Impact of Thermally Inactivated Non-Saccharomyces Yeast Derivatives on White Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains and TIYs Preparation
2.2. Wine Treatment
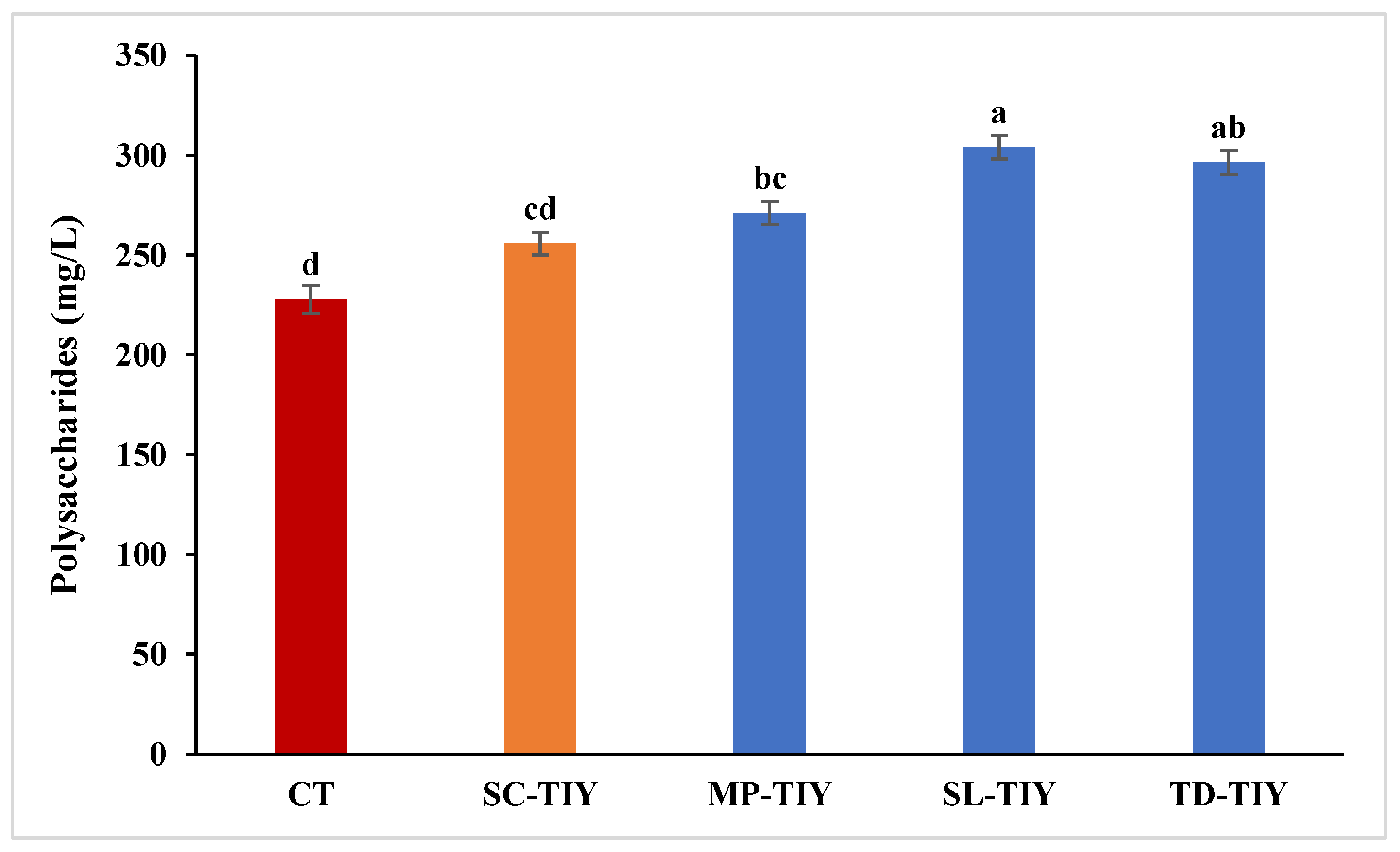
2.3. Total Polysaccharides
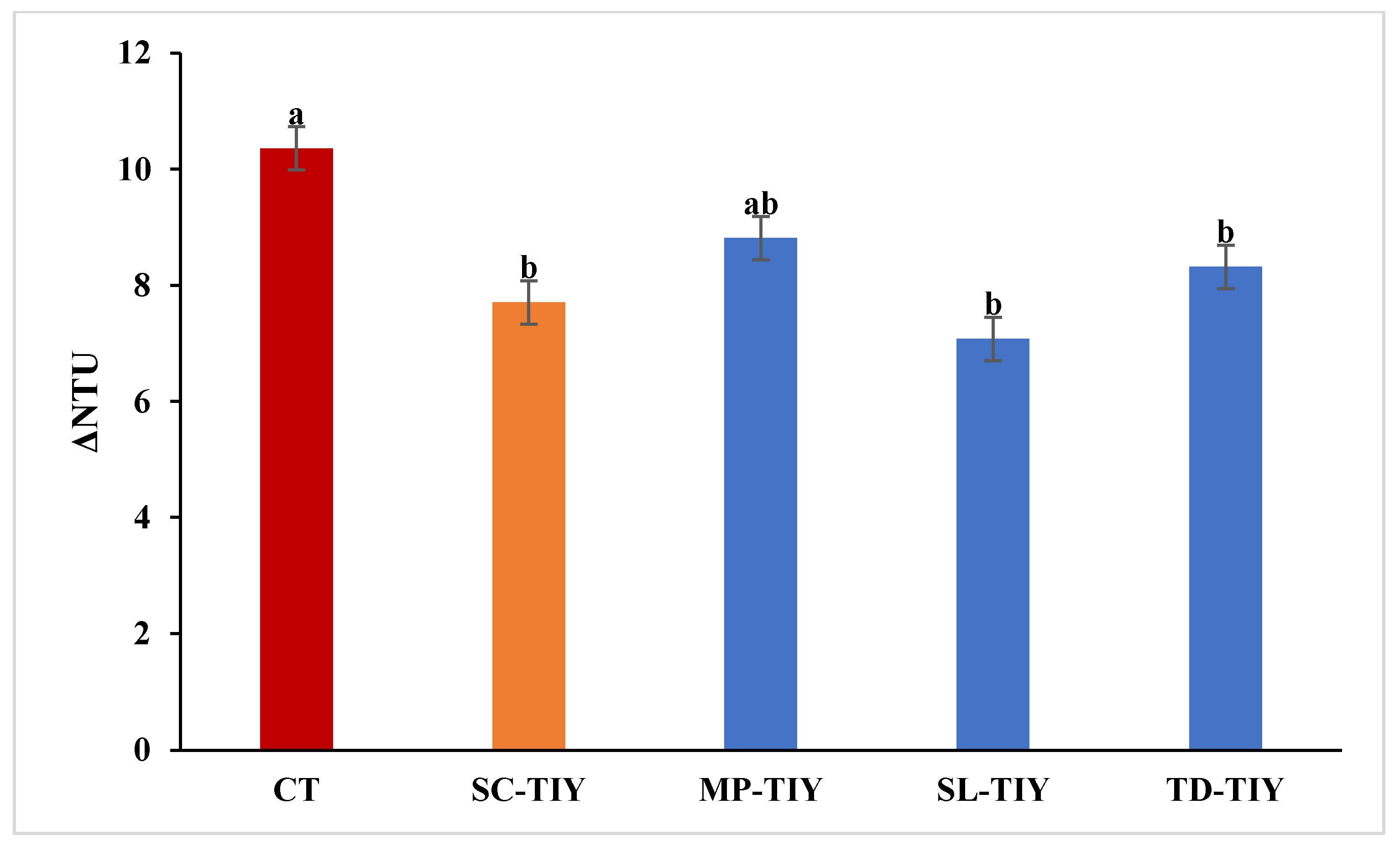
2.4. Heat Test
2.5. Colour Indexes and CIEL*a*b* Trichromatic Coordinates
2.6. Phenolic Compounds
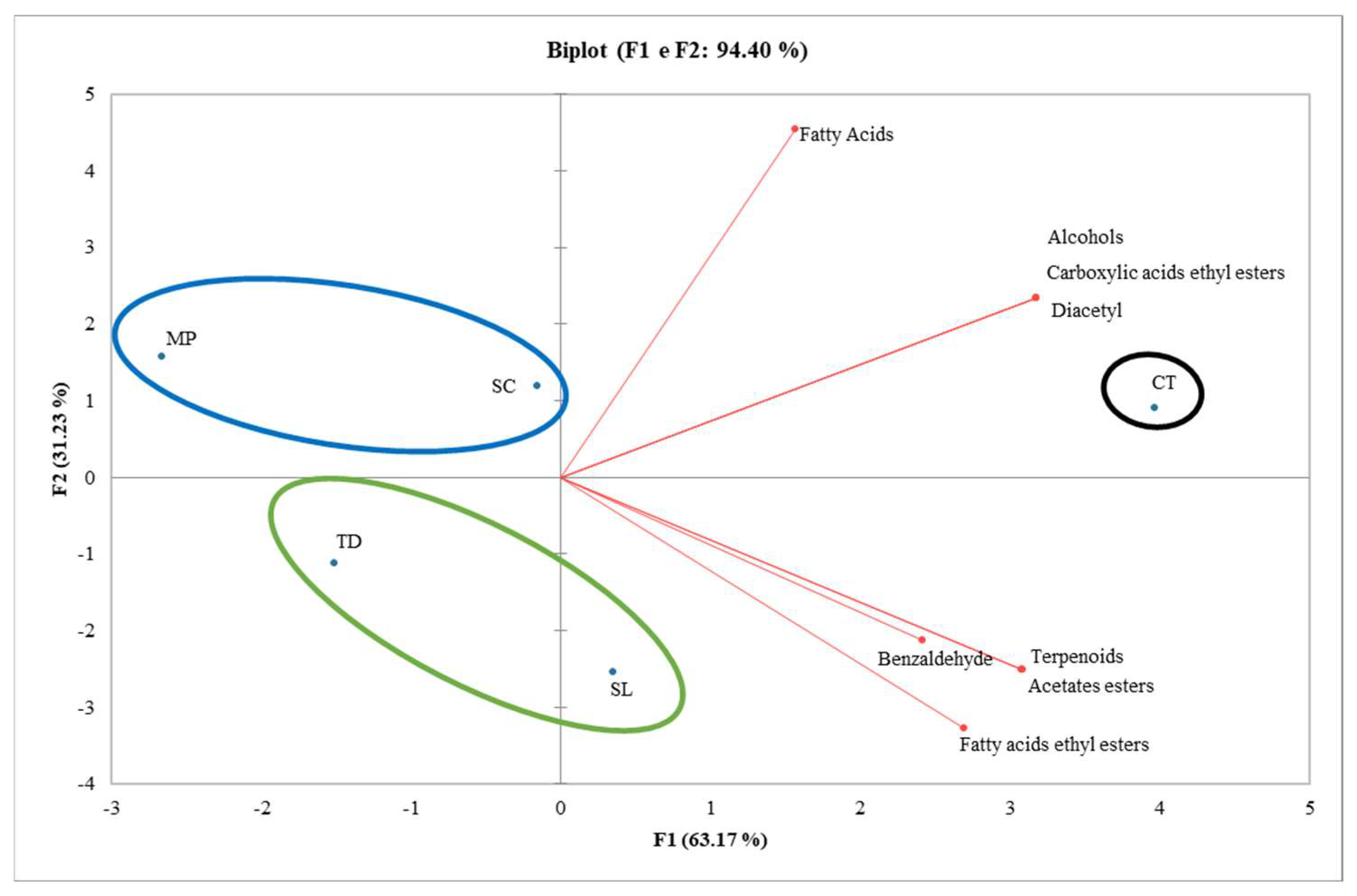
2.7. Volatile Compounds
2.8. Data Analysis
3. Results and Discussion
3.1. Total Polysaccharides content following TIYs treatment
3.2. Protein Stability of TIYs treated wines
3.3. Impact of TIYs on Colour Indexes, CIEL*a*b* Coordinates and Phenol Content
3.4. Effect of TIYs on volatile compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Barrio-Galán, R.; Pérez-Magariño, S.; Ortega-Heras, M.; Williams, P.; Doco, T. Effect of aging on lees and of three different dry yeast derivative products on Verdejo white wine composition and sensorial characteristics. J. Agric. Food Chem. 2011, 59, 12433–12442. [Google Scholar] [CrossRef]
- Bahut, F.; Romanet, R.; Sieczkowski, N.; Schmitt-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Antioxidant activity from inactivated yeast: Expanding knowledge beyond the glutathione-related oxidative stability of wine. Food Chem. 2020, 325, 126941. [Google Scholar] [CrossRef] [PubMed]
- Nioi, C.; Lisanti, M.T.; Meunier, F.; Redon, P.; Massot, A.; Moine, V. Antioxidant activity of yeast derivatives: Evaluation of their application to enhance the oxidative stability of white wine. LWT 2022, 171, 114116. [Google Scholar] [CrossRef]
- Del Barrio-Galán, R.; Úbeda, C.; Gil, M.; Medel-Marabolí, M.; Sieczkowski, N.; Peña-Neira, Á. Evaluation of yeast derivative products developed as an alternative to lees: The effect on the polysaccharide, phenolic and volatile content, and colour and astringency of red wines. Molecules 2019, 24, 1478. [Google Scholar] [CrossRef]
- Rigou, P.; Mekoue, J.; Sieczkowski, N.; Doco, T.; Vernhet, A. Impact of industrial yeast derivative products on the modification of wine aroma compounds and sensorial profile. A review. Food Chem. 2021, 358, 129760. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Baiano, A.; De Gianni, A.; Sinigaglia, M.; Corbo, M.R.; Bevilacqua, A. Differential adsorption of ochratoxin A and anthocyanins by inactivated yeasts and yeast cell walls during simulation of wine aging. Toxins 2015, 7, 4350–4365. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Sorption of volatile phenols by yeast cell walls. Int. J. Wine Res. 2009, 1, 11–18. [Google Scholar] [CrossRef]
