Incorporating Olive By-Products in Bísaro Pig Diets: Effect on Dry-Cured Product Quality
Abstract
1. Introduction
2. Materials and Methods
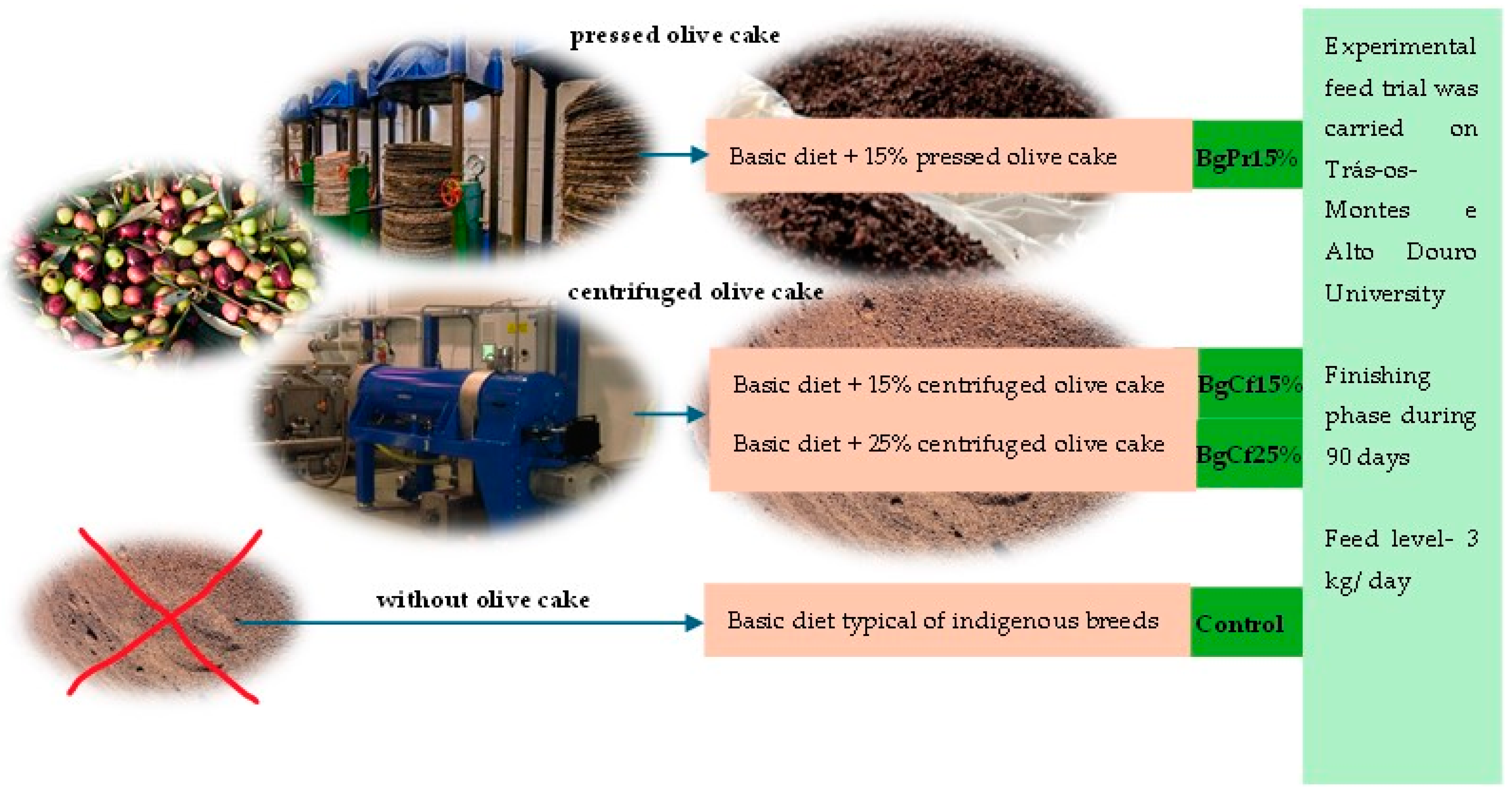
2.1. Experimental Diets and Slaughter Procedure
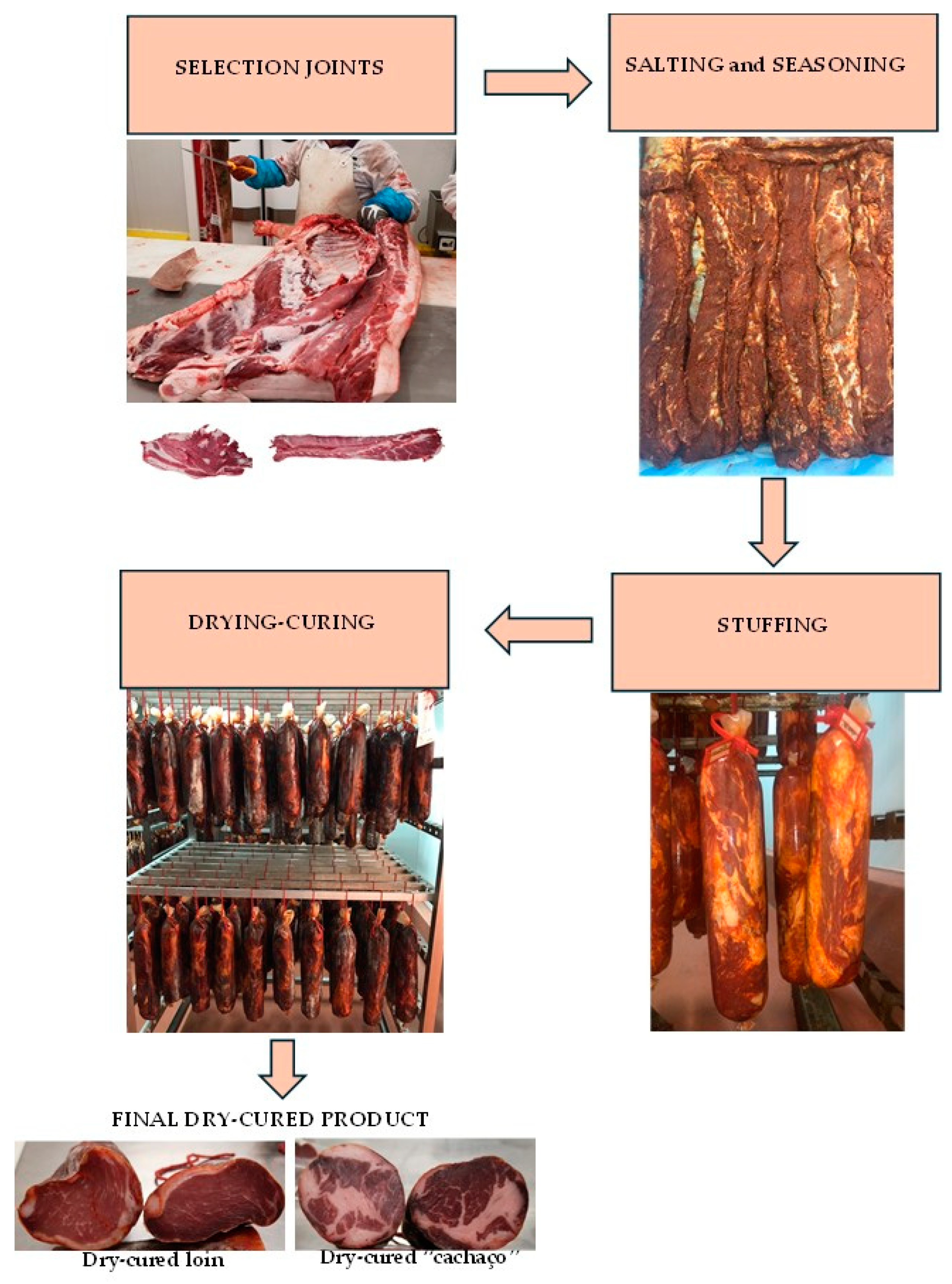
2.2. Dry-Cured Bísaro Loin and “Cachaço”
2.3. Chemical Composition and Physicochemical Analysis of Dry-Cured Loin and Dry-Cured “Cachaço”
2.4. Fatty Acid Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Composition
3.2. Fatty Acids Composition
3.3. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Producing 69% of the World’s Production, the EU Is the Largest Producer of Olive Oil. 2020. Available online: https://ec.europa.eu/info/food-farming-fisheries/plants-and-plant-products/plant-products/olive-oil_en (accessed on 18 April 2024).
