A Review of Ganoderma lucidum Polysaccharide: Preparations, Structures, Physicochemical Properties and Application
Abstract
:1. Introduction of Ganoderma lucidum Polysaccharide
2. Preparation of Ganoderma lucidum Polysaccharide
2.1. Extraction of GLP
2.2. Separation and Purification of GLP
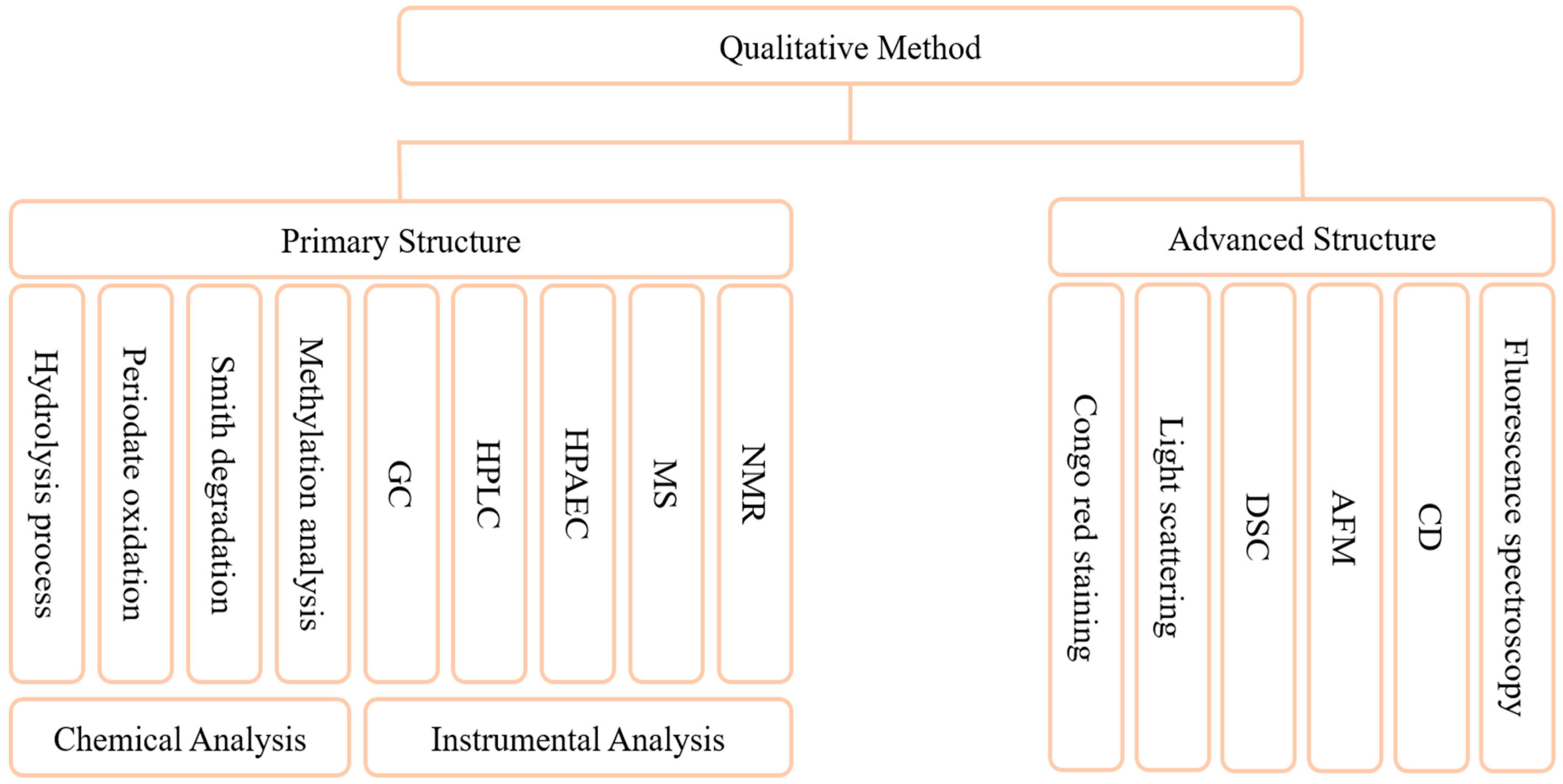
3. Structure of Ganoderma lucidum Polysaccharide
3.1. Structure from Primary to Quaternary
3.2. Structure with or without Activity
| Type | Backbone | Name | Mw | Monosaccharide Composition Ratio | Bioactivity | Raw Source | Reference |
|---|---|---|---|---|---|---|---|
| α | α-(1→6)-D-galactopyranosyl α-(1,2,6)-D-galactopyranosyl | LZ-D-1 | 2.8 × 104 Da | L-Fuc:D-Glc:D-Gal = 1:1:5 | Immunity: Stimulate proliferation of mouse spleen lymphocytes in vitro | Chongming/ Shanghai | [95] |
| α-(1→4)-D-glucan | LB-B1 | 9.3 × 103 Da | Only D-glucose | — | — | [96] | |
| α-(1,6)-Galp | PSG-2 | 6.9 × 104 Da | Galactose:Fucose:Glucose = 8:1:1 | — | Ganzhou/ Jiangxi | [97] | |
| β | β-(1→3)-D-glucan | GL-IV-I | 1.33 × 105 Da | — | — | Longyan/Fujian | [71] |
| GLP20 | 3.75 × 106 Da | — | Immunity: Increase NO production of RAW264.7 macrophages | Shanghai | [72] | ||
| PSGL-I-1A | 7.18 × 105 Da | Only D-glucose | Immunity: Affect T lymphocyte-stimulating activity | Shanxi | [98] | ||
| β- (1→3)-D-glucosyl | LB-NB | 4.7 × 104 Da | Only D-glucose | Immunity: Remarkable stimulation of proliferation of T-cells in vitro | Shanghai | [70] | |
| β-(1→3)-glucose | PSG-1 | — | Glu:Mannose:Galactose = 9:1:1 | — | — | [99] | |
| β-(1→3)-D-glucopyranosyl | SP | 1.0 × 104 Da | — | Immunity: Enhancement of lymphocyte proliferation and antibody production | — | [100] | |
| β-(1→6)-D-glucan | PGL | 1.26 × 105 Da | Only D-glucose | Immunity: Had an immunosuppressive effect on antibody production and lymphocyte proliferation | — | [101] | |
