Effects of Several Tea-like Plants on Liver Injury Induced by Alcohol via Their Antioxidation, Anti-Inflammation, and Regulation of Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tea-like Plants
2.2. Animal and Experimental Design
2.3. Biochemical Analysis of Serum
2.4. Histopathological Analysis of Liver
2.5. Analysis of Oxidative Stress Indices, TG, ADH, and ALDH in Liver
2.6. Analysis of Inflammatory Indices in Liver
2.7. Composition and Structure Analysis of Gut Microbiota
2.8. Determination of Bioactive Compounds in Tea-like Plants
2.9. Statistical Analysis
3. Results
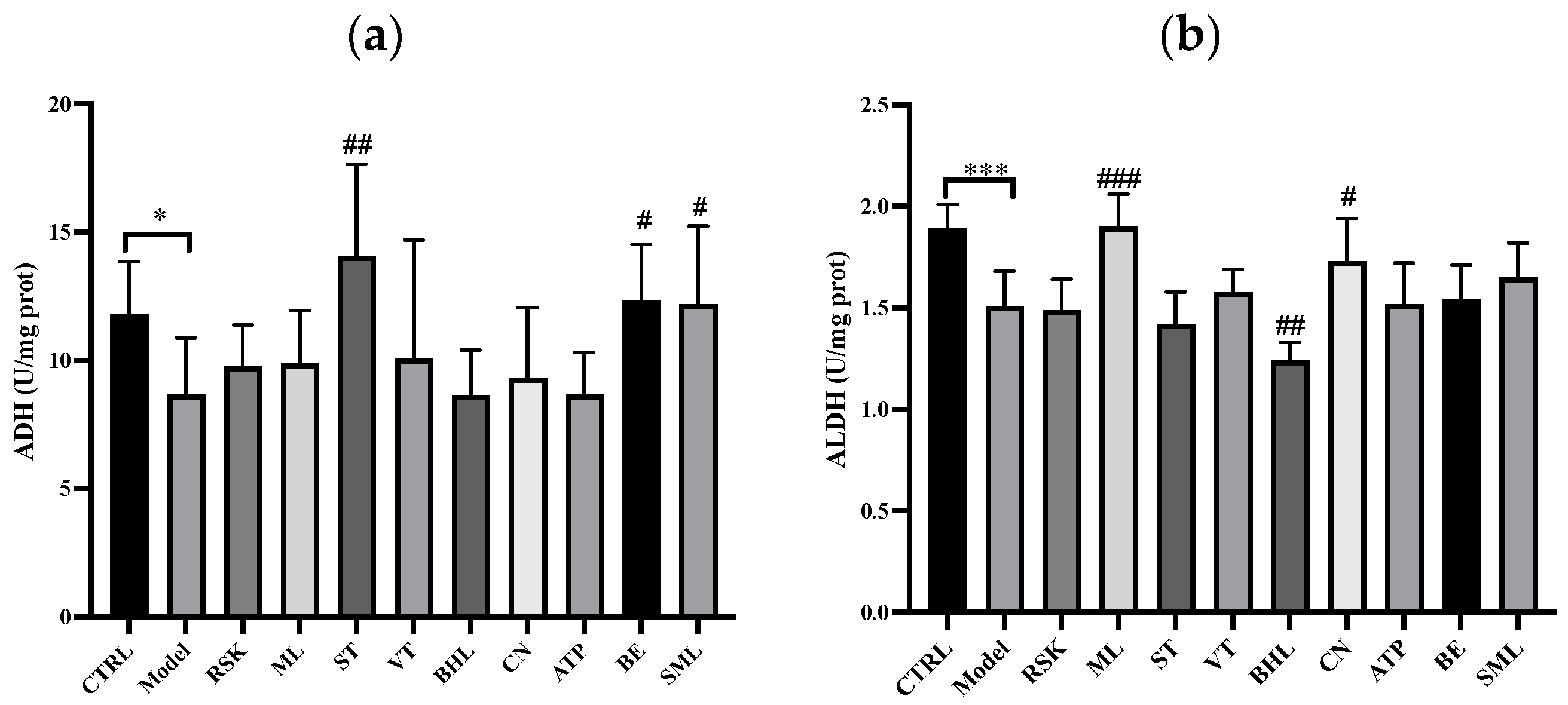
3.1. Effects of Tea-like Plants on Alcohol Metabolism
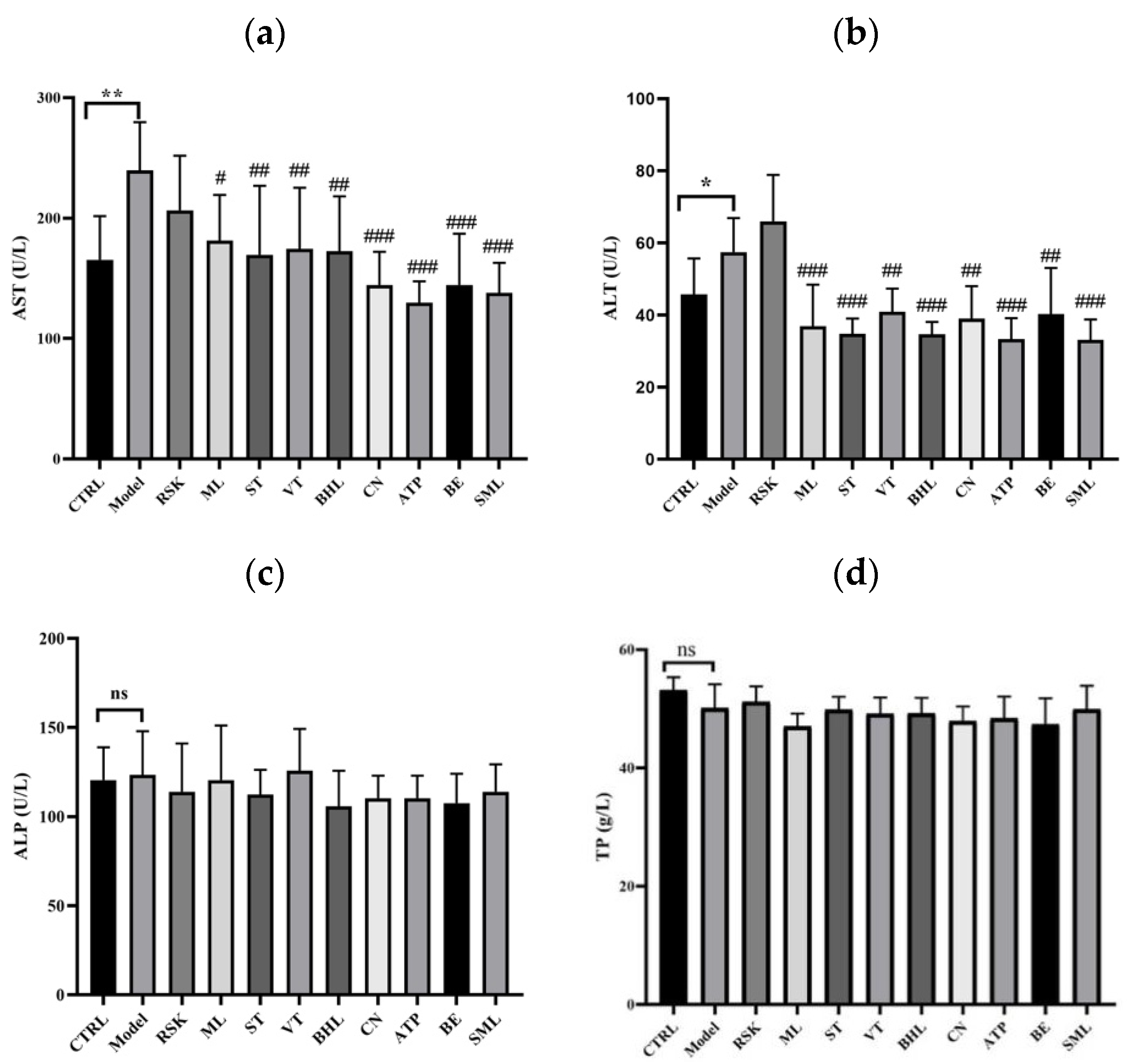
3.2. Effects of Tea-like Plants on Liver Function Indices
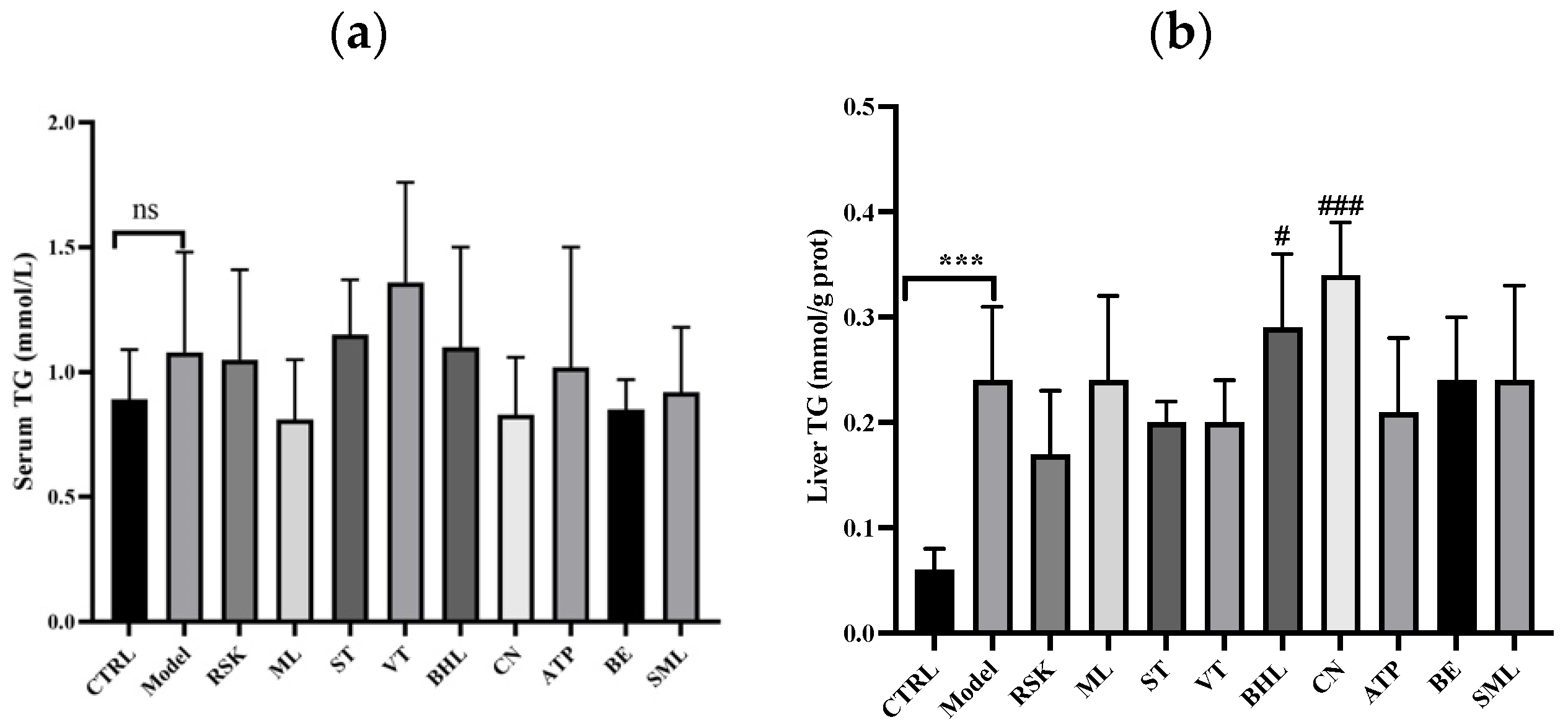
3.3. Effects on Serum Lipid and Hepatic Lipid Indices
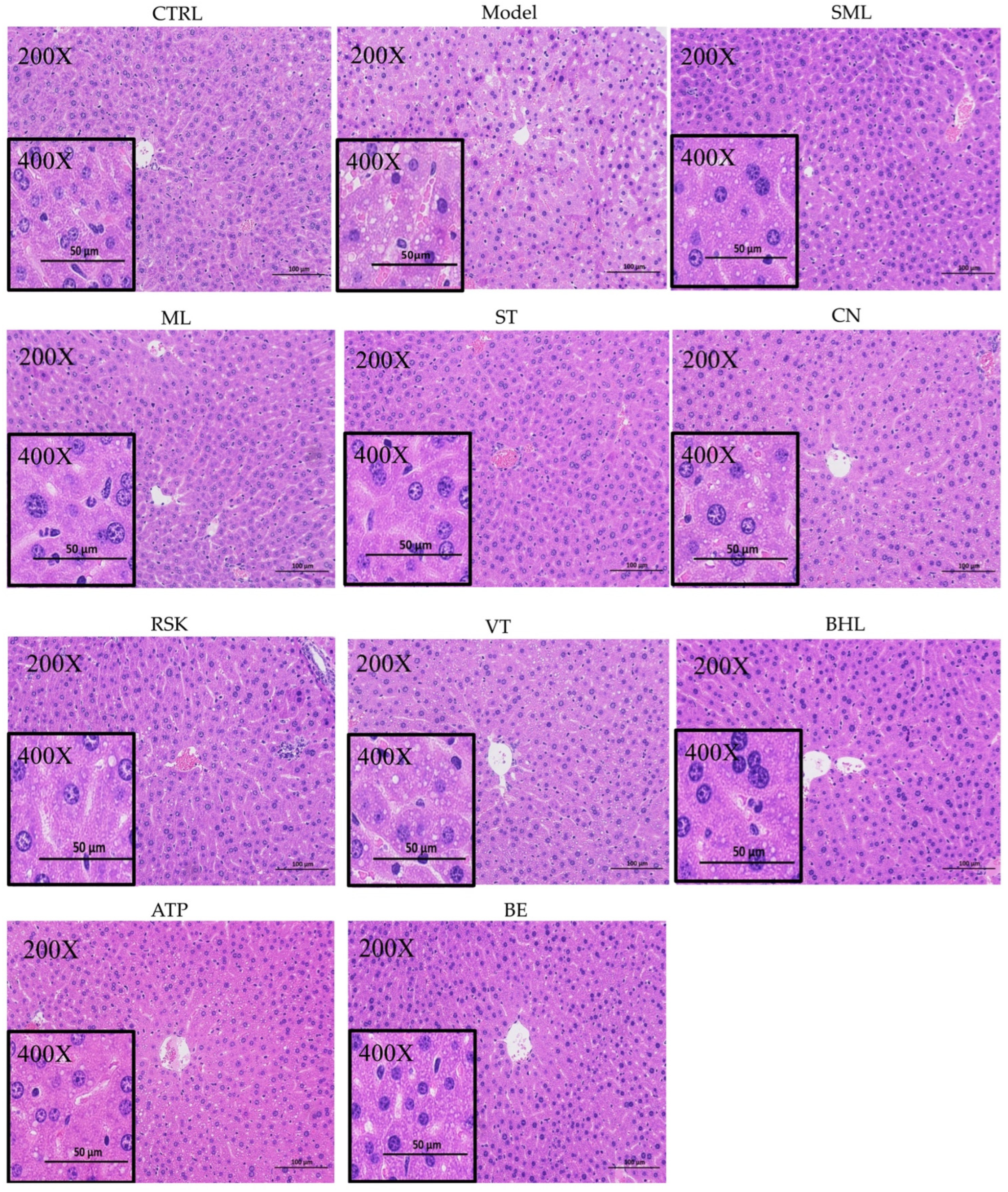
3.4. Hepatic Histopathological Analysis
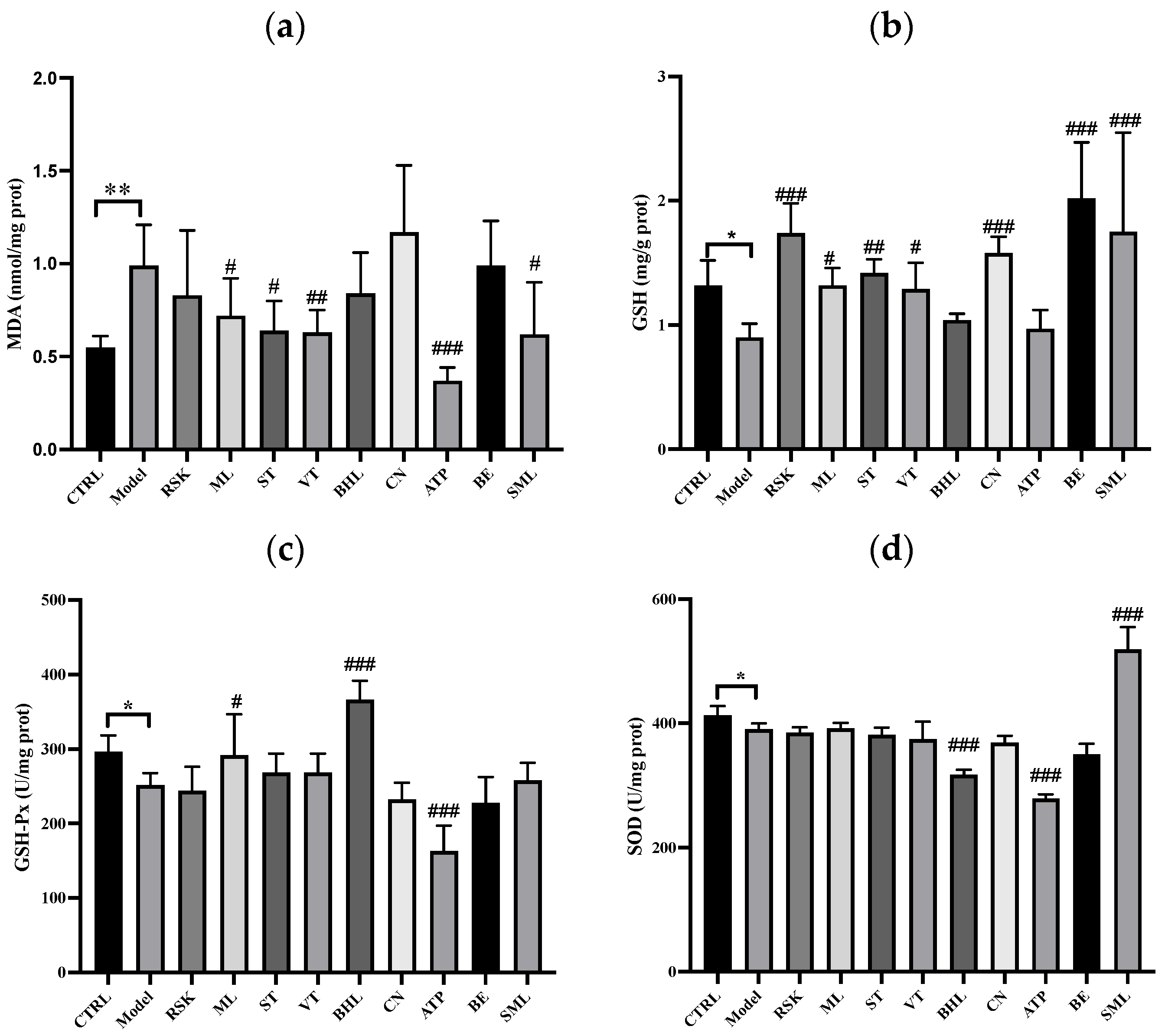
3.5. The Effects of Tea-like Plants on Oxidative Stress in the Liver
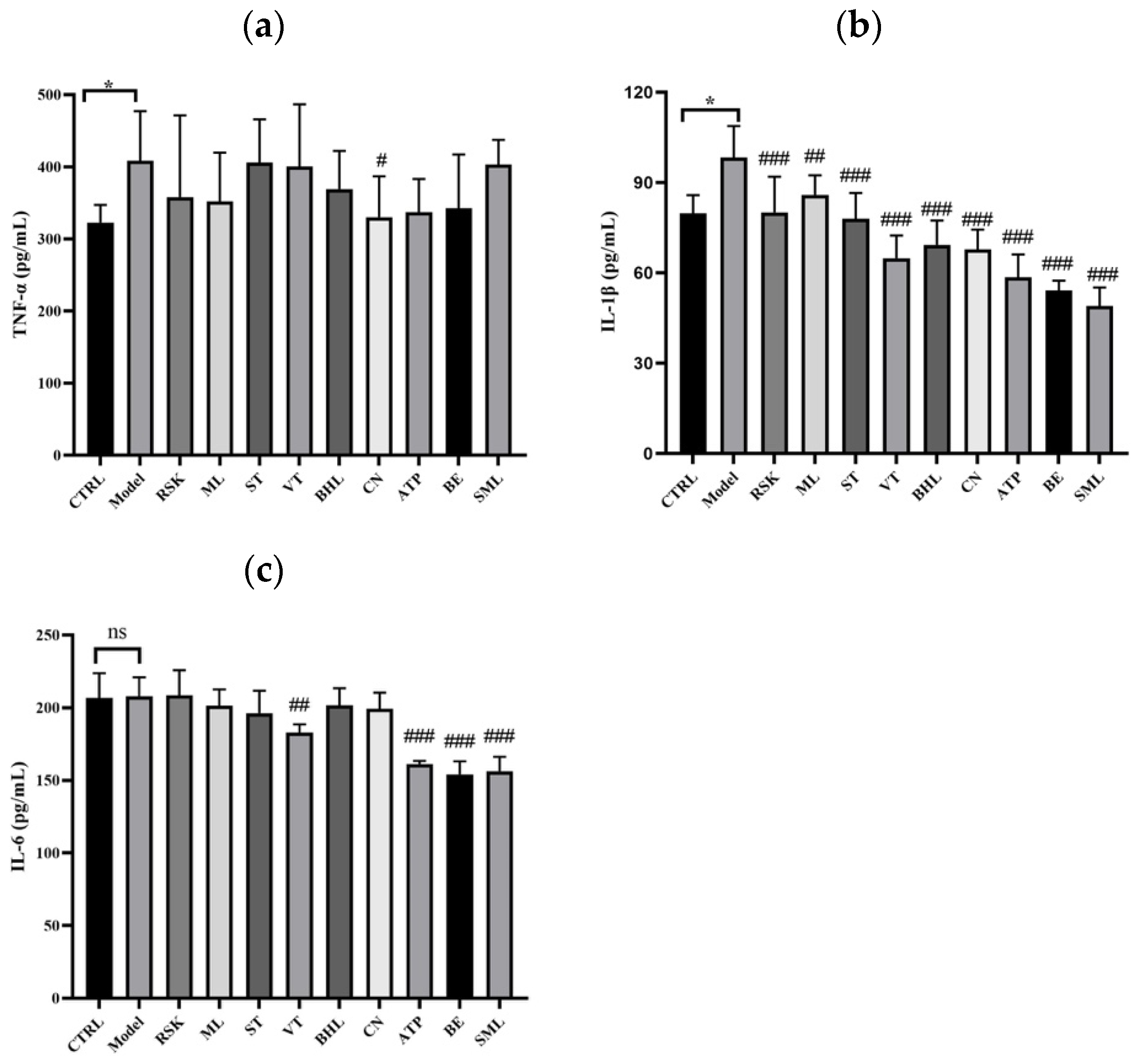
3.6. The Effects of Tea-like Plants on Inflammation in the Liver
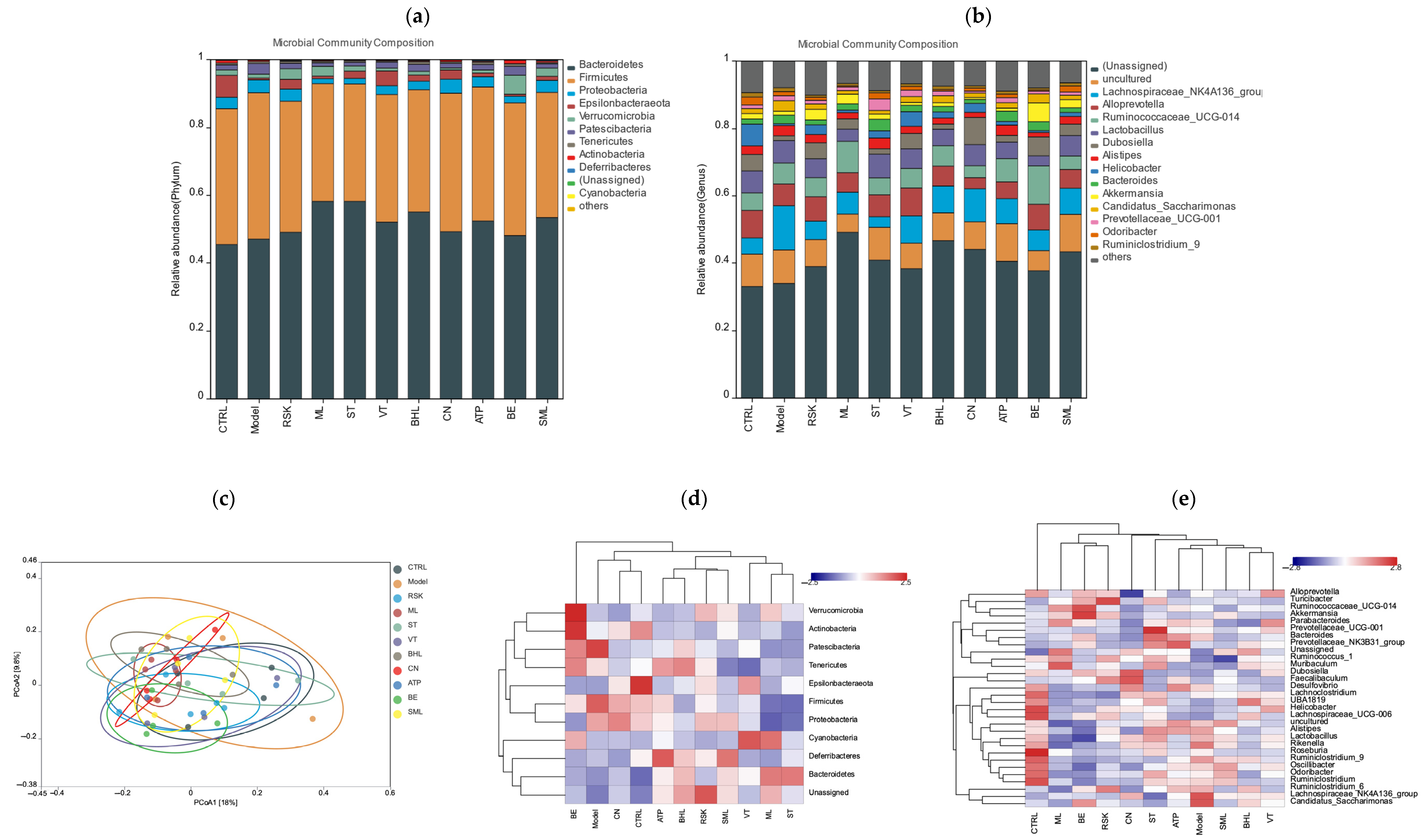
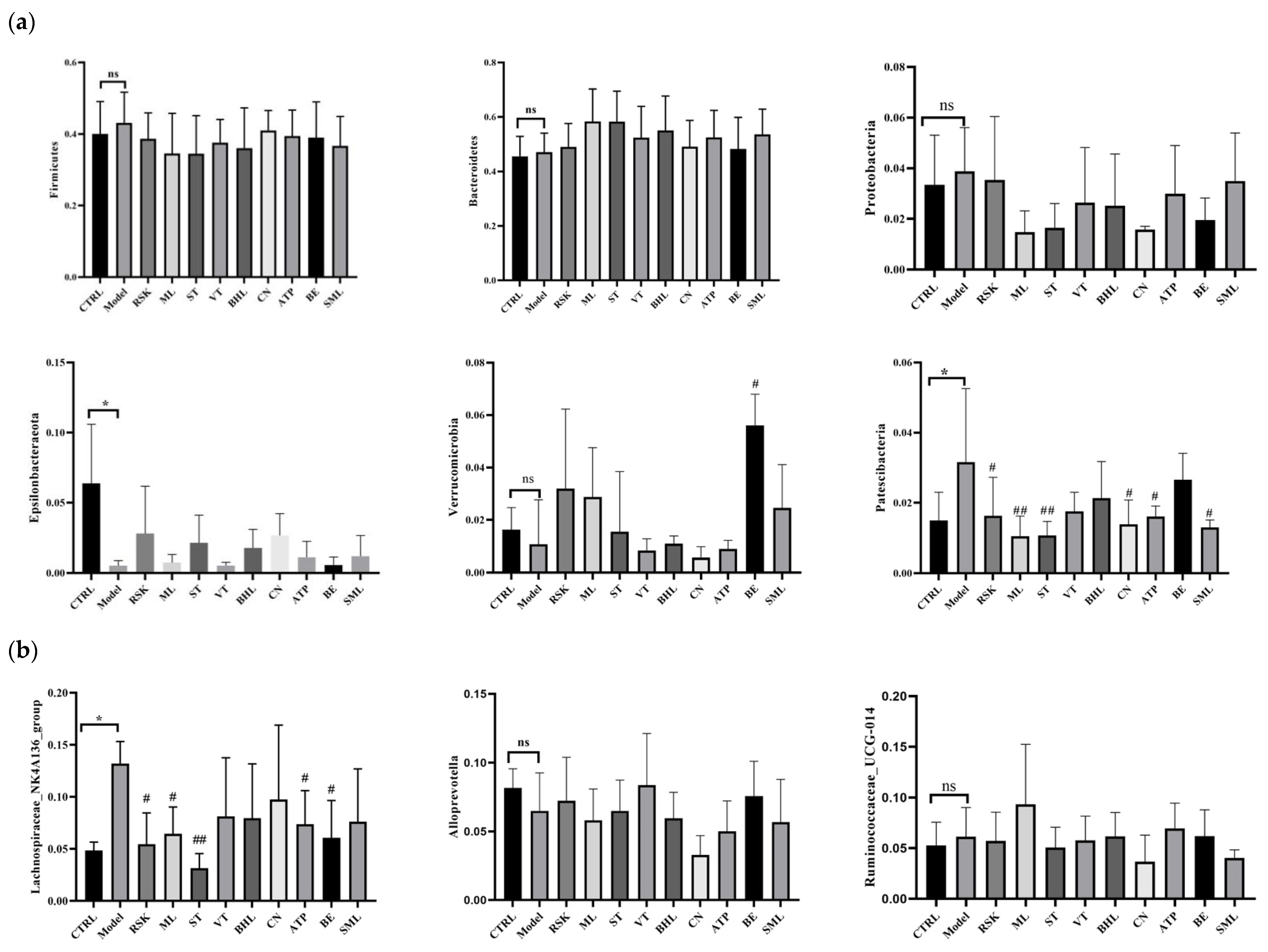
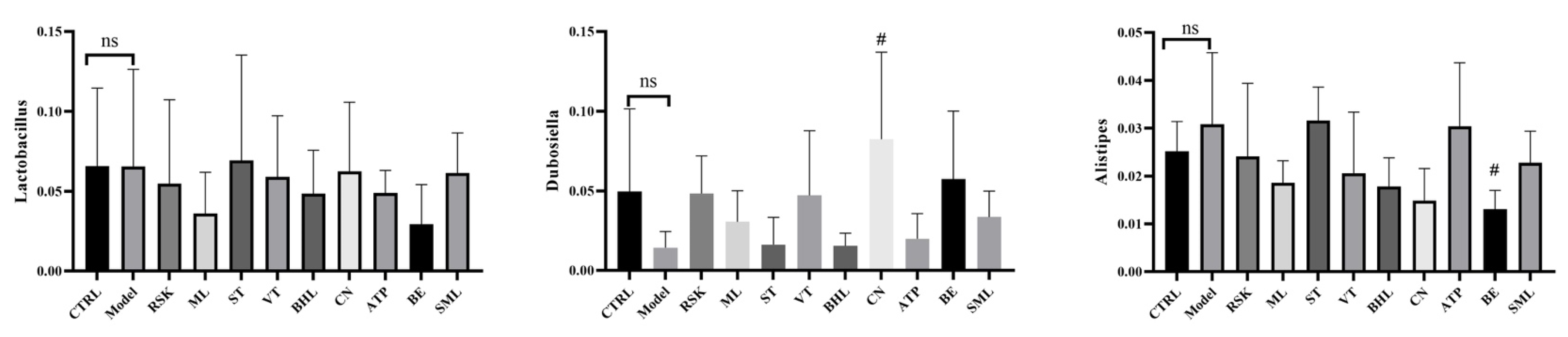
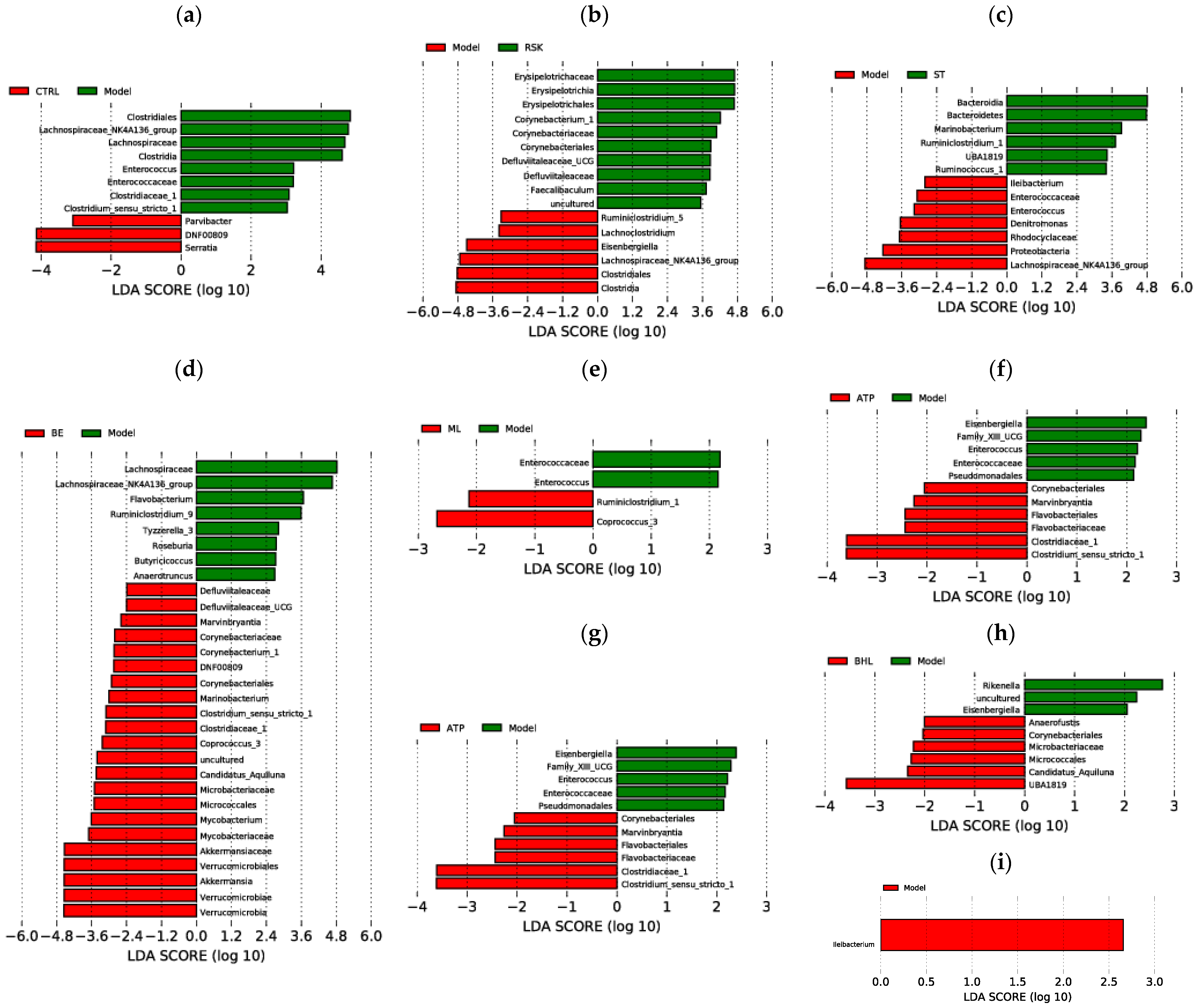
3.7. Effects of Tea-like Plants on Gut Microbiota Diversity and Composition
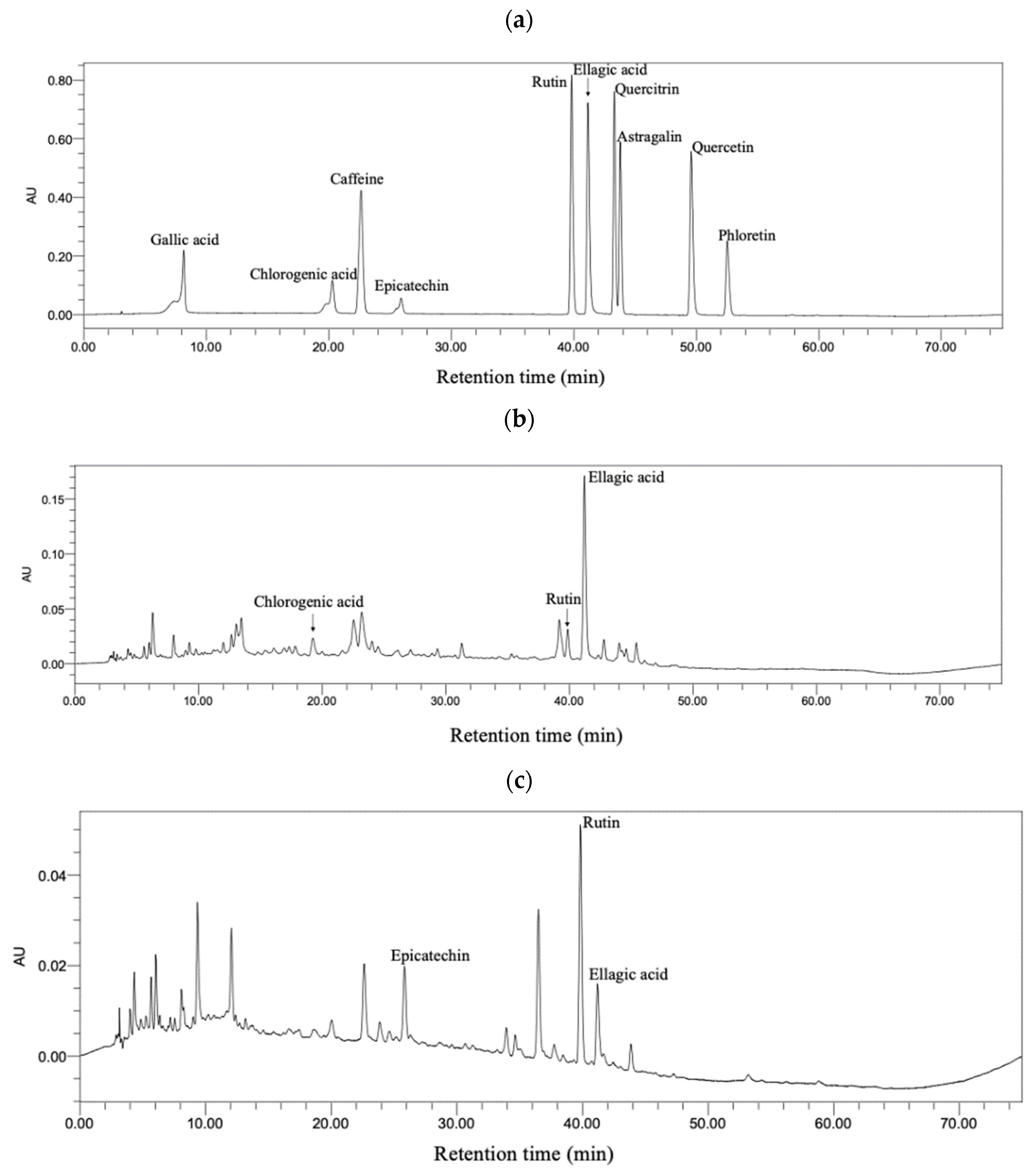
3.8. Bioactive Compounds in Sweet Tea and Camellia nitidissima
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manthey, J.; Shield, K.D.; Rylett, M.; Hasan, O.S.M.; Probst, C.; Rehm, J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: A modelling study. Lancet 2019, 393, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q.; Fan, X.D.; Miyata, T.; Kim, A.; Ross, C.K.C.-D.; Ray, S.; Huang, E.; Taiwo, M.; Arya, R.; Wu, J.G.; et al. Recent advances in understanding of pathogenesis of alcohol-associated liver disease. Annu. Rev. Pathol.-Mech. Dis. 2023, 18, 411–438. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Mathurin, P. Diagnosis and treatment of alcohol-associated liver disease a review. JAMA-J. Am. Med. Assoc. 