Lonicera japonica Fermented by Lactobacillus plantarum Improve Multiple Patterns Driven Osteoporosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Making Honeysuckle Extract
2.2. Quantification of Honeysuckle’s Crude Polysaccharides
2.3. Preparation of Fermentation and Mixed Solution
2.4. Animal Experiments
2.5. Bone Mineral Density (BMD) Assessment
2.6. Analysis of the Tibia Paraffin Section, H&E Staining, and Bone Microarchitecture
2.7. Assessment of Blood Calcium, TRACP-5b, OCN, TNF-α, and IL-6
2.8. Sequencing of 16S rRNA in Colon Content Microbiota
2.9. Metabolite Extraction and LC-MS/MS Analysis
2.10. Statistics
3. Results
3.1. Effect of FS on Bone Structure
3.1.1. FS Significantly Restored BMD Levels
3.1.2. FS Significantly Promoted the Recovery of Bone Microstructure
3.2. Effect of FS on Some Related Factors in Serum
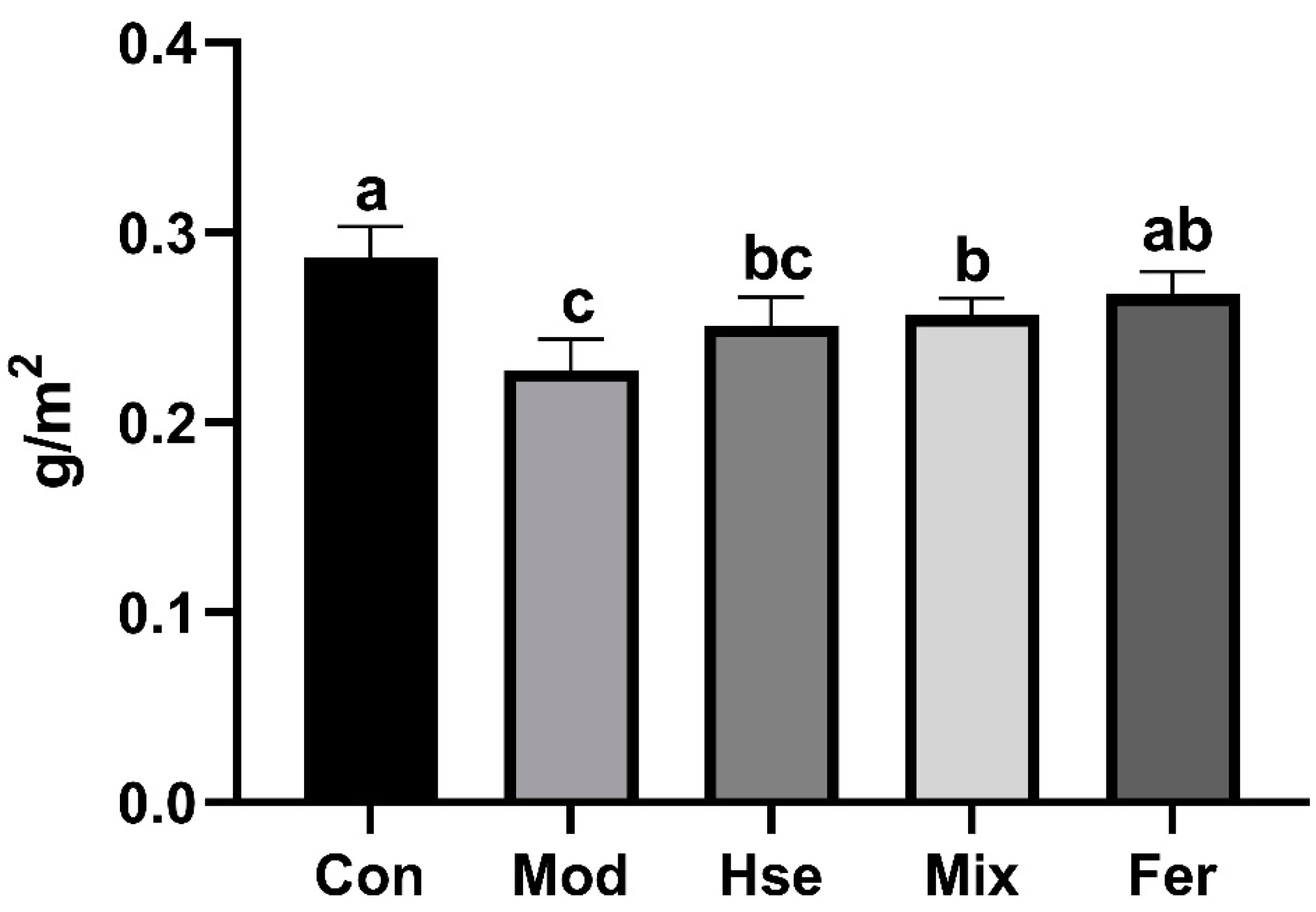
3.2.1. FS More Significantly Improved Calcium Absorption in OP
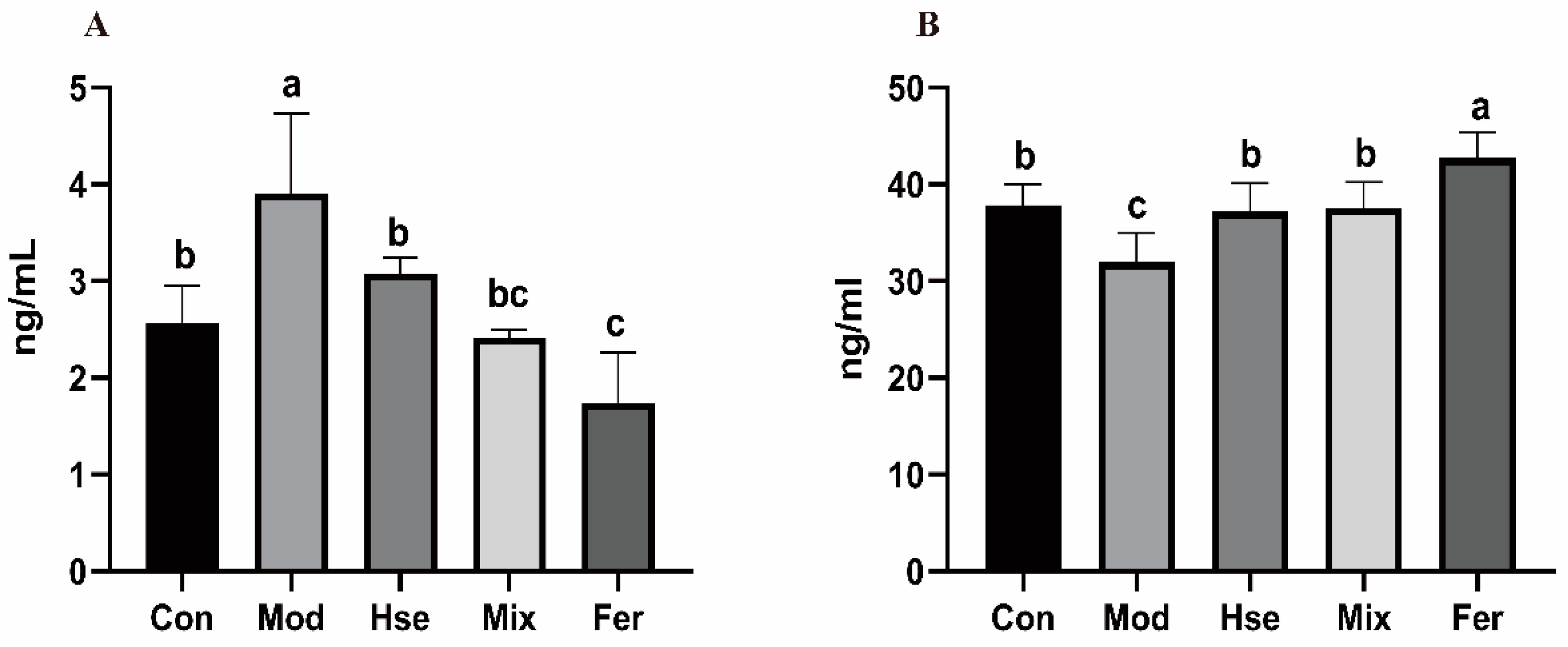
3.2.2. FS Significantly Reduced the Osteoclast Differentiation Biomarker TRACP-5b and Increased the Osteoblast Differentiation Marker OCN
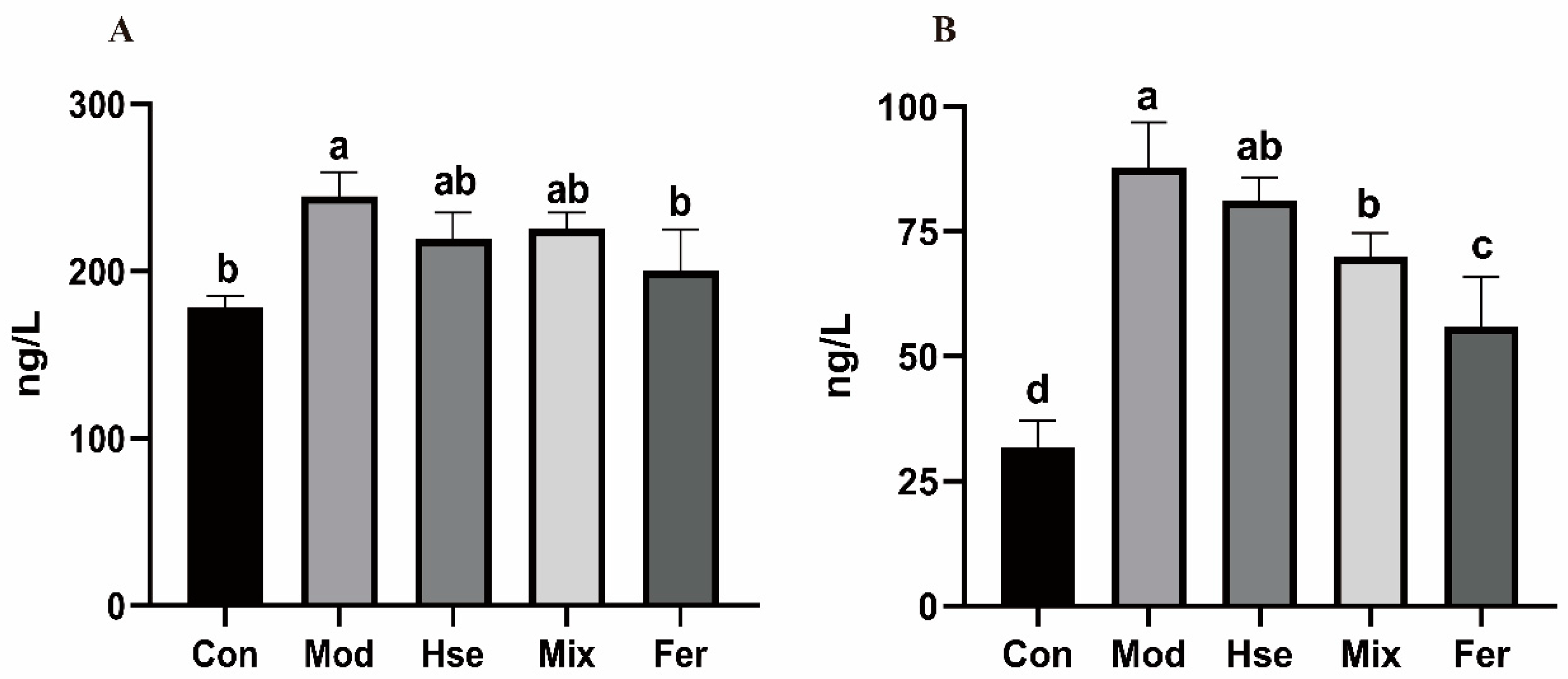
3.2.3. FS More Significantly Reduced the Levels of the Pro-Inflammatory Cytokines TNF-α and IL-6
3.3. Effect of FS on the GM of OP Rats
3.3.1. FS Intervention Significantly Changed the GM of OP Rats
3.3.2. FS Improves OP by Restoring the Abundance of Possible Key GMs
3.4. Influence of FS on Rat Metabolite Levels
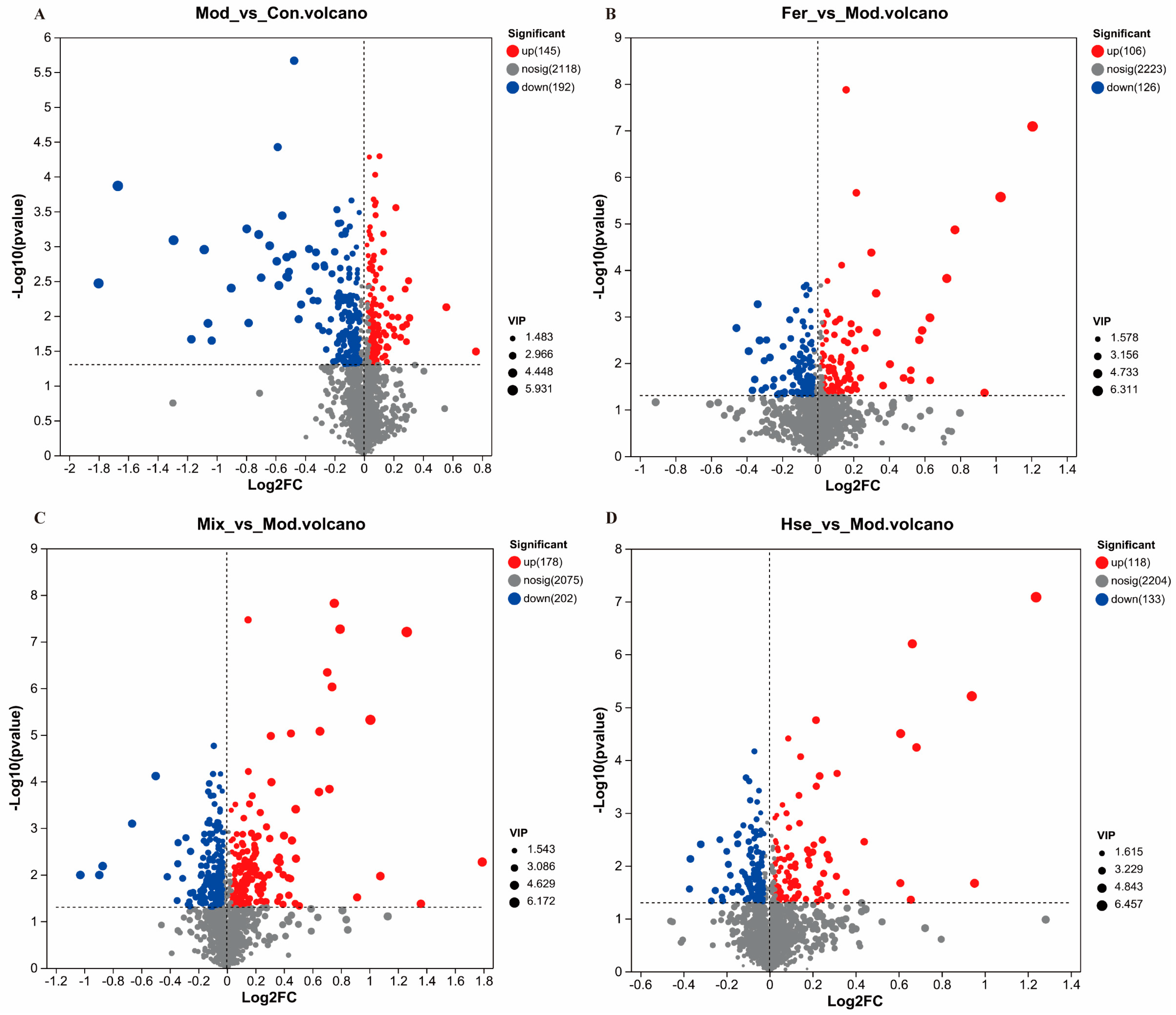
3.4.1. Comparison of Metabolites between FS and MS
3.4.2. Effects of FS on Serum Metabolites in OP Rats
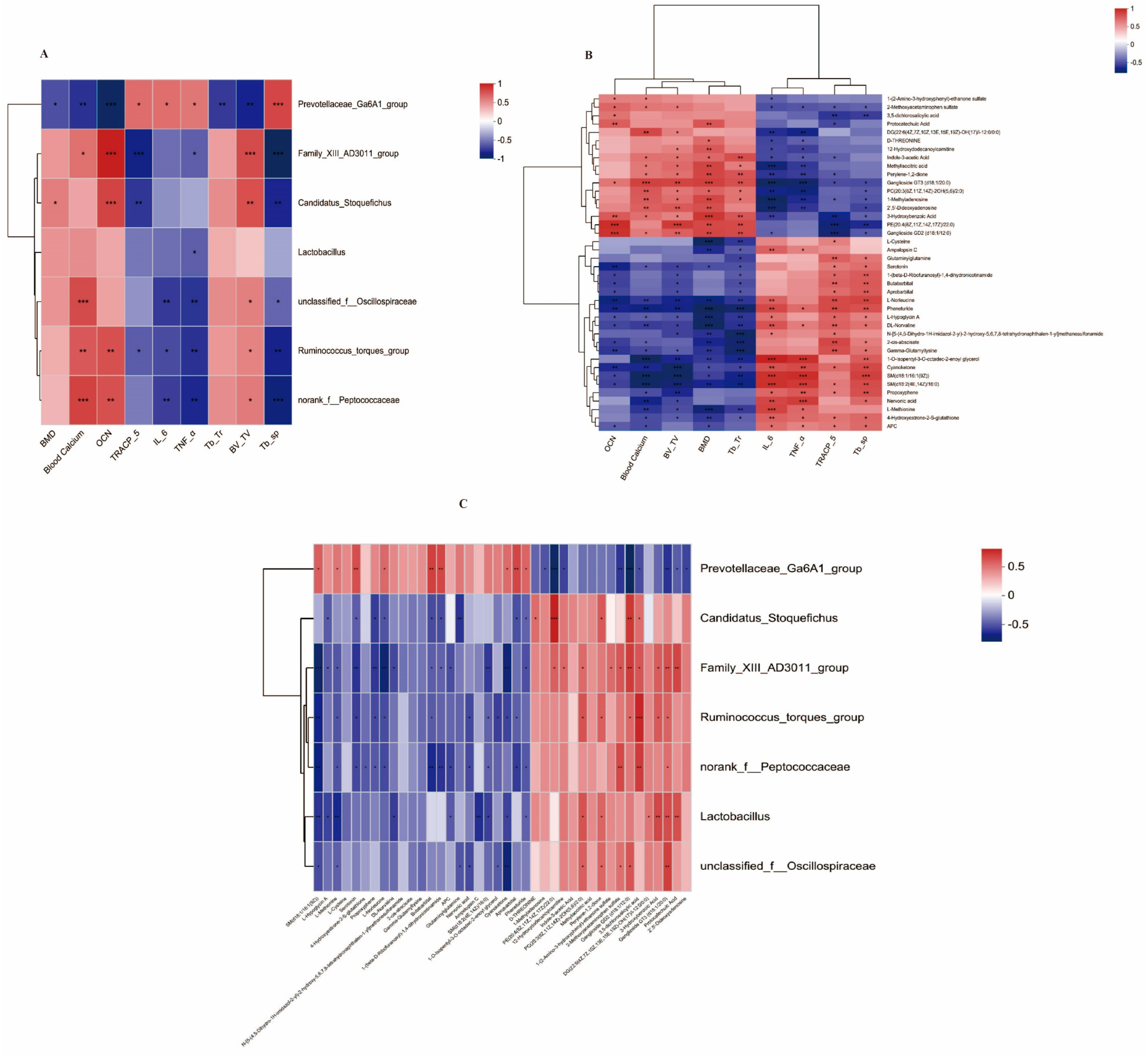
