A Comprehensive Review on the Biogenic Amines in Cheeses: Their Origin, Chemical Characteristics, Hazard and Reduction Strategies
Abstract
:1. Introduction
- (i)
- Mycotoxins (aflatoxin B1, B2, G1 and G2, ochratoxin-A, fumonisin and others) can contaminate milk from animal feeding. The Aflatoxin M1, which arises from the conversion of aflatoxin B1 by ruminants, is very harmful to human health [11].
- (ii)
- Pesticides arising from contaminated animal feed, although their use has been limited or banned in many countries [12].
- (iii)
- Toxins from plants eaten by animals such as pyrrolizidine alkaloids can represent a risk for consumers, although the carry-over towards milk is very low [13].
- (iv)
- Veterinary drugs, such as antibiotics used for animals affected by mastitis, having the affinity to migrate into the milk. Therefore, those animals being under drug treatment need to have a withdrawal period prior to provide milk eligible for human consumption [14].
- (v)
- Dairy sanitizer and detergents used for cleaning-in-place (CIP) and disinfection of cheesemaking production plants [15] that leave residues when not handled properly; their residues can contaminate the product and consequently pose a risk to consumers.
- (vi)
- (vii)
- Undesired microbial metabolites, beyond molecules arising from uncontrolled fermentations (i.e., butyric acid, acetic acid, CO2 and others, which usually provoke defects leading to economy losses), as well as other molecules that are of safety concern such as biogenic amines (BAs).
- (viii)
- New-borne cheese processing/ripening contaminants originating from chemical reactions among milk constituents, i.e., advanced glycation end products (AGEs), representing a risk for human health [18].
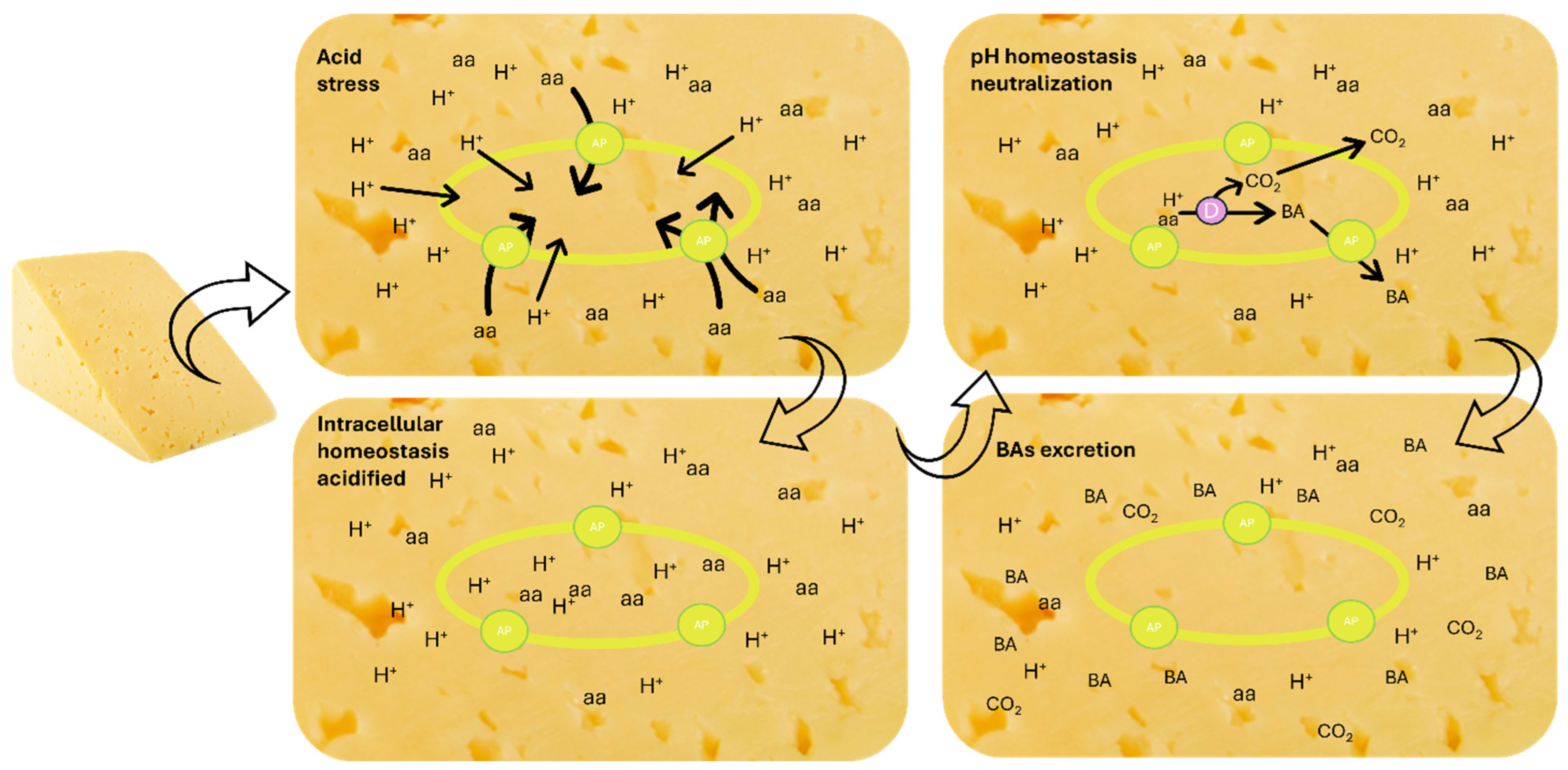
2. Origin—Chemical and Hazard Characteristics of Biogenic Amines
2.1. How Do They Originate
2.2. Chemical Structure and Consumers’ Toxicological Issues
2.3. What Produces Amines in Cheeses
2.4. Factors Influencing BAs Accumulation—Organization of Case Studies
3. Hard and Semi-Hard Cheeses—Case Studies
3.1. Cow’s Milk Cheeses
3.2. Ewe’s Milk Cheeses
3.3. Goat’s Milk Cheeses
3.4. Mixed Milk Cheeses
4. Soft-Ripened Cheeses—Case Studies
4.1. Cow’s Milk Blue Cheeses
4.2. Cow’s Milk Smear-Ripened Cheeses
4.3. Cow’s Milk Surface Mold–Ripened Cheeses
4.4. Others
5. Miscellaneous—Case Studies
6. The Involvement of Cheese Starter Cultures in BAs Production—Case Studies
7. The Involvement of Cheese-Isolated Eukaryotes in BAs Production—Case Studies
8. The Involvement of Microorganisms in Reducing BAs in Cheeses—Case Studies
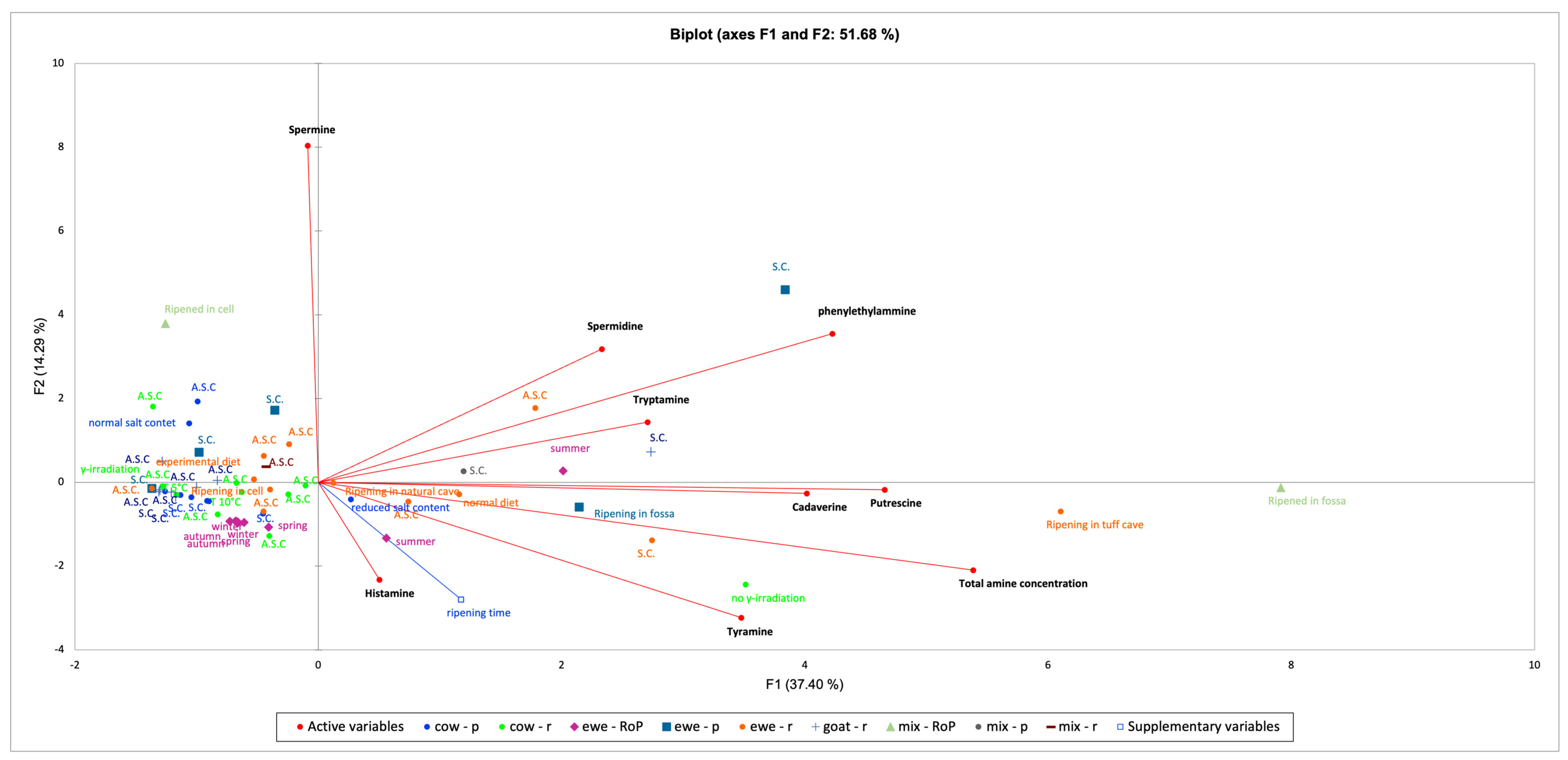
9. Data Analysis
10. Final Considerations and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blažić, M.; Pavić, K.; Zavadlav, S.; Marčac, N. The impact of traditional cheeses and whey on health. Croat. J. Food Sci. Technol. 2017, 9, 198–203. [Google Scholar] [CrossRef]
- Ganesan, P.; Kwak, H.S.; Hong, Y.-H. Nutritional benefits in cheese. In Cheese: Types, Nutrition and Consumption; ResearchGate: Berlin, Germany, 2012; pp. 269–289. [Google Scholar]
- López-Expósito, I.; Amigo, L.; Recio, I. A mini-review on health and nutritional aspects of cheese with a focus on bioactive peptides. Dairy Sci. Technol. 2012, 92, 419–438. [Google Scholar] [CrossRef]
- Nagpal, R.; Behare, P.V.; Kumar, M.; Mohania, D.; Yadav, M.; Jain, S.; Menon, S.; Parkash, O.; Marotta, F.; Minelli, E.; et al. Milk, milk products, and Disease Free Health: An updated overview. Crit. Rev. Food Sci. Nutr. 2012, 52, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Schmid, A.; Sieber, R.; Wehrmüller, K. Cheese in nutrition and health. Dairy Sci. Technol. 2008, 88, 389–405. [Google Scholar] [CrossRef]
- Roselli, M.; Natella, F.; Zinno, P.; Guantario, B.; Canali, R.; Schifano, E.; De Angelis, M.; Nikoloudaki, O.; Gobbetti, M.; Perozzi, G.; et al. Colonization ability and impact on human gut microbiota of foodborne microbes from traditional or Probiotic-Added fermented foods: A Systematic review. Front. Nutr. 2021, 8, 689084. [Google Scholar] [CrossRef]
- Schoder, D.; Melzner, D.; Schmalwieser, A.; Zangana, A.; Winter, P.; Wagner, M. Important Vectors for Listeria monocytogenes Transmission at Farm Dairies Manufacturing Fresh Sheep and Goat Cheese from Raw Milk. J. Food Protect. 2011, 74, 919–924. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, S.; Choi, K.-H. Microbial benefits and risks of raw milk cheese. Food Control 2016, 63, 201–215. [Google Scholar] [CrossRef]
- Motarjemi, Y.; Moy, G.G.; Jooste, P.J.; Anelich, L.E. Milk and dairy products. In Food Safety Management; Elsevier: Amsterdam, The Netherlands, 2014; pp. 83–117. [Google Scholar] [CrossRef]
- Van Asselt, E.D.; Van Der Fels-Klerx, H.J.; Marvin, H.J.P.; Van Bokhorst-van De Veen, H.; Groot, M.N. Overview of food safety hazards in the European dairy supply chain. Compr. Rev. Food Sci. Food Saf. 2016, 16, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Opinion of the Scientific Panel on contaminants in the food chain related to Aflatoxin B1 as undesirable substance in animal feed. EFSA J. 2004, 2, 39. [CrossRef]
- Akhtar, S. Pesticides Residue in milk and milk products: Mini review. Pak. J. Anal. Environ. Chem. 2017, 18, 37–45. [Google Scholar] [CrossRef]
- Hoogenboom, R.L.; Mulder, P.P.J.; Zeilmaker, M.J.; Van Den Top, H.J.; Remmelink, G.J.; Brandon, E.F.A.; Klijnstra, M.D.; Meijer, G.A.L.; Schothorst, R.; Van Egmond, H.P. Carry-over of pyrrolizidine alkaloids from feed to milk in dairy cows. Food Addit. Contam. A 2011, 28, 359–372. [Google Scholar] [CrossRef]
- Oliver, S.P.; Murinda, S.E.; Jayarao, B.M. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: A Comprehensive review. Foodborne Pathog. Dis. 2011, 8, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. Leb.-Wiss Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef]
- Chuchu, N.; Patel, B.; Sebastian, B.; Exley, C. The aluminium content of infant formulas remains too high. BMC Pediatr. 2013, 13, 162. [Google Scholar] [CrossRef]
- Zota, A.R.; Phillips, C.A.; Mitro, S.D. Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003–2010. Environ. Health Perspect. 2016, 124, 1521–1528. [Google Scholar] [CrossRef]
- Li, M.; Shen, M.; Lu, J.; Yang, J.; Huang, Y.; Liu, L.; Fan, H.; Xie, J.; Xie, M. Maillard reaction harmful products in dairy products: Formation, occurrence, analysis, and mitigation strategies. Food Res. Int. 2022, 151, 110839. [Google Scholar] [CrossRef]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Santos, M.H.S. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Ekici, K.; Omer, A.K. Biogenic amines formation and their importance in fermented foods. Bio Web Conf. 2020, 17, 00232. [Google Scholar] [CrossRef]
- Erdag, D.; Merhan, O.; Yildiz, B. Biochemical and pharmacological properties of biogenic amines. In Biogenic Amines; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Perin, L.M.; Nero, L.A. Chapter 7. Occurrence of Biogenic amines in cheese: An Overview. In Food Chemistry, Function and Analysis; Royal Society of Chemistry: London, UK, 2019; pp. 119–132. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2020, 101, 2634–2640. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.-S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Konings, W.N. Microbial transport: Adaptations to natural environments. Antonie Leeuwenhoek 2006, 90, 325–342. [Google Scholar] [CrossRef]
- Landete, J.M.; Pardo, I.; Ferrer, S. Regulation ofhdcexpression and HDC activity by enological factors in lactic acid bacteria. J. Appl. Microbiol. 2008, 105, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Danquah, A.O.; Benjakul, S.; Simpson, B.K. Biogenic Amines in Foods. In Food Biochemistry and Food Processing; Wiley: Hoboken, NJ, USA, 2012; pp. 820–832. [Google Scholar] [CrossRef]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological factors affecting biogenic amine content in foods: A review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Spermine and spermidine are cytotoxic towards intestinal cell cultures, but are they a health hazard at concentrations found in foods? Food Chem. 2018, 269, 321–326. [Google Scholar] [CrossRef]
- Kalač, P. Health effects and occurrence of dietary polyamines: A review for the period 2005–mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef]
- Benkerroum, N. Biogenic amines in dairy products: Origin, incidence, and control means. Compr. Rev. Food Sci. Food Saf. 2016, 15, 801–826. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]
- Wunderlichová, L.; Buňková, L.; Koutný, M.; Jančová, P.; Buňka, F. Formation, degradation, and detoxification of putrescine by foodborne bacteria: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1012–1030. [Google Scholar] [CrossRef]
- Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [CrossRef]
- Fernández, M.; Zúñiga, M. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 2006, 32, 155–183. [Google Scholar] [CrossRef]
- Marcobal, A.; De Las Rivas, B.; Landete, J.M.; Tabera, L.; Muñoz, R. Tyramine and phenylethylamine biosynthesis by food bacteria. Crit. Rev. Food Sci. Nutr. 2012, 52, 448–467. [Google Scholar] [CrossRef]
- Marcobal, A.; De Las Rivas, B.; Muñoz, R. First genetic characterization of a bacterial Î2-phenylethylamine biosynthetic enzyme in Enterococcus faecium RM58. FEMS Microbiol. Lett. 2006, 258, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Trip, H.; Lonvaud-Funel, A.; Lolkema, J.S.; Lucas, P. Evidence of two functionally distinct ornithine decarboxylation systems in lactic acid bacteria. Appl. Environ. Microbiol. 2012, 78, 1953–1961. [Google Scholar] [CrossRef]
