Membrane Damage and Metabolic Disruption as the Mechanisms of Linalool against Pseudomonas fragi: An Amino Acid Metabolomics Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
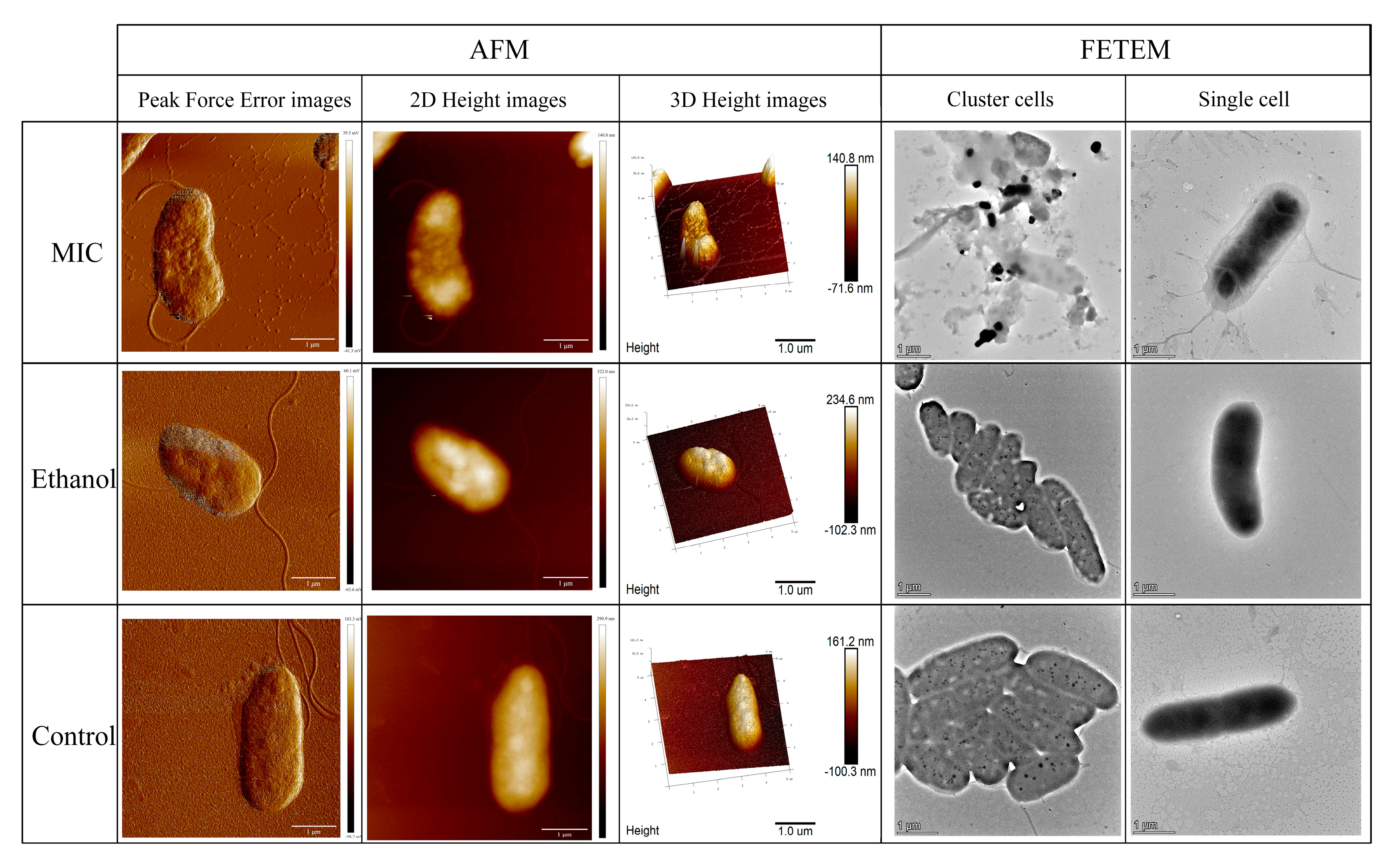
2.2. Investigation of Cell Morphology
2.2.1. AFM Observation
2.2.2. FETEM Observation
2.3. Cell Membrane Integrity
2.3.1. CLSM Observation
2.3.2. FTIR Spectroscopy
2.4. Leakage of Intracellular Material
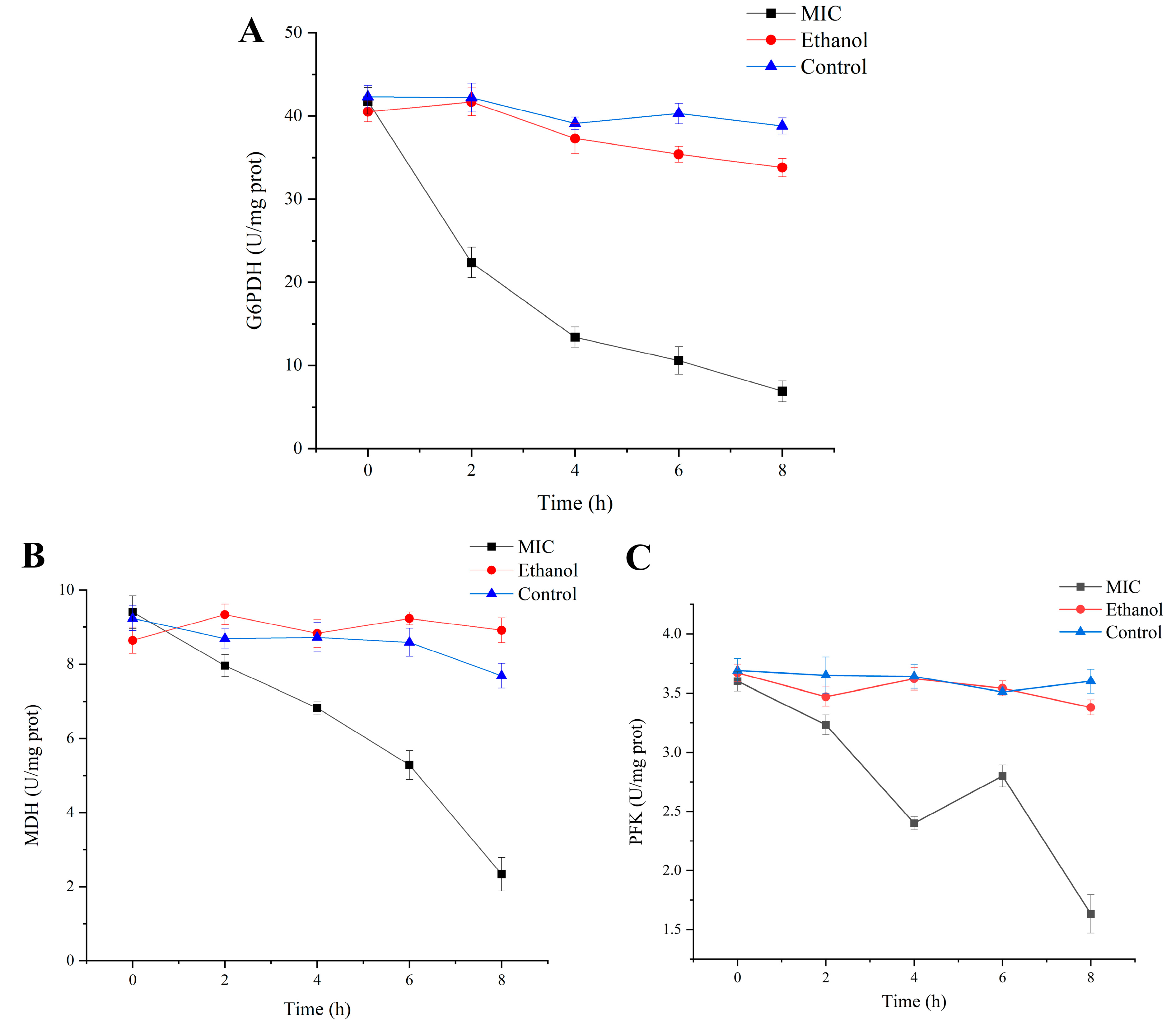
2.5. Measurement of Intracellular Enzyme Activity
2.6. Targeting Amino Acid Metabolomics Technology
2.7. Effects on DNA Expression
2.7.1. Extraction of DNA
2.7.2. Agarose Gel Electrophoresis
2.7.3. DEG Verification Using qRT-PCR
2.8. Molecular Docking
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of Linalool on Cell Morphology of P. fragi
3.2. Effects of Linalool on Cell Membrane Integrity of P. fragi
3.3. Effects of Linalool on Intracellular Proteins and Nucleic of P. fragi
3.4. Effects of Enzyme Activity Related to Respiration and Energy
3.5. Analysis of Amino Acid Metabolism
3.6. qRT-PCR
3.7. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; De Silva, N.R.; Gargouri, N. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar]
- Kabiraz, M.P.; Majumdar, P.R.; Mahmud, M.C.; Bhowmik, S.; Ali, A. Conventional and advanced detection techniques of foodborne pathogens: A comprehensive review. Heliyon 2023, 9, e15482. [Google Scholar] [CrossRef] [PubMed]
- Marchand, S.; Heylen, K.; Messens, W.; Coudijzer, K.; De Vos, P.; Dewettinck, K.; Herman, L.; De Block, J.; Heyndrickx, M. Seasonal influence on heat-resistant proteolytic capacity of Pseudomonas lundensis and Pseudomonas fragi, predominant milk spoilers isolated from Belgian raw milk samples. Environ. Microbiol. 2009, 11, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Caldera, L.; Franzetti, L.v.; Van Coillie, E.; De Vos, P.; Stragier, P.; De Block, J.; Heyndrickx, M. Identification, enzymatic spoilage characterization and proteolytic activity quantification of Pseudomonas spp. isolated from different foods. Food Microbiol. 2016, 54, 142–153. [Google Scholar] [CrossRef]
- An, Z.; Mao, Y.; Du, Y.; Ji, S.; Gao, Z.; Yan, H.; Han, D. Progress in the study of mechanisms related to biological factors of food spoilage and deterioration. J. Food Saf. Qual. 2022, 13, 86–93. [Google Scholar] [CrossRef]
- Ge, R.; Luo, Y.; Ju, N. Progress in the study of microflora on flavour formation in traditional fermented meat products. Microbiol. Bull. 2022, 49, 2295–2307. [Google Scholar]