3.5. Correlation Analysis between Bone Markers and Possible Key Bacterial Genera and Possible Key Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, F.; Zhao, Z.; Gu, Y.; Han, J.; Ye, K.; Zhang, Y. 7,8-Dihydroxyflavone Modulates Bone Formation and Resorption and Ameliorates Ovariectomy-Induced Osteoporosis. eLife 2021, 10, e64872. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Renna, M.D.; Conversano, F.; Casciaro, E.; Di Paola, M.; Quarta, E.; Muratore, M.; Casciaro, S. Major Osteoporotic Fragility Fractures: Risk Factor Updates and Societal Impact. World J. Orthop. 2016, 7, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.A.; El-Hajj Fuleihan, G.; Bauer, D.C.; Camacho, P.M.; Clarke, B.L.; Clines, G.A.; Compston, J.E.; Drake, M.T.; Edwards, B.J.; Favus, M.J.; et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2016, 31, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; Starr, A.Z.; Nunley, J.A. Analysis of Past Secular Trends of Hip Fractures and Predicted Number in the Future 2010–2050. J. Orthop. Trauma 2012, 26, 117. [Google Scholar] [CrossRef]
- Maximov, P.Y.; Lee, T.M.; Craig Jordan, V. The Discovery and Development of Selective Estrogen Receptor Modulators (SERMs) for Clinical Practice. Curr. Clin. Pharmacol. 2013, 8, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.; Hitz, M.; Prieto-Alhambra, D.; Abrahamsen, B. Risks and Benefits of Bisphosphonate Therapies. J. Cell. Biochem. 2016, 117, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-Y.; Tang, C.-H.; Ho, T.-L.; Wang, W.-L.; Yao, C.-H. Chlorogenic Acid Prevents Ovariectomized-Induced Bone Loss by Facilitating Osteoblast Functions and Suppressing Osteoclast Formation. Aging 2024, 16, 4832–4840. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Pang, K.-L.; Chin, K.-Y. Effects of Caffeic Acid and Its Derivatives on Bone: A Systematic Review. Drug Des. Dev. Ther. 2021, 15, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, B.; Shang, J.; Li, R. Ferulic Acid Attenuates Osteoporosis Induced by Glucocorticoid through Regulating the GSK-3β/Lrp-5/ERK Signalling Pathways. Physiol. Int. 2021, 108, 317–341. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Wang, Z.-H.; Deng, L.-H.; Wang, H.; Zheng, Q. Oral Administration of Quercetin or Its Derivatives Inhibit Bone Loss in Animal Model of Osteoporosis. Oxid. Med. Cell. Longev. 2020, 2020, e6080597. [Google Scholar] [CrossRef]
- Lee, H.-H.; Jang, J.-W.; Lee, J.-K.; Park, C.-K. Rutin Improves Bone Histomorphometric Values by Reduction of Osteoclastic Activity in Osteoporosis Mouse Model Induced by Bilateral Ovariectomy. J. Korean Neurosurg. Soc. 2020, 63, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Yang, Y.; Wei, L.; Cao, Y.; Ma, J.; Zheng, X.; Teng, J.; Qin, N. Luteolin Rescues Postmenopausal Osteoporosis Elicited by OVX through Alleviating Osteoblast Pyroptosis via Activating PI3K-AKT Signaling. Phytomedicine 2024, 128, 155516. [Google Scholar] [CrossRef]
- Liu, H.; Yi, X.; Tu, S.; Cheng, C.; Luo, J. Kaempferol Promotes BMSC Osteogenic Differentiation and Improves Osteoporosis by Downregulating miR-10a-3p and Upregulating CXCL12. Mol. Cell. Endocrinol. 2021, 520, 111074. [Google Scholar] [CrossRef]