- Tabor, C.W.; Tabor, H. Polyamines in microorganisms. Microbiol. Rev. 1985, 49, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Burdychova, R.; Komprda, T. Biogenic amine-forming microbial communities in cheese. FEMS Microbiol. Lett. 2007, 276, 149–155. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.J.; Fallico, V.; O’Sullivan, O.; McSweeney, P.L.H.; Sheehan, J.J.; Cotter, P.D.; Giblin, L. High-throughput DNA sequencing to survey bacterial histidine and tyrosine decarboxylases in raw milk cheeses. BMC Microbiol. 2015, 15, 266. [Google Scholar] [CrossRef]
- Tittarelli, F.; Perpetuini, G.; Di Gianvito, P.; Tofalo, R. Biogenic amines producing and degrading bacteria: A snapshot from raw ewes’ cheese. Leb.-Wiss Technol. 2019, 101, 1–9. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic Acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Komprda, T.; Burdychová, R.; Dohnal, V.; Cwiková, O.; Sládková, P.; Dvořáčková, H. Tyramine production in Dutch-type semi-hard cheese from two different producers. Food Microbiol. 2008, 25, 219–227. [Google Scholar] [CrossRef] [PubMed]
- De Llano, D.G.; Cuesta, N.; Rodríguez, N. Biogenic amine production by wild lactococcal and leuconostoc strains. Lett. Appl. Microbiol. 1998, 26, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Mancini, L.; Fox, P.F. Pros and cons for using non-starter lactic acid bacteria (NSLAB) as secondary/adjunct starters for cheese ripening. Trends Food Sci. Technol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Schneller, R.; Good, P.; Jenny, M. Influence of pasteurised milk, raw milk and different ripening cultures on biogenic amine concentrations in semi-soft cheeses during ripening. Z. Leb. Forsch. 1997, 204, 265–272. [Google Scholar] [CrossRef]
- Maifreni, M.; Frigo, F.; Bartolomeoli, I.; Innocente, N.; Biasutti, M.; Marino, M. Identification of the Enterobacteriaceae in Montasio cheese and assessment of their amino acid decarboxylase activity. J. Dairy Res. 2013, 80, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Novella-Rodríguez, S.; Veciana-Nogués, M.T.; Roig-Sagués, A.X.; Trujillo-Mesa, A.J.; Vidal-Carou, M.C. Evaluation of biogenic amines and microbial counts throughout the ripening of goat cheeses from pasteurized and raw milk. J. Dairy Res. 2004, 71, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Schirone, M.; Tofalo, R.; Fasoli, G.; Perpetuini, G.; Corsetti, A.; Manetta, A.C.; Ciarrocchi, A.; Suzzi, G. High content of biogenic amines in Pecorino cheeses. Food Microbiol. 2013, 34, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; Martín, M.; Ladero, V.; Alvarez, M.A.; Fernández, M. Biogenic amines in dairy products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef]
- Ladero, V.; Fernández, M.; Calles-Enríquez, M.; Sánchez-Llana, E.; Cañedo, E.; Martín, M.C.; Alvarez, M.A. Is the production of the biogenic amines tyramine and putrescine a species-level trait in enterococci? Food Microbiol. 2012, 30, 132–138. [Google Scholar] [CrossRef]
- Delbes, C.C.; Pochet, S.S.; Hélinck, S.S.; Veisseire, P.P.; Bord, C.C.; Lebecque, A.; Coton, M.M.; Desmasures, N.N.; Coton, E.E.; Irlinger, F.F.; et al. Impact of Gram-negative bacteria in interaction with a complex microbial consortium on biogenic amine content and sensory characteristics of an uncooked pressed cheese. Food Microbiol. 2012, 30, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Pircher, A.; Bauer, F.; Paulsen, P. Formation of cadaverine, histamine, putrescine and tyramine by bacteria isolated from meat, fermented sausages and cheeses. Eur. Food Res. Technol. 2006, 226, 225–231. [Google Scholar] [CrossRef]
- Ladero, V.; Rattray, F.P.; Mayo, B.; Martín, M.C.; Fernández, M.; Alvarez, M.A. Sequencing and Transcriptional Analysis of the Biosynthesis Gene Cluster of Putrescine-Producing Lactococcus lactis. Appl. Environ. Microbiol. 2011, 77, 6409–6418. [Google Scholar] [CrossRef] [PubMed]
- Gardini, F.; Tofalo, R.; Belletti, N.; Iucci, L.; Suzzi, G.; Torriani, S.; Guerzoni, M.E.; Lanciotti, R. Characterization of yeasts involved in the ripening of Pecorino Crotonese cheese. Food Microbiol. 2006, 23, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; Del Río, B.; Ladero, V.; Martínez, N.; Fernández, M.; Martín, M.C.; Álvarez, M.A. Factors influencing biogenic amines accumulation in dairy products. Front. Microbiol. 2012, 3, 180. [Google Scholar] [CrossRef] [PubMed]
- Chaves-López, C.; Serio, A.; Martuscelli, M.; Paparella, A.; Osorio-Cadavid, E.; Suzzi, G. Microbiological characteristics of kumis, a traditional fermented Colombian milk, with particular emphasis on enterococci population. Food Microbiol. 2011, 28, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Novella-Rodríguez, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Biogenic amines and polyamines in milks and cheeses by Ion-Pair High Performance Liquid Chromatography. J. Agric. Food Chem. 2000, 48, 5117–5123. [Google Scholar] [CrossRef]
- Özdestan, Ö.; Üren, A. Biogenic amine content of kefir: A fermented dairy product. Eur. Food Res. Technol. 2010, 231, 101–107. [Google Scholar] [CrossRef]
- Novella-Rodríguez, S.; Veciana-Nogués, M.T.; Trujillo-Mesa, A.J.; Vidal-Carou, M.C. Profile of Biogenic Amines in Goat Cheese Made from Pasteurized and Pressurized Milks. J. Food Sci. 2002, 67, 2940–2944. [Google Scholar] [CrossRef]
- Arlorio, M.; Coïsson, J.D.; Travaglia, F.; Capasso, M.; Rinaldi, M.; Martelli, A. Proteolysis and production of biogenic amines in toma piemontese pdo cheese during ripening. Ital. J. Food Sci. 2002, 3, 195. [Google Scholar]
- Buňková, L.; Buňka, F.; Mantlová, G.; Čablová, A.; Sedláček, I.; Švec, P.; Pachlová, V.; Kráčmar, S. The effect of ripening and storage conditions on the distribution of tyramine, putrescine and cadaverine in Edam-cheese. Food Microbiol. 2010, 27, 880–888. [Google Scholar] [CrossRef]
- Fernández, M.; Del Río, B.; Linares, D.M.; Martín, M.C.; Alvarez, M.A. Real-Time polymerase chain reaction for quantitative detection of Histamine-Producing bacteria: Use in cheese production. J. Dairy Sci. 2006, 89, 3763–3769. [Google Scholar] [CrossRef]
- Fernández, M.; Linares, D.M.; Del Río, B.; Ladero, V.; Alvarez, M.A. HPLC quantification of biogenic amines in cheeses: Correlation with PCR-detection of tyramine-producing microorganisms. J. Dairy Res. 2007, 74, 276–282. [Google Scholar] [CrossRef]
- Ladero, V.; Calles-Enriquez, M.; Fernandez, M.; Alvarez, M.A. Toxicological effects of dietary biogenic amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Ladero, V.; Linares, D.M.; Fernández, M.; Alvarez, M.A. Real time quantitative PCR detection of histamine-producing lactic acid bacteria in cheese: Relation with histamine content. Food Res. Int. 2008, 41, 1015–1019. [Google Scholar] [CrossRef]
- McSweeney, P.L.H. Biochemistry of cheese ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, Structure, and Digestive Dynamics of Milk from Different Species—A review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef]
- Gantner, V.; Mijić, P.; Baban, M.; Škrtić, Z.; Turalija, A. The overall and fat composition of milk of various species. Mljekarstvo 2015, 65, 223–231. [Google Scholar] [CrossRef]
- Regulation, E.C. No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, 30, 151. [Google Scholar]
- Corrales, J.C.; Sánchez, A.; Luengo, C.; Poveda, J.B.; Contreras, A. Effect of clinical contagious agalactia on the bulk tank milk somatic cell count in Murciano-Granadina Goat Herds. J. Dairy Sci. 2004, 87, 3165–3171. [Google Scholar] [CrossRef]
- Gonzalo, C.; Tardáguila, A.; Ariznabarreta, A.; Romeo, M.; Montoro, V.; Pérez-Guzmán, M.D.; Marco, J.C. Somatic cell counts in dairy livestock and control strategies: Situation in Spain. In Mastitis and Milk Quality; DiegoMarin Publ.: Murcia, Spain, 2000; pp. 145–151. [Google Scholar]
- Silanikove, N.; Merin, U.; Leitner, G. On effects of subclinical mastitis and stage of lactation on milk quality in goats. Small Rumin. Res. 2014, 122, 76–82. [Google Scholar] [CrossRef]
- Quintas, H.; Margatho, G.; Rodríguez-Estévez, V.; Jiménez-Granado, R.; Simões, J. Understanding Mastitis in Goats (II): Microbiological diagnosis and Somatic Cells Count; Springer: Berlin/Heidelberg, Germany, 2017; pp. 335–358. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U.; Prosser, C.G. Recent advances in exploiting goat’s milk: Quality, safety and production aspects. Small Rumin. Res. 2010, 89, 110–124. [Google Scholar] [CrossRef]
- Hernández-Ramos, P.A.; Vivar-Quintana, A.M.; Revilla, I. Estimation of somatic cell count levels of hard cheeses using physicochemical composition and artificial neural networks. J. Dairy Sci. 2019, 102, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Kaskous, S.; Farschtschi, S.; Pfaffl, M.W. Physiological Aspects of milk somatic cell count in Small Ruminants—A review. Dairy 2022, 4, 26–42. [Google Scholar] [CrossRef]
- Zeng, S.S.; Escobar, E.N. Influence of somatic cell count in goat milk on yield and quality of soft cheese. In Proceedings of theIDF/Greek National Committee of IDF/CIRAVAL Seminar on Production and Utilization of EWE and Goat Milk, Crete, Greece, 19–21 October1995; pp. 109–113. [Google Scholar]
- Margetín, M.; Milerski, M.; Apolen, D.; Čapistrák, A.; Oravcov, M.; Debreceni, O. Relationships between production, quality of milk and udder health status of ewes during machine milking. J. Cent. Eur. Agric. 2013, 14, 328–340. [Google Scholar] [CrossRef]
- Tančin, V.; Baranovič, Š.; Uhrinčať, M.; Mačuhová, L.; Vršková, M.; Oravcová, M. Somatic cell counts in raw ewes’ milk in dairy practice: Frequency of distribution and possible effect on milk yield and composition. Mljekarstvo 2017, 67, 253–260. [Google Scholar] [CrossRef]
- Baranovic, S.; Tancin, V.; Tvarozkova, K.; Uhrincat, M.; Macuhova, L.; Palkovic, J. Impact of somatic cell count and lameness on the production and composition of ewe’s milk. Potravinarstvo 2018, 12, 116. [Google Scholar] [CrossRef]
