Evaluating Source Complexity in Blended Milk Cheese: Integrated Strontium Isotope and Multi-Elemental Approach to PDO Graviera Naxos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
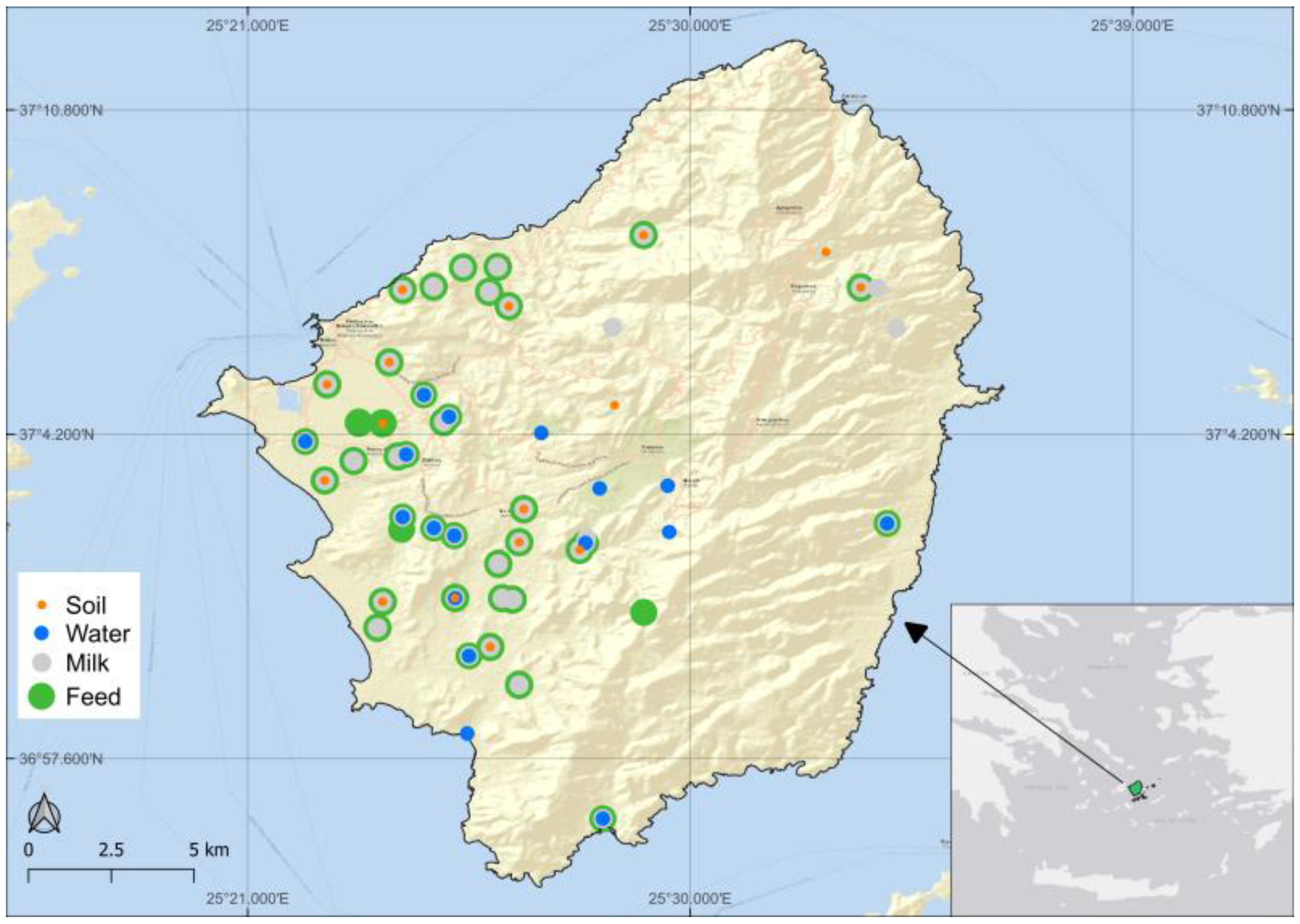
2.2. Study Area
2.3. Sample Collection and Preparation
2.4. Multi-Elemental and Sr Isotopic Analyses
2.5. Statistical Evaluation
3. Results and Discussion
3.1. Elemental Composition of Environmental Samples
3.2. Elemental Composition of Milk and Graviera Naxos Cheese Samples
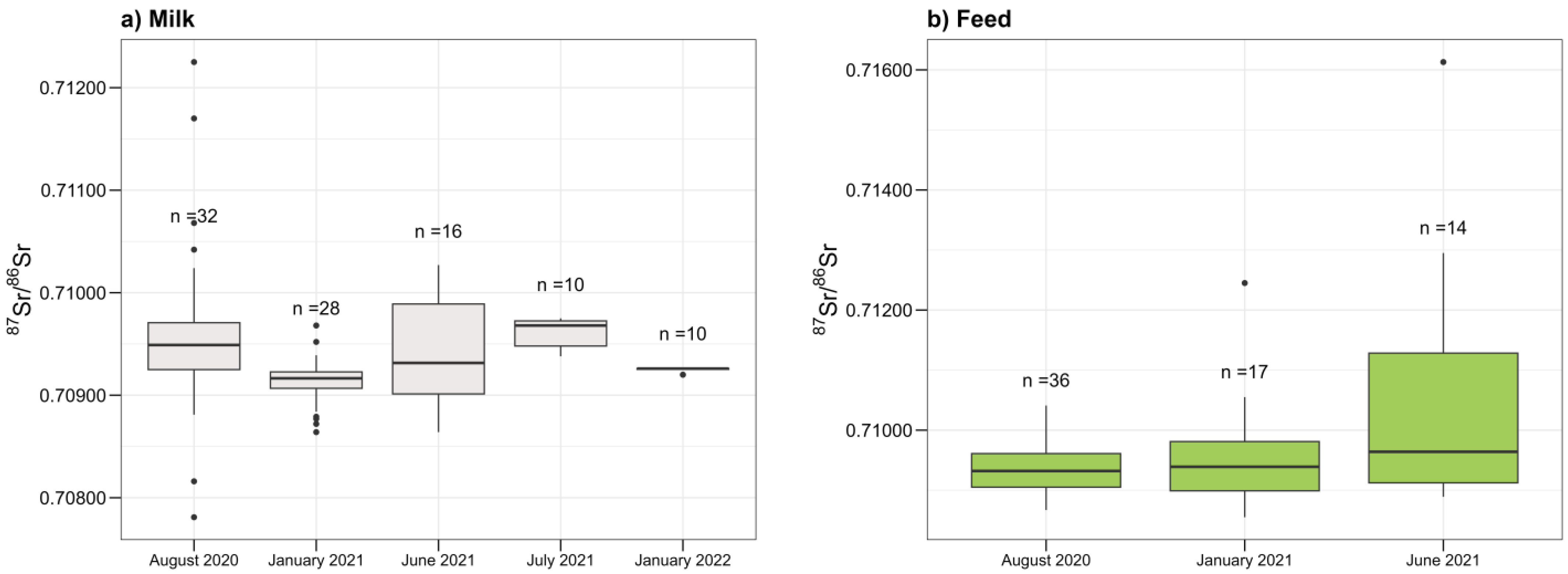
3.3. Distribution of 87Sr/86Sr Values and Statistical Analysis
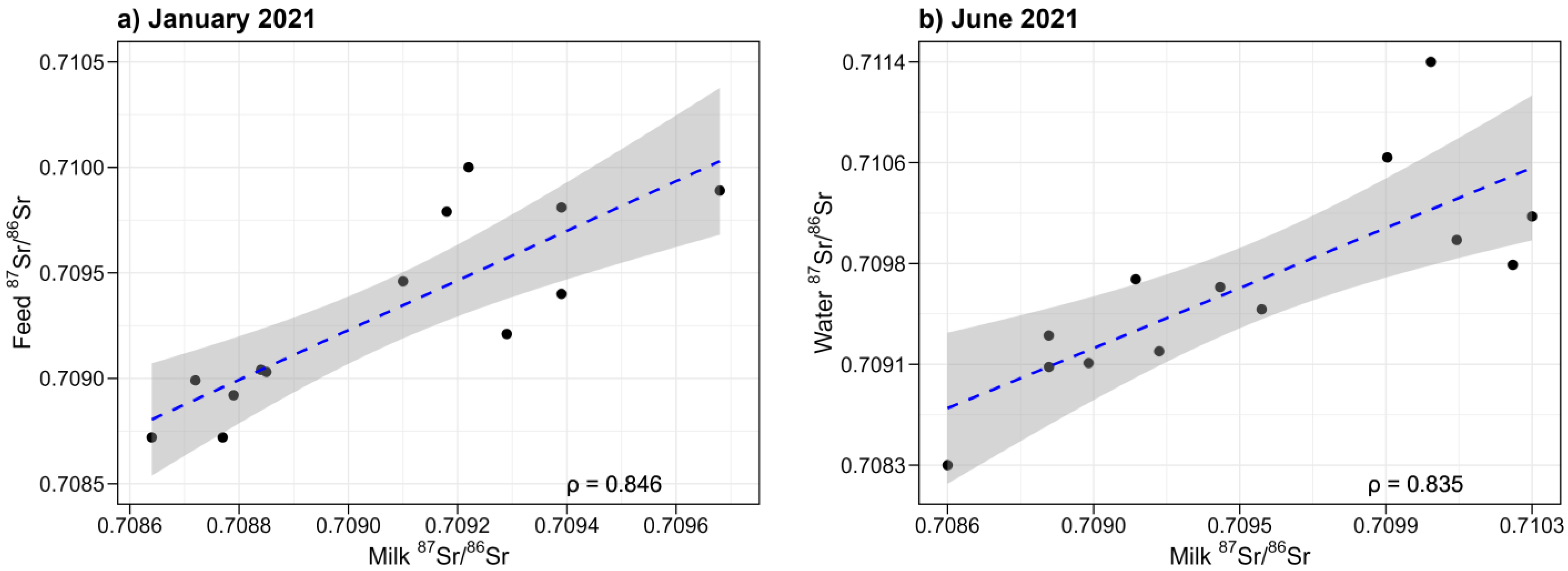
3.4. Contributions to Sr Isotopic Composition of Milk
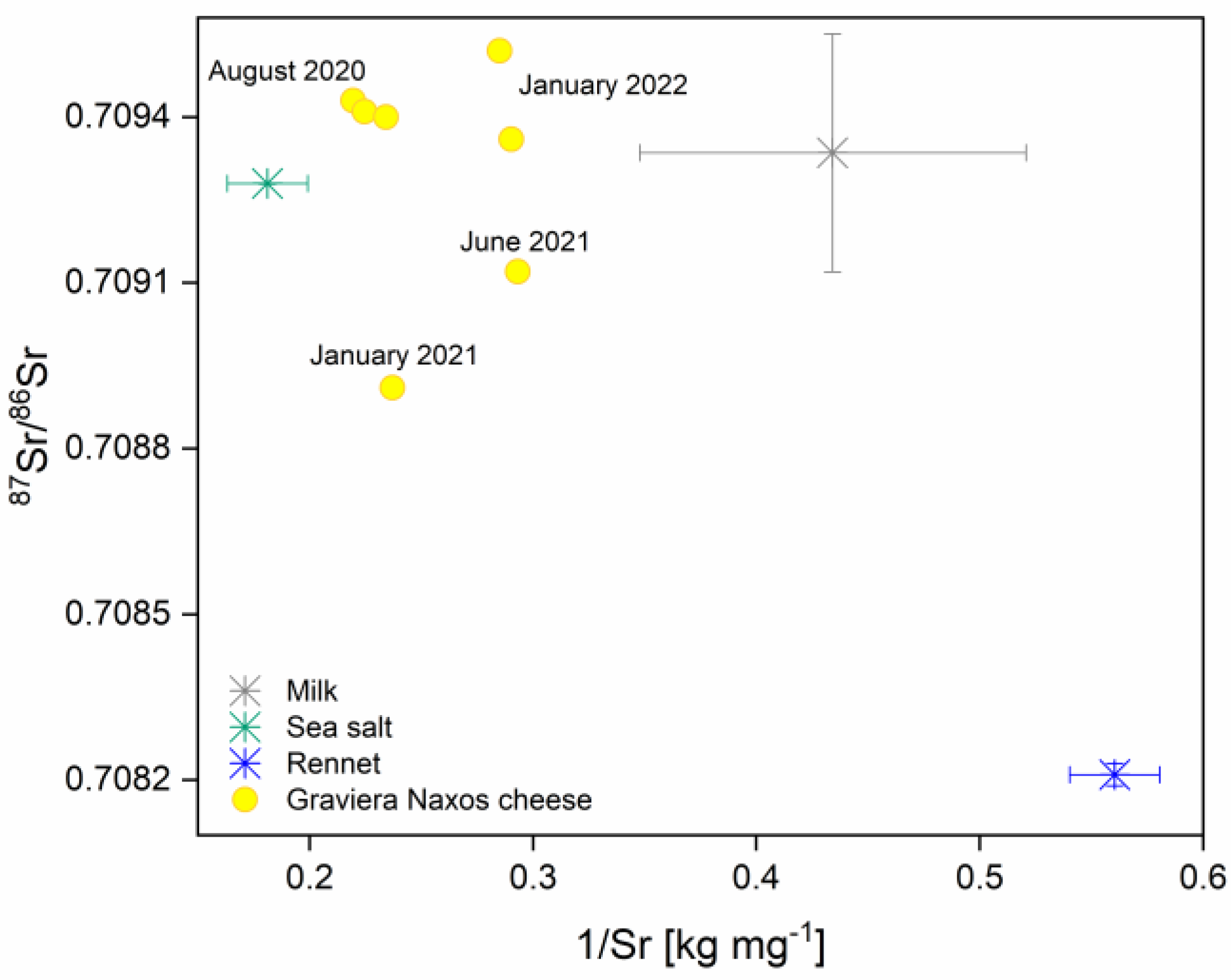
3.5. Mixing Model for Estimating Contributions to Cheese Sr Isotopic Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giannakas, K.; Yiannaka, A. Food Fraud: Causes, Consequences, and Deterrence Strategies. Annu. Rev. Resour. Econ. 2023, 15, 85–104. [Google Scholar] [CrossRef]
- Montgomery, H.; Haughey, S.A.; Elliott, C.T. Recent food safety and fraud issues within the dairy supply chain (2015–2019). Glob. Food Secur. 2020, 26, 100447. [Google Scholar] [CrossRef] [PubMed]
- Dimara, E.; Skuras, D. Consumer demand for informative labeling of quality food and drink products: A European Union case study. J. Consum. Mark. 2005, 22, 90–100. [Google Scholar] [CrossRef]