- Rossi, C.; Chaves-López, C.; Serio, A.; Casaccia, M.; Maggio, F.; Paparella, A. Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2172–2191. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhong, W.; Liu, T.; Zhao, T.; Guo, J. Global Proteomic Analysis of Listeria monocytogenes’ Response to Linalool. Foods 2021, 10, 2449. [Google Scholar] [CrossRef]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Api, A.; Belsito, D.; Bhatia, S.; Bruze, M.; Calow, P.; Dagli, M.; Dekant, W.; Fryer, A.; Kromidas, L.; La Cava, S. RIFM fragrance ingredient safety assessment, Linalool, CAS registry number 78-70-6. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 82, S29–S38. [Google Scholar] [CrossRef]
- An, Q.; Ren, J.-N.; Li, X.; Fan, G.; Qu, S.-S.; Song, Y.; Li, Y.; Pan, S.-Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef]
- Iwasaki, K.; Zheng, Y.-W.; Murata, S.; Ito, H.; Nakayama, K.; Kurokawa, T.; Sano, N.; Nowatari, T.; Villareal, M.O.; Nagano, Y.N. Anticancer effect of linalool via cancer-specific hydroxyl radical generation in human colon cancer. World J. Gastroenterol. 2016, 22, 9765. [Google Scholar] [CrossRef]
- Li, X.-J.; Yang, Y.-J.; Li, Y.-S.; Zhang, W.K.; Tang, H.-B. α-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef]
- Casaburi, A.; Di Martino, V.; Ercolini, D.; Parente, E.; Villani, F. Antimicrobial activity of Myrtus communis L. water-ethanol extract against meat spoilage strains of Brochothrix thermosphacta and Pseudomonas fragi in vitro and in meat. Ann. Microbiol. 2015, 65, 841–850. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, M.; Chen, W.; Yun, Y.-H. Unraveling the antibacterial mechanism of 3-carene against Pseudomonas fragi by integrated proteomics and metabolomics analyses and its application in pork. Int. J. Food Microbiol. 2022, 379, 109846. [Google Scholar] [CrossRef]
- Song, X.; Kang, J.; Wei, X.; Liu, L.; Liu, Y.; Wang, F. Insights into the antibacterial effectiveness of linalool against Shigella flexneri on pork surface: Changes in bacterial growth and pork quality. Int. J. Food Microbiol. 2024, 418, 110718. [Google Scholar] [CrossRef]
- Duarte, A.; Luís, Â.; Oleastro, M.; Domingues, F.C. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Aelenei, P.; Rimbu, C.M.; Guguianu, E.; Dimitriu, G.; Aprotosoaie, A.C.; Brebu, M.; Horhogea, C.E.; Miron, A. Coriander essential oil and linalool—Interactions with antibiotics against Gram-positive and Gram-negative bacteria. Lett. Appl. Microbiol. 2019, 68, 156–164. [Google Scholar] [CrossRef]
