1989. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Potassium. Available online: https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional/#h13 (accessed on 9 December 2023).
- Sodium Reduction. Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 10 December 2023).
- Copper. Available online: https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/ (accessed on 10 December 2023).
- Iron. Available online: https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/ (accessed on 10 December 2023).
- Zinc. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/ (accessed on 10 December 2023).
| Parameter | Setting |
|---|---|
| RF power | 1550 W |
| Plasma gas flow rate (Ar) | 15.0 L min−1 |
| Auxiliary gas flow rate (Ar) | 0.9 L min−1 |
| Nebulizer gas flow rate (Ar) | ~1.0 L min−1 |
| Collision gas flow rate (He) | ~5.0 mL min−1 |
| Sampling/skimmer cones | Nickel |
| Monitored isotopes | No gas mode: 11B, 75As, 111Cd, 137Ba, 208Pb |
| Collision mode (He): 23Na, 24Mg, 27Al, 39K, 44Ca, 52Cr, 55Mn, 56Fe, 59Co, 63Cu, 66Zn, 77Se, 88Sr, 95Mo | |
| Internal standards: 89Y, 185Re, 209Bi |
| Sample Name | Insect | Species | Information | Origin |
|---|---|---|---|---|
| Ants 1 | Columbia ants | Atta laevigata | With salt | Columbia |
| Ants 2 | Columbia ants | Atta laevigata | With salt | Columbia |
| Crickets 1 | Crickets | Acheta domesticus | Whole dehydrated crickets | Toulouse, France |
| Crickets 2 | Crickets | Acheta domesticus | Whole dehydrated crickets | Toulouse, France |
| Crickets 3 | Crickets | Acheta domesticus | Whole dehydrated crickets | Toulouse, France |
| Crickets 4 | Crickets | Gryllus bimaculatus | - | Thailand |
| Crickets 5 | Crickets | N/A | Whole dehydrated crickets | N/A |
| Crickets 6 | Crickets | N/A | Whole dehydrated crickets | N/A |
| Crickets 7 | Crickets | N/A | Whole dehydrated crickets | Netherlands |
| Crickets 8 | Crickets | N/A | Whole dehydrated crickets | Netherlands |
| Locusts 1 | Locusts | Locusta migratoria | With salt | N/A |
| Locusts 2 | Locusts | Locusta migratoria | With salt | N/A |
| Scorpions 1 | Black scorpions | Heterometrus longimanus | With salt | Thailand |
| Scorpions 2 | Black Asian forest scorpions | Heterometrus longimanus | With salt | Thailand |
| Scorpions 3 | Black Asian forest scorpions | Heterometrus longimanus | With salt | Thailand |
| Scorpions 4 | Black Asian forest scorpions | Heterometrus longimanus | With salt | Thailand |
| Tarantulas 1 | Tarantula | Haplopeima albostriatum | - | Thailand |
| Tarantulas 2 | Tarantula | Haplopeima albostriatum | - | Thailand |
| Tarantulas 3 | Tarantula | Haplopeima albostriatum | - | Thailand |
| Water bug 1 | Giant water bug | Nepidae | With oil and salt | N/A |
| Water bug 2 | Giant water bug | Nepidae | With oil and salt | N/A |
| Worms 1 | Mealworms | Tenebrio molitor | - | EU |
| Worms 2 | Silk worms | Bombyx mori | - | Thailand |
| Worms 3 | Morio worms | Zophobas morios | With salt | Thailand |
| Worms 4 | Mealworms | Tenebrio molitor | Whole dehydrated mealworm | Toulouse, France |
| Worms 5 | Mealworms | Tenebrio molitor | Whole dehydrated mealworm | Toulouse, France |
| Worms 6 | Mealworms | Tenebrio molitor | Whole dehydrated mealworm | Toulouse, France |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holowaty, Y.; Leufroy, A.; Mazurais, C.; Beauchemin, D.; Jitaru, P. Multi-Elemental Analysis of Edible Insects, Scorpions, and Tarantulas from French (Online) Market and Human Health Risk Assessment Due to Their Consumption: A Pilot Study. Foods 2024, 13, 2353. https://doi.org/10.3390/foods13152353
Holowaty Y, Leufroy A, Mazurais C, Beauchemin D, Jitaru P. Multi-Elemental Analysis of Edible Insects, Scorpions, and Tarantulas from French (Online) Market and Human Health Risk Assessment Due to Their Consumption: A Pilot Study. Foods. 2024; 13(15):2353. https://doi.org/10.3390/foods13152353
Chicago/Turabian StyleHolowaty, Yulianna, Axelle Leufroy, Clément Mazurais, Diane Beauchemin, and Petru Jitaru. 2024. "Multi-Elemental Analysis of Edible Insects, Scorpions, and Tarantulas from French (Online) Market and Human Health Risk Assessment Due to Their Consumption: A Pilot Study" Foods 13, no. 15: 2353. https://doi.org/10.3390/foods13152353
APA StyleHolowaty, Y., Leufroy, A., Mazurais, C., Beauchemin, D., & Jitaru, P. (2024). Multi-Elemental Analysis of Edible Insects, Scorpions, and Tarantulas from French (Online) Market and Human Health Risk Assessment Due to Their Consumption: A Pilot Study. Foods, 13(15), 2353. https://doi.org/10.3390/foods13152353








