Health Hazard Associated with the Presence of Clostridium Bacteria in Food Products
Abstract
:1. Introduction
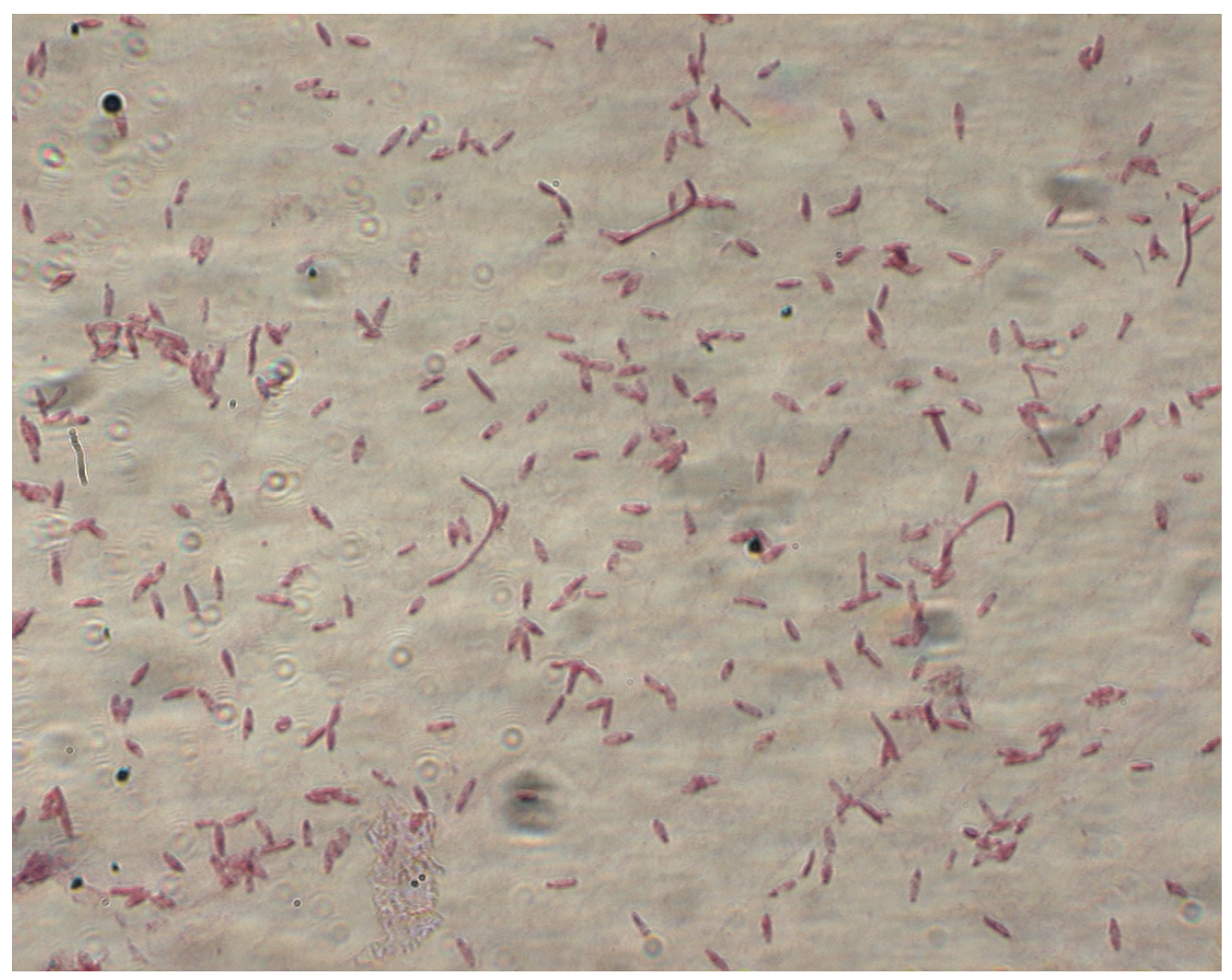
2. General Characteristics of the Genus Clostridium spp.
Pathogenicity
3. Clostridium Bacteria in Food Products
4. Other Aspects of the Presence of Bacteria of the Genus Clostridium in the Environment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mayr, E. Principles of Systematic Zoology; McGraw-Hill: New York, NY, USA, 1969; pp. 34–37. ISBN 978-9383692279. [Google Scholar]
- Dürre, P. From Pandora’s Box to Cornucopia: Clostridia—A Historical Perspective. In Clostridia: Biotechnology and Medical Applications; Bahl, H., Dürre, P., Eds.; Wiley-VCH Verlag GmbH: Berlin, Germany, 2001; pp. 1–6. ISBN 978-3527301751. [Google Scholar]
- Moriishi, K.; Koura, M.; Abe, N.; Fujii, N.; Fujinaga, Y.; Inoue, K.; Ogumad, K. Mosaic structures of neurotoxins produced from Clostridiumbotulinum strain NCTC 2916. FEMS Microbiol. Lett. 1996, 140, 151–158. [Google Scholar]
- Jaroszewska, E.; Pietracha, D.; Misiewicz, A. Patogeny człowieka w żywności pochodzenia roślinnego—Wady i zalety zastosowania techniki Real-Time PCR do ich wykrywania. Postępy Nauk. Technol. Przemysłu Rolno-Spożywczego 2014, 69, 44–54. [Google Scholar]
- Parish, M.E.; Beuchat, L.R.; Suslow, T.V.; Harris, L.J.; Garrett, E.H.; Farber, J.N.; Busta, F.F. Methods to reduce/eliminate pathogens from fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2006, 2, 161–173. [Google Scholar] [CrossRef]
- Erickson, M.C.; Doyle, M.P. The challenges of eliminating or substituting antimicrobial preservatives in foods. Annu. Rev. Food Sci. Technol. 2017, 8, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Jaam, O.E.; Fliss, I.; Aïder, M. Effect of electro-activated aqueous solutions, nisin and moderate heat treatment on the inactivation of Clostridiumsporogenes PA 3679 spores in green beans puree and whole green beans. Anaerobe 2017, 47, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W. Clostridial enzymes and fermentation pathways. In Handbook on Clostridia; Duerre, P., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2005; pp. 81–83. ISBN 9780429205651. [Google Scholar]
- Leja, K.; Myszka, K.; Olkowicz, M.; Juzwa, W.; Czaczyk, K. Clostridiumbifermentans as an aero-tolerant exponent of strictly anaerobe genera. Adv. Microbiol. 2014, 4, 216–224. [Google Scholar] [CrossRef]
- Strus, M.; Pakosz, K.; Gościniak, H.; Przondo-Mordarska, A.; Rożynek, E.; Pituch, H.; Meisel-Mikołajczyk, F.; Heczko, P.B. Antagonistyczne działanie bakterii z rodzaju Lactobacillus wobec beztlenowych i mikroaerofilnych czynników zakażeń przewodu pokarmowego (Helicobacter pylori, Campylobacter coli, Campylobacter jejuni, Clostridiumdifficile). Med. Doświadczalna Mikrobiol. 2001, 53, 133–142. [Google Scholar]