- Dimitrakopoulou, M.E.; Vantarakis, A. Does Traceability Lead to Food Authentication? A Systematic Review from A European Perspective. Food Rev. Int. 2023, 39, 537–559. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Brusic, V.; Georgiou, C.A. Food authentication: State of the art and prospects. Curr. Opin. Food Sci. 2016, 10, 22–31. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Martínez-Salvador, L.E.; Reyes-Jaime, A.; Martínez-Salvador, L.E.; Reyes-Jaime, A. Sustainability in Protected Designations of Origin (PDO) in the European Union: An approach from a Systematic Literature Review. Siembra 2021, 8, e3288. [Google Scholar] [CrossRef]
- Kwasi Bannor, R.; Arthur, K.K.; Oppong, D.; Oppong-Kyeremeh, H. A comprehensive systematic review and bibliometric analysis of food fraud from a global perspective. J. Agric. Food Res. 2023, 14, 100686. [Google Scholar] [CrossRef]
- Griboff, J.; Baroni, M.V.; Horacek, M.; Wunderlin, D.A.; Monferran, M.V. Multielemental + isotopic fingerprint enables linking soil, water, forage and milk composition, assessing the geographical origin of Argentinean milk. Food Chem. 2019, 283, 549–558. [Google Scholar] [CrossRef]
- Potočnik, D.; Nečemer, M.; Perišić, I.; Jagodic, M.; Mazej, D.; Camin, F.; Eftimov, T.; Strojnik, L.; Ogrinc, N. Geographical verification of Slovenian milk using stable isotope ratio, multi-element and multivariate modelling approaches. Food Chem. 2020, 326, 126958. [Google Scholar] [CrossRef]
- Bontempo, L.; Lombardi, G.; Paoletti, R.; Ziller, L.; Camin, F. H, C, N and O stable isotope characteristics of alpine forage, milk and cheese. Int. Dairy J. 2012, 23, 99–104. [Google Scholar] [CrossRef]
- Bontempo, L.; Larcher, R.; Camin, F.; Hölzl, S.; Rossmann, A.; Horn, P.; Nicolini, G. Elemental and isotopic characterisation of typical Italian alpine cheeses. Int. Dairy J. 2011, 21, 441–446. [Google Scholar] [CrossRef]