- Tvarožková, K. The impact of somatic cell count on milk yield and composition. Acta Fytotech. Zootech. 2021, 24, 49–52. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Kelly, A.L.; Caroprese, M.; Marino, R.; Sevi, A. Activities of indigenous proteolytic enzymes in caprine milk of different somatic cell counts. J. Dairy Sci. 2015, 98, 7587–7594. [Google Scholar] [CrossRef]
- Panthi, R.R.; Jordan, K.N.; Kelly, A.L.; Sheehan, J.J. Selection and treatment of milk for cheesemaking. In Cheese; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–50. [Google Scholar] [CrossRef]
- Albenzio, M.; Caroprese, M.; Santillo, A.; Marino, R.; Taibi, L.; Sevi, A. Effects of somatic cell count and stage of lactation on the plasmin activity and Cheese-Making properties of Ewe milk. J. Dairy Sci. 2004, 87, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Marcinkoniene, L.; Ciprovica, I. The influence of milk quality and composition on goat milk suitability for cheese production. Agron. Res. 2020, 18, 1796–1803. [Google Scholar]
- Ducková, V.; Čanigová, M.; Zajác, P.; Remeňová, Z.; Kročko, M.; Nagyová, Ľ. Effect of somatic cell counts occurred in milk on quality of Slovak traditional cheese—Parenica. PotravináRstvo 2019, 13, 675–680. [Google Scholar] [CrossRef]
- Murphy, S.C.; Martin, N.H.; Barbano, D.M.; Wiedmann, M. Influence of raw milk quality on processed dairy products: How do raw milk quality test results relate to product quality and yield? J. Dairy Sci. 2016, 99, 10128–10149. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Pirisi, A.; De Crémoux, R.; Gonzalo, C. Somatic cells of goat and sheep milk: Analytical, sanitary, productive and technological aspects. Small Rumin. Res. 2007, 68, 126–144. [Google Scholar] [CrossRef]
- Li, N.; Richoux, R.; Leconte, N.; Bevilacqua, C.; Maillard, M.-B.; Parayre, S.; Aubert-Frogerais, L.; Warlouzel, J.; Leclair, E.M.; Denis, C.; et al. Somatic cell recovery by microfiltration technologies: A novel strategy to study the actual impact of somatic cells on cheese matrix. Int. Dairy J. 2017, 65, 5–13. [Google Scholar] [CrossRef]
- Olives, A.M.-D.; Navarro-Ríos, M.J.; Rubert-Alemán, J.; Fernández, N.; Molina, M.P. Composition, proteolysis indices and coagulating properties of ewe milk as affected by bulk tank somatic cell count. J. Dairy Res. 2015, 82, 344–349. [Google Scholar] [CrossRef]
- Mazal, G.; Vianna, P.C.B.; Santos, M.V.; Gigante, M.L. Effect of somatic cell count on prato cheese composition. J. Dairy Sci. 2007, 90, 630–636. [Google Scholar] [CrossRef]
- Ubaldo, J.C.S.R.; Carvalho, A.F.; Fonseca, L.M.; Glória, M.B.A. Bioactive amines in Mozzarella cheese from milk with varying somatic cell counts. Food Chem. 2015, 178, 229–235. [Google Scholar] [CrossRef]
- Maréchal, C.L.; Thiéry, R.; Vautor, E.; Loir, Y.L. Mastitis impact on technological properties of milk and quality of milk products—A review. Dairy Sci. Technol. 2011, 91, 247–282. [Google Scholar] [CrossRef]
- Innocente, N.; D’agostin, P. Formation of biogenic amines in a typical semihard Italian cheese. J. Food Prot. 2002, 65, 1498–1501. [Google Scholar] [CrossRef]
- Rank, T.C.; Grappin, R.; Olson, N.F. Secondary proteolysis of cheese during Ripening: A review. J. Dairy Sci. 1985, 68, 801–805. [Google Scholar] [CrossRef]
- Campos-Góngora, E.; González-Martínez, M.T.; López-Hernández, A.A.; Arredondo-Mendoza, G.I.; Ortega-Villarreal, A.S.; González-Martínez, B.E. Histamine and Tyramine in Chihuahua Cheeses during Shelf Life: Association with the Presence of tdc and hdc Genes. Molecules 2023, 28, 3007. [Google Scholar] [CrossRef]
- Innocente, N.; Marino, M.; Marchesini, G.; Biasutti, M. Presence of biogenic amines in a traditional salted Italian cheese. Int. J. Dairy Technol. 2009, 62, 154–160. [Google Scholar] [CrossRef]
- Decadt, H.; Vermote, L.; Díaz-Muñoz, C.; Weckx, S.; De Vuyst, L. Decarboxylase activity of the non-starter lactic acid bacterium Loigolactobacillus rennini gives crack defects in Gouda cheese through the production of γ-aminobutyric acid. Appl. Environ. Microbiol. 2024, 90, e01655-23. [Google Scholar] [CrossRef]
- Kim, S.H.; Na, J.H.; Huh, C.K. Effects of Hwangto coating and grapefruit seed extract treatment on the ripening of Gouda cheese. J. Food Meas. Charact. 2022, 16, 4976–4984. [Google Scholar] [CrossRef]
- Ordóñez, A.I.; Ibáñez, F.C.; Torre, P.; Barcina, Y. Formation of biogenic amines in idiazábal Ewe’s-Milk cheese: Effect of ripening, pasteurization, and starter. J. Food Prot. 1997, 60, 1371–1375. [Google Scholar] [CrossRef]
- Martuscelli, M.; Gardini, F.; Torriani, S.; Mastrocola, D.; Serio, A.; Chaves-López, C.; Schirone, M.; Suzzi, G. Production of biogenic amines during the ripening of Pecorino Abruzzese cheese. Int. Dairy J. 2005, 15, 571–578. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fernández, D.; Arenas, R.; Diezhandino, I.; Tornadijo, M.E.; Fresno, J.M. Biogenic amines in Zamorano cheese: Factors involved in their accumulation. J. Sci. Food Agric. 2015, 96, 295–305. [Google Scholar] [CrossRef]
- Andic, S.; Genccelep, H.; Kose, S. Determination of biogenic amines in herby cheese. Int. J. Food Prop. 2010, 13, 1300–1314. [Google Scholar] [CrossRef]
- Torracca, B.; Nuvoloni, R.; Ducci, M.; Bacci, C.; Pedonese, F. Biogenic amines content of four types of “Pecorino” cheese manufactured in Tuscany. Int. J. Food Prop. 2015, 18, 999–1005. [Google Scholar] [CrossRef]
- Torracca, B.; Pedonese, F.; López, M.B.; Turchi, B.; Fratini, F.; Nuvoloni, R. Effect of milk pasteurisation and of ripening in a cave on biogenic amine content and sensory properties of a pecorino cheese. Int. Dairy J. 2016, 61, 189–195. [Google Scholar] [CrossRef]
- Mascaro, N.; Stocchi, R.; Ricciutelli, M.; Cammertoni, N.; Renzi, F.; Cecchini, S.; Loschi, A.R.; Rea, S. Biogenic amine content and chemical and physical features of italian formaggio di fossa. Ital. J. Food Saf. 2010, 1, 49. [Google Scholar] [CrossRef]
- Schirone, M.; Tofalo, R.; Mazzone, G.; Corsetti, A.; Suzzi, G. Biogenic amine content and microbiological profile of Pecorino di Farindola cheese. Food Microbiol. 2011, 28, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Renes, E.; Fernández, D.; Abarquero, D.; Ladero, V.; Álvarez, M.A.; Tornadijo, M.E.; Fresno, J.M. Effect of forage type, season, and ripening time on selected quality properties of sheep milk cheese. J. Dairy Sci. 2021, 104, 2539–2552. [Google Scholar] [CrossRef] [PubMed]
- Joosten, H.M.L.J. Conditions Allowing the Formation of Biogenic Amines in Cheese. 3. Factors Influencing the Amounts Formed. Ph.D. Thesis, Wageningen University & Research, Wageningen, The Netherlands, 1988. Volume 41. pp. 329–357. [Google Scholar]
- Pintado, A.I.E.; Pinho, O.; Ferreira, I.M.P.L.V.O.; Pintado, M.M.E.; Gomes, A.M.P.; Malcata, F.X. Microbiological, biochemical and biogenic amine profiles of Terrincho cheese manufactured in several dairy farms. Int. Dairy J. 2008, 18, 631–640. [Google Scholar] [CrossRef]
- Calzada, J.; Del Olmo, A.; Picón, A.; Gaya, P.; Nuñez, M. Reducing Biogenic-Amine-Producing bacteria, decarboxylase activity, and biogenic amines in raw milk cheese by High-Pressure treatments. Appl. Environ. Microbiol. 2013, 79, 1277–1283. [Google Scholar] [CrossRef]
- Bennato, F.; Di Domenico, M.; Ianni, A.; Di Gialleonardo, L.; Cammà, C.; Martino, G. Grape pomace in Ewes diet affects metagenomic profile, volatile compounds and biogenic amines contents of ripened cheese. Fermentation 2022, 8, 598. [Google Scholar] [CrossRef]
- Galgano, F.; Suzzi, G.; Favati, F.; Caruso, M.; Martuscelli, M.; Gardini, F.; Salzano, G. Biogenic amines during ripening in ‘Semicotto Caprino’ cheese: Role of enterococci. Int. J. Food Sci. Technol. 2001, 36, 153–160. [Google Scholar] [CrossRef]
- Poveda, J.M.; Molina, G.M.; Gómez-Alonso, S. Variability of biogenic amine and free amino acid concentrations in regionally produced goat milk cheeses. J. Food Compos. Anal. 2016, 51, 85–92. [Google Scholar] [CrossRef]
- Cwiková, O.; Franke, G. Biogenic amines in smear ripened cheeses. PotravináRstvo 2019, 13, 378–384. [Google Scholar] [CrossRef]
- Ladero, V.; Martín, M.C.; Redruello, B.; Mayo, B.; Flórez, A.B.; Fernández, M.; Alvarez, M.A. Genetic and functional analysis of biogenic amine production capacity among starter and non-starter lactic acid bacteria isolated from artisanal cheeses. Eur. Food Res. Technol. 2015, 241, 377–383. [Google Scholar] [CrossRef]
- Pachlová, V.; Charousová, Z.; Šopík, T. Effect of milk origin on proteolysis and accumulation of biogenic amine during ripening of Dutch-type cheese. PotravináRstvo 2017, 11, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Cantor, M.D.; Van Den Tempel, T.; Hansen, T.K.; Ardö, Y. Blue cheese. In Cheese; Elsevier: Amsterdam, The Netherlands, 2017; pp. 929–954. [Google Scholar] [CrossRef]
- Rabie, M.A.; Siliha, H.I.; El-Saidy, S.M.; El-Badawy, A.A.; Malcata, F.X. Effect of γ-irradiation upon biogenic amine formation in blue cheese during storage. Int. Dairy J. 2011, 21, 373–376. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, H.J.; Jo, C.; Park, H.J.; Chung, Y.J.; Byun, M.W. Radiolysis of biogenic amines in model system by gamma irradiation. Food Control 2004, 15, 405–408. [Google Scholar] [CrossRef]
- Reinholds, I.; Rusko, J.; Pugajeva, I.; Berzina, Z.; Jansons, M.; Kirilina-Gutmane, O.; Tihomirova, K.; Bartkevics, V. The occurrence and dietary exposure assessment of mycotoxins, biogenic amines, and heavy metals in Mould-Ripened blue cheeses. Foods 2020, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Standarová, E.; Borkovcová, I.; Dušková, M.; Přidalová, H.; Dračková, M.; Vorlová, L. Effect of some factors on the biogenic amines and polyamines content in Blue-Veined Cheese Niva. Czech J. Food Sci. 2009, 27, S410–S413. [Google Scholar] [CrossRef]