- Zyska, B. Bakterie z rodzaju Clostridium. In Mikrobiologia Techniczna; Libudzisz, Z., Kowal, K., Eds.; Wydawnictwo Politechniki Łódzkiej: Łódź, Poland, 2000; pp. 155–175. [Google Scholar]
- Stackebrandt, E.; Hippe, H.; Dürre, P. Taxonomy and Systematics. In Clostridia: Biotechnology and Medical Applications; Bahl, H., Dürre, P., Eds.; Wiley-VCH Verlag GmbH: Berlin, Germany, 2001; pp. 20–22. [Google Scholar]
- Leja, K.; Myszka, K.; Czaczyk, M. Przemysłowe wykorzystanie bakterii z rodzaju Clostridium. Postep. Mikrobiol. 2014, 53, 15–24. [Google Scholar]
- Kanaan, M.; Tarek, A. Clostridium botulinum, a foodborne pathogen and its impact on public health. Ann. Trop. Med. Public Health 2020, 23, 49–62. [Google Scholar] [CrossRef]
- Bielec, D.; Modrzewska, R. Zatrucie jadem kiełbasianym wczoraj i dziś—Aspekty kliniczne. Przegląd Epidemiol. 2007, 61, 505–512. [Google Scholar]
- Nowak, A.; Ołtuszak-Walczak, E.; Świtoniak, T. Zatrucia i zakażenia pokarmowe. In Mikrobiologia Techniczna; Libudzisz, Z., Kowal, K., Żakowska, Z., Eds.; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2018; pp. 265–288. Volume 2, ISBN 9788301155230. [Google Scholar]
- Franciosa, G.; Ferreira, J.L.; Hatheway, C.L. Detection of type A, B, and E botulism neurotoxin genes in Clostridiumbotulinum and other Clostridiumspecies by PCR: Evidence of unexpressed type B toxin genes in type A toxigenic organisms. J. Clin. Microbiol. 1994, 32, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Bielec, D.; Semczuk, G.; Lis, J.; Firych, J.; Modrzewska, R.; Janowski, R. Epidemiologia i klinika zatruć jadem kiełbasianym chorych leczonych w Klinice Chorób Zakaźnych w Akademii Medycznej w Lublinie w latach 1999–2000. Przegląd Epidemiol. 2002, 56, 435–442. [Google Scholar]
- Lindström, M.; Heikinheimo, A.; Lahti, P.; Korkeala, H. Novel insights into the epidemiology of Clostridiumperfringens type A food poisoning. Food Microbiol. 2011, 28, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ścieżyńska, H.; Maćkiw, E.; Mąka, Ł.; Pawłowska, K. Nowe zagrożenia mikrobiologiczne w żywności. Rocz. Panstw. Zakl. Hig. 2012, 63, 397–402. [Google Scholar] [PubMed]
- Chen, Y.; Li, H.; Yang, L.; Wang, L.; Sun, R.; Shearer, J.E.S.; Sun, F. Rapid Detection of Clostridiumbotulinum in Food Using Loop-Mediated Isothermal Amplification (LAMP). Int. J. Environ. Res. Public Health 2021, 21, 4401. [Google Scholar] [CrossRef]
- Grenda, T.; Jarosz, A.; Sapała, M.; Grenda, A.; Patyra, E.; Kwiatek, K. Clostridium perfringens—Opportunistic Foodborne Pathogen, Its Diversity and Epidemiological Significance. Pathogens 2023, 12, 768. [Google Scholar] [CrossRef]
- Fu, Y.; Alenezi, T.; Sun, X. Clostridium perfringens-Induced Necrotic Diseases: An Overview. Immuno 2022, 2, 387–407. [Google Scholar] [CrossRef]