- Comuzzo, P.; Tat, L.; Tonizzo, A.; Battistutta, F. Yeast derivatives (extracts and autolysates) in winemaking: Release of volatile compounds and effects on wine aroma volatility. Food Chem. 2006, 99, 217–230. [Google Scholar] [CrossRef]
- González-Royo, E.; Esteruelas, M.; Kontoudakis, N.; Fort, F.; Canals, J.M.; Zamora, F. The effect of supplementation with three commercial inactive dry yeasts on the colour, phenolic compounds, polysaccharides and astringency of a model wine solution and red wine. J. Sci. Food Agric. 2016, 97, 172–181. [Google Scholar] [CrossRef]
- Lambert-Royo, M.I.; Ubeda, C.; Del Barrio-Galán, R.; Sieczkowski, N.; Canals, J.M.; Peña-Neira, Á.; Cortiella, M.G. The diversity of effects of yeast derivatives during sparkling wine aging. Food Chem. 2022, 390, 133174. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior, W.J.F.; Torriani, S. Contribution of non-Saccharomyces yeasts to increase glutathione concentration in wine. Aust. J. Grape Wine Res. 2021, 27, 290–294. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef]
- Ballou, C. Structure and biosynthesis of the mannan component of the yeast cell envelope. Adv. Microb. Physiol. 1976, 14, 93–158. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Tan, E.L.; Brown, S.; Nasution, U.J.; Pettolino, F.; Macintyre, O.J.; Anderson, P.A. Hpf2 glycan structure is critical for protection against protein haze formation in white wine. J. Agric. Food Chem. 2009, 57, 3308–3315. [Google Scholar] [CrossRef]
- Mekoue Nguela, J.; Sieczkowski, N.; Roi, S.; Vernhet, A. Sorption of grape proanthocyanidins and wine polyphenols by yeasts, inactivated yeasts, and yeast cell walls. J. Agric. Food Chem. 2015, 63, 660–670. [Google Scholar] [CrossRef]
- Razmkhab, S.; Lopez-Toledano, A.; Ortega, J.M.; Mayen, M.; Merida, J.; Medina, M. Adsorption of phenolic compounds and browning products in white wines by yeasts and their cell walls. J. Agric. Food Chem. 2002, 50, 7432–7437. [Google Scholar] [CrossRef]