- Gurkan, H.; Yilmaztekin, M.; Cakmakci, S.; Hayaloglu, A.A. Volatile compounds and biogenic amines during the ripening of mold-ripened Civil cheese manufactured using three different strains of Penicillium roqueforti. J. Food Saf. 2018, 38, e12568. [Google Scholar] [CrossRef]
- Diezhandino, I.; Fernández, D.; Combarros-Fuertes, P.; Renes, E.; Fresno, J.M.; Tornadijo, M.E. Characteristics and proteolysis of a Spanish blue cheese made with raw or pasteurised milk. Int. J. Dairy Technol. 2022, 75, 630–642. [Google Scholar] [CrossRef]
- Qureshi, T.M.; Vermeer, C.; Vegarud, G.E.; Abrahamsen, R.K.; Skeie, S. Formation of biogenic amines and vitamin K contents in the Norwegian autochthonous cheese Gamalost during ripening. Dairy Sci. Technol. 2013, 93, 303–314. [Google Scholar] [CrossRef]
- Mounier, J.; Coton, M.; Irlinger, F.; Landaud, S.; Bonnarme, P. Smear-Ripened cheeses. In Cheese; Elsevier: Amsterdam, The Netherlands, 2017; pp. 955–996. [Google Scholar] [CrossRef]
- Dugat-Bony, E.; Sarthou, A.-S.; Perello, M.-C.; De Revel, G.; Bonnarme, P.; Hélinck, S. The effect of reduced sodium chloride content on the microbiological and biochemical properties of a soft surface-ripened cheese. J. Dairy Sci. 2016, 99, 2502–2511. [Google Scholar] [CrossRef]
- Komprda, T.; Rejchrtová, E.; Sládková, P.; Zemánek, L.; Vymlátilová, L. Effect of some external factors on the content of biogenic amines and polyamines in a smear-ripened cheese. Dairy Sci. Technol. 2012, 92, 367–382. [Google Scholar] [CrossRef]
- Spinnler, H.-E. Surface Mold–Ripened cheeses. In Cheese; Elsevier: Amsterdam, The Netherlands, 2017; pp. 911–928. [Google Scholar] [CrossRef]
- Metzler, L.; Vali, S.; Paulsen, P.; Seuss-Baum, I. Impact of different starter cultures and cheese coagulants on the formation of biogenic amines in camembert. In Proceedings of the Food Science Conference 2015, Budapest, Hungary, 10–12 November 2015. [Google Scholar]
- Macku, I.; Lazarková, Z.; Bunka, F.; Hrabe, J. Biogenic amine content in mould cheese during storage. Ecol. Chem. Eng. A 2009, 16, 1591–1597. [Google Scholar]
- Rea, S.; Mascaro, N.; Ricciutelli, M.; Cammertoni, N.; Cecchini, S.; Loschi, A.R.; Stocchi, R. Presence of biogenic amines in Ricotta Forte, a traditional Italian dairy product from the Puglia region. Ital. J. Food Sci. 2010, 22, 229–233. [Google Scholar]
- Valsamaki, K.; Michaelidou, A.; Polychroniadou, A. Biogenic amine production in Feta cheese. Food Chem. 2000, 71, 259–266. [Google Scholar] [CrossRef]
- Ma, J.-K.; Raslan, A.A.; Elbadry, S.; El-Ghareeb, W.R.; Mulla, Z.S.; Bin-Jumah, M.; Abdel-Daim, M.M.; Darwish, W.S. Levels of biogenic amines in cheese: Correlation to microbial status, dietary intakes, and their health risk assessment. Environ. Sci. Pollut. Res. Int. 2020, 27, 44452–44459. [Google Scholar] [CrossRef] [PubMed]
- Food Drug Administration (FDA). Fish and Fishery Products Hazards and Controls Guidance; Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition: Washington, DC, USA, 2011. [Google Scholar]
- El-Salam, A.; Adel, Y.; Hassan, M.; El-Aal, A.; Kamal, R.; Ahmed, Y.; Nasseif, M. Determination of Biogenic amines in hard cheeses by High performance liquid chromatography. Benha Vet. Med. J. 2020, 38, 104–109. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.B.; Seol, K.-H.; Ham, J.-S. Quantitative Analysis of Biogenic Amines in Different Cheese Varieties Obtained from the Korean Domestic and Retail Markets. Metabolites 2021, 11, 31. [Google Scholar] [CrossRef]
- Mayer, H.K.; Fiechter, G. UHPLC analysis of biogenic amines in different cheese varieties. Food Control 2018, 93, 9–16. [Google Scholar] [CrossRef]
- Bonczar, G.; Filipczak-Fiutak, M.; Pluta-Kubica, A.; Duda, I.; Walczycka, M.; Staruch, L. The range of protein hydrolysis and biogenic amines content in selected acid- and rennet-curd cheeses. Chem. Pap. 2018, 72, 2599–2606. [Google Scholar] [CrossRef]
- Guarcello, R.; Diviccaro, A.; Barbera, M.; Giancippoli, E.; Settanni, L.; Minervini, F.; Moschetti, G.; Gobbetti, M. A survey of the main technology, biochemical and microbiological features influencing the concentration of biogenic amines of twenty Apulian and Sicilian (Southern Italy) cheeses. Int. Dairy J. 2015, 43, 61–69. [Google Scholar] [CrossRef]
- Buňková, L.; Adamcová, G.; Hudcová, K.; Velichová, H.; Pachlová, V.; Lorencová, E.; Buňka, F. Monitoring of biogenic amines in cheeses manufactured at small-scale farms and in fermented dairy products in the Czech Republic. Food Chem. 2013, 141, 548–551. [Google Scholar] [CrossRef]
- Rohani, S.M.R.; Aliakbarlu, J.; Ehsani, A.; Hassanzadazar, H. Biogenic amines determination in some traditional cheeses in West Azerbaijan province of Iran. DOAJ 2013, 4, 115–118. [Google Scholar]
- Pleva, P.; Buňková, L.; Theimrová, E.; Bartošáková, V.; Buňka, F.; Purevdorj, K. Biogenic amines in smear and mould-ripened cheeses. PotravináRstvo 2014, 8, 321–327. [Google Scholar] [CrossRef]
- Del Valle, L.J.; Ginovart, M.; Gordún, E.; Carbó, R. Histidine decarboxylase-positive lactic acid bacteria strains and the formation of histamine in ripened cheeses. J. Food Process. Preserv. 2018, 42, e13708. [Google Scholar] [CrossRef]
- Leuschner, R.G.; Kurihara, R.; Hammes, W.P. Formation of biogenic amines by proteolytic enterococci during cheese ripening. J. Sci. Food Agric. 1999, 79, 1141–1144. [Google Scholar] [CrossRef]
- Marino, M.; Maifreni, M.; Bartolomeoli, I.; Rondinini, G. Evaluation of amino acid-decarboxylative microbiota throughout the ripening of an Italian PDO cheese produced using different manufacturing practices. J. Appl. Microbiol. 2008, 105, 540–549. [Google Scholar] [CrossRef]
- Zdolec, N.; Bogdanović, T.; Severin, K.; Dobranić, V.; Kazazić, S.; Grbavac, J.; Pleadin, J.; Petričević, S.; Kiš, M. Biogenic Amine Content in Retailed Cheese Varieties Produced with Commercial Bacterial or Mold Cultures. Processes 2021, 10, 10. [Google Scholar] [CrossRef]
- Dobranić, V.; Kazazić, S.; Filipović, I.; Mikulec, N.; Zdolec, N. Composition of raw cow’s milk microbiota and identification of enterococci by MALDI-TOF MS—Short communication. Vet. Arh. 2016, 86, 581–590. [Google Scholar]
- Celano, G.; Calasso, M.; Costantino, G.; Vacca, M.; Ressa, A.; Nikoloudaki, O.; De Palo, P.; Calabrese, F.M.; Gobbetti, M.; De Angelis, M. Effect of Seasonality on Microbiological Variability of Raw Cow Milk from Apulian Dairy Farms in Italy. Microbiol. Spectr. 2022, 10, e00514-22. [Google Scholar] [CrossRef]
- Ladero, V.; Martínez, N.; Martín, M.C.; Fernández, M.; Alvarez, M.A. qPCR for quantitative detection of tyramine-producing bacteria in dairy products. Food Res. Int. 2010, 43, 289–295. [Google Scholar] [CrossRef]
- Poveda, J.M.; Chicón, R.; Cabezas, L. Biogenic amine content and proteolysis in Manchego cheese manufactured with Lactobacillus paracasei subsp. paracasei as adjunct and other autochthonous strains as starters. Int. Dairy J. 2015, 47, 94–101. [Google Scholar] [CrossRef]
- Renes, E.; Ladero, V.; Tornadijo, M.E.; Fresno, J.M. Production of sheep milk cheese with high γ-aminobutyric acid and ornithine concentration and with reduced biogenic amines level using autochthonous lactic acid bacteria strains. Food Microbiol. 2019, 78, 1–10. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Kassem, M.A.; Morsy, O.; Mohamed, N.M. Anti-tyramine potential of Lactobacillus rhamnosus (LGG®) in cheese samples collected from Alexandria, Egypt. Food Biotechnol. 2020, 34, 243–261. [Google Scholar] [CrossRef]
- Madejska, A.; Michalski, M.; Pawul-Gruba, M.; Osek, J. Histamine content in rennet ripening cheeses during storage at different temperatures and times. J. Vet. Res. 2018, 62, 65–69. [Google Scholar] [CrossRef]
- Standarová, E.; Vorlová, L.; Kordiovská, P.; Janštová, B.; Dračková, M.; Borkovcová, I. Biogenic Amine Production in Olomouc Curd Cheese (Olomoucké tvarůžky) at Various Storage Conditions. Acta Vet. Brno 2010, 79, 147–156. [Google Scholar] [CrossRef]
- Bonczar, G.; Filipczak-Fiutak, M.; Pluta-Kubica, A.; Duda, I. Biogenic amines present in cheese—Occurrence and threats. Med. Weter. 2017, 73, 136–143. [Google Scholar] [CrossRef]
- Ovalle-Marmolejo, X.Y.; Redondo-Solano, M.; Granados-Chinchilla, F.; Miranda-Castilleja, D.E.; Arvizu-Medrano, S.M. Effect of stress factors on the production of biogenic amines by lactic acid bacteria isolated from fermented Mexican foods (cheese and beer). Food Control 2023, 146, 109553. [Google Scholar] [CrossRef]
- Van Mastrigt, O.; Egas, R.A.; Abee, T.; Smid, E.J. Aroma formation in retentostat co-cultures of Lactococcus lactis and Leuconostoc mesenteroides. Food Microbiol. 2019, 82, 151–159. [Google Scholar] [CrossRef]
- De Paula, A.T.; Jeronymo-Ceneviva, A.B.; Silva, L.F.; Todorov, S.D.; Franco, B.D.G.M.; Penna, A.L.B. Leuconostoc mesenteroides SJRP55: A potential probiotic strain isolated from Brazilian water buffalo mozzarella cheese. Ann. Microbiol. 2014, 65, 899–910. [Google Scholar] [CrossRef]
- Renes, E.; Diezhandino, I.; Fernández, D.; Ferrazza, R.E.; Tornadijo, M.E.; Fresno, J.M. Effect of autochthonous starter cultures on the biogenic amine content of ewe’s milk cheese throughout ripening. Food Microbiol. 2014, 44, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Novella-Rodríguez, S.; Veciana-Nogués, M.T.; Roig-Sagués, A.X.; Trujillo-Mesa, A.J.; Vidal-Carou, M.C. Influence of starter and nonstarter on the formation of biogenic amine in goat cheese during ripening. J. Dairy Sci. 2002, 85, 2471–2478. [Google Scholar] [CrossRef]
- Flasarová, R.; Pachlová, V.; Buňková, L.; Menšíková, A.; Georgová, N.; Dráb, V.; Buňka, F. Biogenic amine production by Lactococcus lactis subsp. cremoris strains in the model system of Dutch-type cheese. Food Chem. 2016, 194, 68–75. [Google Scholar] [CrossRef]
- Egger, L.; Ménard, O.; Abbühl, L.; Duerr, D.; Stoffers, H.; Berthoud, H.; Meola, M.; Badertscher, R.; Blaser, C.; Dupont, D.; et al. Higher microbial diversity in raw than in pasteurized milk Raclette-type cheese enhances peptide and metabolite diversity after in vitro digestion. Food Chem. 2021, 340, 128154. [Google Scholar] [CrossRef]