| β-(1→3)-(1→6)-D-glucan | GTM5 | 1.76 × 106 Da | Glc | Antitumor | Wuhan | [80] | |
| GTM6 | 1.61 × 106 Da | Glc:Man = 3.83:1 | Antitumor | Wuhan | [80] | ||
| β-(1→3)-(1→4)-(1→6)-D-glucopyranosyl β-(1→6)-D-mannopyranosyl | PL-4 | 2.0 × 105 Da | Mannose:Glc = 1:13 | Immunity: Enhanced the proliferation of T- and B-lymphocytes in vitro | Shanxi | [102] | |
| β-(1→3)-(1→6)-D-glucopyranosyl | Ganoderans B | 7.4 × 103 Da | — | Reduced the blood glucose concentration | Kyoto/Japan | [103] | |
| β-(1→3)-(1→4)-(1→6)-D-glucan | GLSWA-I | 1.57 × 105 Da | — | Significantly promoted dinitrochlorobenzene-induced delayed-type ear swelling in mice | Shanghai | [104] | |
| β-(1,6)-D-Glcp | GLSA50-1B | 1.03 × 105 Da | Only Glucose | — | Shanghai | [105] | |
| α, β | α-(1→4)-D-glucan β-(1→3)-D-glucan | GTM3 | 4.65 × 106 Da | Glc | Antitumor: Exhibited significant inhibition ratio beyond 50% | Wuhan | [80] |
| GTM4 | 4.68 × 106 Da | Glc | Antitumor | Wuhan | [80] | ||
| α-(1→4)-D-glucopyranosyl β-(1→6)-D-galactopyranosyl | PL-1 | 8.3 × 103 Da | Rha:Gal:Glc = 1:4:13 | Immunity: Enhanced the proliferation of T- and B-lymphocytes in vitro | Shanxi | [102] | |
| α-(1→6)-D-galactopyranosyl β-(1→3)-(1→6)-D-glucopyranosyl | Ganoderans C | 5.8 × 103 Da | D-glucose:D-galactose = 24:1 | Reduced the blood glucose concentration | Kyoto/Japan | [103] | |
| α-(1,6)-galactopyranosyl α-(1,2,6)-galactopyranosyl β-(1,3)-glucopyranosyl β-(1,4,6)-glucopyranosyl | LZ-C-1 | 7.0 × 103 Da | L-Fuc, D-Glc, D-Gal | — | Shanghai | [106] | |
| α-(1→6)-D-glucopyranosyl β-(1→3)-D-glucopyranosyl β-(1→3,6)-D-glucopyranosyl | GSG | 1.43 × 105 Da | — | Immunity: Stimulating effects on murine lymphocyte proliferation | Jilin | [107] | |
| Not well-known | 1,2,6-galactose 1,3-glucose 1,6-galactose | GLPCW-II | 1.2 × 104 Da | D-Glc:L-Fuc:D-Gal = 1.00:1.09:4.09 | Stimulated the proliferation of mouse spleen lymphocytes | Shanghai | [108] |