- Ribeiro, T.; Fernandes, C.; Nunes, F.M.; Filipe-Ribeiro, L.; Cosme, F. Influence of the structural features of commercial mannoproteins in white wine protein stabilization and chemical and sensory properties. Food Chem. 2014, 159, 47–54. [Google Scholar] [CrossRef]
- Mekoue Nguela, J.; Teuf, O.; Bicca, S.A.; Vernhet, A. Impact of mannoprotein N-glycosyl phosphorylation and branching on the sorption of wine polyphenols by yeasts and yeast cell walls. Food Chem. 2023, 403, 134326. [Google Scholar] [CrossRef] [PubMed]
- Civa, V.; Chinnici, F.; Picariello, G.; Tarabusi, E.; Bosaro, M.; Mannazzu, I.; Domizio, P. Non-Saccharomyces yeast derivatives: Characterization of novel potential bio-adjuvants for the winemaking process. Curr. Res. Food Sci. 2024, 8, 100774. [Google Scholar] [CrossRef] [PubMed]
- Millarini, V.; Ignesti, S.; Cappelli, S.; Ferraro, G.; Adessi, A.; Zanoni, B.; Domizio, P. Protection of wine from protein haze using Schizosaccharomyces japonicus polysaccharides. Foods 2020, 9, 1407. [Google Scholar] [CrossRef] [PubMed]
- Pocock, K.F.; Waters, E.J. Protein haze in bottled white wines: How well do stability tests and bentonite fining trials predict haze formation during storage and transport? Aust. J. Grape Wine Res. 2006, 12, 212–220. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-Sanjosé, M.L. Application of absorbance values used in wineries for estimating CIELAB parameters in red wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
- Peng, Z.; Iland, P.G.; Oberholster, A.; Sefton, M.A.; Waters, E.J. Analysis of pigmented polymers in red wine by reverse phase HPLC. Aust. J. Grape Wine Res. 2002, 8, 70–75. [Google Scholar] [CrossRef]
- González-Royo, E.; Urtasun, A.; Gil, M.; Kontoudakis, N.; Esteruelas, M.; Fort, F.; Zamora, F. Effect of yeast strain and supplementation with inactive yeast during alcoholic fermentation on wine polysaccharides. Am. J. Enol. Vitic. 2013, 64, 268–273. [Google Scholar] [CrossRef]
- Li, J.; Karboune, S. A comparative study for the isolation and characterization of mannoproteins from Saccharomyces cerevisiae yeast cell wall. Int. J. of Biol. Macromol. 2018, 119, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Palomero, F.; Loira, I.; Suárez-Lepe, J.A. New Trends in Aging on Lees. In Red Wine Technology; Academic Press: London, UK, 2019; pp. 163–176. [Google Scholar] [CrossRef]