- Torracca, B.; Pedonese, F.; Turchi, B.; Fratini, F.; Nuvoloni, R. Qualitative and quantitative evaluation of biogenic amines in vitro production by bacteria isolated from ewes’ milk cheeses. Eur. Food Res. Technol. 2017, 244, 721–728. [Google Scholar] [CrossRef]
- Rodríguez, J.; González-Guerra, A.; Vázquez, L.; Fernández-López, R.; Flórez, A.B.; De La Cruz, F.; Mayo, B. Isolation and phenotypic and genomic characterization of Tetragenococcus spp. from two Spanish traditional blue-veined cheeses made of raw milk. Int. J. Food Microbiol. 2022, 371, 109670. [Google Scholar] [CrossRef]
- De a Møller, C.O.; Castro-Mejía, J.L.; Krych, L.; Rattray, F.P. Histamine-forming ability of Lentilactobacillus parabuchneri in reduced salt Cheddar cheese. Food Microbiol. 2021, 98, 103789. [Google Scholar] [CrossRef]
- Berthoud, H.; Wüthrich, D.; Bruggmann, R.; Wechsler, D.; Fröhlich-Wyder, M.-T.; Irmler, S. Development of new methods for the quantitative detection and typing of Lactobacillus parabuchneri in dairy products. Int. Dairy J. 2017, 70, 65–71. [Google Scholar] [CrossRef]
- Ascone, P.; Maurer, J.; Haldemann, J.; Irmler, S.; Berthoud, H.; Portmann, R.; Fröhlich-Wyder, M.-T.; Wechsler, D. Prevalence and diversity of histamine-forming Lactobacillus parabuchneri strains in raw milk and cheese—A case study. Int. Dairy J. 2017, 70, 26–33. [Google Scholar] [CrossRef]
- Diaz, M.; Del Rio, B.; Sanchez-Llana, E.; Ladero, V.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Histamine-producing Lactobacillus parabuchneri strains isolated from grated cheese can form biofilms on stainless steel. Food Microbiol. 2016, 59, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Ladero, V.; Redruello, B.; Sanchez-Llana, E.; Del Rio, B.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. A PCR-DGGE method for the identification of histamine-producing bacteria in cheese. Food Control 2016, 63, 216–223. [Google Scholar] [CrossRef]
- Dugat-Bony, E.; Bonnarme, P.; Fraud, S.; Catellote, J.; Sarthou, A.-S.; Loux, V.; Rué, O.; Bel, N.; Chuzeville, S.; Hélinck, S. Effect of sodium chloride reduction or partial substitution with potassium chloride on the microbiological, biochemical and sensory characteristics of semi-hard and soft cheeses. Food Res. Int. 2019, 125, 108643. [Google Scholar] [CrossRef]
- Wechsler, D.; Irmler, S.; Berthoud, H.; Portmann, R.; Badertscher, R.; Bisig, W.; Schafroth, K.; Fröhlich-Wyder, M.-T. Influence of the inoculum level of Lactobacillus parabuchneri in vat milk and of the cheese-making conditions on histamine formation during ripening. Int. Dairy J. 2021, 113, 104883. [Google Scholar] [CrossRef]
- Suzzi, G.; Schirone, M.; Martuscelli, M.; Gatti, M.; Fornasari, M.; Neviani, E. Yeasts associated with Manteca. FEMS Yeast Res. 2003, 3, 159–166. [Google Scholar] [CrossRef]
- Wyder, M.-T.; Bachmann, H.-P.; Puhan, Z. Role of selected yeasts in cheese ripening: An evaluation in foil wrapped raclette cheese. Leb.-Wiss. Technol. 1999, 32, 333–343. [Google Scholar] [CrossRef]
- Zeng, J.; Wu, J.; Chen, H.; Ni, S. Review on biological degradation of biogenic amines in food. Int. J. Agric. Sci. Food Technol. 2021, 7, 331–334. [Google Scholar] [CrossRef]
- Callejón, S.; Sendra, R.; Ferrer, S.; Pardo, I. Identification of a novel enzymatic activity from lactic acid bacteria able to degrade biogenic amines in wine. Appl. Microbiol. Biotechnol. 2013, 98, 185–198. [Google Scholar] [CrossRef]
- Tepkasikul, P.; Santiyanont, P.; Booncharoen, A.; Abhisingha, M.; Mhuantong, W.; Chantarasakha, K.; Pitaksutheepong, C.; Visessanguan, W.; Tepaamorndech, S. The functional starter and its genomic insight for histamine degradation in fish sauce. Food Microbiol. 2022, 104, 103988. [Google Scholar] [CrossRef]
- Gardini, F.; Martuscelli, M.; Crudele, M.A.; Paparella, A.; Suzzi, G. Use of Staphylococcus xylosus as a starter culture in dried sausages: Effect on the biogenic amine content. Meat Sci. 2002, 61, 275–283. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Huang, X.; Lan, Q.; Deng, L.; Pei, H.; Wang, Y.; Liu, S.; Yand, Y. Effects of diamine oxidase on the quality and biogenic amine contents of Sichuan-style sausage. Food Ferm. Ind. 2022, 48, 134–140. [Google Scholar] [CrossRef]
- Butor, I.; Jančová, P.; Purevdorj, K.; Klementová, L.; Kluz, M.; Huňová, I.; Pištěková, H.; Buňka, F.; Buňková, L. Effect of Selected Factors Influencing Biogenic Amines Degradation by Bacillus subtilis Isolated from Food. Microorganisms 2023, 11, 1091. [Google Scholar] [CrossRef]
- Saad, M.A.; Abd-Rabou, H.S.; Elkhtab, E.; Rayan, A.M.; Abdeen, A.; Abdelkader, A.; Ibrahim, S.F.; Hussien, H. Occurrence of Toxic Biogenic Amines in Various Types of Soft and Hard Cheeses and Their Control by Bacillus polymyxa D05-1. Fermentation 2022, 8, 327. [Google Scholar] [CrossRef]
- Leuschner, R.G.; Heidel, M.; Hammes, W.P. Histamine and tyramine degradation by food fermenting microorganisms. Int. J. Food Microbiol. 1998, 39, 1–10. [Google Scholar] [CrossRef]
- Herrero-Fresno, A.; Martínez, N.; Sánchez-Llana, E.; Díaz, M.; Fernández, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. Lactobacillus casei strains isolated from cheese reduce biogenic amine accumulation in an experimental model. Int. J. Food Microbiol. 2012, 157, 297–304. [Google Scholar] [CrossRef]
- Adámek, R.; Pachlová, V.; Salek, R.N.; Němečková, I.; Buňka, F.; Buňková, L. Reduction of biogenic amine content in Dutch-type cheese as affected by the applied adjunct culture. Leb.-Wiss Technol. 2021, 152, 112397. [Google Scholar] [CrossRef]
- Klementová, L.; Purevdorj, K.; Butor, I.; Jančová, P.; Bábková, D.; Buňka, F.; Buňková, L. Reduction of histamine, putrescine and cadaverine by the bacteria Lacticaseibacillus casei depending on selected factors in the real condition of the dairy product. Food Microbiol. 2024, 117, 104391. [Google Scholar] [CrossRef] [PubMed]
- Ramos, I.M.; Rodríguez-Sánchez, S.; Palop, M.L.; Poveda, J.M. Reduction in the biogenic amine content of raw milk Manchego cheese by using biogenic-amine-degrading lactic acid bacteria. Food Control 2024, 156, 110133. [Google Scholar] [CrossRef]
- Sarquis, A.; Villarreal, L.A.; Ladero, V.; Maqueda, M.; Del Rio, B.; Alvarez, M.A. Enterocin AS-48 inhibits the growth of—And biofilm formation by—Lactic acid bacteria responsible for the accumulation of biogenic amines in cheese. Int. J. Food Sci. Technol. 2023, 58, 5865–5873. [Google Scholar] [CrossRef]
- Villarreal, L.A.; Ladero, V.; Sarquis, A.; Martinez, B.; Del Rio, B.; Alvarez, M.A. Bacteriocins against biogenic amine-accumulating lactic acid bacteria in cheese: Nisin A shows the broadest antimicrobial spectrum and prevents the formation of biofilms. J. Dairy Sci. 2024, 107, 4277–4287. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Southwick, K.; Reardon, J.; Berg, R.; MacCormack, J.N. Histamine poisoning associated with eating tuna burgers. JAMA 2001, 285, 1327. [Google Scholar] [CrossRef]
- Omer, A.K.; Mohammed, R.R.; Ameen, P.S.M.; Abas, Z.A.; Ekici, K. Presence of biogenic amines in food and their public health implications: A review. J. Food Prot. 2021, 84, 1539–1548. [Google Scholar] [CrossRef]
- Medina, M.A.; Urdiales, J.L.; Rodríguez-Caso, C.; Ramírez, F.J.; Sánchez-Jiménez, F. Biogenic amines and polyamines: Similar biochemistry for different physiological missions and biomedical applications. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 23–59. [Google Scholar] [CrossRef]
- De Mey, E.; De Klerck, K.; De Maere, H.; Dewulf, L.; Derdelinckx, G.; Peeters, M.-C.; Fraeye, I.; Heyden, Y.V.; Paelinck, H. The occurrence of N-nitrosamines, residual nitrite and biogenic amines in commercial dry fermented sausages and evaluation of their occasional relation. Meat Sci. 2014, 96, 821–828. [Google Scholar] [CrossRef]
- Kalač, P.; Krausová, P. A review of dietary polyamines: Formation, implications for growth and health and occurrence in foods. Food Chem. 2005, 90, 219–230. [Google Scholar] [CrossRef]
- Nout, M.J.R. Fermented foods and food safety. Food Res. Int. 1994, 27, 291–298. [Google Scholar] [CrossRef]
- Ekici, K.; Omer, A.K. Biogenic amines in plant food. In Neurotransmitters in Plants: Perspectives and Applications; CRC Press: Boca Raton, FL, USA, 2019; pp. 305–330. [Google Scholar]
- Karovicova, J.; Kohajdova, Z. Biogenic amines in food. Chem. Pap. 2005, 59, 70–79. [Google Scholar] [CrossRef]
- Rauscher-Gabernig, E.; Grossgut, R.; Bauer, F.; Paulsen, P. Assessment of alimentary histamine exposure of consumers in Austria and development of tolerable levels in typical foods. Food Control 2009, 20, 423–429. [Google Scholar] [CrossRef]
- Korn, A.; Da Prada, M.; Raffesberg, W.; Allen, S.; Gasic, S. Tyramine pressor effect in man: Studies with moclobemide, a novel, reversible monoamine oxidase inhibitor. J. Neural Transm. 1988, 26, 57–71. [Google Scholar]
- Korn, A.; Da Prada, M.; Raffesberg, W.; Gasic, S.; Eichler, H.G. Effect of moclobemide, a new reversible monoamine oxidase inhibitor, on absorption and pressor effect of tyramine. J. Cardiovasc. Pharmacol. 1988, 11, 17–23. [Google Scholar] [CrossRef]
- Zimmer, R.; Puech, A.J.; Philipp, F.; Korn, A. Interaction between orally administered tyramine and moclobemide. Acta Psychiatr. Scand. 1990, 82, 78–80. [Google Scholar] [CrossRef]
- Patat, A.; Berlin, I.; Durrieu, G.; Armand, P.; Fitoussi, S.; Molinier, P.; Caille, P. Pressor Effect of Oral Tyramine During Treatment with Befloxatone, a New Reversible Monoamine Oxidase-A Inhibitor, in Healthy Subjects. J. Clin. Pharmacol. 1995, 35, 633–643. [Google Scholar] [CrossRef]