- Wadełek, J. Diagnostyka i leczenie zgorzeli Fourniera w oddziale intensywnej terapii. Nowa Med. 2016, 3, 102–113. [Google Scholar]
- Kądzielska, J.; Obuch-Woszczatyński, P.; Pituch, H.; Młynarczyk, G. Clostridiumperfringens jako czynnik etiologiczny biegunki poantybiotykowej. Postep. Mikrobiol. 2012, 51, 17–25. [Google Scholar]
- Chukwu, E.E.; Nwaokorie, F.O.; Coker, A.O.; Avila-Campos, M.J.; Solis, R.L.; Llanco, L.A.; Ogunsola, F.T. Detection of toxigenic Clostridiumperfringens and Clostridiumbotulinum from food sold in Lagos, Nigeria. Anaerobe 2016, 42, 176–181. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; A Navarro, M.; Li, J.; Shrestha, A.; Uzal, F.; A McClane, B. Pathogenicity and virulence of Clostridiumperfringens. Virulence 2021, 12, 723–753. [Google Scholar] [CrossRef]
- Clancy, C.J.; Buehrle, D.; Vu, M.; Wagener, M.M.; Nguyen, M.H. Impact of revised infectious diseases Society of America and Society for Healthcare Epidemiology of America clinical practice guidelines on the treatment of Clostridium difficile Infections in the United States. Clin. Infect. Dis. 2021, 72, 1944–1949. [Google Scholar] [CrossRef]
- Aguirre, A.M.; Sorg, J.A. Gut associated metabolites and their roles in Clostridioides difficile pathogenesis. Gut Microbes 2022, 14, 2094672. [Google Scholar] [CrossRef]
- Pal, M.; Bulcha, M. Clostridium difficile as an Emerging Foodborne Pathogen of Public Health Significance. Acta Sci. Microbiol. 2021, 4, 46–49. [Google Scholar] [CrossRef]
- Rupnik, M.; Songer, J.G. Chapter 3—Clostridium difficile: Its Potential as a Source of Foodborne Disease. In Advances in Food and Nutrition Research; Taylor, S.L., Ed.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2010; Volume 60, pp. 53–66. ISBN 978-0-12-380944-5. [Google Scholar]
- Bilverstone, T.W.; Garland, M.; Cave, R.J.; Kelly, M.L.; Tholen, M.; Bouley, D.M.; Kehne, S.A.; Melnyk, R.A. The glucosyltransferase activity of C. difficile Toxin B is required for disease pathogenesis. PLoS Pathog. 2020, 16, e1008852. [Google Scholar] [CrossRef]
- Smith, A.; Jenior, M.; Keenan, O.; Hart, J.; Specker, J.; Abbas, A.; Rangel, P.; Di, C.; Green, J.; Bustin, K.; et al. Enterococci enhance Clostridioides difficile pathogenesis. Nature 2022, 611, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. Clostridium difficile in food—Innocent bystander or serious threat? Clin. Microbiol. Infect. 2009, 16, 1. [Google Scholar] [CrossRef]
- Num, S.M.; Useh, N.M. Clostridium: Pathogenic roles, industrial uses and medicinal prospects of natural products as ameliorative agents against pathogenic species. Jordan J. Biol. Sci. 2014, 7, 81–94. [Google Scholar]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2014, 14, 4634. [Google Scholar]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015, 13, 4329. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016, 14, e04634. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, e05077. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar] [CrossRef]
