Exploring the Impact of Fermentation Time and Climate on Quality of Cocoa Bean-Derived Chocolate: Sensorial Profile and Volatilome Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agroclimatic Classification
Statistical Analysis
2.2. Fermentation of Cocoa Seeds
Cocoa Sampling and Drying
2.3. Cocoa Sensory Analysis
2.4. Volatile Compound Analysis in Chocolate
2.5. Data Analysis
3. Results and Discussion
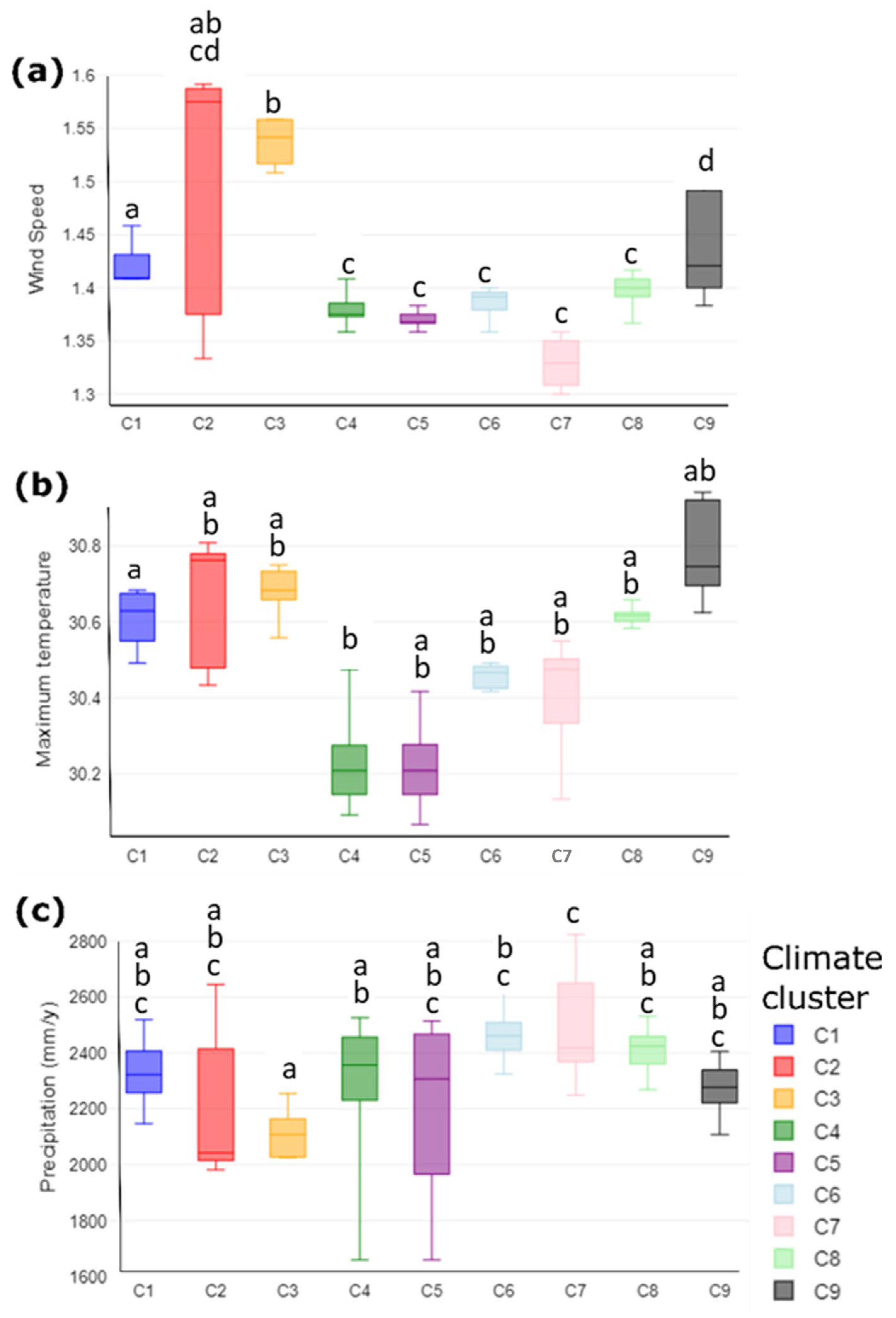
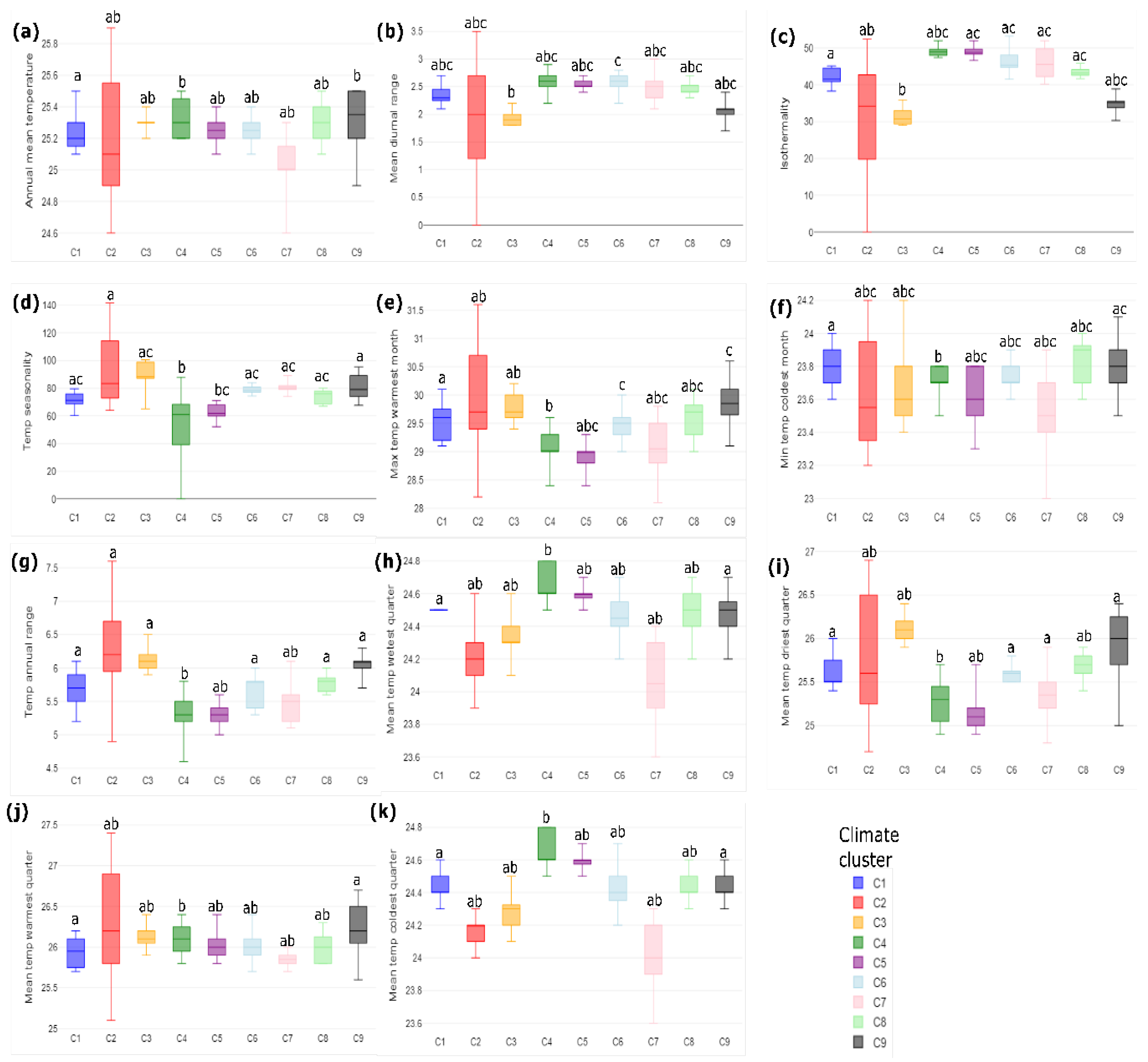
3.1. Climatic Analysis
3.2. Impact of Fermentation Time on the Sensory Profile of Dark Chocolate Samples
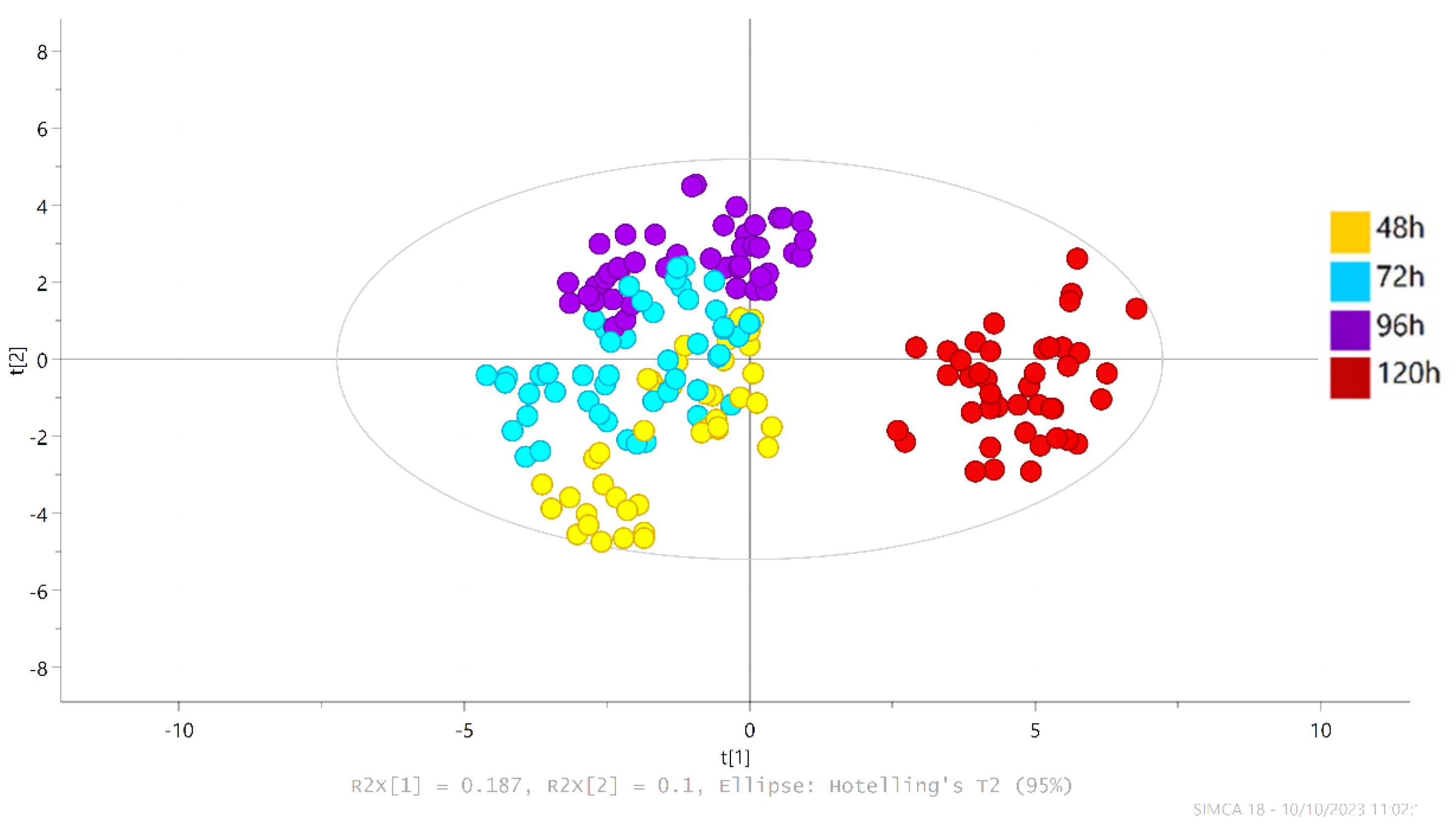
3.3. Identification and Quantification of Volatile Aroma Compounds Present in the Dark Chocolate Samples through Fermentation Time
3.4. Change in Amount of Volatile Compounds Associated with Desirable Aroma Descriptors
3.4.1. Pyrazines
3.4.2. Esters
3.4.3. Aldehyde and Ketones
3.4.4. Alcohols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICCO. Fine Flavor Cocoa—International Cocoa Organization. Available online: https://www.icco.org/fine-or-flavor-cocoa/ (accessed on 3 November 2023).