- Vadillo-Rodríguez, V.; Cavagnola, M.A.; Pérez-Giraldo, C.; Fernández-Calderón, M.C. A physico-chemical study of the interaction of ethanolic extracts of propolis with bacterial cells. Colloids Surf. B Biointerfaces 2021, 200, 111571. [Google Scholar] [CrossRef]
- Trytek, M.; Paduch, R.; Fiedurek, J.; Kandefer-Szerszen, M. Monoterpenes? old compounds, new applications, and biotechnological methods of their production. Biotechnologia 2007, 1, 135–155. [Google Scholar]
- Su, R.; Guo, P.; Zhang, Z.; Wang, J.; Guo, X.; Guo, D.; Wang, Y.; Lü, X.; Shi, C. Antibacterial activity and mechanism of linalool against Shigella sonnei and its application in lettuce. Foods 2022, 11, 3160. [Google Scholar] [CrossRef]
- He, R.; Zhong, Q.; Chen, W.; Zhang, M.; Pei, J.; Chen, H.; Chen, W. Antimicrobial mechanism of linalool against Brochothrix thermosphacta and its application on chilled beef. Food Res. Int. 2022, 157, 111407. [Google Scholar] [CrossRef]
- He, R.; Chen, H.; Chen, W.; Zhang, M.; Pei, J.; Chen, W.; Zhong, Q. Respiratory depression driven by membrane damage as a mechanism for linalool to inhibit Pseudomonas lundensis and its preservation potential for beef. J. Appl. Microbiol. 2023, 134, lxad023. [Google Scholar] [CrossRef]
- Li, Y.; He, R.; Chen, H.; Chen, D.; Chen, W. Respiratory depression as antibacterial mechanism of linalool against Pseudomonas fragi based on metabolomics. Int. J. Mol. Sci. 2022, 23, 11586. [Google Scholar] [CrossRef]
- Li, Y.; Ren, F.; Chen, D.; Chen, H.; Chen, W. Antibacterial Mechanism of Linalool against Pseudomonas fragi: A Transcriptomic Study. Foods 2022, 11, 2058. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Rostamabadi, H.; Assadpour, E.; Jafari, S.M. Morphology and microstructural analysis of bioactive-loaded micro/nanocarriers via microscopy techniques; CLSM/SEM/TEM/AFM. Adv. Colloid Interface Sci. 2020, 280, 102166. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Li, Z.; Wang, K.; Kong, B.; Chen, Q. l-glycine and l-glutamic acid protect Pediococcus pentosaceus R1 against oxidative damage induced by hydrogen peroxide. Food Microbiol. 2022, 101, 103897. [Google Scholar] [CrossRef]
- Wahia, H.; Zhang, L.; Zhou, C.; Mustapha, A.T.; Fakayode, O.A.; Amanor-Atiemoh, R.; Ma, H.; Dabbour, M. Pulsed multifrequency thermosonication induced sonoporation in Alicyclobacillus acidoterrestris spores and vegetative cells. Food Res. Int. 2022, 156, 111087. [Google Scholar] [CrossRef]
- Shailaja, A.; Bruce, T.F.; Gerard, P.; Powell, R.R.; Pettigrew, C.A.; Kerrigan, J.L. Comparison of cell viability assessment and visualization of Aspergillus niger biofilm with two fluorescent probe staining methods. Biofilm 2022, 4, 100090. [Google Scholar] [CrossRef]