- Rauscher-Gabernig, E.; Gabernig, R.; Brueller, W.; Grossgut, R.; Bauer, F.; Paulsen, P. Dietary exposure assessment of putrescine and cadaverine and derivation of tolerable levels in selected foods consumed in Austria. Eur. Food Res. Technol. 2012, 235, 209–220. [Google Scholar] [CrossRef]
| Name | Precursor | Structure | Body Function * | Health Issues |
|---|---|---|---|---|
| Histamine | Histidine | 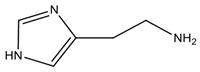 Aromatic | Balancing the body temperature and regulating the stomach volume, stomach pH, cerebral activities, neurotransmitter and inflammation mediators; it also initiates allergic reactions | Responsible for “scombrid poisoning”, hypotension, headache, abdominal cramps, diarrhea and vomiting |
| Tyramine | Tyrosine | 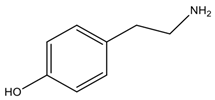 Aromatic | Vasoconstriction *, increases blood pressure, active noradrenalin secretion, inflammation mediators | Responsible for the “cheese reaction”, hypertensive crises and headache; it can inhibit some oxidase enzymes |
| Tryptamine | Tryptophan | 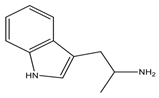 Aromatic | Vasoconstriction *, increases blood pressure, neurotransmitter and neuromodulator | Increases blood pressure, hypertension |
| Phenylethylamine | Phenylalanine | 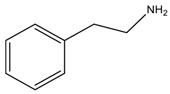 Aromatic | Neurotransmitter | Hypertensive crises and headache |
| Putrescine | Ornithine, glutamine, arginine, agmatine | 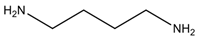 Diamine | Found in high concentration in brain; a deficiency led to depression syndrome *; regulation of gene expression, maturation of intestine, cell growth and differentiation | Promotes the formation of nitrosamines, cadaverine and polyamines; inhibits oxidase enzymes, enhancing the toxicity of histamine and tyramine; causes hypotension, bradycardia and lockjaw |
| Cadaverine | Lysine | 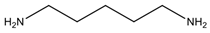 Diamine | Regulation of gene expression, maturation of intestine, cell growth and differentiation | Promotes the formation of nitrosamines; inhibits oxidase enzymes, enhancing the toxicity of histamine and tyramine |
| Spermidine | Putrescine + propylamine residue from S-adenosyl-methionine | 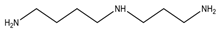 Polyamine | Growth regulation; regulation of membrane-linked enzymes * | Associated with food allergies; could promote colon cancer. |
| Spermine | spermidine + propylamine residue from S-adenosyl-methionine | 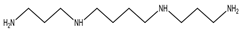 Polyamine | Growth regulation; regulation of membrane-linked enzymes | Associated with food allergies; could promote colon cancer |
| Cheese Name | Species | Milk Heat Treatment | Variable Considered | Ripening Time (Days) | His | Tyr | Put | Cad | Try | Sme | Spd | Phe | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Montasio | Cow | Raw | 60 | 1.9 (1.7) | 3.33 (3.6) | 19.19 (36.9) | 3.22 (6.1) | 0.19 (0.3) | 0 (0) | 0 (0) | 0.46 (0.5) | [99] | |
| 90 | 3.7 (1.6) | 5.19 (2.8) | 1.15 (1.2) | 0.49 (0.4) | 0.14 (0.1) | 0 (0) | 0 (0) | 0.42 (0.6) | |||||
| 120 | 6.71 (5) | 6.09 (3.5) | 0.68 (0.6) | 0.22 (0.2) | 0.12 (0.1) | 0 (0) | 0 (0) | 0.47 (0.4) | |||||
| 150 | 21.66 (11) | 24.89 (10.1) | 36.36 (33.1) | 1.3 (1.1) | 0.4 (0.2) | 0 (0) | 0 (0) | 1.42 (0.4) | |||||
| Toma Piemontese PDO | Cow | Raw | not commercial | 4 | 12.00 | 10.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | [65] |
| 19 | 14.00 | 26.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 29 | 15.00 | 34.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 39 | 22.00 | 60.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 49 | 37.00 | 108.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| commercial | 0.00 | 39.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 0.00 | 178.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 40.00 | 95.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 67.00 | 58.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 29.00 | 79.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 0.00 | 146.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 54.00 | 165.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 27.00 | 31.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| Cheddar | Cow | 33.40 | 52.90 | 19.40 | 13.90 | 6.90 | 0.00 | 0.00 | 0.00 | [141] | |||
| Ras | 82.80 | 43.20 | 74.40 | 36.80 | 28.30 | 24.00 | 0.00 | 0.00 | |||||
| Chihuahua cheese | Cow | 0 | 0 (0) | 33 (47.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | [101] | ||
| 60 | 7.5 (18.4) | 110.83 (66.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| 120 | 50.33 (74.7) | 173.17 (36.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Pecorino Abruzzese | Ewe | Raw | autochthonous starter culture | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | [106] |
| 14 | 20.00 | 100.00 | 55.00 | 10.00 | 5.00 | 5.00 | 6.00 | 50.00 | |||||
| 30 | 100.00 | 80.00 | 10.00 | 10.00 | 20.00 | 5.00 | 5.00 | 20.00 | |||||
| 60 | 280.00 | 190.00 | 50.00 | 60.00 | 25.00 | 5.00 | 50.00 | 30.00 | |||||
| Past. | commercial starter cultures (Streptococcus thermophilus, Lactobacillus casei and Lb. delbruekii subsp. bulgaricus) | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| 14 | 30.00 | 10.00 | 10.00 | 2.00 | 10.00 | 5.00 | 4.00 | 5.00 | |||||
| 30 | 120.00 | 10.00 | 10.00 | 10.00 | 20.00 | 10.00 | 15.00 | 10.00 | |||||
| 60 | 110.00 | 280.00 | 190.00 | 100.00 | 30.00 | 20.00 | 20.00 | 300.00 | |||||
| Formaggio di Fossa | Mix | ripened in Fossa | 100 | 24.00 | 461.00 | 579.60 | 1302.86 | 0.00 | 0.00 | 16.49 | 173.00 | [111] | |
| ripened in cell | 100 | 0.00 | 24.92 | 18.57 | 0.00 | 0.00 | 27.58 | 0.00 | 0.00 | ||||
| Ewe cheese | Ewe | Raw | Commercial starter (Lactococcus lactis, Lactococcus cremoris) | 1 | 13.80 | 27.40 | 30.10 | 41.00 | 22.10 | 0.00 | 20.50 | 13.00 | [105] |
| 15 | 25.60 | 40.30 | 66.40 | 70.90 | 31.00 | 0.00 | 29.90 | 22.20 | |||||
| 30 | 11.50 | 35.10 | 63.60 | 70.70 | 12.30 | 0.00 | 15.30 | 11.20 | |||||
| 90 | 0.00 | 88.60 | 98.40 | 78.10 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 120 | 0.00 | 101.00 | 70.40 | 65.70 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 180 | 0.00 | 238.00 | 103.30 | 77.10 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| Herby cheese | Ewe | 360 | 197.9 (155.32) | 360.39 (253.66) | 192.51 (209.23) | 288.46 (435.40) | 103.3 (37.09) | 0 (0) | 0 (0) | 33.58 (31.79) | [108] | ||
| Pecorino di farindola | Ewe | 90 | 5.18 (7.27) | 417.73 (362.49) | 119.82 (145.16) | 100.46 (78.53) | 0 (0) | 0 (0) | 78.94 (37.93) | 31.74 (39.01) | [112] | ||
| Terrincho cheese | Ewe | market cheese | 30 | 4.04 (5.56) | 79.86 (117.24) | 218.48 (142.18) | 135.6 (78.61) | 67.68 (59.11) | 0 (0) | 0 (0) | 65.84 (95.88) | [115] | |
| Pecorino | Ewe | Past. | natural cave ripening | 150 | 0.00 | 420.00 | 22.00 | 2.00 | 11.00 | 2.00 | 0.00 | 56.00 | [109] |
| Raw | ripening room | 60 | 0.00 | 147.00 | 54.00 | 37.00 | 12.00 | 0.00 | 0.00 | 24.00 | |||
| Past. | ripened in fossa | 180 | 10.00 | 1040.00 | 39.00 | 54.00 | 12.00 | 1.00 | 0.00 | 127.00 | |||
| Raw | tuff cave | 150 | 23.00 | 1132.00 | 512.00 | 262.00 | 88.00 | 0.00 | 0.00 | 144.00 | |||
| Canestrato di Castel del Monte | Ewe | Past. | no starter culture | 240 | 10.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | [53] |
| Pecorino di fossa | Mix | Raw | 90 | 743.30 | 1771.30 | 986.00 | 2127.00 | 0.00 | 0.00 | 0.00 | 232.40 | ||
| Pecorino sotto fieno | Ewe | Past. | 180 | 200.00 | 58.90 | 8.30 | 36.90 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Pecorino di grotta | Mix | Past. | 120 | 235.40 | 312.10 | 8.90 | 0.00 | 0.00 | 0.00 | 0.00 | 26.00 | ||
| Pecorino abruzzese sott’olio | Raw | 240 | 13.10 | 394.50 | 36.30 | 57.30 | 0.00 | 0.00 | 0.00 | 62.10 | |||
| Pecorino di Atri or Abruzzese | Ewe | Raw | 150 | 0.00 | 10.60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Pecorino d’Abruzzo | 300 | 130.70 | 140.90 | 128.10 | 33.60 | 0.00 | 0.00 | 0.00 | 12.70 | ||||
| Pecorino d’Abruzzo | 90 | 266.70 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Pecorino d’Abruzzo | 186.00 | 702.40 | 377.70 | 172.40 | 0.00 | 0.00 | 0.00 | 44.40 | |||||
| Pecorino d’Abruzzo | 14.50 | 224.40 | 369.00 | 116.40 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| Pecorino d’Abruzzo | 761.00 | 7.70 | 55.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| Pecorino di Farindola | 11.20 | 397.70 | 127.00 | 110.60 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| Zamorano PDO | Ewe | Raw | 1 | 10.00 | 0.00 | 15.00 | 35.00 | 0.00 | 0.00 | 0.00 | 8.00 | [107] | |
| 60 | 10.00 | 30.00 | 40.00 | 36.00 | 10.00 | 3.00 | 30.00 | 14.00 | |||||
| 120 | 18.00 | 41.00 | 48.00 | 40.00 | 15.00 | 10.00 | 38.00 | 51.00 | |||||
| 180 | 20.00 | 48.00 | 51.00 | 41.00 | 20.00 | 21.00 | 50.00 | 55.00 | |||||
| 240 | 40.00 | 52.00 | 100.00 | 44.00 | 27.00 | 20.00 | 53.00 | 130.00 | |||||
| 300 | 42.00 | 76.00 | 131.00 | 45.00 | 44.00 | 21.00 | 100.00 | 149.00 | |||||
| Past. | 1 | 10.00 | 1.00 | 14.00 | 35.00 | 3.00 | 1.00 | 0.00 | 2.00 | ||||
| 60 | 11.00 | 19.00 | 13.00 | 35.00 | 4.00 | 21.00 | 1.00 | 4.00 | |||||
| 120 | 11.00 | 19.00 | 14.00 | 36.00 | 11.00 | 21.00 | 2.00 | 12.00 | |||||
| 180 | 12.00 | 31.00 | 14.00 | 38.00 | 11.00 | 22.00 | 3.00 | 13.00 | |||||
| 240 | 18.00 | 39.00 | 18.00 | 37.00 | 18.00 | 28.00 | 3.00 | 21.00 | |||||
| 300 | 25.00 | 42.00 | 19.00 | 37.00 | 22.00 | 31.00 | 4.00 | 25.00 | |||||
| Ewe cheese | Ewe | Past. | summer | 100 | 134.77 | 185.19 | 35.26 | 191.59 | 124.17 | 0.00 | 0.00 | 69.68 | [113] |
| summer | 180 | 233.42 | 501.65 | 6.61 | 149.44 | 0.00 | 0.00 | 0.00 | 19.63 | ||||
| autumn | 100 | 234.14 | 152.61 | 0.00 | 6.39 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| autumn | 180 | 211.19 | 210.90 | 0.00 | 1.28 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| winter | 100 | 237.59 | 183.48 | 0.00 | 6.39 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| winter | 180 | 204.24 | 234.92 | 0.00 | 7.66 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| spring | 100 | 202.85 | 209.20 | 0.00 | 1.28 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| spring | 180 | 202.85 | 338.24 | 0.00 | 1.28 | 2.00 | 0.00 | 0.00 | 0.00 | ||||