- Ríos, F.; Ruiz, A.; Lecaro, J.; Rehpani, C. Estrategias País Para la Oferta de Cacaos Especiales—Políticas e Iniciativas Privadas Exitosas en el Perú, Ecuador, Colombia y Republica Dominicana. Swisscontact Colombia Foundation. 2017. Available online: https://www.swisscontact.org/_Resources/Persistent/b/7/9/0/b7909c63ce01d3c0333defd340188b15376359d7/Estrategias_pais_para_la_oferta_de_cacaos_especiales.pdf (accessed on 20 June 2023).
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products-An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Biancolillo, A.; Preys, S.; Gaci, B.; Le-Quere, J.L.; Laboure, H.; Deuscher, Z. Multi-block classification of chocolate and cocoa samples into sensory poles. Food Chem. 2021, 340, 127904. [Google Scholar] [CrossRef]
- Gil, M.; Llano, S.; Jaramillo, Y.; Quijano, J.; Londono-Londono, J. Matrix effect on quantification of sugars and mannitol developed during the postharvest of cocoa: An alternative method for traceability of aroma precursors by liquid chromatography with an evaporative detector. J. Food Sci. Technol. 2020, 57, 210–221. [Google Scholar] [CrossRef]
- Mendoza Salazar, M.M.; Lizarazo-Medina, P.X. Assessment of the fungal community associated with cocoa bean fermentation from two regions in Colombia. Food Res. Int. 2021, 149, 110670. [Google Scholar] [CrossRef]
- Kongor, J.E.; Hinneh, M.; de Walle DVan Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- del Pilar López-Hernández, M.; Sandoval-Aldana, A.P.; García-Lozano, J.; Criollo-Nuñez, J. Cacao materials (Theobroma cacao L.) from different production areas in Colombia: A morphological study. Trends Hortic. 2022, 5, 64. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Prandi, B.; Palla, G.; Sforza, S. Influence of fermentation level and geographical origin on cocoa bean oligopeptide pattern. Food Chem. 2016, 211, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Escobar, S.; Santander, M.; Zuluaga, M.; Chacón, I.; Rodríguez, J.; Vaillant, F. Fine cocoa beans production: Tracking aroma precursors through a comprehensive analysis of flavor attributes formation. Food Chem. 2021, 365, 130627. [Google Scholar] [CrossRef] [PubMed]
- Hinneh, M.; Abotsi, E.E.; Van de Walle, D.; Tzompa-Sosa, D.A.; De Winne, A.; Simonis, J. Pod storage with roasting: A tool to diversifying the flavor profiles of dark chocolates produced from ‘bulk’ cocoa beans? (part I: Aroma profiling of chocolates). Food Res. Int. 2019, 119, 84–98. [Google Scholar] [CrossRef]
- Santander, M.; Vaillant, F.; Sinuco, D.; Rodríguez, J.; Escobar, S. Enhancement of fine flavour cocoa attributes under a controlled postharvest process. Food Res. Int. 2021, 143, 110236. [Google Scholar] [CrossRef] [PubMed]
- Santander Muñoz, M.; Rodríguez Cortina, J.; Vaillant, F.E.; Escobar Parra, S. An overview of the physical and biochemical transformation of cocoa seeds to beans and to chocolate: Flavor formation. Crit. Rev. Food Sci. Nutr. 2020, 60, 1593–1613. [Google Scholar] [CrossRef]