- Cybulska, J.; Cieśla, J.; Kurzyna-Szklarek, M.; Szymańska-Chargot, M.; Pieczywek, P.M.; Zdunek, A. Influence of pectin and hemicelluloses on physical properties of bacterial cellulose. Food Chem. 2023, 429, 136996. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Sun, X.-h.; Zhou, T.-t.; Wei, C.-h.; Lan, W.-q.; Zhao, Y.; Pan, Y.-j.; Wu, V.C. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Zhang, S.-B.; Qin, Y.-L.; Li, S.-F.; Lv, Y.-Y.; Zhai, H.-C.; Hu, Y.-S.; Cai, J.-P. Antifungal mechanism of 1-nonanol against Aspergillus flavus growth revealed by metabolomic analyses. Appl. Microbiol. Biotechnol. 2021, 105, 7871–7888. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Izano, E.A.; Gopal, P.; Karwacki, M.T.; Kim, S.; Bose, J.L.; Bayles, K.W.; Horswill, A.R. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. MBio 2012, 3, 112. [Google Scholar] [CrossRef]
- Xue, Q.; Liu, X.; Russell, P.; Li, J.; Pan, W.; Fu, J.; Zhang, A. Evaluation of the binding performance of flavonoids to estrogen receptor alpha by Autodock, Autodock Vina and Surflex-Dock. Ecotoxicol. Environ. Saf. 2022, 233, 113323. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef]
- Kahli, H.; Béven, L.; Grauby-Heywang, C.; Debez, N.; Gammoudi, I.; Moroté, F.; Sbartai, H.; Cohen-Bouhacina, T. Impact of Growth Conditions on Pseudomonas fluorescens Morphology Characterized by Atomic Force Microscopy. Int. J. Mol. Sci. 2022, 23, 9579. [Google Scholar] [CrossRef]
- Hammerschmidt, S.; Rohde, M. Electron Microscopy to Study the Fine Structure of the Pneumococcal Cell. Methods Mol. Biol. 2019, 1968, 13–33. [Google Scholar] [CrossRef]
- Sonker, N.; Pandey, A.K.; Singh, P. Efficiency of Artemisia nilagirica (Clarke) Pamp. essential oil as a mycotoxicant against postharvest mycobiota of table grapes. J. Sci. Food Agric. 2015, 95, 1932–1939. [Google Scholar] [CrossRef]
- Kalily, E.; Hollander, A.; Korin, B.; Cymerman, I.; Yaron, S. Mechanisms of resistance to linalool in Salmonella Senftenberg and their role in survival on basil. Environ. Microbiol. 2016, 18, 3673–3688. [Google Scholar] [CrossRef]
- Li, J.; Suo, Y.; Liao, X.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Analysis of Staphylococcus aureus cell viability, sublethal injury and death induced by synergistic combination of ultrasound and mild heat. Ultrason. Sonochem. 2017, 39, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Shewanella putrefaciens. Molecules 2021, 26, 245. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, Q.; Wu, D.; Al-Qadiri, H.M.; Al-Alami, N.I.; Kang, D.