| Pecorino | Ewe | Raw | normal diet | 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | [117] |
| 10% grape pomace-enriched diet | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| normal diet | 60 | 0.00 | 32.53 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 38.11 | ||||
| 10% grape pomace-enriched diet | 0.00 | 42.72 | 7.63 | 18.33 | 0.00 | 0.00 | 0.00 | 13.35 | |||||
| normal diet | 90 | 0.00 | 40.18 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 46.28 | ||||
| 10% grape pomace-enriched diet | 0.00 | 34.55 | 5.34 | 10.67 | 0.00 | 0.00 | 0.00 | 15.04 | |||||
| normal diet | 120 | 0.00 | 101.23 | 0.00 | 15.74 | 0.00 | 0.00 | 0.00 | 85.37 | ||||
| 10% grape pomace-enriched diet | 0.00 | 103.03 | 14.11 | 20.00 | 0.00 | 0.00 | 0.00 | 63.51 | |||||
| Semicotto Caprino | Goat | Raw | 15 | 0.00 | 50.00 | 100.00 | 20.00 | 30.00 | 0.00 | 0.00 | 10.00 | [118] | |
| 30 | 50.00 | 120.00 | 220.00 | 40.00 | 90.00 | 0.00 | 40.00 | 40.00 | |||||
| 60 | 130.00 | 230.00 | 940.00 | 100.00 | 380.00 | 20.00 | 70.00 | 140.00 | |||||
| Goat cheese | Goat | Raw | 1 | 3.05 | 4.49 | 10.00 | 53.47 | 1.29 | 0.00 | 0.00 | 0.80 | [52] | |
| 14 | 3.45 | 29.25 | 33.97 | 72.04 | 3.38 | 0.00 | 0.00 | 2.62 | |||||
| 30 | 5.37 | 93.47 | 42.86 | 87.15 | 7.00 | 0.00 | 0.00 | 6.26 | |||||
| 60 | 27.99 | 245.32 | 74.15 | 177.81 | 9.05 | 0.00 | 0.00 | 19.64 | |||||
| 90 | 43.06 | 324.67 | 86.40 | 196.47 | 12.15 | 0.00 | 0.00 | 27.34 | |||||
| Past. | 1 | 0.00 | 0.00 | 0.79 | 1.29 | 0.00 | 0.00 | 0.00 | 0.70 | ||||
| 14 | 1.19 | 0.96 | 3.86 | 1.93 | 2.21 | 0.00 | 0.00 | 1.48 | |||||
| 30 | 2.16 | 2.54 | 7.83 | 5.77 | 5.42 | 0.00 | 0.00 | 4.46 | |||||
| 60 | 3.50 | 7.22 | 8.17 | 15.52 | 6.48 | 0.00 | 0.00 | 7.97 | |||||
| 90 | 6.34 | 10.90 | 14.61 | 32.73 | 7.56 | 0.00 | 0.00 | 8.89 | |||||
| 41.90 | 43.60 | 5.60 | 74.80 | 3.00 | 0.00 | 0.00 | 6.20 | [119] | |||||
| 26.40 | 37.00 | 10.60 | 2.60 | 3.80 | 0.00 | 0.00 | 22.50 | ||||||
| 10.20 | 50.70 | 2.20 | 0.50 | 11.90 | 0.00 | 0.00 | 3.80 | ||||||
| 21.00 | 14.10 | 0.80 | 1.10 | 0.00 | 0.00 | 0.00 | 1.90 | ||||||
| 40.90 | 45.20 | 2.80 | 0.70 | 1.20 | 0.00 | 0.00 | 1.30 | ||||||
| 60.50 | 22.40 | 1.60 | 7.20 | 0.00 | 0.00 | 0.00 | 0.80 | ||||||
| 18.90 | 4.20 | 0.90 | 1.50 | 0.00 | 0.00 | 0.00 | 1.00 | ||||||
| 28.50 | 45.30 | 21.70 | 1.30 | 0.00 | 0.00 | 0.00 | 2.20 | ||||||
| Ricotta Forte | Mix | control (no sorbic acid) | 1256.96 | 2797.78 | 1469.10 | 3759.50 | 0.00 | 19.34 | 12.00 | 491.33 | [137] | ||
| Ewe | with preservative (sorbic acid) | 201.44 | 387.77 | 431.68 | 1044.61 | 0.00 | 21.12 | 0.00 | 0.00 | ||||
| Ewe | control (no sorbic acid) | 1273.51 | 2142.08 | 1468.51 | 4641.05 | 0.00 | 29.79 | 17.27 | 721.80 | ||||
| Blue cheese | Cow | Raw | no γ-irradiation | 0 | 15.70 | 980.00 | 14.80 | 7.20 | 0.00 | 0.00 | 5.40 | 0.00 | [124] |
| γ-irradiation 2kGy | 0 | 16.50 | 985.00 | 15.70 | 9.20 | 0.00 | 0.00 | 10.30 | 0.00 | ||||
| γ-irradiation 4kGy | 0 | 16.80 | 969.00 | 17.00 | 6.70 | 0.00 | 0.00 | 10.80 | 0.00 | ||||
| γ-irradiation 6kGy | 0 | 0.00 | 950.00 | 7.60 | 3.30 | 0.00 | 0.00 | 15.40 | 0.00 | ||||
| no γ-irradiation | 30 | 22.80 | 1021.00 | 18.90 | 16.10 | 0.00 | 0.00 | 6.10 | 0.00 | ||||
| γ-irradiation 2kGy | 30 | 18.50 | 877.00 | 14.50 | 7.50 | 0.00 | 0.00 | 4.00 | 0.00 | ||||
| γ-irradiation 4kGy | 30 | 16.90 | 588.00 | 13.10 | 4.30 | 0.00 | 0.00 | 6.00 | 0.00 | ||||
| γ-irradiation 6kGy | 30 | 0.00 | 563.00 | 7.30 | 3.80 | 0.00 | 0.00 | 7.00 | 0.00 | ||||
| no γ-irradiation | 60 | 23.20 | 1775.00 | 21.70 | 9.20 | 0.00 | 0.00 | 8.40 | 0.00 | ||||
| γ-irradiation 2kGy | 60 | 22.50 | 834.00 | 15.10 | 8.30 | 0.00 | 0.00 | 6.00 | 0.00 | ||||
| γ-irradiation 4kGy | 60 | 9.10 | 646.00 | 12.20 | 2.30 | 0.00 | 0.00 | 12.80 | 0.00 | ||||
| γ-irradiation 6kGy | 60 | 0.00 | 439.00 | 9.50 | 2.40 | 0.00 | 0.00 | 10.10 | 0.00 | ||||
| no γ-irradiation | 90 | 35.70 | 2219.00 | 26.50 | 12.30 | 0.00 | 0.00 | 15.90 | 0.00 | ||||
| γ-irradiation 2kGy | 90 | 29.00 | 978.00 | 22.60 | 9.30 | 0.00 | 0.00 | 14.80 | 0.00 | ||||
| γ-irradiation 4kGy | 90 | 1.80 | 626.00 | 19.20 | 3.20 | 0.00 | 0.00 | 15.00 | 0.00 | ||||
| γ-irradiation 6kGy | 90 | 0.00 | 403.00 | 9.10 | 3.90 | 0.00 | 0.00 | 15.40 | 0.00 | ||||
| Niva | Cow | market | 30 | 17.33 (4.91) | 77.9 (66.88) | 24.55 (7.06) | 188 (185.88) | 3.45 (2.07) | 2.23 (0.41) | 3.5 (1.46) | 0 (0) | [127] | |
| Gamalost | Cow | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | [130] | ||
| 10 | 0.00 | 0.00 | 11.87 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 20 | 0.00 | 0.00 | 25.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 30 | 0.00 | 0.00 | 24.57 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 60 | 0.00 | 0.00 | 16.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| Norvegia cheese | 90 | 1.43 | 5.56 | 0.00 | 0.00 | 0.00 | 1.09 | 0.00 | 0.00 | ||||
| Surface-ripened cheeses | Cow | reduced salt content | 27 | 0.00 | 0.00 | 54.10 | 460.30 | 0.00 | 0.00 | 0.00 | 0.00 | [132] | |
| normal salt content | 27 | 0.00 | 0.00 | 11.40 | 153.80 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Olomouc curd cheese | Cow | Past. | storage temperature 5 °C | 150.00 | 320.00 | 290.00 | 160.00 | 20.00 | 10.00 | 20.00 | 10.00 | [133] | |
| storage temperature 20 °C | 160.00 | 1350.00 | 800.00 | 1430.00 | 160.00 | 10.00 | 10.00 | 160.00 | |||||
| shape disk | 200.00 | 840.00 | 360.00 | 550.00 | 100.00 | 10.00 | 20.00 | 150.00 | |||||
| shape bar | 75.00 | 415.00 | 370.00 | 490.00 | 180.00 | 10.00 | 10.00 | 100.00 | |||||
| 8 | 0.00 | 160.00 | 160.00 | 80.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 10 | 0.00 | 390.00 | 160.00 | 120.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 42 | 0.00 | 790.00 | 410.00 | 640.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 66 | 0.00 | 880.00 | 610.00 | 830.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| winter | 60.00 | 620.00 | 280.00 | 710.00 | 200.00 | 5.00 | 10.00 | 110.00 | |||||
| spring | 240.00 | 740.00 | 430.00 | 300.00 | 90.00 | 10.00 | 20.00 | 99.00 | |||||
| summer | 90.00 | 480.00 | 390.00 | 550.00 | 110.00 | 3.00 | 15.00 | 180.00 | |||||
| Civil cheese | Cow | Raw | 0 | 0.00 | 0.95 | 0.34 | 2.31 | 0.52 | 0.26 | 0.26 | 0.66 | [128] | |
| ripening temperature 5 °C | 30 | 0.00 | 0.84 | 0.13 | 1.40 | 0.04 | 0.07 | 0.27 | 0.75 | ||||
| ripening temperature 10 °C | 30 | 0.00 | 5.34 | 1.80 | 2.54 | 0.07 | 0.10 | 0.37 | 0.54 | ||||
| ripening temperature 5 °C | 60 | 0.00 | 2.22 | 0.40 | 2.25 | 0.06 | 0.26 | 0.21 | 0.76 | ||||
| ripening temperature 10 °C | 60 | 0.00 | 18.55 | 32.20 | 106.42 | 0.60 | 0.12 | 0.31 | 2.93 | ||||
| ripening temperature 5 °C | 90 | 0.00 | 4.12 | 1.85 | 2.87 | 0.07 | 0.04 | 0.36 | 6.24 | ||||
| ripening temperature 10 °C | 90 | 0.00 | 19.93 | 47.79 | 151.41 | 0.89 | 0.10 | 0.43 | 1.06 | ||||
| Camembert | Cow | Past. | mold strain type 1 | 14 | 12.34 | 0.00 | 15.60 | 12.61 | 0.00 | 0.00 | 18.13 | 0.00 | [135] |
| mold strain type 2 | 11.57 | 0.00 | 15.83 | 11.91 | 0.00 | 0.00 | 17.32 | 0.00 | |||||
| mold strain type 3 | 11.23 | 0.00 | 14.55 | 12.21 | 0.00 | 0.00 | 20.43 | 0.00 | |||||
| coagulant type 1 | 10.59 | 0.00 | 15.40 | 13.55 | 0.00 | 0.00 | 20.45 | 0.00 | |||||
| coagulant type 2 | 10.75 | 19.38 | 15.46 | 13.27 | 0.00 | 0.00 | 20.02 | 0.00 | |||||
| coagulant type 3 | 11.23 | 20.38 | 16.12 | 14.71 | 0.00 | 0.00 | 21.69 | 0.00 | |||||
| Burrata | Cow | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 9.27 | 0.00 | 0.00 | [142] | |||
| Mascarpone | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 7.24 | 0.00 | 0.00 | |||||
| Cream | 0.00 | 25.66 | 9.57 | 41.07 | 0.00 | 8.85 | 0.00 | 24.47 | |||||
| Ricotta | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 11.16 | 0.00 | 12.12 | |||||
| Pecorino Romano | Ewe | 1468.46 | 125.00 | 99.43 | 108.20 | 0.00 | 88.65 | 0.00 | 0.00 | ||||
| Parmigiano Reggiano | Cow | 0.00 | 31.81 | 0.00 | 5.07 | 0.00 | 90.11 | 0.00 | 0.00 | ||||
| Grana Padano | 68.51 | 48.36 | 49.84 | 4.13 | 0.00 | 66.95 | 0.00 | 0.00 | |||||
| Asiago | 7.92 | 110.93 | 23.06 | 18.27 | 14.00 | 15.53 | 0.00 | 15.02 | |||||
| Mimolette | 0.00 | 96.32 | 0.00 | 65.19 | 17.88 | 104.01 | 8.71 | 16.75 | |||||
| Gouda | 24.15 | 12.70 | 0.00 | 152.92 | 0.00 | 23.11 | 5.25 | 9.63 | |||||
| Cheddar | 176.87 | 63.03 | 7.86 | 18.88 | 14.67 | 56.34 | 0.00 | 15.94 | |||||
| Gruyere | 22.25 | 52.47 | 0.00 | 17.04 | 33.27 | 125.53 | 0.00 | 0.00 | |||||
| Emmental | 0.00 | 81.98 | 22.64 | 13.26 | 0.00 | 50.48 | 4.27 | 12.63 | |||||
| Smoked | 0.00 | 30.99 | 8.22 | 13.06 | 0.00 | 42.74 | 0.00 | 0.00 | |||||
| Comte | 8.69 | 149.13 | 0.00 | 10.27 | 0.00 | 101.29 | 0.00 | 10.03 | |||||
| Edam | 12.79 | 209.20 | 20.61 | 41.83 | 0.00 | 73.05 | 10.80 | 26.56 | |||||
| Appenzeller | 225.69 | 101.69 | 13.77 | 58.08 | 0.00 | 153.23 | 0.00 | 0.00 | |||||
| Camembert | 7.23 | 11.53 | 6.92 | 0.00 | 0.00 | 15.20 | 26.65 | 22.25 | |||||
| Brie | 8.67 | 12.81 | 7.43 | 0.00 | 0.00 | 17.12 | 15.87 | 9.90 | |||||
| Feta | Goat | 14.27 | 310.00 | 100.00 | 3.00 | 9.82 | 12.80 | 17.35 | 5.22 | ||||
| Chevre | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 11.28 | 0.00 | 0.00 | |||||
| Caprice des dieux | Cow | 0.00 | 19.40 | 8.01 | 0.00 | 0.00 | 59.97 | 14.39 | 0.00 | ||||
| Bleu d’Auvergne | 4.30 | 22.93 | 13.57 | 0.00 | 0.00 | 105.99 | 14.70 | 0.00 | |||||
| Fauquet Maroilles | 0.00 | 90.66 | 15.35 | 14.18 | 0.00 | 132.11 | 13.67 | 8.90 | |||||
| Extra-hard cheeses | 51 | 4.6 | 1.7 | 2.7 | 3.3 | 0.0 | 0.0 | 0.0 | [143] | ||||
| Hard cheeses | 204.9 | 97.5 | 18.8 | 16.5 | 9.3 | 0.0 | 0.0 | 0.0 | |||||