2021, 326, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global epidemiology of alcohol-associated cirrhosis and HCC: Trends, projections and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G.; Ponziani, F.R.; Dionisi, T.; Mosoni, C.; Vassallo, G.A.; Sestito, L.; Petito, V.; Picca, A.; Marzetti, E.; Tarli, C.; et al. Gut microbiota compositional and functional fingerprint in patients with alcohol use disorder and alcohol-associated liver disease. Liver Int. 2020, 40, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, S.; Li, Y.; Gan, R.Y.; Li, H.B. Gut microbiota’s relationship with liver disease and role in hepatoprotection by dietary natural products and probiotics. Nutrients 2018, 10, 1457. [Google Scholar] [CrossRef]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y.; et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, Y.X.; Pan, C.Q.; Xing, H.C. Gut microbiota in alcohol-related liver disease: Pathophysiology and gut-brain cross talk. Front. Pharmacol. 2023, 14, 1258062. [Google Scholar] [CrossRef]
- Long, P.; Cui, Z.H.; Wang, Y.L.; Zhang, C.H.; Zhang, N.; Li, M.H.; Xiao, P.G. Commercialized non-Camellia tea: Traditional function and molecular identification. Acta Pharm. Sin. B 2014, 4, 227–237. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, R.G.; Zhou, B.F.; Shen, Z.; Chen, X.Y.; Gao, J.; Wang, B.S. Chromosome-scale genome assembly of sweet tea (Lithocarpus polystachyus Rehder). Sci. Data 2023, 10, 873. [Google Scholar] [CrossRef]
- Saimaiti, A.; Huang, S.Y.; Xiong, R.G.; Wu, S.X.; Zhou, D.D.; Yang, Z.J.; Luo, M.; Gan, R.Y.; Li, H.B. Antioxidant capacities and polyphenol contents of kombucha beverages based on vine tea and sweet tea. Antioxidants 2022, 11, 1655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Zhao, Y.F.; Zhang, M.Y.; Zhang, Y.L.; Ji, H.F.; Shen, L. Recent advances in research on vine tea, a potential and functional herbal tea with dihydromyricetin and myricetin as major bioactive compounds. J. Pharm. Anal. 2021, 11, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Zhao, H.F.; Dong, Y.; Yang, B.; Zhao, M.M. Macroporous resin purification behavior of phenolics and rosmarinic acid from Rabdosia serra (MAXIM.) HARA leaf. Food Chem. 2012, 130, 417–424. [Google Scholar] [CrossRef]
- Liu, G.L.; Xu, W.; Liu, X.J.; Yan, X.L.; Chen, J. Two new abietane diterpenoids from the leaves of Rabdosia serra. J. Asian Nat. Prod. Res. 2020, 22, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Chemical composition, bioactivity and safety aspects of kuding tea-from beverage to herbal extract. Nutrients 2020, 12, 2796. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Chen, Y.L.; Yu, Y.Y.; Zang, J.; Wu, Y.K.; He, Z. Ilex latifolia Thunb protects mice from HFD-induced body weight gain. Sci. Rep. 2017, 7, 14660. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Xie, M.H.; Dai, Z.Q.; Wan, P.; Ye, H.; Zeng, X.X.; Sun, Y. Kudingcha and Fuzhuan Brick Tea Prevent Obesity and Modulate Gut Microbiota in High-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, 1700485. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.Y.; Luo, K.Y.; Xiao, Q.; Sun, Z.Y.; Wang, Y.F.; Cui, C.F.; Chen, F.C.; Xu, B.; Shen, W.J.; Wan, F.C.; et al. Effect of mulberry leaf or mulberry leaf extract on glycemic traits: A systematic review and meta-analysis. Food Funct. 2023, 14, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.Q.; Chai, X.Y.; Hou, G.G.; Zhao, F.L.; Meng, Q.G. Phytochemistry, bioactivities and future prospects of mulberry leaves: A review. Food Chem. 2022, 372, 131335. [Google Scholar] [CrossRef]
- Xiong, R.G.; Wu, S.X.; Cheng, J.; Saimaiti, A.; Liu, Q.; Shang, A.; Zhou, D.D.; Huang, S.Y.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic compounds, and sensory acceptability of kombucha-fermented beverages from bamboo leaf and mulberry leaf. Antioxidants 2023, 12, 1573. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Wan, S.Q.; Yao, L.N.; Lin, D.; Wu, T.; Chen, Y.J.; Zhang, A.L.; Lu, C.F. Bamboo leaf: A review of traditional medicinal property, phytochemistry, pharmacology, and purification technology. J. Ethnopharmacol. 2023, 306, 116166. [Google Scholar] [CrossRef] [PubMed]
- Koide, C.L.K.; Collier, A.C.; Berry, M.J.; Panee, J. The effect of bamboo extract on hepatic biotransforming enzymes—Findings from an obese-diabetic mouse model. J. Ethnopharmacol. 2011, 133, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Lin, B.H.; Mo, J.G.; Chen, Q.H.; Su, L.; Li, Y.J.; Yang, K.D. Composition and antioxidant and antibacterial activities of essential oils from three yellow Camellia species. Trees-Struct. Funct. 2019, 33, 205–212. [Google Scholar] [CrossRef]
- He, D.Y.; Li, X.Y.; Sai, X.; Wang, L.L.; Li, S.Y.; Xu, Y.P. Camellia nitidissima CW Chi: A review of botany, chemistry, and pharmacology. Phytochem. Rev. 2018, 17, 327–349. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Peng, X.; Tang, Q.; Yu, D.Y.; Luo, Y.Y.; Shi, L.Y.; Huang, L.D.; Mou, H.M.; Tang, L.; Feng, B.M. Active fraction of IgE-mediated type I allergy from Camellia nitidissima. Cent. South Pharm. 2009, 7, 721–724. [Google Scholar]