| — | PSG-1 | 1.013 × 106 Da | Glucose:Mannose:Galactose = 4.91:1:1.28 | — | Ganzhou/ Jiangxi | [97] | |
| 1,3-glucosyl | GSG | 8.0 × 103 Da | Only D-glucose | Immunity: Potentiated the Con A-induced proliferative response of splenocytes | — | [109] | |
| — | GLIS | — | D-glucose:D-galactose:D-mannose = 3.0:1:1 | Immunity | — | [110] | |
| — | SeGLP-2B-1 | 1.06 × 106 Da | Glucose:Rhamnose:Xylose:Galactose = 1.000:0.652:0.443:0.227 | Anticancer | — | [111] | |
| Glucan | PL-3 | 6.3 × 104 Da | Glucan | Immunity: Enhanced the proliferation of T- and B-lymphocytes in vitro | Shanxi | [102] | |
| — | GTM1 | 6.28 × 105 Da | Galactose:Mannose = 1.85:1 | Antitumor | Wuhan | [80] | |
| — | GTM2 | 8.18 × 105 Da | Galactose:Glc = 1:1.36 | Antitumor | Wuhan | [80] |
4. Analysis of Ganoderma lucidum Polysaccharide
4.1. Quantitative Analysis of GLP
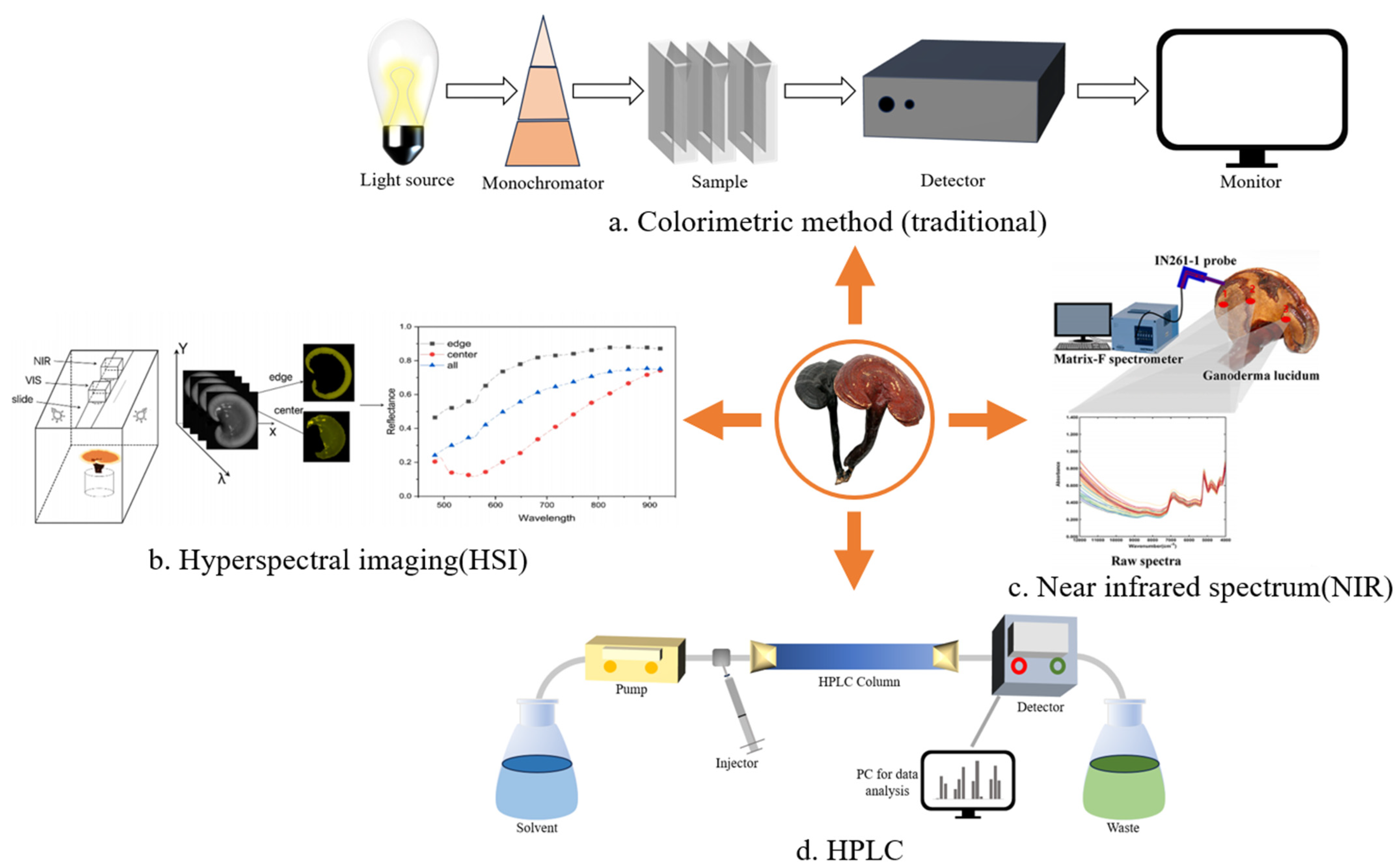
4.2. Qualitative Analysis of GLP
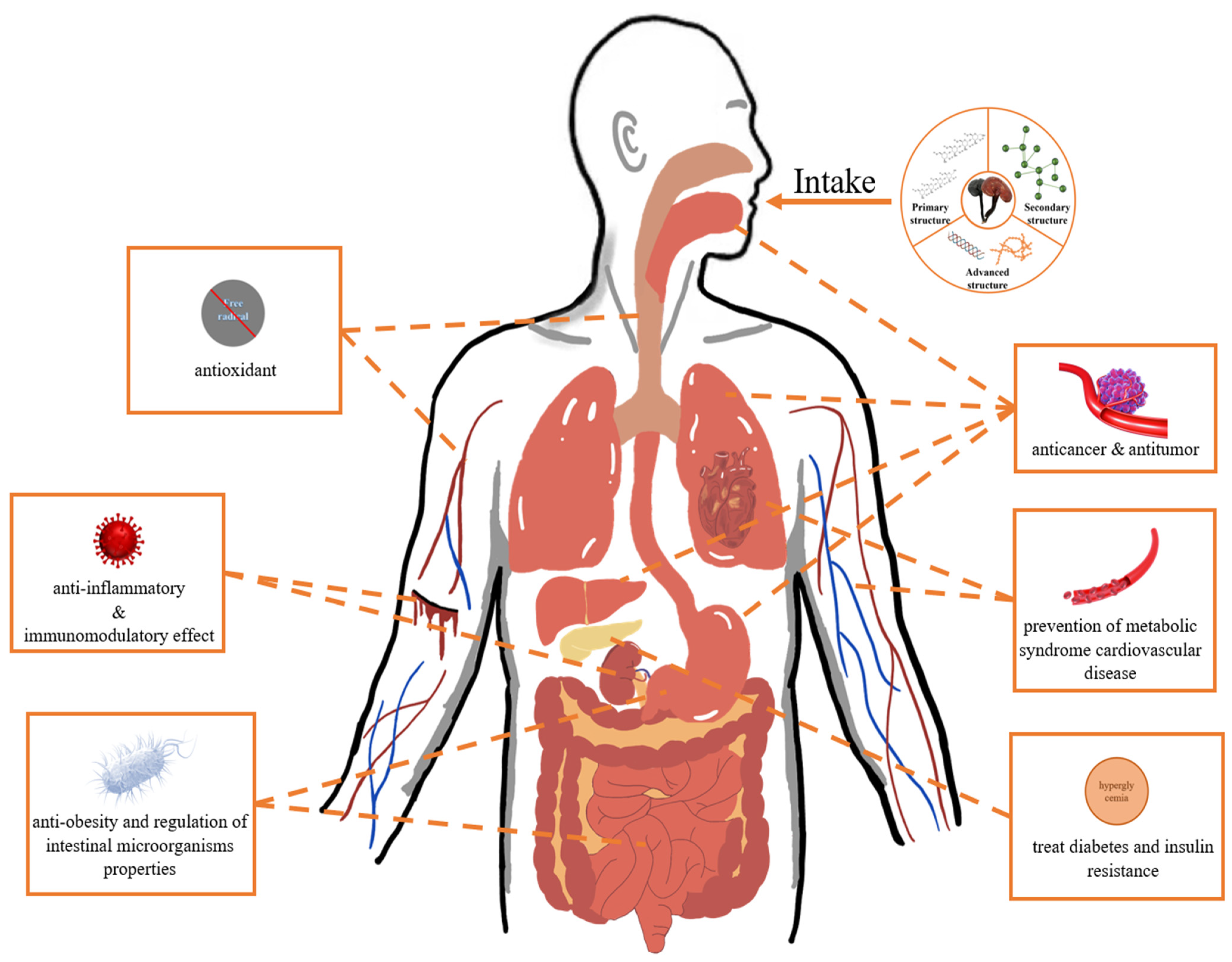
5. Conclusions and Future Perspectives
- Some GLPs are insoluble in water, so it is difficult to satisfy all the chemical properties as well as exhibit satisfactory biological activity [17]. In order to solve this problem, their physical properties and chemical structure can be changed by chemical modification [138,139]. Nanoparticles prepared from natural polysaccharides have a special structure that enables the active ingredient to be encapsulated in a polymer matrix and precisely transported to a specific site for releasing [17]. GLPs can also be combined with other materials to form composite materials to improve their biological activity [140,141]. The study of chemical modification and nano delivery systems can make some GLPs show more obvious biological activity and increase their applications.