- Crittenden, R.G.; Andrew, A.S.; Lefournour, M.; Young, M.D.; Middleton, H.; Stockmann, R. Determining the geographic origin of milk in Australasia using multi-element stable isotope ratio analysis. Int. Dairy J. 2007, 17, 421–428. [Google Scholar] [CrossRef]
- Fortunato, G.; Mumic, K.; Wunderli, S.; Pillonel, L.; Bosset, J.O.; Gremaud, G. Application of strontium isotope abundance ratios measured by MC-ICP-MS for food authentication. J. Anal. At. Spectrom. 2004, 19, 227–234. [Google Scholar] [CrossRef]
- Horacek, M.; Lage, M.; Vakhlu, J. Exploring the Potential of 87Sr/86Sr Isotope Ratio with Strontium and Rubidium Levels to Assess the Geographic Origin of Saffron. Foods 2023, 12, 2830. [Google Scholar] [CrossRef] [PubMed]
- Cellier, R.; Bérail, S.; Barre, J.; Epova, E.; Ronzani, A.-L.; Van Leeuwen, C.; Milcent, S.; Ors, P.; Donard, O.F.X. Specificity and Origin of the Stability of the Sr Isotopic Ratio in Champagne Wines. Molecules 2021, 26, 5104. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, S.; Brunner, M.; Boulyga, S.F.; Galler, P.; Horacek, M.; Prohaska, T. Identification of Marchfeld asparagus using Sr isotope ratio measurements by MC-ICP-MS. Anal. Bioanal. Chem. 2008, 390, 487–494. [Google Scholar] [CrossRef]
- Techer, I.; Medini, S.; Janin, M.; Arregui, M. Impact of agricultural practice on the Sr isotopic composition of food products: Application to discriminate the geographic origin of olives and olive oil. Appl. Geochem. 2017, 82, 1–14. [Google Scholar] [CrossRef]