- EFSA; ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 5926. [Google Scholar] [CrossRef]
- EFSA; ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar] [CrossRef]
- EFSA; ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 6971. [Google Scholar] [CrossRef]
- EFSA; ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 7666. [Google Scholar] [CrossRef]
- EFSA; ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Peñuelas, M.; Guerrero-Vadillo, M.; Valdezate, S.; Zamora, M.J.; Leon-Gomez, I.; Flores-Cuéllar, Á.; Carrasco, G.; Díaz-García, O.; Varela, C. Botulism in Spain: Epidemiology and Outcomes of Antitoxin Treatment, 1997–2019. Toxins 2023, 15, 2. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Oszmiański, J. Zagrożenia powstające w żywności minimalnie przetworzonej i skuteczne metody ich eliminacji. Zywn-Nau Technol. J. 2014, 2, 5–18. [Google Scholar]
- Przetaczek-Rożnowska, I.; Kuźniak, M. Źródła zanieczyszczeń mikrobiologicznych ziół leczniczych i przypraw oraz metody ich dekontaminacji. Postępy Fitoter. 2016, 1, 59–62. [Google Scholar]
- Kręgiel, D.; Piątkiewicz, A.; Żakowska, Z.; Kunicka-Styczyńska, A. Zanieczyszczenia mikrobiologiczne surowców. In Mikrobiologia Techniczna; Libudzisz, Z., Kowal, K., Żakowska, Z., Eds.; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2018; Volume 2, pp. 235–252. ISBN 9788301155230. [Google Scholar]
- Pinto, C.A.; Mousakhani Ganjeh, A.; Barba, F.J.; Saraiva, J.A. Impact of pH and High-Pressure Pasteurization on the Germination and Development of Clostridiumperfringens Spores under Hyperbaric Storage versus Refrigeration. Foods 2024, 13, 1832. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.H.D.; Vazquez, A.J.; Hill, K.; Smith, T.J.; Nottingham, R.; Stone, N.E.; Sobek, C.J.; Cocking, J.H.; Fernández, R.A.; Caballero, P.A.; et al. Differentiating botulinum neurotoxin-producing Clostridia with a simple, multiplex PCR assay. Appl. Environ. Microbiol. 2017, 83, e00806-17. [Google Scholar] [CrossRef]
- Popoff, M.R.; Brüggemann, H. Regulatory Networks Controlling Neurotoxin Synthesis in Clostridiumbotulinum and Clostridiumtetani. Toxins 2022, 14, 364. [Google Scholar] [CrossRef] [PubMed]
- Dahlsten, E.; Lindström, M.; Korkeala, H. Mechanism of food processing and storage-related stress tolerance in Clostridiumbotulinum. Res. Microbiol. 2015, 166, 344–352. [Google Scholar] [CrossRef]
- Rodgers, S. Survival of Clostridiumbotulinum in hot-fill meals. Food Serv. Technol. 2002, 2, 69–79. [Google Scholar] [CrossRef]