- The structure and activity of polysaccharides are closely related [142,143], but most of the current studies are on the relationship between primary structures and biological activity, and there are few studies on advanced structures and conformations. In future studies, we need to characterize these advanced structures and conformations with more novel methods and advanced instruments and elucidate their relationship with activity. This will provide us with more information about the biology of GLPs and scientific basis for their potential applications.
- As the main active substance of GL, polysaccharide has attracted more and more attention due to its remarkable biological activity. However, the scarcity of natural resources, the restriction of growth conditions, and the difficulty of controlling the stability of yield hinder its development and application. It would be a good solution to obtain GL strains with high polysaccharide content by artificial breeding with gene technology [144,145,146]. Of course, it is also possible to cultivate disease-resistant and pest-resistant GL through these technologies to improve the overall quality of GL.
- GL has different species, and their growth environments are not the same, which causes great differences in the content, efficacy, and structure of their bioactive ingredients. In order to facilitate researchers to understand information more quickly and clearly, it is essential to establish a shared database. This database can collect experimental data on GLPs from the whole world, and visitors can browse these data to explore the principles and mechanisms behind it and further promote the vigorous development of GL research.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, S.; Zhang, S.; Peng, B.; Tan, D.; Wu, M.; Wei, J.; Wang, Y.; Luo, H. Ganoderma lucidum: A comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci. Hum. Wellness 2024, 13, 568–596. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, Q.; Wang, H.; Zhao, M.; Liu, S.; Nie, S.; Xie, M. Immunomodulatory effect of Ganoderma atrum polysaccharides on Th17/Treg balance. J. Funct. Foods 2018, 45, 215–222. [Google Scholar] [CrossRef]
- Tan, W.-C.; Kuppusamy, U.R.; Phan, C.-W.; Tan, Y.-S.; Raman, J.; Anuar, A.M.; Sabaratnam, V. Ganoderma neo-japonicum Imazeki revisited: Domestication study and antioxidant properties of its basidiocarps and mycelia. Sci. Rep. 2015, 5, 12515. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Q.; He, Y.-M. The effect of Ganoderma lucidum extract on immunological function and identify its anti-tumor immunostimulatory activity based on the biological network. Sci. Rep. 2018, 8, 12680. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shi, L.; Ding, K. Structure elucidation and anti-tumor activity in vivo of a polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Int. J. Biol. Macromol. 2019, 141, 693–699. [Google Scholar] [CrossRef]
- de Camargo, M.R.; Inacio, K.K.; Frazon, T.F.; Amôr, N.G.; Corrêa, L.E.; Costa, F.C.; Quagliato, E.N.; Rodini, C.O.; Lara, V.S. Ganoderma lucidum polysaccharides associated with 5-Fluorouracil impair OSCC tumorigenesis in vitro. Pharmacol. Res.-Mod. Chin. Med. 2023, 9, 100310. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, R.; Yao, J.; Xi, C.; Du, S. Ganoderma lucidum Polysaccharide Enhanced the Antitumor Effects of 5-Fluorouracil against Gastric Cancer through Its Upregulation of NKG2D/MICA. Int. J. Polym. Sci. 2019, 2019, 4564213. [Google Scholar] [CrossRef]
- Han, W.; Chen, H.; Zhou, L.; Zou, H.; Luo, X.; Sun, B.; Zhuang, X. Polysaccharides from Ganoderma sinense-rice bran fermentation products and their anti-tumor activities on non-small-cell lung cancer. BMC Complement. Med. Ther. 2021, 21, 169. [Google Scholar] [CrossRef]
- Li, G.-L.; Tang, J.-F.; Tan, W.-L.; Zhang, T.; Zeng, D.; Zhao, S.; Ran, J.-H.; Li, J.; Wang, Y.-P.; Chen, D.-L. The anti-hepatocellular carcinoma effects of polysaccharides from Ganoderma lucidum by regulating macrophage polarization via the MAPK/NF-κB signaling pathway. Food Funct. 2023, 14, 3155–3168. [Google Scholar] [CrossRef]
- de Camargo, M.R.; Frazon, T.F.; Inacio, K.K.; Smiderle, F.R.; Amôr, N.G.; Dionísio, T.J.; Santos, C.F.; Rodini, C.O.; Lara, V.S. Ganoderma lucidum polysaccharides inhibit in vitro tumorigenesis, cancer stem cell properties and epithelial-mesenchymal transition in oral squamous cell carcinoma. J. Ethnopharmacol. 2022, 286, 114891. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Gabryelska, K.; Grabarczyk, M. Mushrooms of the Genus Ganoderma Used to Treat Diabetes and Insulin Resistance. Molecules 2019, 24, 4075. [Google Scholar] [CrossRef] [PubMed]
- Klupp, N.L.; Kiat, H.; Bensoussan, A.; Steiner, G.Z.; Chang, D.H. A double-blind, randomised, placebo-controlled trial of Ganoderma lucidum for the treatment of cardiovascular risk factors of metabolic syndrome. Sci. Rep. 2016, 6, 29540. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, L.; Zhai, Q.; Chu, C.; Wang, S.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Combined Ganoderma lucidum polysaccharide and ciprofloxacin therapy alleviates Salmonella enterica infection, protects the intestinal barrier, and regulates gut microbiota. Food Funct. 2023, 14, 6896–6913. [Google Scholar] [CrossRef]
- Kou, F.; Ge, Y.; Wang, W.; Mei, Y.; Cao, L.; Wei, X.; Xiao, H.; Wu, X. A review of Ganoderma lucidum polysaccharides: Health benefit, structure-activity relationship, modification, and nanoparticle encapsulation. Int. J. Biol. Macromol. 2023, 243, 125199. [Google Scholar] [CrossRef]