- Tchaikovsky, A.; Zitek, A.; Irrgeher, J.; Opper, C.; Scheiber, R.; Moder, K.; Congiu, L.; Prohaska, T. Chemometric tools for determining site-specific elemental and strontium isotopic fingerprints in raw and salted sturgeon caviar. Eur. Food Res. Technol. 2019, 245, 2515–2528. [Google Scholar] [CrossRef]
- Andriessen, P.A.M.; Boelrijk, N.A.I.M.; Hebeda, E.H.; Priem, H.N.A.; Verdurnen, E.A.T.; Verschure, R.H. Dating the events of metamorphism and granitic magmatism in the Alpine orogen of Naxos (Cyclades, Greece). Contrib. Mineral. Petrol. 1979, 69, 215–225. [Google Scholar] [CrossRef]
- Voerkelius, S.; Lorenz, G.D.; Rummel, S.; Quétel, C.R.; Heiss, G.; Baxter, M.; Brach-Papa, C.; Deters-Itzelsberger, P.; Hoelzl, S.; Hoogewerff, J.; et al. Strontium isotopic signatures of natural mineral waters, the reference to a simple geological map and its potential for authentication of food. Food Chem. 2010, 118, 933–940. [Google Scholar] [CrossRef]
- Nafplioti, A. Tracing population mobility in the Aegean using isotope geochemistry: A first map of local biologically available 87 Sr/86 Sr signatures. J. Archaeol. Sci. 2011, 38, 1560–1570. [Google Scholar] [CrossRef]
- Prevedorou, E.A. The Role of Kin Relations and Residential Mobility during the Transition from Final Neolithic to Early Bronze Age in Attica, Greece. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2015. [Google Scholar]
- Hoogewerff, J.A.; Reimann, C.; Ueckermann, H.; Frei, R.; Frei, K.M.; van Aswegen, T.; Stirling, C.; Reid, M.; Clayton, A.; Ladenberger, A.; et al. Bioavailable 87Sr/86Sr in European soils: A baseline for provenancing studies. Sci. Total Environ. 2019, 672, 1033–1044. [Google Scholar] [CrossRef]