- Comuzzo, P.; Calligaris, S.; Iacumin, L.; Ginaldi, F.; Paz, A.E.P.; Zironi, R. Potential of high pressure homogenization to induce autolysis of wine yeasts. Food Chem. 2015, 185, 340–348. [Google Scholar] [CrossRef]
- Waters, E.J.; Pellerin, P.; Brillouet, J.M. A Saccharomyces mannoprotein that protects wine from protein haze. Carbohydr. Polym. 1994, 23, 185–191. [Google Scholar] [CrossRef]
- Lomolino, G.; Curioni, A. Protein haze formation in white wines: Effect of Saccharomyces cerevisiae cell wall components prepared with different procedures. J. Agric. Food Chem. 2007, 55, 8737–8744. [Google Scholar] [CrossRef]
- Dupin, I.V.; McKinnon, B.M.; Ryan, C.; Boulay, M.; Markides, A.J.; Jones, G.P.; Waters, E.J. Saccharomyces cerevisiae mannoproteins that protect wine from protein haze: Their release during fermentation and lees contact and a proposal for their mechanism of action. J. Agric. Food Chem. 2000, 48, 3098–3105. [Google Scholar] [CrossRef]
- Recamales, Á.F.; Sayago, A.; González-Miret, M.L.; Hernanz, D. The effect of time and storage conditions on the phenolic composition and colour of white wine. Food Res. Int. 2006, 39, 220–229. [Google Scholar] [CrossRef]
- Marquez, T.; Millan, C.; Salmon, J.M. Plasma membrane sterols are involved in yeast’s ability to adsorb polyphenolic compounds resulting from wine model solution browning. J. Agric. Food Chem. 2009, 57, 8026–8032. [Google Scholar] [CrossRef] [PubMed]
- Comuzzo, P.; Tat, L.; Fenzi, D.; Brotto, L.; Battistutta, F.; Zironi, R. Interactions between yeast autolysates and volatile compounds in wine and model solution. Food Chem. 2011, 127, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Bayón, M.A.; Andujar-Ortiz, I.; Alcaide-Hidalgo, J.M.; Martin-Alvarez, P.J.; Moreno-Arribas, M.V. Characterization of commercial inactive dry yeast preparations for enological use based on their ability to release soluble compounds and their behavior toward aroma compounds in model wines. J. Agric. Food Chem. 2009, 57, 10784–10792. [Google Scholar] [CrossRef] [PubMed]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Lubbers, S.; Charpentier, C.; Feuillat, M.; Voilley, A. Influence of yeast walls on the behavior of aroma compounds in a model wine. Am. J. Enol. Vitic. 1994, 45, 29–33. [Google Scholar] [CrossRef]