- Li, F.; Liu, T.; Liu, X.; Han, C.; Li, L.; Zhang, Q.; Sui, X. Ganoderma lucidum polysaccharide hydrogel accelerates diabetic wound healing by regulating macrophage polarization. Int. J. Biol. Macromol. 2024, 260, 129682. [Google Scholar] [CrossRef]
- Lo, H.-C.; Lin, T.-E.; Lin, C.-Y.; Wang, W.-H.; Chen, Y.-C.; Tsai, P.-H.; Su, J.-C.; Lu, M.-K.; Hsu, W.-H.; Lin, T.-Y. Targeting TGFβ receptor-mediated snail and twist: WSG, a polysaccharide from Ganoderma lucidum, and it-based dissolvable microneedle patch suppress melanoma cells. Carbohydr. Polym. 2024, 341, 122298. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Li, G.; Jiang, Y.; Zhang, G.; Ling, J. A novel promising neuroprotective agent: Ganoderma lucidum polysaccharide. Int. J. Biol. Macromol. 2023, 229, 168–180. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Cui, F.-J.; Sun, L.; Zan, X.-Y.; Sun, W.-J. Recent advances in Ganoderma lucidum polysaccharides: Structures/bioactivities, biosynthesis and regulation. Food Biosci. 2023, 56, 103281. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, J.; Wang, C.; Liu, L.; Li, B.; Zhou, J. Optimization of enzymatic extraction of polysaccharides from Ganoderma lucidum using response surface methodology. Sci. Technol. Food Ind. 2016, 37, 238–244. [Google Scholar] [CrossRef]
- Yin, Y.; Yao, Q. Extraction Process of Ganoderma lucidum Polysaccharides. Adv. Microbiol. 2018, 7, 26–37. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, J.; Zheng, F.; Yao, J.; Wang, T.; Jiang, S.; Pang, M. Study on extraction of polysaccharides from Ganoderma lucidum by hot compressed water and its antioxidant activities. J. Food Sci. Technol. 2018, 36, 58–62. [Google Scholar] [CrossRef]
- Kang, Q.; Chen, S.; Li, S.; Wang, B.; Liu, X.; Hao, L.; Lu, J. Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int. J. Biol. Macromol. 2019, 124, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhang, W.; Liu, S. Optimization of ultrasonic-assisted extraction of polysaccharides and triterpenoids from the medicinal mushroom Ganoderma lucidum and evaluation of their in vitro antioxidant capacities. PLoS ONE 2020, 15, e0244749. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Smiderle, F.R.; Morales, D.; Iacomini, M.; Soler-Rivas, C. Strengths and weaknesses of the aniline-blue method used to test mushroom (1→3)-β-d-glucans obtained by microwave-assisted extractions. Carbohydr. Polym. 2019, 217, 135–143. [Google Scholar] [CrossRef]
- Zhang, J.J.; Chen, F.F.; Yan, Z.P.; Li, C.; Sun, E.; Luo, Y.; Tan, X.B. Alkali extraction of Ganoderma lucidum β-glucan and its anti-tumor immune regulation. Acta Pharm. Sin. 2020, 55, 512–521. [Google Scholar] [CrossRef]
- Zheng, Y.; Shuangshi, L.; Guowei, Y.; Yuepeng, W.; Zhongzhi, L.; Limin, H. Effect of twin-screw extrusion on polysaccharide extraction from Ganoderma lucidum. Sci. Technol. Food Ind. 2017, 8, 280–283. [Google Scholar] [CrossRef]
- Sun, Y.; He, H.; Wang, Q.; Yang, X.; Jiang, S.; Wang, D. A Review of Development and Utilization for Edible Fungal Polysaccharides: Extraction, Chemical Characteristics, and Bioactivities. Polymers 2022, 14, 4454. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.-C.; Chang, J.-S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Do, D.T.; Lam, D.H.; Nguyen, T.; Phuong Mai, T.T.; Phan, L.T.M.; Vuong, H.T.; Nguyen, D.V.; Linh, N.T.T.; Hoang, M.N.; Mai, T.P.; et al. Utilization of Response Surface Methodology in Optimization of Polysaccharides Extraction from Vietnamese Red Ganoderma lucidum by Ultrasound-Assisted Enzymatic Method and Examination of Bioactivities of the Extract. Sci. World J. 2021, 2021, 7594092. [Google Scholar] [CrossRef]
- Xu, N.; Sun, Y.H.; Guo, X.L.; Liu, C.; Mao, Q.; Hou, J.M. Optimization of ultrasonic-microwave synergistic extraction of polysaccharides from Morchella conica. J. Food Process. Preserv. 2017, 42, 13423. [Google Scholar] [CrossRef]
- Song, C.F.; Wang, S.G.; Yang, J.; Cui, Z.W.; Gu, Y.H. Optimization of Vacuum-Microwave Radiation Pretreatment on Extraction of Ganoderma Polysaccharides. Math. Probl. Eng. 2015, 2015, 792832. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Q.; Chai, N. 灵芝多糖的发酵提取方法、灵芝多糖组合物及其应用. CN114672527A, 28 June 2022. [Google Scholar]
- Li, R.; Shi, G.; Chen, L.; Liu, Y. Polysaccharides extraction from Ganoderma lucidum using a ternary deep eutectic solvents of choline chloride/guaiacol/lactic acid. Int. J. Biol. Macromol. 2024, 263, 130263. [Google Scholar] [CrossRef]
- Cai, C.; Wang, Y.; Yu, W.; Wang, C.; Li, F.; Tan, Z. Temperature-responsive deep eutectic solvents as green and recyclable media for the efficient extraction of polysaccharides from Ganoderma lucidum. J. Clean. Prod. 2020, 274, 123047. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Hou, T.; An, S.; Guo, B.; Liu, C.; Hu, L.; Huang, Y.; Zhang, S.; Song, M.; et al. Extraction kinetics, physicochemical properties and immunomodulatory activity of the novel continuous phase transition extraction of polysaccharides from Ganoderma lucidum. Food Funct. 2021, 12, 9708–9718. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, W. Optimization of Extraction Process of Polysaccharide from Lucidum Spores Using Response Surface Analysis. Food Res. Dev. 2017, 38, 51–55. [Google Scholar] [CrossRef]
- Huang, S.-Q.; Li, J.-W.; Wang, Z.; Pan, H.-X.; Chen, J.-X.; Ning, Z.-X. Optimization of Alkaline Extraction of Polysaccharides from Ganoderma lucidum and Their Effect on Immune Function in Mice. Molecules 2010, 15, 3694–3708. [Google Scholar] [CrossRef]