- Frank, A.B.; Frei, R.; Moutafi, I.; Voutsaki, S.; Orgeolet, R.; Kristiansen, K.; Frei, K.M. The geographic distribution of bioavailable strontium isotopes in Greece—A base for provenance studies in archaeology. Sci. Total Environ. 2021, 791, 148156. [Google Scholar] [CrossRef]
- Kizos, T.; Vakoufaris, H. Alternative Agri-Food Geographies? Geographic Indications in Greece. Tijdschr. Voor Econ. Soc. Geogr. 2011, 102, 220–235. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1107/96 of 12 June 1996 on the Registration of Geographical Indications and Designations of Origin under the Procedure Laid down in Article 17 of Council Regulation (EEC) No 2081/92. 1996. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31996R1107 (accessed on 8 April 2024).
- Greek Government Gazette. Joint Ministerial Decision 313071/18.01.94—Conditions, Terms, and Procedure for Establishing Designations of Origin for Agricultural Products. 1994. Available online: https://www.et.gr/api/DownloadFeksApi/?fek_pdf=19940200023 (accessed on 26 March 2024).
- Cao, S.; Neubauer, F.; Bernroider, M.; Liu, J. The lateral boundary of a metamorphic core complex: The Moutsounas shear zone on Naxos, Cyclades, Greece. J. Struct. Geol. 2013, 54, 103–128. [Google Scholar] [CrossRef]
- Georgia, P.P.; Kotopouli, C.N.; Piper, D.J.W. Granitoid rocks of Naxos, Greece: Regional geology and petrology. Geol. J. 1997, 32, 153–171. [Google Scholar]
- Partsinevelou, A.S.; Lozios, S.; Stournaras, G. Fracture pattern description and analysis of the hard rock hydrogeological environment in Naxos Island, Hellas. In Advances in the Research of Aquatic Environment; Springer: Berlin/Heidelberg, Germany, 2011; pp. 45–52. [Google Scholar] [CrossRef]