- Molina-Alcaide, R.; Yáñez-Ruiz, D.R. Potential use of olive by-products in ruminant feeding: A review. Anim. Feed. Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Albuquerque, J.A.; Gonzalez, J.; García, D.; Cegarra, J. Agrochemical characterization of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Oktay, Z. Olive Cake as a Biomass Fuel for Energy Production. Energy Sources Part A Recovery Util. Environ. Eff. 2006, 28, 329–339. [Google Scholar] [CrossRef]
- Coelho, L.; Portela, C.; Cravo, A.; Reis, M. Valorização do bagaço de azeitona por compostagem, para utilização agrícola. V Simpósio Nacional de Olivicultura, 14:133-140. Escola Superior Agrária de Santarém, 25 e 26 de Setembro. Actas Port. Hortic. 2009, 14, 133–140. [Google Scholar]
- Teixeira, A.; Rodrigues, S. Consumer perceptions towards healthier meat products. Curr. Opin. Food Sci. 2021, 38, 147–154. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Montes, R.; Purriños, L.; Cobas, N.; Franco, D. Fatty acid composition of Celta pig breed as influenced by sex and location of fat in the carcass. J. Sci. Food Agric. 2012, 92, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, J.; Teixeira, A. Slaughter weight rather than sex affects carcass cuts and tissue composition of Bísaro pigs. Meat Sci. 2019, 154, 54–60. [Google Scholar] [CrossRef]
- Council Regulation (EC)-Official Journal of the European Communities No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. 2009, pp. 1–30. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1099 (accessed on 23 April 2024).
- AOAC International; Cunniff, P. AOAC Official Methods of Analysis of AOAC International, 16th ed.; The Association: Washington, DC, USA, 1995; ISBN 9780935584547. [Google Scholar]
- NP 1614/2002; Determination of Moisture Content. Reference Method (ISO 1442:1197). In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- NP-ISO-1615/2002; Determination of Total Ashes. Reference Method. In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- NP 1612/2002; Determination of Total Nitrogen Content. Reference Method (ISO 937:1978). In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- NP 1987/2002; Carnes e Produtos Cárneos; Determinação do teor em Hidroxiprolina. (Método de Referência). In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- Hornsey, H.C. The colour of cooked cured pork. I-Estimation of the nitric oxide-haem pigments. J. Sci. Food Agric. 1956, 7, 534–540. [Google Scholar] [CrossRef]
- NP 1845/1982; Meat and Meat Products—Determination of Chloride Content—Standard Method. Portuguese Institute of Quality, Ministry of Economy Innovation: Caparica, Portugal, 1982.
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dominguez, R.; Borrajo, P.; Lorenzo, J.M. The effect of cooking methods on nutritional value of foal meat. J. Food Compos. Anal. 2015, 43, 61–67. [Google Scholar] [CrossRef]
- Vieira, C.; Sarmiento-García, A.; García, J.J.; Rubio, B.; Martínez, B. Quality and Shelf Life of Fresh Meat from Iberian Pigs as Affected by a New Form of Presentation of Oleic Acid and an Organic-Acid Mix in the Diet. Foods 2021, 10, 985. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Leite, A.; Vasconcelos, L.; Ferreira, I.; Domínguez, R.; Pereira, E.; Rodrigues, S.; Lorenzo, J.M.; Teixeira, A. Effect of the inclusion of olive cake in the diet on the physicochemical characteristics of dry-cured loin and dry-cured “cachaço” of Bísaro pig. Appl. Sci. 2023, 13, 1439. [Google Scholar] [CrossRef]