- Wu, S.X.; Xiong, R.G.; Cheng, J.; Xu, X.Y.; Tang, G.Y.; Huang, S.Y.; Zhou, D.D.; Saimaiti, A.; Gan, R.Y.; Li, H.B. Preparation, antioxidant activities and bioactive components of kombucha beverages from golden-flower tea (Camellia petelotii) and honeysuckle-flower tea (Lonicera japonica). Foods 2023, 12, 3010. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, D.D.; Shang, A.; Gan, R.Y.; Li, H.B. Influences of microwave-assisted extraction parameters on antioxidant activity of the extract from Akebia trifoliata peels. Foods 2021, 10, 1432. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, H.; Xu, Q.L.; Zhou, Z.Y.; Wu, P.; Wei, X.Y.; Cao, Y.; Chen, X.X.; Tan, J.W. Antibacterial oleanane-type triterpenoids from pericarps of Akebia trifoliata. Food Chem. 2015, 168, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yu, N.X.; Wang, Z.L.; Qiu, T.T.; Jiang, L.; Zhu, X.M.; Sun, Y.; Xiong, H. Akebia trifoliata pericarp extract ameliorates inflammation through NF-κB/MAPK signaling pathways and modifies gut microbiota. Food Funct. 2020, 11, 4682–4696. [Google Scholar] [CrossRef]
- Afifi, N.A.; Ibrahim, M.A.; Galal, M.K. Hepatoprotective influence of quercetin and ellagic acid on thioacetamide-induced hepatotoxicity in rats. Can. J. Physiol. Pharmacol. 2018, 96, 624–629. [Google Scholar] [CrossRef]
- Lee, J.H.; Won, J.H.; Choi, J.M.; Cha, H.H.; Jang, Y.J.; Park, S.; Kim, H.G.; Kim, H.C.U.; Kim, D.K. Protective effect of ellagic acid on concanavalin A-induced hepatitis via toll-like receptor and mitogen-activated protein kinase/nuclear factor kb signaling pathways. J. Agric. Food Chem. 2014, 62, 10110–10117. [Google Scholar] [CrossRef]
- Srigopalram, S.; Jayraaj, I.A.; Kaleeswaran, B.; Balamurugan, K.; Ranjithkumar, M.; Kumar, T.S.; Park, J.I.; Nou, I.S. Ellagic acid normalizes mitochondrial outer membrane permeabilization and attenuates inflamma-tion-mediated cell proliferation in experimental liver cancer. Appl. Biochem. Biotechnol. 2014, 173, 2254–2266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, J.J.; Sheng, L.; Yuan, M.; Wu, Y.; Chen, L.; Wang, G.H.; Qiu, Z.P. Ellagic acid ameliorates AKT-driven hepatic steatosis in mice by suppressing de novo lipogenesis via the AKT/SREBP-1/FASN pathway. Food Funct. 2019, 10, 3410–3420. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Y.; Li, S.; Gan, R.Y.; Li, H.B. Natural Products for Prevention and Treatment of Chemical-Induced Liver Injuries. Compr. Rev. Food Sci. Food Saf. 2018, 17, 472–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.J.; Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Zhang, J.J.; Li, S.; Xu, D.P.; Li, H.B. Effects of Beverages on Alcohol Metabolism: Potential Health Benefits and Harmful Impacts. Int. J. Mol. Sci. 2016, 17, 354. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Mao, Q.Q.; Zhou, D.D.; Luo, M.; Gan, R.Y.; Li, H.Y.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Li, H.B. Effects of tea against alcoholic fatty liver disease by modulating gut microbiota in chronic alcohol-exposed mice. Foods 2021, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.Y.; Li, B.Y.; Gan, R.Y.; Mao, Q.Q.; Wang, Y.F.; Shang, A.; Meng, J.M.; Xu, X.Y.; Wei, X.L.; Li, H.B. The in vivo antioxidant and hepatoprotective actions of selected Chinese teas. Foods 2020, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.C.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.H.; Liangpunsakul, S. Alcohol metabolizing enzymes, microsomal ethanol oxidizing system, cytochrome P450 2E1, catalase, and aldehyde dehydrogenase in alcohol-associated liver disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef]
- Tran, S.; Nowicki, M.; Chatterjee, D.; Gerlai, R. Acute and chronic ethanol exposure differentially alters alcohol dehydrogenase and aldehyde dehydrogenase activity in the zebrafish liver. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 56, 221–226. [Google Scholar] [CrossRef]
- Pohl, K.; Moodley, P.; Dhanda, A.D. Alcohol’s impact on the gut and liver. Nutrients 2021, 13, 3170. [Google Scholar] [CrossRef]
- Zhang, X.M.; Feng, J.; Su, S.F.; Huang, L. Hepatoprotective effects of Camellia nitidissima aqueous ethanol extract against CCl4-induced acute liver injury in SD rats related to Nrf2 and NF-κB signalling. Pharm. Biol. 2020, 58, 239–246. [Google Scholar] [CrossRef]
- Liu, X.K.; Wang, K.Y.; Cai, G.Z.; Li, H.T.; Guo, Y.L.; Gong, J.Y. Comparative chemical diversity and antioxidant activities of three species of Akebia herbal medicines. Arab. J. Chem. 2023, 16, 104549. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yu, N.X.; Peng, H.L.; Hu, Z.Y.; Sun, Y.; Zhu, X.M.; Jiang, L.; Xiong, H. The profiling of bioactives in Akebia trifoliata pericarp and metabolites, bioavailability and in vivo anti-inflammatory activities in DSS-induced colitis mice. Food Funct. 2019, 10, 3977–3991. [Google Scholar] [CrossRef]