| Semi-hard cheeses | 38.3 | 151.7 | 36.5 | 47.3 | 49.3 | 0.0 | 0.0 | 0.0 | |||||
| Blue-veined cheeses | 36.6 | 13.9 | 15.9 | 9.5 | 3.5 | 0.0 | 0.0 | 0.0 | |||||
| Mold-ripened soft cheeses | 4.0 | 3.1 | 26.8 | 67.2 | 12.7 | 0.0 | 0.0 | 0.0 | |||||
| Smear-ripened cheeses | 17.9 | 47.1 | 23.0 | 8.1 | 5.4 | 0.0 | 0.0 | 0.0 | |||||
| Acid-curd cheeses | 28.8 | 156.4 | 118.3 | 86.9 | 14.9 | 0.0 | 0.0 | 0.0 | |||||
| Cheddar | Cow | rennet curd | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 20.20 | 25.10 | 0.00 | [144] | ||
| Emmentaler | rennet curd | 5.80 | 5.78 | 2.67 | 1.60 | 0.90 | 2.79 | 2.29 | 2.08 | ||||
| Camembert | rennet curd | 2.45 | 67.58 | 67.02 | 4.33 | 1.21 | 1.89 | 1.67 | 8.76 | ||||
| Tvorog | acid-curd | 1.08 | 6.23 | 6.47 | 2.43 | 0.92 | 1.02 | 2.82 | 0.87 | ||||
| Harzer | acid-curd | 1.08 | 6.23 | 6.47 | 2.43 | 0.92 | 1.02 | 2.82 | 0.87 | ||||
| Fried | acid-curd | 24.08 | 275.50 | 281.33 | 377.50 | 48.91 | 5.70 | 7.74 | 5.30 | ||||
| Vastedda della Valle del Belice PDO | Ewe | Raw | no starter culture | 2 | 5.70 | 5.90 | 1.10 | 1.00 | 1.40 | 1.10 | 0.30 | 0.50 | [145] |
| Caprino di Castel Fiorentin | Goat | natural whey starter | 30 | 0.00 | 25.00 | 9.00 | 0.00 | 0.00 | 4.70 | 0.00 | 0.00 | ||
| Caciocavallo Podolico Dauno | Cow | natural whey starter | 30 | 0.00 | 7.00 | 0.00 | 1.40 | 0.00 | 13.60 | 0.00 | 0.00 | ||
| Fior di Capra | Goat | no starter culture | 90 | 18.00 | 29.30 | 15.40 | 0.30 | 0.80 | 0.00 | 0.00 | 0.00 | ||
| Caprino Girgentano | no starter culture | 90 | 89.00 | 1.70 | 6.20 | 0.90 | 0.40 | 0.00 | 0.00 | 4.00 | |||
| Caprino di Biccari | Thermised | starter cultures | 210 | 44.00 | 227.00 | 298.00 | 27.00 | 73.00 | 0.00 | 5.00 | 145.00 | ||
| Caciocavallo Palermitano | Cow | Raw | no starter culture | 120 | 149.00 | 4.00 | 12.00 | 0.00 | 4.00 | 0.00 | 13.00 | 0.00 | |
| Ragusano PDO | no starter culture | 120 | 54.80 | 23.90 | 7.60 | 2.90 | 0.00 | 0.00 | 0.00 | 4.50 | |||
| Caciocavallo Silano PDO | natural whey starter | 150 | 435.00 | 33.00 | 37.00 | 17.00 | 6.00 | 0.00 | 0.00 | 0.00 | |||
| Cacioricotta | Past. | no starter culture | 30 | 19.00 | 13.00 | 14.00 | 18.00 | 0.00 | 10.00 | 5.00 | 0.00 | ||
| Fiore Sicano | Raw | no starter culture | 60 | 155.00 | 32.00 | 16.00 | 32.00 | 53.00 | 0.00 | 0.00 | 2.60 | ||
| Cacio | Mix | Past. | starter cultures | 60 | 17.00 | 305.00 | 53.00 | 111.00 | 18.00 | 0.00 | 0.00 | 143.00 | |
| Vaccino | Cow | no starter culture | 90 | 14.90 | 4.30 | 62.00 | 28.00 | 0.00 | 15.00 | 0.00 | 0.00 | ||
| Provola dei Nebrodi | Thermised | no starter culture | 30 | 1.00 | 1.00 | 0.75 | 0.40 | 0.10 | 0.00 | 0.10 | 0.10 | ||
| Pecorino Foggiano | Ewe | starter cultures | 120 | 253.00 | 303.00 | 594.00 | 129.00 | 0.00 | 0.00 | 0.00 | 25.00 | ||
| Canestrato Pugliese PDO | Raw | no starter culture | 90 | 3.90 | 273.00 | 208.00 | 199.00 | 0.00 | 0.00 | 4.00 | 46.00 | ||
| Maiorchino | Mix | Thermised | no starter culture | 180 | 2.00 | 61.00 | 5.00 | 1.30 | 1.90 | 0.00 | 3.50 | 82.00 | |
| Pecorino Siciliano PDO | Ewe | Raw | no starter culture | 120 | 219.00 | 86.00 | 60.00 | 5.40 | 2.80 | 0.00 | 2.00 | 9.00 | |
| Piacentinu Ennese | no starter culture | 270 | 243.00 | 134.00 | 204.00 | 9.50 | 6.00 | 0.00 | 10.00 | 28.50 | |||
| Tuma Persa | Cow | Thermised | no starter culture | 270 | 250.00 | 14.00 | 23.00 | 0.00 | 2.30 | 0.00 | 0.00 | 4.00 | |
| Bryndza | Ewe | Raw | 24.2 | 107.4 | 60.9 | 42.6 | 0.0 | 9.7 | 0.00 | 0.0 | [146] | ||
| Smoked cheese | 0.0 | 38.3 | 99.9 | 80.7 | 0.0 | 0.0 | 0.0 | 0.0 | |||||
| Fresh cheese | 0.0 | 0.0 | 20.7 | 19.6 | 0.0 | 0.0 | 0.0 | 0.0 | |||||
| Unripened (fresh) cheese | Past. | 0.0 | 11.1 | 118.2 | 35.8 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Pasta filata-type cheese | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 13.2 | 0.0 | 0.0 | |||||
| Brined cheese | 0.0 | 174.6 | 229.5 | 125.6 | 0.0 | 14.0 | 0.0 | 0.0 | |||||
| Flavored cheese | 0.0 | 114.7 | 108.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||||
| Ripened cheese | Goat | Raw | 0.0 | 207.1 | 0.0 | 149.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| Unripened (fresh) cheese (unflavored) | Past. | 0.0 | 11.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Unripened (fresh) cheese (flavored) | 0.0 | 10.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||||
| Pasta filata type cheese | 0.0 | 8.5 | 41.1 | 40.3 | 0.0 | 0.0 | 0.0 | 0.0 | |||||
| Unripened (fresh) cheese (unflavored) | Cow | Past. | 0.0 | 15.2 | 111.4 | 8.9 | 0.0 | 17.9 | 0.0 | 0.0 | |||
| Unripened (fresh) cheese (flavored) | 0.0 | 22.7 | 0.0 | 22.4 | 0.0 | 15.5 | 0.0 | 0.0 | |||||
| Ripened cheese | 0.0 | 101.4 | 70.1 | 72.6 | 0.0 | 97.9 | 0.0 | 0.0 | |||||
| Pasta filata type cheese (unflavored) | 19.3 | 25.8 | 37.3 | 0.0 | 0.0 | 12.7 | 0.0 | 0.0 | |||||
| Pasta filata type cheese (flavored) | 0.0 | 0.0 | 14.7 | 0.0 | 0.0 | 12.0 | 0.0 | 0.0 | |||||
| Pasta filata type cheese (unflavored, smoked) | 18.8 | 0.0 | 0.0 | 10.7 | 0.0 | 9.8 | 0.0 | 0.0 | |||||
| Koopeh | Cow | 70.80 | 8.48 | 156.09 | 282.34 | 0.00 | 0.00 | 0.00 | 0.00 | [147] | |||
| Lighvan | 37.59 | 351.12 | 277.53 | 342.74 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| Red Salmas | 105.21 | 182.62 | 438.03 | 701.05 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| White-veined cheese | Cow | 0 (0) | 15.69 (8.53) | 25.22 (35.14) | 34.57 (103.7) | 0 (0) | 37.23 (17.43) | 1.83 (2.76) | 0 (0) | [148] | |||
| Blue-veined cheese | 0 (0) | 101.02 (65.09) | 12.62 (6.75) | 1.7 (4.16) | 0 (0) | 152.27 (32.6) | 12.92 (4.93) | 0 (0) | |||||
| Feta | Cow | 49.00 | 77.00 | 60.00 | 70.00 | 0.00 | 2.00 | 5.00 | 0.00 | [139] | |||
| Karish | 145.00 | 160.00 | 80.00 | 230.00 | 0.00 | 8.00 | 12.00 | 0.00 | |||||
| Mozzarella | 140.00 | 159.00 | 165.00 | 290.00 | 0.00 | 7.80 | 16.00 | 0.00 | |||||
| Rumi | 125.00 | 520.00 | 100.00 | 400.00 | 0.00 | 10.00 | 17.00 | 0.00 | |||||
| Mish | 230.00 | 900.00 | 140.00 | 590.00 | 0.00 | 9.00 | 16.00 | 0.00 | |||||
| Goat cheese | Goat | Raw | no starter culture | 150 | 12 (11.31) | 30 (4.24) | 10 (7.07) | 18.5 (13.44) | 0 (0) | 0 (0) | 0 (0) | 34 (22.63) | [149] |
| starter cultures | 22 (9.9) | 4.5 (0.71) | 2.5 (0.71) | 1 (1.41) | 0 (0) | 0 (0) | 0 (0) | 1 (1.41) | |||||
| Cheese | Cow | Raw | short ripening time | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | [68] | |
| Goat | short ripening time | 110.00 | 63.94 | 38.75 | 38.90 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Ewe | short ripening time | 102.00 | 233.33 | 10.40 | 48.40 | 0.00 | 0.00 | 0.00 | 48.40 | ||||
| Mix | short ripening time | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Cow | Past. | short ripening time | 0.00 | 4.40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Goat | short ripening time | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Ewe | short ripening time | 60.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Mix | short ripening time | 20.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Cow | Raw | long ripening time | 22.84 | 67.66 | 3.27 | 10.34 | 0.00 | 12.40 | 0.00 | 4.81 | |||
| Goat | long ripening time | 171.30 | 152.60 | 160.99 | 44.81 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Ewe | long ripening time | 49.84 | 132.66 | 138.36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Mix | long ripening time | 65.18 | 216.85 | 127.03 | 85.80 | 0.00 | 5.70 | 0.00 | 0.00 | ||||
| Cow | Past. | long ripening time | 21.80 | 26.96 | 97.68 | 194.20 | 0.00 | 2.20 | 0.00 | 0.00 | |||
| Goat | long ripening time | 13.84 | 15.24 | 58.46 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Ewe | long ripening time | 0.00 | 150.53 | 9.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Mix | long ripening time | 10.62 | 4.34 | 3.31 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Blue cheese | Cow | Raw | 210.00 | 188.82 | 15.40 | 137.63 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Mix | 462.00 | 508.89 | 236.07 | 320.85 | 0.00 | 0.00 | 0.00 | 27.42 | |||||
| Past. | 56.51 | 117.16 | 46.74 | 61.15 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 253.87 | 229.59 | 95.25 | 173.57 | 0.00 | 0.00 | 0.00 | 2.10 | ||||||
| Gouda | Cow | Past. | starter cultures (E. faecalis; E. faecium) | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | [150] |
| 14 | 0.00 | 56.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 28 | 0.00 | 122.85 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 42 | 0.00 | 170.83 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 56 | 0.00 | 233.96 | 2.28 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 70 | 0.00 | 242.27 | 1.54 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| 84 | 0.00 | 476.82 | 1.93 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natrella, G.; Vacca, M.; Minervini, F.; Faccia, M.; De Angelis, M. A Comprehensive Review on the Biogenic Amines in Cheeses: Their Origin, Chemical Characteristics, Hazard and Reduction Strategies. Foods 2024, 13, 2583. https://doi.org/10.3390/foods13162583
Natrella G, Vacca M, Minervini F, Faccia M, De Angelis M. A Comprehensive Review on the Biogenic Amines in Cheeses: Their Origin, Chemical Characteristics, Hazard and Reduction Strategies. Foods. 2024; 13(16):2583. https://doi.org/10.3390/foods13162583
Chicago/Turabian StyleNatrella, Giuseppe, Mirco Vacca, Fabio Minervini, Michele Faccia, and Maria De Angelis. 2024. "A Comprehensive Review on the Biogenic Amines in Cheeses: Their Origin, Chemical Characteristics, Hazard and Reduction Strategies" Foods 13, no. 16: 2583. https://doi.org/10.3390/foods13162583
APA StyleNatrella, G., Vacca, M., Minervini, F., Faccia, M., & De Angelis, M. (2024). A Comprehensive Review on the Biogenic Amines in Cheeses: Their Origin, Chemical Characteristics, Hazard and Reduction Strategies. Foods, 13(16), 2583. https://doi.org/10.3390/foods13162583