- Langourieux, S.; Crouzet, J. Study of aroma compounds-polysaccharides interactions by dynamic exponential dilution. LWT 1994, 27, 544–549. [Google Scholar] [CrossRef]
| Strain | Species | Origin | TIY Code |
|---|---|---|---|
| EC1118 | Saccharomyces cerevisiae | Lallemand a | SC-TIY |
| 46 | Metschnikowia pulcherrima | DAGRI b | MP-TIY |
| 64 | Saccharomycodes ludwigii | DAGRI b | SL-TIY |
| 92 | Torulaspora delbrueckii | DAGRI b | TD-TIY |
| Wine | Alcohol % (v/v) | Titratable Acidity (Tartaric Acid g/L) | Volatile Acidity (Acetic Acid g/L) | pH | ΔNTU |
|---|---|---|---|---|---|
| Trebbiano Toscano | 12.18 ± 0.02 | 5.76 ± 0.02 | 0.13 ± 0.02 | 3.14 ± 0.01 | 10.40 ± 0.54 |
| CT | SC-TIY | MP-TIY | SL-TIY | TD-TIY | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A420 | 0.099 a | ± | 0.001 | 0.084 b | ± | 0.005 | 0.075 d | ± | 0.001 | 0.084 b | ± | 0.002 | 0.080 c | ± | 0.002 |
| A520 | 0.038 a | ± | 0.001 | 0.029 b | ± | 0.004 | 0.019 c | ± | 0.001 | 0.028 b | ± | 0.001 | 0.027 b | ± | 0.001 |
| A620 | 0.011 a | ± | 0.001 | 0.012 a | ± | 0.004 | 0.003 b | ± | 0.001 | 0.011 a | ± | 0.001 | 0.011 a | ± | 0.001 |
| CI | 0.149 a | ± | 0.003 | 0.125 b | ± | 0.013 | 0.098 c | ± | 0.001 | 0.122 b | ± | 0.004 | 0.118 b | ± | 0.004 |
| Tone | 2.620 c | ± | 0.037 | 2.991 b | ± | 0.252 | 3.965 a | ± | 0.037 | 2.986 b | ± | 0.07 | 2.982 b | ± | 0.074 |
| Chroma* | 6.633 a | ± | 0.018 | 5.564 b | ± | 0.098 | 5.303 c | ± | 0.048 | 5.584 b | ± | 0.054 | 5.354 c | ± | 0.032 |
| Hue (°) | 87.536 d | ± | 0.479 | 93.073 b | ± | 0.414 | 94.097 a | ± | 0.251 | 92.40 c | ± | 0.285 | 92.947 b | ± | 0.251 |
| L* | 97.411 c | ± | 0.078 | 97.911 b | ± | 0.362 | 98.44 d | ± | 0.053 | 97.967 b | ± | 0.1 | 98.011 b | ± | 0.093 |
| a* | 0.287 a | ± | 0.04 | −0.299 c | ± | 0.036 | −0.384 d | ± | 0.013 | −0.231 b | ± | 0.028 | −0.272 c | ± | 0.015 |
| b* | 6.626 a | ± | 0.018 | 5.554 b | ± | 0.098 | 5.288 c | ± | 0.047 | 5.579 b | ± | 0.051 | 5.346 c | ± | 0.034 |
| ΔE | 1.32 | 2.00 | 1.29 | 1.52 | |||||||||||
| TP 1 | 66.680 a | ± | 0.574 | 66.080 ab | ± | 0.529 | 61.025 bc | ± | 2.286 | 60.196 bc | ± | 1.806 | 55.898 d | ± | 3.339 |