- Xu, H.; Yin, D.; Liu, T.; Chen, F.; Chen, Y.; Wang, X.; Sheng, J. Tea Polysaccharide Inhibits RANKL-Induced Osteoclastogenesis in RAW264.7 Cells and Ameliorates Ovariectomy-Induced Osteoporosis in Rats. Biomed. Pharmacother. 2018, 102, 539–548. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Liu, L.; Zhang, G.; Zhou, A.; Peng, X. The Gut Microbiota Alteration and the Key Bacteria in Astragalus Polysaccharides (APS)-Improved Osteoporosis. Food Res. Int. 2020, 138, 109811. [Google Scholar] [CrossRef]
- Cénit, M.C.; Matzaraki, V.; Tigchelaar, E.F.; Zhernakova, A. Rapidly Expanding Knowledge on the Role of the Gut Microbiome in Health and Disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2014, 1842, 1981–1992. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Li, Y.-J.; Lu, P.-P.; Dai, G.-C.; Chen, X.-X.; Rui, Y.-F. The Modulatory Effect and Implication of Gut Microbiota on Osteoporosis: From the Perspective of “Brain–Gut–Bone” Axis. Food Funct. 2021, 12, 5703–5718. [Google Scholar] [CrossRef]
- Yan, J.; Takakura, A.; Zandi-Nejad, K.; Charles, J.F. Mechanisms of Gut Microbiota-Mediated Bone Remodeling. Gut Microbes 2018, 9, 84–92. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Bézie, S.; Charreau, B.; Vimond, N.; Lasselin, J.; Gérard, N.; Nerrière-Daguin, V.; Bellier-Waast, F.; Duteille, F.; Anegon, I.; Guillonneau, C. Human CD8+ Tregs Expressing a MHC-Specific CAR Display Enhanced Suppression of Human Skin Rejection and GVHD in NSG Mice. Blood Adv. 2019, 3, 3522–3538. [Google Scholar] [CrossRef]