-H.; Shin, J.-H.; Tang, J.; Jabal, J.M.; Aston, E.D. Using of infrared spectroscopy to study the survival and injury of Escherichia coli O157: H7, Campylobacter jejuni and Pseudomonas aeruginosa under cold stress in low nutrient media. Food Microbiol. 2011, 28, 537–546. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, J.; Qi, Y.; Xu, J.; Wei, X.; Fan, M. New insights into thermo-acidophilic properties of Alicyclobacillus acidoterrestris after acid adaptation. Food Microbiol. 2021, 94, 103657. [Google Scholar] [CrossRef] [PubMed]
- Medić, A.; Stojanović, K.; Izrael-Živković, L.; Beškoski, V.; Lončarević, B.; Kazazić, S.; Karadžić, I. A comprehensive study of conditions of the biodegradation of a plastic additive 2,6-di-tert-butylphenol and proteomic changes in the degrader Pseudomonas aeruginosa san ai. RSC Adv. 2019, 9, 23696–23710. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Liu, L.; Liu, Y.; Wang, X. Ferulic acid inactivates Shigella flexneri through cell membrane destruction, biofilm retardation, and altered gene expression. J. Agric. Food Chem. 2020, 68, 7121–7131. [Google Scholar] [CrossRef]
- Yuxiao, Z.; Ying, C.; Yanyin, G.; Yangli, M.; Mei, Y.; Ruiqing, F.; Yupeng, S.; Jing, Q. Proteomics revealed the effects of nucleic acid metabolism, transcription and translation on postharvest broccoli senescence under elevated O2 stress. Postharvest Biol. Technol. 2023, 195, 112110. [Google Scholar] [CrossRef]
- Kaila, V.R.I.; Wikström, M. Architecture of bacterial respiratory chains. Nat. Rev. Microbiol. 2021, 19, 319–330. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Shi, C.; Hu, W.; Cui, H.; Lin, L. Characterization of controlled-release Eucalyptus citriodora oil/Zinc ions nanoparticles with enhanced antibacterial properties against E. coli O157:H7 in fruit juice. Food Res. Int. 2022, 162, 112138. [Google Scholar] [CrossRef]
- Tao, Y.; Qian, L.-H.; Xie, J. Effect of chitosan on membrane permeability and cell morphology of Pseudomonas aeruginosa and Staphyloccocus aureus. Carbohydr. Polym. 2011, 86, 969–974. [Google Scholar] [CrossRef]
- Venkat, S.; Gregory, C.; Sturges, J.; Gan, Q.; Fan, C. Studying the lysine acetylation of malate dehydrogenase. J. Mol. Biol. 2017, 429, 1396–1405. [Google Scholar] [CrossRef]
- Abd Majid, R. Molecular and Biochemical Pharmacology of Mitochondrial Enzymes in the Malaria Parasite Plasmodium Falciparum; The University of Liverpool: Liverpool, UK, 2011. [Google Scholar]