| Compound | CT | SC-TIY | MP-TIY | SL-TIY | TD-TIY | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols (mg/L) | |||||||||||||||
| 2-Methylpropanol | 13.60 a | ± | 0.20 | 13.58 a | ± | 1.00 | 13.91 a | ± | 0.55 | 14.66 a | ± | 0.26 | 13.36 a | ± | 0.13 |
| n-Butanol | 0.07 a | ± | 0.00 | 0.06 a | ± | 0.00 | 0.06 a | ± | 0.00 | 0.07 a | ± | 0.01 | 0.07 a | ± | 0.01 |
| 2-Methylbutanol | 15.93 a | ± | 0.26 | 15.00 a | ± | 0.73 | 15.39 a | ± | 1.07 | 14.17 a | ± | 0.84 | 14.59 a | ± | 0.51 |
| 3-Methylbutanol | 99.51 a | ± | 15.53 | 92.03 a | ± | 1.08 | 82.54 a | ± | 0.44 | 84.22 a | ± | 2.65 | 80.92 a | ± | 2.06 |
| 2-Phenylethanol | 49.18 a | ± | 5.61 | 41.08 ab | ± | 1.9 | 41.52 ab | ± | 0.4 | 39.76 b | ± | 2.42 | 41.79 ab | ± | 1.48 |
| 1-Hexanol | 1.03 a | ± | 0.05 | 0.89 b | ± | 0.01 | 0.85 b | ± | 0.00 | 0.88 b | ± | 0.05 | 0.97 ab | ± | 0.01 |
| Benzyl alcohol | 0.41 a | ± | 0.01 | 0.34 b | ± | 0.00 | 0.33 b | ± | 0.01 | 0.33 b | ± | 0.02 | 0.35 b | ± | 0.02 |
| Trans-(E)-3-Hexenol | 0.05 a | ± | 0.00 | 0.04 c | ± | 0.00 | 0.04 c | ± | 0.00 | 0.04 c | ± | 0.00 | 0.05 b | ± | 0.00 |
| Cis-(Z)-3-Hexenol | 0.24 a | ± | 0.02 | 0.21 a | ± | 0.00 | 0.20 a | ± | 0.00 | 0.21 a | ± | 0.01 | 0.23 a | ± | 0.00 |
| 3-Ethoxypropanol | 0.09 a | ± | 0.01 | 0.07 ab | ± | 0.01 | 0.07 ab | ± | 0.00 | 0.06 b | ± | 0,00 | 0.07 ab | ± | 0.00 |
| 3-Methylthiopropanol | 2.30 a | ± | 0.01 | 2.13 b | ± | 0.00 | 2.30 a | ± | 0.02 | 2.09 bc | ± | 0.02 | 2.03 c | ± | 0.02 |
| Total alcohols | 182.41 | 165.41 | 157.20 | 156.50 | 154.42 | ||||||||||
| Terpenoids (μg/L) | |||||||||||||||
| Linalool | 16.04 a | ± | 0.40 | 14.24 a | ± | 2.04 | 13.16 a | ± | 0.06 | 13.16 a | ± | 0.85 | 14.96 a | ± | 0.23 |
| α-Terpineol | 6.44 a | ± | 0.51 | 4.56 b | ± | 0.23 | 4.48 b | ± | 0.34 | 4.20 b | ± | 0.28 | 5.08 b | ± | 0.06 |
| β-Citronellol | 20.33 a | ± | 4.68 | 18.29 a | ± | 5.84 | 17.99 a | ± | 0.75 | 18.14 a | ± | 0.57 | 15.55 a | ± | 4.20 |
| Nerol | 32.36 | ± | 1.98 | 29.80 a | ± | 2.43 | 29.20 a | ± | 1.92 | 27.92 a | ± | 1.92 | 27.24 a | ± | 2.55 |
| Geraniol | 12.06 a | ± | 0.49 | 9.00 b | ± | 0.03 | 9.35 b | ± | 0.04 | 10.05 ab | ± | 0.31 | 10.71 ab | ± | 1.17 |
| β-Damascenone | 11.32 a | ± | 2.43 | 7.68 a | ± | 0.68 | 8.68 a | ± | 0.40 | 7.92 a | ± | 0.45 | 8.20 a | ± | 0.06 |