- Pateiro, M.; Franco, D.; Carril, J.A.; Lorenzo, J.M. Changes on physico-chemical properties, lipid oxidation and volatile compounds during the manufacture of Celta dry-cured loin. J. Food Sci. Technol. 2015, 52, 4808–4818. [Google Scholar] [CrossRef]
- Seong, P.N.; Park, K.M.; Kang, G.H.; Cho, S.H.; Park, B.Y.; Ba, H.V. The impact of ripening time on Technological quality traits, chemical change and Sensory characterisictics of dry-cured loin. Asian Australas. J. Animal Sci. 2015, 28, 677–687. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Libera, J.; Kononiuk, A.; Keska, P.; Wójciak, K. Use of grape seed extract as a natural antioxidant additive in dry-cured pork neck technology. Biotechnol. Food Sci. 2018, 82, 141–150. [Google Scholar]
- Lorenzo, J.M.; Carballo, J. Changes in physico-chemical properties and volatile compounds throughout the manufacturing process of dry-cured foal loin. Meat Sci. 2015, 99, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Asensio, M.; Silva, A.; Armenteros, M.; Caballero, D.; Martín, N.; Lorido, L.; Sánchez-Montero, L.; Fernandez, C.; Noguera, J.L.; Ramos, M. Quality evaluation of dry-cured shoulder from different genetic lines of Iberian pigs. Arch. Zootec. 2018, 67, 155–160. [Google Scholar] [CrossRef]
- Caballero, D.; Asensio, M.; Fernández, C.; Reina, R.; García, M.J.; Noguera, J.L.; Silva, A. Effects of genotypes and crossbreeding on the quality parameters of dry-cured shoulders from different Iberian genetic pig lines. J. Food Meas. Charact. 2020, 14, 818–829. [Google Scholar] [CrossRef]
- Cardoso-Toset, F.; Luque, I.; Morales-Partera, A.; Galán-Relaño, A.; Barrero-Domínguez, B.; Hernández, M.; Gómez-Laguna, J. Survival of Streptococcus suis, Streptococcus dysgalactiae and Trueperella pyogenes in dry-cured Iberian pork shoulders and loins. Food Microbiol. 2017, 61, 66–71. [Google Scholar] [CrossRef]
- Leite, A.; Vasconcelos, L.; Ferreira, I.; Sarmiento-García, A.; Domínguez, R.; Santos, E.M.; Campagnol, P.C.B.; Rodrigues, S.; Lorenzo, J.M.; Teixeira, A. Chemical, physicochemical and sensorial characterization of nitrite-free dry Bísaro shoulders. Foods 2022, 11, 3079. [Google Scholar] [CrossRef]
- Barbora-Cánovas, G.V.; Fontana, A.J.; Schmidt, S.-J.; Labuza, T.P. Water Activity in Foods: Fundamentals and Aplications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; p. 640. [Google Scholar]
- Dimakopoulou-Papazoglou, D.; Katsanidis, E. Effect of maltodextrin, sodium chloride, and liquid smoke on the mass transfer kinetics and storage stability of osmotically dehydrated beef meat. Food Bioprocess Tech. 2017, 10, 2034–2045. [Google Scholar] [CrossRef]
- Andres, A.I.; Ventanas, S.; Ventanas, J.; Cava, R.; Ruiz, J. Physicochemcial changes throughout the ripening of dry-cured hams with different salt content and processing conditions. Eur. Food Res. Technol. 2005, 221, 30–35. [Google Scholar] [CrossRef]
- Sánchez-Parra, M.; Ordónez-Díaz, J.L.; Pérez-Aparicio, J.; Moreno-Rojas, J. Physicochemical and microbiological changes associated with processing in dry-cured Tuna. Appl. Sci. 2023, 13, 5900. [Google Scholar] [CrossRef]
- Di Rosa, A.; Zumbo, A.; Furfaro, M.E.; Carcione, G.; D’Angelo, G.; Chiofalfo, V. Effect of two different rearing systems on quality of dry cured “Coppa” of Nero Siliciano pig. In Proceedings of the 8th International Symposium on the Mediterranean Pig, Ljubljana, Slovenia, 10–12 October 2013. [Google Scholar]