- Babul, J. Phosphofructokinases from Escherichia coli. Purification and characterization of the nonallosteric isozyme. J. Biol. Chem. 1978, 253, 4350–4355. [Google Scholar] [CrossRef]
- Liu, M.; Feng, M.; Yang, K.; Cao, Y.; Zhang, J.; Xu, J.; Hernández, S.H.; Wei, X.; Fan, M. Transcriptomic and metabolomic analyses reveal antibacterial mechanism of astringent persimmon tannin against Methicillin-resistant Staphylococcus aureus isolated from pork. Food Chem. 2020, 309, 125692. [Google Scholar] [CrossRef]
- Horinouchi, T.; Tamaoka, K.; Furusawa, C.; Ono, N.; Suzuki, S.; Hirasawa, T.; Yomo, T.; Shimizu, H. Transcriptome analysis of parallel-evolved Escherichia coli strains under ethanol stress. BMC Genom. 2010, 11, 579. [Google Scholar] [CrossRef] [PubMed]
- Batson, S.; de Chiara, C.; Majce, V.; Lloyd, A.J.; Gobec, S.; Rea, D.; Fülöp, V.; Thoroughgood, C.W.; Simmons, K.J.; Dowson, C.G.; et al. Inhibition of D-Ala:D-Ala ligase through a phosphorylated form of the antibiotic D-cycloserine. Nat. Commun. 2017, 8, 1939. [Google Scholar] [CrossRef]
- Hao, M.; Wang, M.; Tang, T.; Zhao, D.; Yin, S.; Shi, Y.; Liu, X.; Wudong, G.; Yang, Y.; Zhang, M.; et al. Regulation of the Gene for Alanine Racemase Modulates Amino Acid Metabolism with Consequent Alterations in Cell Wall Properties and Adhesive Capability in Brucella spp. Int. J. Mol. Sci. 2023, 24, 16145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, R.; Song, Z.; Fu, W.; Yun, L.; Gao, J.; Hu, G.; Wang, Z.; Wu, H.; Zhang, G.; et al. Iris lactea var. chinensis plant drought tolerance depends on the response of proline metabolism, transcription factors, transporters and the ROS-scavenging system. BMC Plant Biol. 2023, 23, 17. [Google Scholar] [CrossRef]
- Chandel, N.S. Amino Acid Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040584. [Google Scholar] [CrossRef]
- Holeček, M. Roles of malate and aspartate in gluconeogenesis in various physiological and pathological states. Metabolism 2023, 145, 155614. [Google Scholar] [CrossRef]
- He, R.; Chen, W.; Zhong, Q.; Zhang, M.; Pei, J.; Chen, W.; Chen, H. Targeted amino acid consumption and respiratory depression to study the antibacterial mechanism of linalool against Shigella sonnei. Food Control 2024, 155, 110058. [Google Scholar] [CrossRef]
- Guitart Font, E.; Sprenger, G.A. Opening a novel biosynthetic pathway to dihydroxyacetone and glycerol in Escherichia coli mutants through expression of a gene variant (fsaA A129S) for fructose 6-phosphate aldolase. Int. J. Mol. Sci. 2020, 21, 9625. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, D.; van der Rest, M.E.; Petrović, S. Biochemical and genetic characterization of the membrane-associated malate dehydrogenase (acceptor) from Corynebacterium glutamicum. Eur. J. Biochem. 1998, 254, 395–403. [Google Scholar] [CrossRef]