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [CrossRef]
- Du, B.; Jeepipalli, S.P.K.; Xu, B. Critical review on alterations in physiochemical properties and molecular structure of natural polysaccharides upon ultrasonication. Ultrason. Sonochem. 2022, 90, 106170. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, J.; Shen, M.; Chen, Y.; Yu, Q.; Xie, J. Structure, function and advance application of microwave-treated polysaccharide: A review. Trends Food Sci. Technol. 2022, 123, 198–209. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Miao, S.; Lu, X. Advances in Enzymatic Extraction of Polysaccharides. Sci. Technol. Food Ind. 2021, 42, 351–358. [Google Scholar] [CrossRef]
- Lazou, A.E. Food extrusion: An advanced process for innovation and novel product development. Crit. Rev. Food Sci. Nutr. 2022, 64, 4532–4560. [Google Scholar] [CrossRef]
- Zhou, J.; Qin, L.; Zhang, C.; Luo, S.; Cui, S. Study on Extraction of Lentinan by Orthogonal Optimization of Twin-Screw Extrusion Pretreatment and Microwave-Assisted Extraction. Process Technol. 2020, 5, 89–94+99. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, J.; Lan, W.; Yu, L.; Bi, Y.; Song, S.; Xiong, B.; Wang, H. Polysaccharide decolorization: Methods, principles of action, structural and functional characterization, and limitations of current research. Trends Food Sci. Technol. 2023, 138, 284–296. [Google Scholar] [CrossRef]
- Ke, L.; Duan, X.; Cui, J.; Song, X.; Ma, W.; Zhang, W.; Liu, Y.; Fan, Y. Research progress on the extraction technology and activity study of Epimedium polysaccharides. Carbohydr. Polym. 2023, 306, 120602. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Yang, X.; Zhou, H. The Deproteinization, Antioxidant Acticities and Inhibitory Effect on α-Amylase of Polysaccharides from Corn Silk. Am. J. Biochem. Biotechnol. 2019, 15, 83–90. [Google Scholar] [CrossRef]
- Zeng, X.; Li, P.; Chen, X.; Kang, Y.; Xie, Y.; Li, X.; Xie, T.; Zhang, Y. Effects of deproteinization methods on primary structure and antioxidant activity of Ganoderma lucidum polysaccharides. Int. J. Biol. Macromol. 2019, 126, 867–876. [Google Scholar] [CrossRef]
- Mengting, L.; Metsawur, M.; Tong, L.; Ping, X.; Shulan, S.; Jinao, D. Study on the Enzymatic Deproteinization Technology, Composition Analysis and Immunomodulatory Activity of Isatidis Radix Polysaccharides. J. Nanjing Univ. Tradit. Chin. Med. 2024, 40, 379–390. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, R.; Sun, J.; Duan, Y.; Zhou, H.; Zhou, W.; Li, G. Static decolorization of polysaccharides from the leaves of Rhododendron dauricum: Process optimization, characterization and antioxidant activities. Process Biochem. 2022, 121, 113–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Campbell, R.; Drake, M.; Zhong, Q. Decolorization of Cheddar cheese whey by activated carbon. J. Dairy Sci. 2015, 98, 2982–2991. [Google Scholar] [CrossRef]
- Shao, L.; Sun, Y.; Liang, J.; Li, M.; Li, X. Decolorization affects the structural characteristics and antioxidant activity of polysaccharides from Thesium chinense Turcz: Comparison of activated carbon and hydrogen peroxide decolorization. Int. J. Biol. Macromol. 2020, 155, 1084–1091. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, H.; Li, Y.; Wu, M.; Yu, M.; Sun, X. Optimized purification process of polysaccharides from Carex meyeriana Kunth by macroporous resin, its characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2019, 132, 76–86. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, B.X. Ganoderma and Health; Springer: Singapore, 2019. [Google Scholar]
- Kang, J.; Hua, X.; Yang, R.; Chen, Y.; Yang, H. Characterization of natural low-methoxyl pectin from sunflower head extracted by sodium citrate and purified by ultrafiltration. Food Chem. 2015, 180, 98–105. [Google Scholar] [CrossRef]
- Du, J.; Li, J.; Zhu, J.; Huang, C.; Bi, S.; Song, L.; Hu, X.; Yu, R. Structural characterization and immunomodulatory activity of a novel polysaccharide from Ficus carica. Food Funct. 2018, 9, 3930–3943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Z.; Mei, H.; Xu, J.; Zhou, T.; Cheng, F.; Wang, K. Angelica sinensis polysaccharide nanoparticles as a targeted drug delivery system for enhanced therapy of liver cancer. Carbohydr. Polym. 2019, 219, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cao, D.; Ji, H.; Xu, H.; Feng, Y.; Liu, A. Physicochemical characterization, rheological properties, and hypolipidemic and antioxidant activities of compound polysaccharides in Chinese herbal medicines by fractional precipitation. Int. J. Biol. Macromol. 2023, 242, 124838. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Sudharsan, S.; Sivasankar, P.; Sivakumar, L.; Vairamani, S.; Shanmugam, A. Isolation and chemical characteristics of rhamnose enriched polysaccharide from Grateloupia lithophila. Carbohydr. Polym. 2018, 195, 486–494. [Google Scholar] [CrossRef]