- Chetschik, I.; Kneubühl, M.; Chatelain, K.; Schlüter, A.; Bernath, K.; Hühn, T. Investigations on the Aroma of Cocoa Pulp (Theobroma cacao L.) and Its Influence on the Odor of Fermented Cocoa Beans. J. Agric. Food Chem. 2018, 66, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, A.; Hühn, T.; Kneubühl, M.; Chatelain, K.; Rohn, S.; Chetschik, I. Comparison of the Aroma Composition and Sensory Properties of Dark Chocolates Made with Moist Incubated and Fermented Cocoa Beans. J. Agric. Food Chem. 2022, 70, 4057–4065. [Google Scholar] [CrossRef]
- Guzmán Penella, S.; Boulanger, R.; Maraval, I.; Kopp, G.; Corno, M.; Fontez, B. Link between Flavor Perception and Volatile Compound Composition of Dark Chocolates Derived from Trinitario Cocoa Beans from Dominican Republic. Molecules 2023, 28, 3805. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Contreras-Ramos, S.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Kadow, D.; Bohlmann, J.; Phillips, W.; Lieberei, R. Identification of main fine or flavour components in two genotypes of the cocoa tree (Theobroma cacao L.). J. Appl. Bot. Food Qual. 2013, 86, 90–98. [Google Scholar]
- Lemarcq, V.; Van de Walle, D.; Monterde, V.; Sioriki, E.; Dewettinck, K. Assessing the flavor of cocoa liquor and chocolate through instrumental and sensory analysis: A critical review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5523–5539. [Google Scholar] [CrossRef] [PubMed]
- Rottiers, H.; Tzompa Sosa, D.A.; Lemarcq, V.; De Winne, A.; De Wever, J.; Everaert, H. A multipronged flavor comparison of Ecuadorian CCN51 and Nacional cocoa cultivars. Eur. Food Res. Technol. 2019, 245, 2459–2478. [Google Scholar] [CrossRef]
- Tuenter, E.; Delbaere, C.; De Winne, A.; Bijttebier, S.; Custers, D.; Foubert, K. Non-volatile and volatile composition of West African bulk and Ecuadorian fine-flavor cocoa liquor and chocolate. Food Res. Int. 2020, 130, 108943. [Google Scholar] [CrossRef]
- Fanning, E.; Eyres, G.; Frew, R.; Kebede, B. Linking cocoa quality attributes to its origin using geographical indications. Food Control 2023, 151, 109825. [Google Scholar] [CrossRef]
- González-Orozco, C.E.; Porcel, M.; Yockteng, R.; Caro-Quintero, A.; Rodriguez-Medina, C.; Santander, M. Integrating new variables into a framework to support cacao denomination of origin: A case study in Southwest Colombia. J. Sci. Food Agric. 2023, 3, 1367–1381. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Orozco, C.; Porcel, M.; Escobar, S.; Bravo, D.; Lopez, Y.G.; Yockteng, R. Cacao (Theobroma cacao L.) climate zones and its associated agrobiodiversity in Arauca, Colombia. Biodivers. Data J. 2023, 11, e112771. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; van den Hoogen, J.; Aalto, J.; Ashcroft, M.B.; De Frenne, P.; Kemppinen, J. Global maps of soil temperature. Glob. Chang. Biol. 2022, 28, 3110–3144. [Google Scholar] [CrossRef]