- Ortiz, A.; Tejerina, D.; Contador, R.; Andrés, A.I.; Petrón, M.J.; Cáceres-Nevado, J.M.; García-Torres, S. Quality traits of dry-cured loins from Iberian pigs reared in Montanera system as affected by pre- freezing cure. Foods 2021, 10, 48. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Domínguez, R.; Pateiro, M.; Franco, D.; Barba, J.F.; Lorenzo, J.M. Chemical and physico-chemical changes during the dry-cured processing of deer loin. Int. J. Food Sci. Technol. 2020, 55, 1025–1031. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, I.; Franco, D.; Carballo, J.; Lorenzo, J.M. Physochemical changes during manufacture and final sensory characteristics of dry-cured Celta ham. Effect of muscle type. Food Control 2014, 43, 263–269. [Google Scholar] [CrossRef]
- Soto, E.; Hoz, L.; Ordóñez, J.A.; Hierro, E.; Herranz, B.; López-Bote, C.; Cambero, M.I. Impact of feeding and rearing systems of Iberian pigs ion volatile profile and sensory characteristics of dry-cured loin. Meat Sci. 2008, 79, 666–676. [Google Scholar] [CrossRef]
- Gençcelep, H.; Ihtiyar, B.; Yüzer, M.O. Determination of quality properties of kastamonu pastirma: A dry-cured meat product. Harran Tarim. ve Gida Bilimleri Derg. 2022, 26, 491–500. [Google Scholar] [CrossRef]
- Zanardi, E.; Novelli, E.; Ghiretti, G.P.; Chizzolini, R. Oxidative stability of lipids and cholesterol in salame Milano, coppa and Parma ham: Dietary supplementation with vitamin E and oleic acid. Meat Sci. 2000, 55, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Cittadini, A.; Domínguez, R.; Gómez, B.; Pateiro, M.; Pérez-Santaescolástica, C.; López-Fernández, O.; Sarriés, M.V.; Lorenzo, J.M. Effect of NaCl replacement by other chloride salts on physicochemical parameters, proteolysis and lipolysis of dry-cured foal “Cecina”. J. Food Sci. Technol. 2019, 57, 1628–1635. [Google Scholar] [CrossRef]
- Leite, A.; Domínguez, R.; Vasconcelos, L.; Ferreira, I.; Pereira, E.; Pinheiro, V.; Outor-Monteiro, D.; Rodrigues, S.; Lorenzo, J.M.; Santos, E.M.; et al. Can the Introduction of Different Olive Cakes Affect the Carcass, Meat and Fat Quality of Bísaro Pork? Foods 2022, 11, 1650. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, I.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of inclusion of chestnut in the finishing diet on fatty acid profile of dry-cured ham from Celta pig breed. Meat Sci. 2012, 92, 394–399. [Google Scholar] [CrossRef]
- Gómez, M.; Cachaldora, A.; Fonseca, S.; Domínguez, R.; Carballo, J.; Franco, I. Biochemical, oxidative, and lipolytic changes during vacuum-packed storage of dry-cured loin: Effect of chestnuts intake by Celta pigs. J. Food Qual. 2018, 2018, 7690501. [Google Scholar] [CrossRef]
- Contador, R.; Ortiz, A.; Ramírez, M.R.; García-Torres, S.; López-Parra, M.M.; Tejerina, D. Physico-chemical and sensory qualities of Iberian sliced dry-cured loins from various commercial categories and the effect of the type of packaging and refrigeration time. LWT Food Sci. Technol. 2021, 141, 110876. [Google Scholar] [CrossRef]
- Pleadin, J.; Lesic, T.; Vujacic, V.; Milicevic, D.; Buneta, A.; Susnic, S.; Lukanic, I.; Kresic, G. Comparison of Chemical composition and fatty acid profile traditional meat products from Croatian and Montenegro. J. Food Qual. 2021, 2021, 5586436. [Google Scholar] [CrossRef]
- Colonna, M.A.; Tarricone, S.; Giannico, F.; Selvaggi, M.; Carriero, F.; Crupi, P.; Corbo, F.; Clodoveo, M.L. Dietary effects of extra virgin olive oil extracted by ultrasound technology or refined olive oil on the quality traits of pork and “Capocollo di Martina Franca” dry-cured met. Animals 2021, 11, 954. [Google Scholar] [CrossRef]