- Tang, W.; Liu, D.; Yin, J.-Y.; Nie, S.-P. Consecutive and progressive purification of food-derived natural polysaccharide: Based on material, extraction process and crude polysaccharide. Trends Food Sci. Technol. 2020, 99, 76–87. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Cui, S.-H.; Zha, X.-Q.; Bansal, V.; Xue, L.; Li, X.-L.; Hao, R.; Pan, L.-H.; Luo, J.-P. Jellyfish skin polysaccharides: Extraction and inhibitory activity on macrophage-derived foam cell formation. Carbohydr. Polym. 2014, 106, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Huang, S.; Chen, J.; Wang, B.; He, L.; Zhang, L.; Li, S.; Wang, J.; Wu, J.; Lai, X.; et al. A novel green method for deproteinization of polysaccharide from Cipangopaludina chinensis by freeze-thaw treatment. J. Clean. Prod. 2017, 142, 3409–3418. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Zheng, Y.; He, Z.; Guan, P.; He, X.; Hui, L.; Dai, Y. Study of Schiff base formation between dialdehyde cellulose and proteins, and its application for the deproteinization of crude polysaccharide extracts. Ind. Crops Prod. 2018, 112, 532–540. [Google Scholar] [CrossRef]
- Guo, Y.; Ye, H.; Wang, H.; Wang, Q.; Fan, S.; Dou, H. Asymmetrical flow field-flow fractionation combined with ultrafiltration: A novel and high-efficiency approach for separation, purification, and characterization of Ganoderma lucidum polysaccharides. Talanta 2023, 253, 124053. [Google Scholar] [CrossRef]
- Diener, M.; Adamcik, J.; Sánchez-Ferrer, A.; Jaedig, F.; Schefer, L.; Mezzenga, R. Primary, Secondary, Tertiary and Quaternary Structure Levels in Linear Polysaccharides: From Random Coil, to Single Helix to Supramolecular Assembly. Biomacromolecules 2019, 20, 1731–1739. [Google Scholar] [CrossRef]
- Jin, Y.; Li, P.; Wang, F. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- Bao, X.; Dong, Q.; Fang, J. Structure and Conformation Behavior of a Glucan from Spores of Ganoderma lucidum (Fr.) Karst. Acta Biochim. Biophys. Sin. 2000, 32, 557–561. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L. Structure and chain conformation of five water-soluble derivatives of a β-d-glucan isolated from Ganoderma lucidum. Carbohydr. Res. 2009, 344, 105–112. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Tang, Q.; Yang, Y.; Guo, Q.; Wang, Q.; Wu, D.; Cui, S.W. Physicochemical characterization of a high molecular weight bioactive β-d-glucan from the fruiting bodies of Ganoderma lucidum. Carbohydr. Polym. 2014, 101, 968–974. [Google Scholar] [CrossRef]
- Li, J.; Gu, F.; Cai, C.; Hu, M.; Fan, L.; Hao, J.; Yu, G. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2020, 143, 806–813. [Google Scholar] [CrossRef]
- Luo, H.-j.; Zhang, Y.-k.; Wang, S.-z.; Lin, S.-q.; Wang, L.-f.; Lin, Z.-x.; Lu, G.-d.; Lin, D.-m. Structural characterization and anti-oxidative activity for a glycopeptide from Ganoderma lucidum fruiting body. Int. J. Biol. Macromol. 2024, 261, 129793. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Kan, Q.; Song, M.; Hou, T.; An, S.; Lin, H.; Chen, H.; Hu, L.; Xiao, J.; et al. Extraction, Structural Characterization, and Immunomodulatory Activity of a High Molecular Weight Polysaccharide from Ganoderma lucidum. Front. Nutr. 2022, 9, 846080. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ren, Q.; Wang, S.; Gao, J.; Shen, C.; Zhang, S.; Wang, Y.; Guan, F. Chemical Modification of Polysaccharides: A Review of Synthetic Approaches, Biological Activity and the Structure–Activity Relationship. Molecules 2023, 28, 6073. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Wu, X. Ganoderma lucidum polysaccharide (GLP) enhances antitumor immune response by regulating differentiation and inhibition of MDSCs via a CARD9-NF-κB-IDO pathway. Biosci. Rep. 2020, 40, BSR20201170. [Google Scholar] [CrossRef] [PubMed]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.A.; Wani, A.H.; War, J.M.; Bhat, M.Y. Major Bioactive Properties of Ganoderma Polysaccharides: A Review. Asian J. Pharm. Clin. Res. 2021, 14, 11–24. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, L.; Zeng, F.; Kennedy, J.F. Structure and antitumor activities of the water-soluble polysaccharides from Ganoderma tsugae mycelium. Carbohydr. Polym. 2005, 59, 385–392. [Google Scholar] [CrossRef]
- Kao, P.-F.; Wang, S.-H.; Hung, W.-T.; Liao, Y.-H.; Lin, C.-M.; Yang, W.-B. Structural Characterization and Antioxidative Activity of Low-Molecular-Weights Beta-1,3-Glucan from the Residue of Extracted Ganoderma lucidum Fruiting Bodies. J. Biomed. Biotechnol. 2012, 2012, 673764. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Liang, Y.-C.; Lee, S.-S.; Chiang, B.-L. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-κB and p38 mitogen-activated protein kinase pathways. J. Leukoc. Biol. 2005, 78, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Firenzuoli, F.; Gori, L.; Lombardo, G. The Medicinal Mushroom Agaricus blazei Murrill: Review of Literature and Pharmaco-Toxicological Problems. Evid.-Based Complement. Altern. Med. 2007, 5, 3–15. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, W.; Yi, Y.; Yang, G.; Wu, Z.; Yi, J.; Kong, F. Synthesis of β-(1→6)-branched β-(1→3) glucohexaose and its analogues containing an α-(1→3) linked bond with antitumor activity. Bioorgan. Med. Chem. 2003, 11, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Wiater, A.; Paduch, R.; Choma, A.; Pleszczyńska, M.; Siwulski, M.; Dominik, J.; Janusz, G.; Tomczyk, M.; Szczodrak, J. Biological study on carboxymethylated (1→3)-α-d-glucans from fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2012, 51, 1014–1023. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.-Q.; Nie, S.-P.; Wang, Y.-X.; Cui, S.W.; Xie, M.-Y. Sulfated modification, characterization and property of a water-insoluble polysaccharide from Ganoderma atrum. Int. J. Biol. Macromol. 2015, 79, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Duan, J.; Fang, X.; Fang, J. Chemical modifications of the (1→3)-α-D-glucan from spores of Ganoderma lucidum and investigation of their physicochemical properties and immunological activity. Carbohydr. Res. 2001, 336, 127–140. [Google Scholar] [CrossRef]