- Ciucci, T.; Ibáñez, L.; Boucoiran, A.; Birgy-Barelli, E.; Pène, J.; Abou-Ezzi, G.; Arab, N.; Rouleau, M.; Hébuterne, X.; Yssel, H.; et al. Bone Marrow Th17 TNFα Cells Induce Osteoclast Differentiation, and Link Bone Destruction to IBD. Gut 2015, 64, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota Modulate Anxiety-Like Behavior and Endocrine Abnormalities in Hypothalamic-Pituitary-Adrenal Axis. Front. Cell. Infect. Microbiol. 2017, 7, 489. [Google Scholar] [CrossRef]
- Yadav, V.K.; Balaji, S.; Suresh, P.S.; Liu, X.S.; Lu, X.; Li, Z.; Guo, X.E.; Mann, J.J.; Balapure, A.K.; Gershon, M.D.; et al. Pharmacological Inhibition of Gut-Derived Serotonin Synthesis Is a Potential Bone Anabolic Treatment for Osteoporosis. Nat. Med. 2010, 16, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, X.; Liu, N.; Chang, J.; Liu, G.; Hu, S. Lactobacillus Plantarum Attenuates Glucocorticoid-Induced Osteoporosis by Altering the Composition of Rat Gut Microbiota and Serum Metabolic Profile. Front. Immunol. 2024, 14, 1285442. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ao, H.; Peng, C.; Yan, D. Gut Microbiota, a New Frontier to Understand Traditional Chinese Medicines. Pharmacol. Res. 2019, 142, 176–191. [Google Scholar] [CrossRef]
- Zhao, Y.; Hong, K.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Lactobacillus fermentum and Its Potential Immunomodulatory Properties. J. Funct. Foods 2019, 56, 21–32. [Google Scholar] [CrossRef]
- Xi, X.; Wei, X.; Wang, Y.; Chu, Q.; Xiao, J. Determination of Tea Polysaccharides in Camellia Sinensis by a Modified Phenol-Sulfuric Acid Method. Arch. Biol. Sci. 2010, 62, 669–676. [Google Scholar] [CrossRef]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone Histomorphometry: Standardization of Nomenclature, Symbols, and Units: Report of the Asbmr Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]