- Assi-Clair, B.J.; Koné, M.K.; Kouamé, K.; Lahon, M.C.; Berthiot, L.; Durand, N. Effect of aroma potential of Saccharomyces cerevisiae fermentation on the volatile profile of raw cocoa and sensory attributes of chocolate produced thereof. Eur. Food Res. Technol. 2019, 245, 1459–1471. [Google Scholar] [CrossRef]
- Fisher, J.B.; Whittaker, R.J.; Malhi, Y. ET come home: Potential evapotranspiration in geographical ecology. Glob. Ecol. Biogeogr. 2011, 20, 1–18. [Google Scholar] [CrossRef]
- Adejuwon, J.O.; Tewogbade, K.E.; Oguntoke, O.; Ufoegbune, G.C. Comparing farmers’ perception of climate effect on cocoa yield with climate data in the Humid zone of Nigeria. Heliyon 2023, 9, e23155. [Google Scholar] [CrossRef]
- Black, E.; Pinnington, E.; Wainwright, C.; Lahive, F.; Quaife, T.; Allan, R.P. Cocoa plant productivity in West Africa under climate change: A modelling and experimental study. Environ. Res. Lett. 2020, 16, 14009. [Google Scholar] [CrossRef]
- Chizari, A.; Mohamed, Z.; Shamsudin, M.N.; Seng, K.W.K. The Effects of Climate Change Phenomena on Cocoa Production in Malaysia. Int. J. Environ. Agric. Biotechnol. 2017, 2, 2599–2604. [Google Scholar] [CrossRef]
- Akoa, S.P.; Boulanger, R.; Effa Onomo, P.; Lebrun, M.; Ondobo, M.L.; Lahon, M.C. Sugar profile and volatile aroma composition in fermented dried beans and roasted nibs from six controlled pollinated Cameroonian fine-flavor cocoa (Theobroma cacao L.) hybrids. Food Biosci. 2023, 53, 102603. [Google Scholar] [CrossRef]
- Batista, N.N.; Ramos, C.L.; Dias, D.R.; Pinheiro, A.C.M.; Schwan, R.F. The impact of yeast starter cultures on the microbial communities and volatile compounds in cocoa fermentation and the resulting sensory attributes of chocolate. J. Food Sci. Technol. 2016, 53, 1101–1110. [Google Scholar] [CrossRef]
- Qin, X.W.; Lai, J.X.; Tan, L.H.; Hao, C.Y.; Li, F.P.; He, S.Z. Characterization of volatile compounds in Criollo, Forastero, and Trinitario cocoa seeds (Theobroma cacao L.) in China. Int. J. Food Prop. 2017, 20, 2261–2275. [Google Scholar] [CrossRef]
- Bastos, V.S.; Uekane, T.M.; Bello, N.A.; de Rezende, C.M.; Flosi Paschoalin, V.M.; Del Aguila, E.M. Dynamics of volatile compounds in TSH 565 cocoa clone fermentation and their role on chocolate flavor in Southeast Brazil. J. Food Sci. Technol. 2019, 56, 2874–2887. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.M.; Botina, B.L.; García, M.C.; Cardona, W.A.; Montenegro, A.C.; Criollo, J. Dynamics of cocoa fermentation and its effect on quality. Sci. Rep. 2021, 11, 16746. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.; Costa, J.; Vilela, L.; Lima, N.; Santos, C.; Schwan, R. Influence of S. cerevisiae and P. kluyveri as starters on chocolate flavour. J. Sci. Food Agric. 2021, 101, 4409–4419. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor formation and character in cocoa and chocolate: A critical review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef]
- Seyfried, C.; Granvogl, M. Characterization of the Key Aroma Compounds in Two Commercial Dark Chocolates with High Cocoa Contents by Means of the Sensomics Approach. J. Agric. Food Chem. 2019, 67, 5827–5837. [Google Scholar] [CrossRef]