| β-Ionone | 0.80 a | ± | 0.00 | 0.70 a | ± | 0.14 | 0.60 a | ± | 0.00 | 0.80 a | ± | 0.00 | 0.90 a | ± | 0.14 |
| Total terpenoids | 99.35 | 84.27 | 83.46 | 82.19 | 82.64 | ||||||||||
| Carbonyl compounds (mg/L) | |||||||||||||||
| Benzaldehyde | 93.46 a | ± | 6.31 | 89.76 a | ± | 4.07 | 75.32 b | ± | 0.40 | 101.94 a | ± | 1.50 | 75.44 b | ± | 2.04 |
| Diacetyl | 0.18 ab | ± | 0.02 | 0.14 b | ± | 0.01 | 0.19 a | ± | 0.00 | 0.18 ab | ± | 0.01 | 0.22 a | ± | 0.01 |
| Total carbonyl compounds | 93.64 | 89.90 | 75.51 | 102.12 | 75.66 | ||||||||||
| Fatty acids (mg/L) | |||||||||||||||
| 2-Methylpropanoic acid | 1.98 a | ± | 0.08 | 1.82 abc | ± | 0.02 | 1.87 ab | ± | 0.00 | 1.68 c | ± | 0.03 | 1.71 bc | ± | 0.01 |
| Butanoic acid | 1.26 a | ± | 0.00 | 1.01 b | ± | 0.02 | 1.01 b | ± | 0.08 | 0.93 b | ± | 0.03 | 0.96 b | ± | 0.02 |
| 3-Methylbutanoic acid | 0.37 a | ± | 0.02 | 0.31 ab | ± | 0.03 | 0.31 ab | ± | 0.00 | 0.28 b | ± | 0.02 | 0.30 b | ± | 0.00 |
| Hexanoic acid | 9.38 a | ± | 1.11 | 10.16 a | ± | 0.49 | 9.37 a | ± | 0.84 | 9.39 a | ± | 0.25 | 8.39 a | ± | 0.88 |
| Octanoic acid | 14.36 a | ± | 1.85 | 13.78 a | ± | 0.78 | 13.67 a | ± | 0.46 | 12.55 a | ± | 0.44 | 12.72 a | ± | 0.02 |
| Decanoic acid | 1.05 a | ± | 0.11 | 0.76 a | ± | 0.11 | 0.90 a | ± | 0.11 | 0.92 a | ± | 0.10 | 0.80 a | ± | 0.08 |
| Total fatty acids | 28.41 | 27.84 | 27.12 | 24.71 | 24.99 | ||||||||||
| Fatty acids ethyl esters (μg/L) | |||||||||||||||
| Ethyl isobutyrate | 42.04 | ± | 2.39 | 38.13 | ± | 2.64 | 37.20 | ± | 2.07 | 41.91 | ± | 1.70 | 40.40 | ± | 0.57 |
| Ethyl butanoate | 588.63 | ± | 64.71 | 537.24 | ± | 7.07 | 491.88 | ± | 20.31 | 543.56 | ± | 7.64 | 530.92 | ± | 13.18 |
| Ethyl 3-methylbutanoate | 17.20 a | ± | 1.36 | 14.60 ab | ± | 0.17 | 13.32 b | ± | 1.19 | 14.64 ab | ± | 0.23 | 16.48 ab | ± | 0.57 |
| Ethyl hexanoate | 905.08 | ± | 129.49 | 740.52 | ± | 40.56 | 789.64 | ± | 31.17 | 825.32 | ± | 14.99 | 856.24 | ± | 35.07 |
| Ethyl octanoate | 1058.88 ab | ± | 53.51 | 1026.12 ab | ± | 102.11 | 880.80 b | ± | 48.08 | 1233.04 a | ± | 40.28 | 1064.68 a | ± | 18.27 |
| Ethyl decanoate | 214.22 a | ± | 13.83 | 139.11 b | ± | 18.60 | 106.40 b | ± | 22.88 | 163.20 ab | ± | 0.38 | 152.53 b | ± | 3.14 |