- Brichau, S. Constraining the Tectonic Evolution of Extensional Fault Systems in the Cyclades (Greece) Using Low-Temperature Thermochronology. Ph.D. Thesis, Johannes Gutenberg-Universität Mainz, Mainz, Germany, 2004. Available online: https://theses.hal.science/tel-00006814 (accessed on 26 March 2024).
- Leonidopolou, D.; Evelpidou, N.; Vasilopoulos, A.; Stournaras, G. Geomorphologic Factors Affecting Intrinsic Vulnerability in Fissured Media. Application on Falatados—Livada area, SE Tinos island (Cyclades—Greece). In Proceedings of the 8th International Hydrogeological Congress of Greece, Athens, Greece, 8–10 October 2008; Available online: https://www.researchgate.net/publication/339272023_Geomorphologic_factors_affecting_intrinsic_vulnerability_in_fissured_media_Application_on_Falatados_-_Livada_area_SE_Tinos_island_Cyclades_-_Greece (accessed on 26 March 2024).
- Theocharatos, G. The Climate of Cyclades. Ph.D. Thesis, Universitry of Athens, Athens, Greece, 1978. [Google Scholar]
- Maroukian, H.; Leonidopoulou, D.; Skarpelis, N.; Stournaras, G. Effects of Lithology, Mineralogy, and Weathering on Particle Size Variability of Sediments in the Coastal Environment of Livada Bay in SE Tinos Island, Greece. J. Coast. Res. 2010, 2010, 167–175. [Google Scholar] [CrossRef]
- Zuliani, T.; Kanduč, T.; Novak, R.; Vreča, P. Characterization of bottled waters by multielemental analysis, stable and radiogenic isotopes. Water 2020, 12, 2454. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- European Parliament. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002L0032 (accessed on 17 May 2024).