- Jeon, S.; Carr, R. Alcohol effects on hepatic lipid metabolism. J. Lipid Res. 2020, 61, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Oku, H.; Ogawa, Y.; Iwaoka, E.; Yamaguchi, Y.; Kagota, S.; Kazumasa, S.; Kunitomo, M.; Ishiguro, K. Preventive effects of the extract of kinka-cha, a folk tea, on a rat model of metabolic syndrome. J. Nat. Med. 2011, 65, 610–616. [Google Scholar] [CrossRef]
- Li, B.Y.; Li, H.Y.; Zhou, D.D.; Huang, S.Y.; Luo, M.; Gan, R.Y.; Mao, Q.Q.; Saimaiti, A.; Shang, A.; Li, H.B. Effects of Different Green Tea Extracts on Chronic Alcohol Induced-Fatty Liver Disease by Ameliorating Oxidative Stress and Inflammation in Mice. Oxidative Med. Cell. Longev. 2021, 2021, 5188205. [Google Scholar] [CrossRef]
- Munkong, N.; Somnuk, S.; Jantarach, N.; Ruxsanawet, K.; Nuntaboon, P.; Kanjoo, V.; Yoysungnoen, B. Red Rice Bran Extract Alleviates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease and Dyslipidemia in Mice. Nutrients 2023, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Ran, B.B.; Guo, C.E.; Li, W.D.; Li, W.S.; Wang, Q.; Qian, J.X.; Li, H.L. Sea buckthorn (Hippophae rhamnoides L.) fermentation liquid protects against alcoholic liver disease linked to regulation of liver metabolome and the abundance of gut microbiota. J. Sci. Food Agric. 2021, 101, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Xie, Z.Y.; Zhang, Y.; Liu, Y.; Niu, A.J.; Liu, Y.Y.; Zhang, L.B.; Guan, L.L. Rosa rugosa polysaccharide attenuates alcoholic liver disease in mice through the gut-liver axis. Food Biosci. 2021, 44, 101385. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology—Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.W.; Liu, X.F.; Liang, L.H.; Wang, G.Q.; Xiong, Z.Q.; Zhang, H.; Song, X.; Ai, L.Z.; Xia, Y.J. Antrodin A from Antrodia camphorata modulates the gut microbiome and liver metabolome in mice exposed to acute alcohol intake. Food Funct. 2021, 12, 2925–2937. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Z.; Li, R.; Jiang, Y.M.; Zhao, F.; Yu, Z.S.; Wang, Y.Q.; Dong, Z.X.; Liu, P.; Li, X.K. Bifidobacterium breve ATCC15700 pretreatment prevents alcoholic liver disease through modulating gut microbiota in mice exposed to chronic alcohol intake. J. Funct. Food. 2020, 72, 104045. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.J.; Zhang, S.Y.; Li, Y.S.; Yang, X.; Li, N.; Zeng, G.F.; Chen, F.P.; Tuo, X. Insight into the binding characteristics of rutin and alcohol dehydrogenase: Based on the biochemical method, spectroscopic experimental and molecular model. J. Photochem. Photobiol. B-Biol. 2022, 228, 112394. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, V.; Solaipriya, S.; Sivaramakrishnan, V. Role of ellagic acid for the prevention and treatment of liver diseases. Phytother. Res. 2021, 35, 2925–2944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mehmood, A.; Soliman, M.M.; Iftikhar, A.; Iftikhar, M.; Aboelenin, S.M.; Wang, C.T. Protective effects of ellagic acid against alcoholic liver disease in mice. Front. Nutr. 2021, 8, 744520. [Google Scholar] [CrossRef] [PubMed]
- Siroma, T.K.; Machate, D.J.; Zorgetto-Pinheiro, V.A.; Figueiredo, P.S.; Marcelino, G.; Hiane, P.A.; Bogo, D.; Pott, A.; Cury, E.R.J.; Guimaraes, R.D.A.; et al. Polyphenols and ω-3 pUFAs: Beneficial outcomes to obesity and its related metabolic diseases. Front. Nutr. 2022, 8, 781622. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sim, Y.; Hwang, J.H.; Kwun, I.S.; Lim, J.H.; Kim, J.; Kim, J.I.; Baek, M.C.; Akbar, M.; Seo, W.; et al. Ellagic acid prevents binge alcohol-induced leaky gut and liver injury through inhibiting gut dysbiosis and oxidative stress. Antioxidants 2021, 10, 1386. [Google Scholar] [CrossRef]
- Kim, H.; Pan, J.H.; Kim, S.H.; Lee, J.H.; Park, J.W. Chlorogenic acid ameliorates alcohol-induced liver injuries through scavenging reactive oxygen species. Biochimie 2018, 150, 131–138. [Google Scholar] [CrossRef]
- Zhu, H.K.; Jiang, W.H.; Liu, C.; Wang, C.; Hu, B.; Guo, Y.H.; Cheng, Y.L.; Qian, H. Ameliorative effects of chlorogenic acid on alcoholic liver injury in mice via gut microbiota informatics. Eur. J. Pharmacol. 2022, 928, 175096. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Das, D.K.; Manna, K.; Datta, S.; Ray, T.; Sil, A.K.; Dey, S. Epicatechin ameliorates ionising radiation-induced oxidative stress in mouse liver. Free Radic. Res. 2012, 46, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, Y.N.; Xu, Y.L.; Hu, Z.H.; Wan, X.; Huang, H.C.; Huang, D.B. Protective effect of epicatechin on APAP-induced acute liver injury of mice through anti-inflammation and apoptosis inhibition. Nat. Prod. Res. 2020, 34, 855–858. [Google Scholar] [CrossRef] [PubMed]
| No. | Herbal Tea | Scientific Name | Place of Origin |
|---|---|---|---|
| 1 | Xi Huang Cao | Rabdosia serra (Maxim.) Kudo | Zhaoqing, Guangdong |
| 2 | Mulberry leaf | Morus alba Linn | Bozhou, Anhui |
| 3 | Sweet tea | Rubus suavissimus S. Lee | Jinxiu, Guangxi |
| 4 | Vine tea | Ampelopsis grossedentata (Hand.-Mazz.) W. T. Wang | Enshi, Hubei |
| 5 | Broadleaf holly leaf | Ilex latifolia Thunb. | Yuqing, Guizhou |