| Ethyl 2-phenylacetate | 9.96 a | ± | 0.62 | 8.12 b | ± | 0.28 | 8.48 ab | ± | 0.57 | 7.64 b | ± | 0.17 | 8.36 ab | ± | 0.06 |
| Total fatty acids ethyl esters | 2836.35 | 2503.84 | 2327.72 | 2829.31 | 2669.61 | ||||||||||
| Carboxylic acid ethyl esters (mg/L) | |||||||||||||||
| Ethyl lactate | 2,06 a | ± | 0.24 | 1.91 a | ± | 0.13 | 2.03 a | ± | 0.11 | 1.98 a | ± | 0.07 | 2.01 a | ± | 0.01 |
| Monoethyl succinate | 49.90 a | ± | 1.38 | 34.25 c | ± | 2.37 | 41.12 b | ± | 1.49 | 39.76 bc | ± | 0.08 | 41.25 b | ± | 0.39 |
| Diethyl succinate | 2.58 a | ± | 0.13 | 2.28 b | ± | 0.07 | 2.28 b | ± | 0.01 | 2.13 b | ± | 0.03 | 2.20 b | ± | 0.02 |
| Total carboxylic acids esters | 54.54 | 38.45 | 45.44 | 43.86 | 45.46 | ||||||||||
| Acetate esters (μg/L) | |||||||||||||||
| Isobutyl acetate | 50.81 a | ± | 1.13 | 48.2 a | ± | 2.98 | 50.25 a | ± | 0.36 | 52.18 a | ± | 1.73 | 51.42 a | ± | 0.77 |
| n-butyl acetate | 9.07 b | ± | 0.91 | 9.01 b | ± | 0.96 | 27.55 a | ± | 0.40 | 26.59 a | ± | 0.68 | 12.56 b | ± | 0.74 |
| Isoamyl acetate | 2305.81 a | ± | 78.30 | 2263.41 a | ± | 76.22 | 2241.14 a | ± | 7.68 | 2350.00 a | ± | 28.11 | 2216.17 a | ± | 83.90 |
| n-hexyl acetate | 33.64 a | ± | 2.60 | 28.08 a | ± | 3.39 | 29.12 a | ± | 0.51 | 33.08 a | ± | 1.19 | 29.76 a | ± | 1.92 |
| 2-phenylethyl acetate | 990.84 a | ± | 106.86 | 1023.73 a | ± | 23.53 | 1010.64 a | ± | 38.52 | 999.56 a | ± | 7.52 | 962.96 a | ± | 4.53 |
| Total acetates | 3390.16 | 3372.43 | 3358.69 | 3461.53 | 3272.87 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Civa, V.; Maioli, F.; Canuti, V.; Pietrini, B.M.; Bosaro, M.; Mannazzu, I.; Domizio, P. Impact of Thermally Inactivated Non-Saccharomyces Yeast Derivatives on White Wine. Foods 2024, 13, 2640. https://doi.org/10.3390/foods13162640
Civa V, Maioli F, Canuti V, Pietrini BM, Bosaro M, Mannazzu I, Domizio P. Impact of Thermally Inactivated Non-Saccharomyces Yeast Derivatives on White Wine. Foods. 2024; 13(16):2640. https://doi.org/10.3390/foods13162640
Chicago/Turabian StyleCiva, Valentina, Francesco Maioli, Valentina Canuti, Bianca Maria Pietrini, Matteo Bosaro, Ilaria Mannazzu, and Paola Domizio. 2024. "Impact of Thermally Inactivated Non-Saccharomyces Yeast Derivatives on White Wine" Foods 13, no. 16: 2640. https://doi.org/10.3390/foods13162640