- World Health Organization. Fourth Edition Incorporating the First and Second Addenda Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Council of the European Union. Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. 1998. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:1998:330:TOC (accessed on 17 June 2024).
- Khan, I.T.; Nadeem, M.; Imran, M.; Ayaz, M.; Ajmal, M.; Ellahi, M.Y.; Khalique, A. Antioxidant capacity and fatty acids characterization of heat treated cow and buffalo milk. Lipids Health Dis. 2017, 16, 163. [Google Scholar] [CrossRef]
- Nečemer, M.; Potočnik, D.; Ogrinc, N. Discrimination between Slovenian cow, goat and sheep milk and cheese according to geographical origin using a combination of elemental content and stable isotope data. J. Food Compos. Anal. 2016, 52, 16–23. [Google Scholar] [CrossRef]
- Bontempo, L.; Barbero, A.; Bertoldi, D.; Camin, F.; Larcher, R.; Perini, M.; Sepulcri, A.; Zicarelli, L.; Piasentier, E. Isotopic and elemental profiles of Mediterranean buffalo milk and cheese and authentication of Mozzarella di Bufala Campana PDO: An initial exploratory study. Food Chem. 2019, 285, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.L.d.P.; Baptista, D.P.; Orlando, E.A.; Gigante, M.L.; Pallone, J.A.L. Effect of processing on the bioaccessibility of essential minerals in goat and cow milk and dairy products assessed by different static in vitro digestion models. Food Chem. 2022, 374, 131739. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. 2023. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32023R0915 (accessed on 17 June 2024).
- Choi HBin Lee, K.S.; Park, S.; Jeong, E.K.; Gautam, M.K.; Shin, W.J. National-scale distribution of strontium isotope ratios in environmental samples from South Korea and its implications for provenance studies. Chemosphere 2023, 317, 137895. [Google Scholar] [CrossRef]
- Aguzzoni, A.; Bassi, M.; Robatscher, P.; Scandellari, F.; Tirler, W.; Tagliavini, M. Intra- And intertree variability of the 87Sr/86Sr ratio in apple orchards and its correlation with the soil 87Sr/86Sr ratio. J. Agric. Food Chem. 2019, 67, 5728–5735. [Google Scholar] [CrossRef]
- Gregorčič, S.H.; Ogrinc, N.; Frew, R.; Nečemer, M.; Strojnik, L.; Zuliani, T. The provenance of slovenian milk using 87Sr/86Sr isotope ratios. Foods 2021, 10, 1729. [Google Scholar] [CrossRef]
- Cardot, V.; Le Roux, Y.; Jurjanz, S. Drinking Behavior of Lactating Dairy Cows and Prediction of Their Water Intake. J. Dairy Sci. 2008, 91, 2257–2264. [Google Scholar] [CrossRef]
- Govan, E.; Jackson, A.L.; Inger, R.; Bearhop, S.; Parnell, A.C. simmr: A package for fitting Stable Isotope Mixing Models in R. arXiv 2023, arXiv:2306.07817. [Google Scholar] [CrossRef]
- Lengfelder, F.; Grupe, G.; Stallauer, A.; Huth, R.; Söllner, F. Modelling strontium isotopes in past biospheres—Assessment of bioavailable 87Sr/86Sr ratios in local archaeological vertebrates based on environmental signatures. Sci. Total Environ. 2019, 648, 236–252. [Google Scholar] [CrossRef]
- Montgomery, J.; Evans, J.A.; Cooper, R.E. Resolving archaeological populations with Sr-isotope mixing models. Appl. Geochem. 2007, 22, 1502–1514. [Google Scholar] [CrossRef]
- Toncala, A.; Trautmann, B.; Velte, M.; Kropf, E.; McGlynn, G.; Peters, J.; Harbeck, M. On the premises of mixing models to define local bioavailable 87Sr/86Sr ranges in archaeological contexts. Sci. Total Environ. 2020, 745, 140902. [Google Scholar] [CrossRef] [PubMed]