- Danyluk, B.; Bilska, A.; Kirklo, P. Ocena mikrobiologiczna wybranych produktów drobiowych z grupy żywności wygodnej. Nauka Przyr. Technol. 2015, 9, 3. [Google Scholar] [CrossRef]
- Munir, M.T.; Mtimet, N.; Guillier, L.; Meurens, F.; Fravalo, P.; Federighi, M.; Kooh, P. Physical Treatments to Control Clostridiumbotulinum Hazards in Food. Foods 2023, 12, 1580. [Google Scholar] [CrossRef]
- Pernu, N.; Keto-Timonen, R.; Lindström, M.; Korkeala, H. High prevalence of Clostridiumbotulinum in vegetarian sausages. Food Microbiol. 2020, 91, 103512. [Google Scholar] [CrossRef]
- Duc, H.M.; Hoa, T.T.K.; Ha, C.T.T.; Van Hung, L.; Van Thang, N.; Minh Son, H.; Flory, G.A. Prevalence and Antibiotic Resistance Profile of Clostridiumperfringens Isolated from Pork and Chicken Meat in Vietnam. Pathogens 2024, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, E.; Mauriz, E.; Sanz-Gómez, J.; González-Paramás, A.M.; Adanero-Jorge, F.; García-Fernández, C. Exploring Propolis as a Sustainable Bio-Preservative Agent to Control Foodborne Pathogens in Vacuum-Packed Cooked Ham. Microorganisms 2024, 12, 914. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Foster, N.F.; Riley, T.V. Susceptibility of Clostridium difficile to the food preservatives sodium nitrite, sodium nitrate and sodium metabisulphite. Anaerobe 2016, 31, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Warriner, K.; Xu, C.; Habash, M.; Sultan, S.; Weese, S.J. Dissemination of Clostridium difficile in food and the environment: Significant sources of C. difficile community-acquired infection? J. Appl. Microbiol. 2016, 122, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Nalepa, B.; Markiewicz, L.H. PCR-DGGE markers for qualitative profiling of microbiota in raw milk and ripened cheeses. Food Sci. Technol. 2017, 84, 168–174. [Google Scholar] [CrossRef]
- Libudzisz, Z. Bakterie fermentacji mlekowej. In Mikrobiologia Techniczna; Libudzisz, Z., Kowal, K., Żakowska, Z., Eds.; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2018; Volume 2, pp. 25–58. ISBN 9788301155230. [Google Scholar]
- Nowak, A.; Piątkiewicz, A. Mikrobiologiczne psucie żywności. In Mikrobiologia Techniczna; Libudzisz, Z., Kowal, K., Żakowska, Z., Eds.; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2018; Volume 2, pp. 253–264. ISBN 9788301155230. [Google Scholar]
- Hoang, L.H.; Nga, T.T.; Tram, N.T.; Trang, L.T.; Ha, H.T.T.; Hoang, T.H.; Anh, D.D.; Yen, P.B.; Nguyen, N.T.; Morita, M.; et al. First report of foodborne botulism due to Clostridiumbotulinum type A(B) from vegetarian home-canned pate in Hanoi, Vietnam. Anaerobe 2022, 77, 102514. [Google Scholar] [CrossRef] [PubMed]