- Spada, F.P.; de Alencar, S.M.; Purgatto, E. Comprehensive chocolate aroma characterization in beverages containing jackfruit seed flours and cocoa powder. Future Foods 2022, 6, 100158. [Google Scholar] [CrossRef]
- Purbaningrum, K.; Hidayat, C.; Witasari, L.D.; Utami, T. Flavor Precursors and Volatile Compounds Improvement of Unfermented Cocoa Beans by Hydrolysis Using Bromelain. Foods 2023, 12, 820. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Fleet, G.H.; Zhao, J. Unravelling the contribution of lactic acid bacteria and acetic acid bacteria to cocoa fermentation using inoculated organisms. Int. J. Food Microbiol. 2018, 279, 43–56. [Google Scholar] [CrossRef]
- Ducki, S.; Miralles-Garcia, J.; Zumbé, A.; Tornero, A.; Storey, D.M. Evaluation of solid-phase micro-extraction coupled to gas chromatography-mass spectrometry for the headspace analysis of volatile compounds in cocoa products. Talanta 2008, 74, 1166–1174. [Google Scholar] [CrossRef]


| No. | Volatile Compounds | Odor/ Flavor Descriptions | Chemical Group | RT (min) | KI Lit. | KI Cal. |
|---|---|---|---|---|---|---|
| Fruity | ||||||
| 1 | Acetaldehyde | Tart (acidic), fruity (α) | Aldehyde | 2.96 | 702 ± 12 | 702 |
| 2 | 2-p entanone | Fruity, thinner, acetone (η) | Ketone | 7.00 | 686 | 690 |
| 3 | Nonanal | Tallowy, fruity (η) | Aldehyde | 30.14 | 1390 | 1398 |
| 4 | Hexanal | Green, woody (σ) | Aldehyde | 11.20 | 798 | 800 |
| 5 | Ethyl acetate | Pineapple (η) | Ester | 4.55 | 872 | 888 |
| 6 | Methyl acetate | Fruity (σ) | Ester | 3.78 | 813 | 828 |
| 7 | Butyl acetate | Fruity banana, sharp (ω) | Ester | 10.26 | 1075 | 1070 |
| 8 | Hexyl acetate | Pear - like (μ) | Ester | 22.00 | 1273 ± 7 | 1279 |
| 9 | 1-m ethoxy -2-propyl acetate | Sweet (υ) | Ester | 42.20 | 1233 | 1238 |
| 10 | 2-heptanone | Fruity sweet, coconut (η) | Ketone | 15.57 | 1181 | 1182 |
| 11 | 2,3-b utanediol (isomer A) | Sweet (α) | Alcohol | 39.61 | 1492–1582 | 1533 |
| 12 | 2,3-b utanediol (isomer B) | Sweet (α) | Alcohol | 39.61 | 1492–1582 | 1533 |
| 13 |
2,3-b
utanediol
monoacetate | – | Ester | 41.30 | 1560–1570 | 1568 |
| 14 | 2-pentanol | Green, fruity, sweet (η) | Alcohol | 13.12 | 1122 | 1119 |
| 15 | Limonene | Citrus (η) | 17.10 | 1026 | 1030 | |
| 16 | 2-phenylacetaldehyde | Berry, geranium, honey (α) | Aldehyde | 44.90 | 1634 | 1640 |
| Nutty/chocolate | ||||||
| 17 | 2-m ethylbutanal | Malty and chocolate (γ) | Aldehyde | 5.00 | 910 | 914 |
| 18 | 2-m ethyl -propanal | Malty and chocolate (β) | Aldehyde | 3.60 | 817 | 819 |
| 19 | Benzaldehyde | Nutty, almond (β) | Aldehyde | 36.28 | 1516 | 1520 |
| 20 | 3-m ethylbutanal | Malty and chocolate (β) | Aldehyde | 5.10 | 912 | 918 |
| 21 | 2,3,5,6-t etramethylpyrazine | Milk-coffee, roasted, chocolate (δ) | Pyrazine | 34.19 | 1489 | 1469 |
| 22 | 2,3,5-t rimethylpyrazine | Cocoa, roasted, cooked peanut (δ) | Pyrazine | 29.82 | 1408 | 1402 |
| 23 | Methyl pyrazine | Nutty-chocolate (ε) | Pyrazine | 21.70 | 1266 ± 10 | 1270 |