- Kanis, J.A. Diagnosis of Osteoporosis and Assessment of Fracture Risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Brandi, M.L. Microarchitecture, the Key to Bone Quality. Rheumatology 2009, 48, iv3–iv8. [Google Scholar] [CrossRef]
- Heaney, R.P. Calcium, Dairy Products and Osteoporosis. J. Am. Coll. Nutr. 2000, 19, 83S–99S. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Takahashi, N. Regulatory Mechanisms of Osteoblast and Osteoclast Differentiation. Oral. Dis. 2002, 8, 147–159. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R. Proinflammatory Cytokines and Osteoporosis. Curr. Osteoporos. Rep. 2009, 7, 134–139. [Google Scholar] [CrossRef]
- Warabi, S.; Tachibana, Y.; Kumegawa, M.; Hakeda, Y. Dexamethasone Inhibits Bone Resorption by Indirectly Inducing Apoptosis of the Bone-Resorbing Osteoclasts via the Action of Osteoblastic Cells. Cytotechnology 2001, 35, 25–34. [Google Scholar] [CrossRef]
- Briot, K.; Roux, C. Glucocorticoid-Induced Osteoporosis. RMD Open 2015, 1, e000014. [Google Scholar] [CrossRef]
- Lukert, B.P.; Raisz, L.G. Glucocorticoid-Induced Osteoporosis: Pathogenesis and Management. Ann. Intern. Med. 1990, 112, 352–364. [Google Scholar] [CrossRef]
- Ding, K.; Hua, F.; Ding, W. Gut Microbiome and Osteoporosis. Aging Dis. 2020, 11, 438–447. [Google Scholar] [CrossRef]
- Nilsson, A.G.; Sundh, D.; Bäckhed, F.; Lorentzon, M. Lactobacillus Reuteri Reduces Bone Loss in Older Women with Low Bone Mineral Density: A Randomized, Placebo-Controlled, Double-Blind, Clinical Trial. J. Intern. Med. 2018, 284, 307–317. [Google Scholar] [CrossRef]