- Fua’di, M.T.; Er, B.; Lee, S.; Chan, P.P.; Khoo, J.; Tan, D.; Li, H.; Muhammad, I.R.; Raj, P.; Kurupatham, L.; et al. Characteristics of Gastroenteritis Outbreaks Investigated in Singapore: 2018–2021. Int. J. Environ. Res. Public Health 2024, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Leja, K.; Czaczyk, K.; Myszka, K. Biotechnological synthesis of 1,3-propanediol using Clostridium ssp. Afr. J. Biotechnol. 2011, 10, 11093–11101. [Google Scholar]
- Leja, K.; Czaczyk, K.; Myszka, K. The use of microorganisms in 1,3-propanediol production. Afr. J. Microbiol. Res. 2011, 5, 4652–4658. [Google Scholar]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Samul, D.; Worsztynowicz, P.; Leja, K.; Grajek, W. Beneficial and harmful roles of bacteria from the Clostridium genus. Acta Biochim. 2013, 60, 515–521. [Google Scholar] [CrossRef]
- Grenda, T.; Grenda, A.; Domaradzki, P.; Krawczyk, P.; Kwiatek, K. Probiotic Potential of Clostridium spp.—Advantages and Doubts. Curr. Issues Mol. Biol. 2022, 44, 3118–3130. [Google Scholar] [CrossRef] [PubMed]
- Bigalke, H.; Shoer, L.F. Clostridial neurotoxins. In Bacterial Protein Toxins; Handbook of Experimental Pharmacology; Aktories, K., Just, I., Eds.; Springer: Berlin, Germany, 2000; pp. 407–443. [Google Scholar]
- Schiavo, G.; Matteoli, M.; Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000, 80, 717–766. [Google Scholar] [CrossRef] [PubMed]
- Kreydon, O.P.; Geiges, M.L.; Boni, R.; Burg, G. Botulinum toxin: From poison to medicine. A historical review. Hautarzt 2000, 51, 733–737. [Google Scholar]
- Heap, J.T.; Theys, J.; Ehsaan, M.; Kubiak, A.M.; Dubois, L.; Paesmans, K.; Van Mellaert, L.; Knox, R.; Kuehne, S.A.; Lambin, P.; et al. Spores of Clostridium engineered for clinical efficacy and safety cause regression and cure of tumors in vivo. Oncotarget 2014, 5, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Hallet, M. Therapy with Botulinum Toxin; Marcel Dekker, Inc.: New York, NY, USA, 1994; pp. 43–46. ISBN 978-0824788247. [Google Scholar]
- Brin, M.F. Botulinum toxin: Chemistry, pharmacology, toxicity, and immunology. Muscle Nerve 1997, 20, 156–168. [Google Scholar] [CrossRef]
- Eklund, F.T.; Poysky, L.M.; Mseitif, T.; Strom, M.T. Evidence for plasmid-mediated toxin and bacteriocin production in Clostridiumbotulinum type G. Appl. Environ. Microbiol. 1988, 54, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.M.; Robb, F.T.; Webster, J.R.; Woods, D.R. Bacteriocin production by Clostridium acetobutylicum in an industrial fermentation process. Appl. Environ. Microbiol. 1979, 37, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.J.; Moyra, R.R.; Morris, J.G. Purification of two Clostridium bacteriocins by procedures appropriate to hydrophobic proteins. Antimicrob. Agents Chemother. 1975, 3, 256–264. [Google Scholar] [CrossRef]