| 24 | 2,6 -d imethylpyrazine | Vegetal. roasted (η) | Pyrazine | 24.55 | 1300 | 1370 |
| 25 | 3,5 -dimethyl -2-ethylpyrazine | Earthy roasty (σ) | Pyrazine | 34.00 | 1464 | 1470 |
| 26 | 4H- pyran -4-one-2-3-dihydro-3,5-dihydroxy-6-methyl | – | Pyrazine | 64.187 | 2271 ± 14 | 2281 |
| 27 | 2-formylpyrrole | Malty /dark chocolate (ε) | Pyrrole | 61.00 | 2030 ± 14 | 2041 |
| 28 | 2-a cetyl -pyrrole | Bread, cocoa, hazelnut, licorice, walnut (δ) | Pyrrole | 58.84 | 1985 | 1973 |
| 29 | furan-2-phentyl | Sweet rum, caramel and butter (χ) | Furan | 20.00 | 1232 ± 9 | 1150 |
| Floral | ||||||
| 30 | 2-phenylethyl acetate | Dried fruit–like, flowery (γ) | Ester | 53.14 | 1810 | 1813 |
| 31 | Benzyl alcohol | Floral, rose, phenolic (φ) | Alcohol | 57.00 | 1872 | 1870 |
| 32 | Phenylmethyl butanoate | Sweet, floral, strawberry, (ε) | Ester | 57.30 | 1860 ± 9 | 1869 |
| 33 | 2-p henylethanol | Flowery (γ) | Alcohol | 57.39 | 1891 | 1906 |
| Creamy/buttery | ||||||
| 34 | Acetoin | Buttery, sour cream (δ) | Ketone | 22.01 | 1250 | 1284 |
| 35 | Butyrolactone | Sweet, caramel-like (δ) | Lactone | 42.18 | 1618 | 1632 |
| Undesirable | ||||||
| 36 | 3-m ethylbutanoic acid | Pungent, sweaty (γ) | Acid | 45.70 | 1676 | 1666 |
| 37 | Acetic acid | Sour, astringent, vinegar (γ) | Acid | 31.80 | 1452 | 1449 |
| 38 | Ethanol | Alcoholic (η) | Alcohol | 5.59 | 929 | 932 |
| 39 | 2-furanmethanol | Bitter (α) | Furan | 46.80 | 1661 ± 9 | 1650 |
| 40 | Ethylbenzene | Off -flavor (ε) | Hydrocarbon | 13.11 | 857 | 856 |
| 41 | Dimethyl sulfide | Unpleasant, cabbage-like (γ) | Organosulfur | 3.30 | 716 | 754 |
| 42 | Toluene | Caramel, synthetic (off-flavor) Fuel-like (α) | Hydrocarbon | 9.10 | 1042 ± 11 | 1025 |
| Others | ||||||
| 43 | Unidentified compound 1 | Unspecified | Unspecified | 2.70 | – | – |
| 44 | Unidentified compound 2 | Unspecified | Unspecified | 28.10 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llano, S.; Vaillant, F.; Santander, M.; Zorro-González, A.; González-Orozco, C.E.; Maraval, I.; Boulanger, R.; Escobar, S. Exploring the Impact of Fermentation Time and Climate on Quality of Cocoa Bean-Derived Chocolate: Sensorial Profile and Volatilome Analysis. Foods 2024, 13, 2614. https://doi.org/10.3390/foods13162614
Llano S, Vaillant F, Santander M, Zorro-González A, González-Orozco CE, Maraval I, Boulanger R, Escobar S. Exploring the Impact of Fermentation Time and Climate on Quality of Cocoa Bean-Derived Chocolate: Sensorial Profile and Volatilome Analysis. Foods. 2024; 13(16):2614. https://doi.org/10.3390/foods13162614
Chicago/Turabian StyleLlano, Sandra, Fabrice Vaillant, Margareth Santander, Andrés Zorro-González, Carlos E. González-Orozco, Isabelle Maraval, Renaud Boulanger, and Sebastián Escobar. 2024. "Exploring the Impact of Fermentation Time and Climate on Quality of Cocoa Bean-Derived Chocolate: Sensorial Profile and Volatilome Analysis" Foods 13, no. 16: 2614. https://doi.org/10.3390/foods13162614