- Baum, S.J.; Kris-Etherton, P.M.; Willett, W.C.; Lichtenstein, A.H.; Rudel, L.L.; Maki, K.C.; Whelan, J.; Ramsden, C.E.; Block, R.C. Fatty acids in cardiovascular health and disease: A comprehensive update. J. Clin. Lipidol. 2012, 6, 216–234. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Ventanas, J.; Toldrá, F. Nutritional composition of dry-cured ham and its role in a healthy diet. Meat Sci. 2010, 84, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Cavas, R.; Ruiz, J.; Ventanas, J.; Antequera, T. Effect of α-tocopheryl acetate supplementation and the extensive feeding of pigs on the volatile aldehydes during the maturation of Iberian ham. Food Sci. Technol. Int. 1999, 5, 235–241. [Google Scholar] [CrossRef]
- De Jesús, M.C.; Domínguez, R.; Cantalapiedra, J.; Iglesias, A.; Lorenzo, J.M.; Lorenzo, J.M. Effect of the amount of chestnuts in the diet of Celta pigs on the fatty acid profile of dry-cured lacon. Grasas Y Aceites 2016, 67, 119. [Google Scholar]
- Ortiz, A.; González, E.; García-Torres, S.; Gaspar, P.; Tejerina, D. Do animal slaughter age and pre-cure freezing have a significant impact on the quality of Iberian dry-cured pork loin? Meat Sci. 2021, 179, 108531. [Google Scholar] [CrossRef]
- Keys, A.; Mienotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H. The Diet and 15-Year Death Rate in the Seven Countries Study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef]
- Keys, A. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease; Harvard University Press: Cambridge, MA, USA, 1980; pp. xi+381. ISBN 0-674-80237-3. [Google Scholar]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- WHO and FAO. Diet, Nutrition, and the Prevention of Chronic Diseases (Report of a joint WHO and FAO Expert Consulation). WHO Tech. Rep. Ser. 2003, 916, 1–160. [Google Scholar]
- Bayés-García, L.; Tres, A.; Vichi, S.; Calvet, T.; Cuevas-Diarte, M.A.; Codony, R.; Boatella, J.; Caixach, J.; Ueno, S.; Guardiola, F. Authentication of Iberian dry-cured ham: New approaches by polymorphic fingerprint and ultrahigh resolution mass spectrometry. Food Control 2016, 60, 370–377. [Google Scholar] [CrossRef]
- UK Department of Health. Report on health and social subjects No. 46. In Nutritional Aspects of Cardiovascular Disease; HMSO: London, UK, 1994; p. 51. [Google Scholar]
- World Health Organization. Interim summary of conclusions and dietary recommendations on total fat and fatty acids. In Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition, 10 to 14 November; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- British Nutrition Foundation. Unsaturated Fatty Acids: Nutritional and Physiological Significance: The Report from the British Nutrition Foundation’s Task Force; Chapman and Hall, Ltd.: London, UK, 1992. [Google Scholar]
- Simopoulos, A. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of the dietary Omega-6:Omega-3 fatty acid ratio: Medical implications. World Rev. Nutr. Diet. 2009, 100, 1–21. [Google Scholar]
- Simopoulos, A.P. ω-3 fatty acids in the prevention management of cardiovascular disease. Can. J. Physiol. Pharmacol. 1997, 75, 234–239. [Google Scholar] [PubMed]
- Maekawa, S.; Takada, S.; Nambu, H.; Furihata, T.; Kakutani, N.; Setoyama, D.; Ueyanagi, Y.; Kang, D.; Sabe, H.; Kinugawa, S. Linoleic acid improves assembly of the CII subunit and CIII2/CIV complex of the mitochondrial oxidative phosphorylation system in heart failure. Cell Commun. Signal. 2019, 17, 128. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Liu, G.; Willett, W.C.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Hu, F.B.; Sun, Q. Associations between Linoleic Acid Intake and Incident Type 2 Diabetes among U.S. Men and Women. Diabetes Care 2019, 42, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Lands, W.E.M. Fish, Omega-3, and Human Health, 2nd ed.AOCS Press: Urbana, IL, USA, 2005. [Google Scholar]