- Dar, H.Y.; Shukla, P.; Mishra, P.K.; Anupam, R.; Mondal, R.K.; Tomar, G.B.; Sharma, V.; Srivastava, R.K. Lactobacillus acidophilus Inhibits Bone Loss and Increases Bone Heterogeneity in Osteoporotic Mice via Modulating Treg-Th17 Cell Balance. Bone Rep. 2018, 8, 46–56. [Google Scholar] [CrossRef]
- Duscha, A.; Hegelmaier, T.; Dürholz, K.; Desel, C.; Gold, R.; Zaiss, M.M.; Haghikia, A. Propionic Acid Beneficially Modifies Osteoporosis Biomarkers in Patients with Multiple Sclerosis. Ther. Adv. Neurol. Disord. 2022, 15, 175628642211039. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Chen, S.-Y.; Wu, Y.-H.; Liao, Y.-L.; Yen, G.-C. Ameliorative Effect of Buckwheat Polysaccharides on Colitis via Regulation of the Gut Microbiota. Int. J. Biol. Macromol. 2023, 227, 872–883. [Google Scholar] [CrossRef]
- Yang, X.; He, Z.; Hu, R.; Yan, J.; Zhang, Q.; Li, B.; Yuan, X.; Zhang, H.; He, J.; Wu, S. Dietary β-Carotene on Postpartum Uterine Recovery in Mice: Crosstalk Between Gut Microbiota and Inflammation. Front. Immunol. 2021, 12, 744425. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Szeto, I.M.-Y.; Li, N.; Yang, H.; Zhou, Y.; Liu, B.; He, F.; Zhang, L.; Duan, S.; Chen, J. Effects of Menaquinone-7 on the Bone Health of Growing Rats under Calcium Restriction: New Insights from Microbiome-Metabolomics. Nutrients 2023, 15, 3398. [Google Scholar] [CrossRef]
- Li, F.; Han, X.; Tang, T.; Wang, Z.; Zhao, Q.; Wang, Z.; Xu, Z.; Chang, Y.; Gou, Z.; Li, Y. Mendelian Randomization Distinguished the Changes in Gut Microbiota Caused by IBD from Those That Lead to IBD 2024. Available online: https://www.researchsquare.com/article/rs-3897238/v1 (accessed on 25 January 2024).