| 2013 EFSA [36] | 2014 EFSA [37] | 2015 EFSA [38] | 2016 EFSA [39] | 2017 EFSA [40] | 2018 EFSA [41] | 2019 EFSA [42] | 2020 EFSA [43] | 2021 EFSA [44] | 2022 EFSA [45] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of outbreaks of food poisoning caused by bacterial toxins | 834 | 840 | 849 | 848 | 818 | 950 | 997 | 527 | 679 | 1141 |
| Number of cases (hospitalizations/fatalities) | 9203 (452/1) | 8610 (586/5) | 8847 (497/3) | 8967 (401/1) | 8468 (583/7) | 9726 (534/6) | 10,555 (361/14) | 4517 (182/6) | 6378 (310/7) | 13,902 (416/11) |
| Number of outbreaks of food poisoning caused by Clostridium spp. toxins | 170 no data | 160 including: Clostridium botulinum—9 Clostridium perfringens—124 Others Clostridium spp.—27 | 122 including: Clostridium botulinum—24 Clostridium perfringens—96 Others Clostridium spp.—2 | Clostridium botulinum—18 | Clostridium botulinum—9 | 86 incuding: Clostridium botulinum—15 Clostridium perfringens—71 | 82 incuding: Clostridium botulinum—7 Clostridium perfringens—75 | 41 including: Clostridium botulinum—9 Clostridium perfringens—32 | 47 including: Clostridium botulinum—7 Clostridium perfringens—40 | 62 including: Clostridium botulinum—7 Clostridium perfringens—55 |
| Number of cases (hospitalizations/fatalities) | 3530 (66/1) no data | 3285 (65/3) no data | 2074 (68/3) incuding: Clostridium botulinum—60 (43/0) Clostridium perfringens—2014 (25/3) Others Clostridium spp.—4 (no data) | Clostridium botulinum—49 (39/0) | Clostridium botulinum—26 (26/2) | 1831 (53/4) incuding: Clostridium botulinum—48 (35/2) Clostridium perfringens—1783 (18/2) | 2443 (42/4) incuding: Clostridium botulinum—17 (15/1) Clostridium perfringens—2426 (27/3) | 716 (44/2) incuding: Clostridium botulinum—34 (34/0) Clostridium perfringens—682 (10/2) | 802 (40/4) incuding: Clostridium botulinum—24 (15/0) Clostridium perfringens—778 (25/4) | 2917 (21/3) incuding: Clostridium botulinum—20 (10/0) Clostridium perfringens—2897 (11/3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilska, A.; Wochna, K.; Habiera, M.; Serwańska-Leja, K. Health Hazard Associated with the Presence of Clostridium Bacteria in Food Products. Foods 2024, 13, 2578. https://doi.org/10.3390/foods13162578
Bilska A, Wochna K, Habiera M, Serwańska-Leja K. Health Hazard Associated with the Presence of Clostridium Bacteria in Food Products. Foods. 2024; 13(16):2578. https://doi.org/10.3390/foods13162578
Chicago/Turabian StyleBilska, Agnieszka, Krystian Wochna, Małgorzata Habiera, and Katarzyna Serwańska-Leja. 2024. "Health Hazard Associated with the Presence of Clostridium Bacteria in Food Products" Foods 13, no. 16: 2578. https://doi.org/10.3390/foods13162578
APA StyleBilska, A., Wochna, K., Habiera, M., & Serwańska-Leja, K. (2024). Health Hazard Associated with the Presence of Clostridium Bacteria in Food Products. Foods, 13(16), 2578. https://doi.org/10.3390/foods13162578