- Singh, M. Essential fatty acids, DHA and human brain. Indian J. Pediatr. 2005, 72, 239–242. [Google Scholar] [CrossRef]
- Wertz, P.W. Essential fatty acids and dietary stress. Toxicol. Ind. Health 2009, 25, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Razmaite, V.; Švirmickas, G.J.; Šiukšcius, A.U. Effect of Weight, Sex and Hunting Period on Fatty Acid Composition of Intramuscular and Subcutaneous Fat from Wild Boar. Ital. J. Anim. Sci. 2012, 11, 174–179. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Liotta, L.; Chiofalo, V.; Presti, L.V.; Chiofalo, B. In vivo performances, carcass traits, and meat quality of pigs fed olive cake processing waste. Animals 2019, 9, 1155. [Google Scholar] [CrossRef]
- Cava, R.; Estévez, M.; Ruiz, J.; Morcuende, D. Physicochemical characteristics of three muscles from free-range reared Iberian pigs slaughtered at 90 kg live weight. Meat Sci. 2003, 63, 533–541. [Google Scholar] [CrossRef]
- Covaciu, F.D.; Feher, I.; Cristea, G.; Dehelean, A. Nutritional quality and safety assessment of pork meat cut from Romania: Fatty acids and Elemental profile. Foods 2024, 13, 804. [Google Scholar] [CrossRef]
- Greenacre, M.; Groenen, P.J.F.; Hastie, T.; d’Enza, A.I.; Markos, A.; Tuzhilina, E. Principal component analysis. Nat. Rev. Methods Primers 2022, 2, 100. [Google Scholar] [CrossRef]

| Diets | ||||
|---|---|---|---|---|
| Control | BgCf15 | BgCf25 | BgPr15 | |
| Chemical composition of the diet | ||||
| DM | 86.35 | 83.76 | 90.77 | 88.40 |
| OM | 94.73 | 95.35 | 95.03 | 95.00 |
| NDF | 20.06 | 27.47 | 36.63 | 27.50 |
| ADF | 7.62 | 12.31 | 23.67 | 13.97 |
| ADL | 2.49 | 4.78 | 9.62 | 5.75 |
| PB | 12.31 | 11.18 | 13.78 | 10.94 |
| GB | 5.74 | 6.75 | 4.51 | 5.86 |
| Fatty acids (g/100 g) | ||||
| ΣSFA | 15.27 | 12.88 | 16.28 | 11.91 |
| ΣMUFA | 23.80 | 59.32 | 41.71 | 56.69 |
| ΣPUFA | 22.25 | 9.18 | 25.11 | 7.27 |
| n-6/n-3 | 17.18 | 7.77 | 14.08 | 7.57 |
| Physicochemical Composition (g/100 g) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aw | Moisture | Ash | Total Fat | Protein | Collagen | Haem Pigments | NaCl | |||||||||
| L | C | L | C | L | C | L | C | L | C | L | C | L | C | L | C | |
| Control | 0.893 | 0.857 | 41.27 | 31.24 | 5.07 | 4.97 | 21.40 | 41.09 | 32.61 | 24.52 | 3.44 | 3.15 | 2.31 | 4.17 | 3.75 | 4.24 |
| Cf15 | 0.873 | 0.839 | 42.73 | 33.43 | 4.98 | 5.96 | 19.03 | 36.59 | 32.24 | 24.99 | 3.35 | 2.55 | 2.15 | 4.33 | 3.55 | 5.01 |
| Cf 25 | 0.866 | 0.862 | 40.02 | 31.84 | 7.21 | 5.36 | 24.62 | 40.03 | 29.73 | 23.40 | 3.66 | 2.74 | 2.72 | 4.04 | 6.53 | 4.01 |
| Pr15 | 0.896 | 0.815 | 41.69 | 30.99 | 4.25 | 6.13 | 16.90 | 43.37 | 34.26 | 24.39 | 2.83 | 2.87 | 2.41 | 3.84 | 2.76 | 5.36 |
| SEM | 0.01 | 1.37 | 0.57 | 3.46 | 1.13 | 0.56 | 0.29 | 0.53 | ||||||||
| Significance product | *** | *** | ns | *** | *** | ns | *** | ns | ||||||||
| Significance diet | ns | ns | ns | ns | ns | ns | ns | * | ||||||||
| Significance product x diet | * | ns | ** | ns | ns | ns | ns | *** | ||||||||
| Fatty Acids | L | C | SEM | p | D | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Cf15 | Cf25 | Pr15 | Control | Cf15 | Cf25 | Pr15 | ||||
| C10:0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.004 | ns | ns |
| C12:0 | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 | 0.05 | 0.04 | 0.05 | 0.003 | ns | ns |
| C14:0 | 1.14 | 1.15 | 1.13 | 1.11 | 1.13 | 1.13 | 1.12 | 1.11 | 0.03 | ns | ns |
| C14:1 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.005 | ns | ns |
| C15:0 | 0.05 | 0.05 | 0.05 | 0.07 | 0.02 | 0.03 | 0.02 | 0.01 | 0.008 | *** | ns |