- Qi, T.; Li, L.; Weidong, T. The Role of Sphingolipid Metabolism in Bone Remodeling. Front. Cell Dev. Biol. 2021, 9, 752540. [Google Scholar] [CrossRef]
- Zhang, H.; Li, K.; Zhao, Y.; Zhang, Y.; Sun, J.; Li, S.; Lin, G. Long-Term Use of Fluoxetine Accelerates Bone Loss through the Disruption of Sphingolipids Metabolism in Bone Marrow Adipose Tissue. Transl. Psychiatry 2020, 10, 138. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The Metabolism and Significance of Homocysteine in Nutrition and Health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef]
- Soyupek, F.; Cerci, S.; Yildiz, S.; Yildiz, M.; Gumus, B. Effect of Homocysteine on Bone Mineral Density of Rats. Biol. Trace Elem. Res. 2007, 118, 255–259. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Iii, J.F.R.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut Microbes Promote Colonic Serotonin Production through an Effect of Short-Chain Fatty Acids on Enterochromaffin Cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Karsenty, G.; Yadav, V.K. Regulation of Bone Mass by Serotonin: Molecular Biology and Therapeutic Implications. Annu. Rev. Med. 2011, 62, 323–331. [Google Scholar] [CrossRef]
- Bliziotes, M. Update in Serotonin and Bone. J. Clin. Endocrinol. Metab. 2010, 95, 4124–4132. [Google Scholar] [CrossRef]
- Hellou, J.; Ross, N.W.; Moon, T.W. Glutathione, Glutathione S-Transferase, and Glutathione Conjugates, Complementary Markers of Oxidative Stress in Aquatic Biota. Environ. Sci. Pollut. Res. 2012, 19, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Jie, J.; Li, W.; Wang, G.; Xu, X. FK506 Ameliorates Osteoporosis Caused by Osteoblast Apoptosis via Suppressing the Activated CaN/NFAT Pathway during Oxidative Stress. Inflamm. Res. 2021, 70, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Koenigstein, A.; Joseph, J.; Sun, L.; Kalyanaraman, B.; Zaidi, M.; Avadhani, N.G. Role of Mitochondrial Reactive Oxygen Species in Osteoclast Differentiation. Ann. N. Y. Acad. Sci. 2010, 1192, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The Role of Oxidative Stress during Inflammatory Processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Jie, L.; Ma, Z.; Gao, Y.; Shi, X.; Yu, L.; Mao, J.; Wang, P. The Mechanism of Palmatine-Mediated Intestinal Flora and Host Metabolism Intervention in OA-OP Comorbidity Rats. Front. Med. 2023, 10, 1153360. [Google Scholar] [CrossRef]
- Wu, Y.-X.; Wu, T.; Xu, B.; Xu, X.; Chen, H.; Li, X.; Wang, G. Protocatechuic Acid Inhibits Osteoclast Differentiation and Stimulates Apoptosis in Mature Osteoclasts. Biomed. Pharmacother. 2016, 82, 399–405. [Google Scholar] [CrossRef]
- Khan, S.A.; Chatterjee, S.S.; Kumar, V. Low Dose Aspirin like Analgesic and Anti-Inflammatory Activities of Mono-Hydroxybenzoic Acids in Stressed Rodents. Life Sci. 2016, 148, 53–62. [Google Scholar] [CrossRef]
- Shen, J.; Yang, L.; You, K.; Chen, T.; Su, Z.; Cui, Z.; Wang, M.; Zhang, W.; Liu, B.; Zhou, K.; et al. Indole-3-Acetic Acid Alters Intestinal Microbiota and Alleviates Ankylosing Spondylitis in Mice. Front. Immunol. 2022, 13, 762580. [Google Scholar] [CrossRef]
- Sasaki, E.; Hamamura, K.; Mishima, Y.; Furukawa, K.; Nagao, M.; Kato, H.; Hamajima, K.; Sato, T.; Miyazawa, K.; Goto, S.; et al. Attenuation of Bone Formation through a Decrease in Osteoblasts in Mutant Mice Lacking the GM2/GD2 Synthase Gene. Int. J. Mol. Sci. 2022, 23, 9044. [Google Scholar] [CrossRef]
- Santilli, F.; Fabrizi, J.; Pulcini, F.; Santacroce, C.; Sorice, M.; Delle Monache, S.; Mattei, V. Gangliosides and Their Role in Multilineage Differentiation of Mesenchymal Stem Cells. Biomedicines 2022, 10, 3112. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Xu, W.; Luo, J.; Liu, L.; Peng, X. Lonicera japonica Fermented by Lactobacillus plantarum Improve Multiple Patterns Driven Osteoporosis. Foods 2024, 13, 2649. https://doi.org/10.3390/foods13172649
Chen Z, Xu W, Luo J, Liu L, Peng X. Lonicera japonica Fermented by Lactobacillus plantarum Improve Multiple Patterns Driven Osteoporosis. Foods. 2024; 13(17):2649. https://doi.org/10.3390/foods13172649
Chicago/Turabian StyleChen, Zimin, Weiye Xu, Jianming Luo, Liu Liu, and Xichun Peng. 2024. "Lonicera japonica Fermented by Lactobacillus plantarum Improve Multiple Patterns Driven Osteoporosis" Foods 13, no. 17: 2649. https://doi.org/10.3390/foods13172649
APA StyleChen, Z., Xu, W., Luo, J., Liu, L., & Peng, X. (2024). Lonicera japonica Fermented by Lactobacillus plantarum Improve Multiple Patterns Driven Osteoporosis. Foods, 13(17), 2649. https://doi.org/10.3390/foods13172649