| 6 | Golden-flower tea | Camellia nitidissima C. W. Chi | Fangchenggang, Guangxi |
| 7 | Akebia pericarp | Akebia trifoliata (Thunb.) Koidz. | Haikou, Hainan |
| 8 | Bamboo leaf | Lophatherum gracile Brongn. | Chengdu, Sichuan |
| Group | ACE Index | Chao1 Index | Simpson Index | Shannon’s E Index |
|---|---|---|---|---|
| CTRL | 1053.55 ± 24.94 | 8.63 ± 2.38 | 0.40 ± 0.05 | 1.13 ± 0.11 |
| Model | 1081.08 ± 27.69 | 5.88 ± 1.52 | 0.43 ± 0.02 | 1.01 ± 0.07 |
| RSK | 1081.00 ± 36.24 | 7.40 ± 2.22 | 0.41 ± 0.03 | 1.09 ± 0.08 |
| ML | 1091.98 ± 19.95 | 8.63 ± 4.08 | 0.49 ± 0.06 | 0.93 ± 0.12 |
| ST | 1072.50 ± 31.70 | 6.25 ± 0.83 | 0.49 ± 0.05 | 0.89 ± 0.08 |
| VT | 1033.98 ± 47.87 | 6.63 ± 2.41 | 0.44 ± 0.07 | 0.98 ± 0.15 |
| BHL | 1086.60 ± 21.53 | 8.88 ± 2.63 | 0.47 ± 0.04 | 0.98 ± 0.08 |
| CN | 1022.45 ± 65.62 | 8.91 ± 3.71 | 0.43 ± 0.04 | 1.04 ± 0.08 |
| ATP | 1097.35 ± 5.02 | 8.88 ± 2.77 | 0.45 ± 0.04 | 0.99 ± 0.12 |
| BE | 1054.68 ± 34.84 | 6.68 ± 1.26 | 0.41 ± 0.03 | 1.13 ± 0.09 |
| SML | 1070.85 ± 6.23 | 6.28 ± 1.06 | 0.44 ± 0.05 | 1.02 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Luo, M.; Zhou, D.-D.; Huang, S.; Xiong, R.; Wu, S.; Saimaiti, A.; Li, B.; Shang, A.; Tang, G.-Y.; et al. Effects of Several Tea-like Plants on Liver Injury Induced by Alcohol via Their Antioxidation, Anti-Inflammation, and Regulation of Gut Microbiota. Foods 2024, 13, 2521. https://doi.org/10.3390/foods13162521
Cheng J, Luo M, Zhou D-D, Huang S, Xiong R, Wu S, Saimaiti A, Li B, Shang A, Tang G-Y, et al. Effects of Several Tea-like Plants on Liver Injury Induced by Alcohol via Their Antioxidation, Anti-Inflammation, and Regulation of Gut Microbiota. Foods. 2024; 13(16):2521. https://doi.org/10.3390/foods13162521
Chicago/Turabian StyleCheng, Jin, Min Luo, Dan-Dan Zhou, Siyu Huang, Ruogu Xiong, Sixia Wu, Adila Saimaiti, Bangyan Li, Ao Shang, Guo-Yi Tang, and et al. 2024. "Effects of Several Tea-like Plants on Liver Injury Induced by Alcohol via Their Antioxidation, Anti-Inflammation, and Regulation of Gut Microbiota" Foods 13, no. 16: 2521. https://doi.org/10.3390/foods13162521
APA StyleCheng, J., Luo, M., Zhou, D.-D., Huang, S., Xiong, R., Wu, S., Saimaiti, A., Li, B., Shang, A., Tang, G.-Y., & Li, H. (2024). Effects of Several Tea-like Plants on Liver Injury Induced by Alcohol via Their Antioxidation, Anti-Inflammation, and Regulation of Gut Microbiota. Foods, 13(16), 2521. https://doi.org/10.3390/foods13162521