| C16:0 | 26.12 | 25.98 | 26.05 | 25.48 | 26.38 | 26.17 | 26.37 | 25.80 | 0.30 | ns | ns |
| C16:1n-7 | 2.50 | 2.32 | 2.29 | 2.36 | 2.53 | 2.34 | 2.32 | 2.15 | 0.08 | ns | ** |
| C17:0 | 0.17 | 0.20 | 0.21 | 0.12 | 0.24 | 0.23 | 0.24 | 0.22 | 0.02 | ** | ns |
| C17:1n-7 | 0.23 | 0.22 | 0.22 | 0.19 | 0.24 | 0.23 | 0.23 | 0.20 | 0.02 | ns | ns |
| C18:0 | 13.1 | 13.41 | 13.30 | 12.96 | 12.68 | 12.72 | 12.93 | 12.84 | 0.32 | * | ns |
| 9t-C18:1 | 0.21 | 0.23 | 0.19 | 0.21 | 0.20 | 0.22 | 0.18 | 0.22 | 0.01 | ns | ** |
| C18:1n-9 | 47.82 | 47.40 | 47.49 | 48.43 | 47.97 | 48.01 | 47.43 | 48.49 | 0.55 | ns | ns |
| C18:2n-6 | 6.50 | 6.79 | 6.76 | 6.74 | 6.62 | 6.81 | 7.02 | 6.90 | 0.26 | ns | ns |
| C20:0 | 0.21 | 0.19 | 0.21 | 0.22 | 0.17 | 0.17 | 0.18 | 0.18 | 0.01 | *** | ns |
| C18:3n-6 | 0.011 | 0.011 | 0.013 | 0.006 | 0.003 | 0.006 | 0.004 | 0.003 | 0.002 | *** | ns |
| C20:1n-9 | 0.77 | 0.80 | 0.91 | 0.82 | 0.76 | 0.80 | 0.86 | 0.79 | 0.03 | ns | *** |
| C18:3n-3 | 0.20 | 0.24 | 0.24 | 0.22 | 0.21 | 0.24 | 0.24 | 0.25 | 0.01 | ns | *** |
| C20:2n-6 | 0.24 | 0.24 | 0.2 | 0.25 | 0.23 | 0.24 | 0.28 | 0.24 | 0.01 | ns | ** |
| C22:0 | 0.04 | 0.05 | 0.03 | 0.05 | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 | * | ns |
| C20:3n-6 | 0.05 | 0.05 | 0.05 | 0.06 | 0.04 | 0.05 | 0.05 | 0.04 | 0.006 | ns | ns |
| C22:1n-9 | 0.03 | 0.04 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.02 | 0.004 | ns | ns |
| C23:0 | 0.35 | 0.40 | 0.33 | 0.43 | 0.27 | 0.32 | 0.22 | 0.29 | 0.05 | *** | ns |
| C24:1n-9 | 0.06 | 0.07 | 0.06 | 0.07 | 0.07 | 0.07 | 0.07 | 0.06 | 0.007 | ns | ns |
| C22:6n-3 | 0.03 | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.02 | 0.005 | ns | ns |
| SFA | 41.31 | 41.52 | 41.38 | 40.52 | 41.01 | 40.88 | 41.16 | 40.55 | 0.56 | ns | ns |
| MUFA | 51.65 | 51.11 | 51.23 | 52.15 | 51.83 | 51.73 | 51.15 | 51.95 | 0.56 | ns | ns |
| PUFA | 7.04 | 7.38 | 7.39 | 7.33 | 7.16 | 7.40 | 7.70 | 7.49 | 0.28 | ns | ns |
| n-6 | 6.80 | 7.10 | 7.11 | 7.06 | 6.90 | 7.11 | 7.36 | 7.18 | 0.27 | ns | ns |
| n-3 | 0.22 | 0.27 | 0.28 | 0.26 | 0.26 | 0.28 | 0.33 | 0.31 | 0.02 | ** | * |
| n-6/n-3 | 31.75 | 27.39 | 27.37 | 28.24 | 30.85 | 26.74 | 24.83 | 24.64 | 1.88 | ns | ** |
| IA | 0.52 | 0.52 | 0.52 | 0.50 | 0.53 | 0.52 | 0.53 | 0.51 | 0.01 | ns | ns |
| IT | 1.35 | 1.36 | 1.35 | 1.30 | 1.33 | 1.32 | 1.34 | 1.3 | 0.03 | ns | ns |
| h/H | 2.00 | 2.01 | 2.01 | 2.09 | 2.00 | 2.02 | 2.00 | 2.07 | 0.04 | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, A.; Vasconcelos, L.; Lopez, S.; Outor-Monteiro, D.; Pinheiro, V.; Rodrigues, S.; Teixeira, A. Incorporating Olive By-Products in Bísaro Pig Diets: Effect on Dry-Cured Product Quality. Foods 2024, 13, 2579. https://doi.org/10.3390/foods13162579
Leite A, Vasconcelos L, Lopez S, Outor-Monteiro D, Pinheiro V, Rodrigues S, Teixeira A. Incorporating Olive By-Products in Bísaro Pig Diets: Effect on Dry-Cured Product Quality. Foods. 2024; 13(16):2579. https://doi.org/10.3390/foods13162579
Chicago/Turabian StyleLeite, Ana, Lia Vasconcelos, Sergio Lopez, Divanildo Outor-Monteiro, Victor Pinheiro, Sandra Rodrigues, and Alfredo Teixeira. 2024. "Incorporating Olive By-Products in Bísaro Pig Diets: Effect on Dry-Cured Product Quality" Foods 13, no. 16: 2579. https://doi.org/10.3390/foods13162579
APA StyleLeite, A., Vasconcelos, L., Lopez, S., Outor-Monteiro, D., Pinheiro, V., Rodrigues, S., & Teixeira, A. (2024). Incorporating Olive By-Products in Bísaro Pig Diets: Effect on Dry-Cured Product Quality. Foods, 13(16), 2579. https://doi.org/10.3390/foods13162579