- Spooner, E.T.C. The strontium isotopic composition of seawater, and seawater-oceanic crust interaction. Earth Planet. Sci. Lett. 1976, 31, 167–174. [Google Scholar] [CrossRef]
- Angino, E.E.; Billings, G.K.; Andersen, N. Observed variations in the strontium concentration of sea water. Chem. Geol. 1966, 1, 145–153. [Google Scholar] [CrossRef]
- Epova, E.N.; Bérail, S.; Zuliani, T.; Malherbe, J.; Sarthou, L.; Valiente, M.; Donard, O.F. 87Sr/86Sr isotope ratio and multielemental signatures as indicators of origin of European cured hams: The role of salt. Food Chem. 2018, 246, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Alonzi, E.; Pacheco-Forés, S.I.; Gordon, G.W.; Kuijt, I.; Knudson, K.J. New understandings of the sea spray effect and its impact on bioavailable radiogenic strontium isotope ratios in coastal environments. J. Archaeol. Sci. Rep. 2020, 33, 102462. [Google Scholar] [CrossRef]


| Sample Type | Location | Range of 87Sr/86Sr Ratio Values | Reference |
|---|---|---|---|
| Whole-rock schist (n = 1) | Naxos, Greece (southeast) | 0.70930 | Andriessen et al. (1979) [20] |
| Whole-rock granite (n = 9) | Naxos, Greece (north) | 0.71418–0.72282 | |
| Granodiorite (n = 21) | Naxos, Greece (west) | 0.71056–0.71724 | |
| Natural mineral water (n = 11) | Greece (w/o South Aegean) | 0.70782–0.70946 | Voerkelius et al. (2010) [21] |
| Pig and cow enamel, archaeological human bone, modern snail shell (n = 8) | Naxos, Greece (central and western island) | 0.70903–0.71004 | Nafplioti (2011) [22] |
| Modern hare mandible (n = 6) | Naxos, Greece | 0.70881–0.71068 | Prevedorou et al. (2015) [23] |
| Grazing and agricultural soil (n = 6) | South Aegean, Greece | 0.70500–0.71000 | Hoogewerff et al. (2019) * [24] |
| Previous studies (n = 28) | South Aegean, Greece | 0.70806–0.70972 | Frank et al. (2021) ** [25] |
| Matrix | Sampling Campaign | Number of Samples | 87Sr/86Sr | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | Min. | Max. | SD | |||
| Bulk soil | August 2020 | 16 | 0.71303 | 0.71182 | 0.70865 | 0.72276 | 0.00373 |
| Bioavailable soil fraction | August 2020 | 16 | 0.70967 | 0.70956 | 0.70859 | 0.71150 | 0.00067 |
| Water | January 2021 | 18 | 0.70996 | 0.70978 | 0.70831 | 0.71209 | 0.00100 |
| Feed | August 2020, January 2021, June 2021 | 67 | 0.70963 | 0.70935 | 0.70855 | 0.71613 | 0.00109 |
| Milk | August 2020, January 2021, June 2021, July 2021, January 2022 | 96 | 0.70940 | 0.70926 | 0.70781 | 0.71225 | 0.00058 |
| Graviera Naxos cheese | August 2020, January 2021, June 2021, January 2022 | 7 | 0.70928 | 0.70938 | 0.70891 | 0.70952 | 0.00021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikezić, M.; Chantzi, P.; Irrgeher, J.; Zuliani, T. Evaluating Source Complexity in Blended Milk Cheese: Integrated Strontium Isotope and Multi-Elemental Approach to PDO Graviera Naxos. Foods 2024, 13, 2540. https://doi.org/10.3390/foods13162540
Nikezić M, Chantzi P, Irrgeher J, Zuliani T. Evaluating Source Complexity in Blended Milk Cheese: Integrated Strontium Isotope and Multi-Elemental Approach to PDO Graviera Naxos. Foods. 2024; 13(16):2540. https://doi.org/10.3390/foods13162540
Chicago/Turabian StyleNikezić, Majda, Paraskevi Chantzi, Johanna Irrgeher, and Tea Zuliani. 2024. "Evaluating Source Complexity in Blended Milk Cheese: Integrated Strontium Isotope and Multi-Elemental Approach to PDO Graviera Naxos" Foods 13, no. 16: 2540. https://doi.org/10.3390/foods13162540