- O’Brien, R.; Taylor, B.L. Formation and dissimilation of oxalacetate and pyruvate Pseudomonas citronellolis grown on noncarbohydrate substrates. J. Bacteriol. 1977, 130, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Snáel, J.; Machová, I.; Olínová, V.; Kaika, V.; Pichová, I. Phosphofructokinases A and B from Mycobacterium tuberculosis Display Different Catalytic Properties and Allosteric Regulation. Int. J. Mol. Sci. 2021, 22, 1483. [Google Scholar] [CrossRef]
- Dai, S.; Lian, Z.; Qi, W.; Chen, Y.; Tong, X.; Tian, T.; Lyu, B.; Wang, M.; Wang, H.; Jiang, L. Non-covalent interaction of soy protein isolate and catechin: Mechanism and effects on protein conformation. Food Chem. 2022, 384, 132507. [Google Scholar] [CrossRef]
- Zhou, C.; Li, C.; Siva, S.; Cui, H.; Lin, L. Chemical composition, antibacterial activity and study of the interaction mechanisms of the main compounds present in the Alpinia galanga rhizomes essential oil. Ind. Crops Prod. 2021, 165, 113441. [Google Scholar] [CrossRef]







| Gene Name | Nucleotide Sequence (5′→3′) | Direction Primer | Location (nt) | Product Length (bp) |
|---|---|---|---|---|
| 16s RNA | CGGGAACATTGAGACAGG | Forward | 1006 | 121 |
| AGAGTGCCCACCATTACG | Reverse | 1126 | ||
| zwf | GCTGCTGTGCCTGATTGC GTCGCTCTGGGTGTTGGA | Forward Reverse | 720 930 | 211 |
| mqo | AAAGACGGCAGCACAACC ACGGGCCAAACAGGATGA | Forward Reverse | 745 1021 | 277 |
| pfkB | GCAGGGTGTCGAGCATGTAGT CGCTGAGCAAACCGTGGA | Forward Reverse | 639 799 | 161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Chen, H.; Wang, R.; Zhong, Q.; Chen, W.; Zhang, M.; He, R.; Chen, W. Membrane Damage and Metabolic Disruption as the Mechanisms of Linalool against Pseudomonas fragi: An Amino Acid Metabolomics Study. Foods 2024, 13, 2501. https://doi.org/10.3390/foods13162501
Cai J, Chen H, Wang R, Zhong Q, Chen W, Zhang M, He R, Chen W. Membrane Damage and Metabolic Disruption as the Mechanisms of Linalool against Pseudomonas fragi: An Amino Acid Metabolomics Study. Foods. 2024; 13(16):2501. https://doi.org/10.3390/foods13162501
Chicago/Turabian StyleCai, Jiaxin, Haiming Chen, Runqiu Wang, Qiuping Zhong, Weijun Chen, Ming Zhang, Rongrong He, and Wenxue Chen. 2024. "Membrane Damage and Metabolic Disruption as the Mechanisms of Linalool against Pseudomonas fragi: An Amino Acid Metabolomics Study" Foods 13, no. 16: 2501. https://doi.org/10.3390/foods13162501