- Zhang, S.; Nie, S.; Huang, D.; Huang, J.; Feng, Y.; Xie, M. A Polysaccharide from Ganoderma atrum Inhibits Tumor Growth by Induction of Apoptosis and Activation of Immune Response in CT26-Bearing Mice. J. Agric. Food Chem. 2014, 62, 9296–9304. [Google Scholar] [CrossRef]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Tan, Y.; Wang, S.; Chen, H.; Zhou, A. Progress in understanding the structure-activity relationship and hypoglycemic mechanism of polysaccharides. Food Sci. 2021, 42, 355–363. [Google Scholar] [CrossRef]
- Meng, Y.; Lyu, F.; Xu, X.; Zhang, L. Recent Advances in Chain Conformation and Bioactivities of Triple-Helix Polysaccharides. Biomacromolecules 2020, 21, 1653–1677. [Google Scholar] [CrossRef]
- Liang, Z.; Yin, Z.; Liu, X.; Ma, C.; Wang, J.; Zhang, Y.; Kang, W. A glucomannogalactan from Pleurotus geesteranus: Structural characterization, chain conformation and immunological effect. Carbohydr. Polym. 2022, 287, 119346. [Google Scholar] [CrossRef]
- Guo, X.; Kang, J.; Xu, Z.; Guo, Q.; Zhang, L.; Ning, H.; Cui, S.W. Triple-helix polysaccharides: Formation mechanisms and analytical methods. Carbohydr. Polym. 2021, 262, 117962. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, J.; Zhou, K.; Yang, Y.; Zhou, S.; Jia, W.; Hao, R.; Pan, Y. Purification, NMR Study and Immunostimulating Property of a Fucogalactan from the Fruiting Bodies of Ganoderma lucidum. Planta Medica 2008, 74, 1730–1734. [Google Scholar] [CrossRef]
- Bao, X.; Fang, J. Islation and Structural Determination of a Glucan from the Spores of Ganoderma lucidum. Acta Bot. Sin. 2001, 43, 312–315. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, S.-P.; Yin, J.-Y.; Wang, Y.-X.; Xie, M.-Y. Structural characterization of a heterogalactan purified from fruiting bodies of Ganoderma atrum. Food Hydrocoll. 2014, 36, 339–347. [Google Scholar] [CrossRef]
- Bao, X.-F.; Zhen, Y.; Ruan, L.; Fang, J.-N. Purification, Characterization, and Modification of T Lymphocyte Stimulating Polysaccharide from Spores of Ganoderma lucidum. Chem. Pharm. Bull. 2002, 50, 623–629. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, S.; Cui, S.W.; Xu, M.; Ding, H.; Xie, M. Characterization of a bioactive polysaccharide from Ganoderma atrum: Re-elucidation of the fine structure. Carbohydr. Polym. 2017, 158, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Liu, C.; Fang, J.; Li, X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydr. Res. 2001, 332, 67–74. [Google Scholar] [CrossRef]
- Bao, X.; Fang, J.; Li, X. Structural Characterization and Immunomodulating Activity of a Complex Glucan from Spores of Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2014, 65, 2384–2391. [Google Scholar] [CrossRef]
- Bao, X.-F.; Wang, X.-S.; Dong, Q.; Fang, J.-N.; Li, X.-Y. Structural features of immunologically active polysaccharides from Ganoderma lucidum. Phytochemistry 2002, 59, 175–181. [Google Scholar] [CrossRef]
- Tomoda, M.; Gonda, R.; Kasahara, Y.; Hikino, H. Glycan Structures Of Ganoderans B And C, Hypoglycemic Glycans Of Ganoderma lucidum Fruit Bodies. Phytochemistry 1986, 25, 2817–2820. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yu, H.; Zhou, S.; Zhang, Z.; Wu, D.; Yan, M.; Tang, Q.; Zhang, J. Structural characterization and immuno-enhancing activity of a highly branched water-soluble β-glucan from the spores of Ganoderma lucidum. Carbohydr. Polym. 2017, 167, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wang, Y.; Shi, L.; Yao, J.; Li, J.; Ma, F.; Ding, K. A novel water-soluble β-d-glucan isolated from the spores of Ganoderma lucidum. Carbohydr. Res. 2012, 353, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, J.; Yang, Y.; Zhou, S.; Liu, Y.; Tang, Q.; Du, X.; Chen, H.; Pan, Y. Structural characterisation of a heteropolysaccharide by NMR spectra. Food Chem. 2009, 112, 962–966. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Zhang, L. Structure and immunological activity of a novel polysaccharide from the spores of Ganoderma lucidum. Afr. J. Biotechnol. 2011, 10, 10923–10929. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, J.; Ye, X.; Tang, Q.; Liu, Y.; Gong, C.; Du, X.; Pan, Y. Structural elucidation of the polysaccharide moiety of a glycopeptide (GLPCW-II) from Ganoderma lucidum fruiting bodies. Carbohydr. Res. 2008, 343, 746–752. [Google Scholar] [CrossRef]
- Guo, L.; Xie, J.; Ruan, Y.; Zhou, L.; Zhu, H.; Yun, X.; Jiang, Y.; Lü, L.; Chen, K.; Min, Z.; et al. Characterization and immunostimulatory activity of a polysaccharide from the spores of Ganoderma lucidum. Int. Immunopharmacol. 2009, 9, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, Q.; Zimmerman-Kordmann, M.; Reutter, W.; Fan, H. Activation of B lymphocytes by GLIS, a bioactive proteoglycan from Ganoderma lucidum. Life Sci. 2002, 71, 623–638. [Google Scholar] [CrossRef]
- Shang, D.; Zhang, J.; Wen, L.; Li, Y.; Cui, Q. Preparation, Characterization, and Antiproliferative Activities of the Se-Containing Polysaccharide SeGLP-2B-1 from Se-Enriched Ganoderma lucidum. J. Agric. Food Chem. 2009, 57, 7737–7742. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Wu, J.; Weng, L.; Zhang, H.; Zhang, J.; Wu, A. An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydr. Polym. 2020, 227, 115332. [Google Scholar] [CrossRef]
- Zhao, H.; Lai, C.-J.-S.; Yu, Y.; Wang, Y.-n.; Zhao, Y.-J.; Ma, F.; Hu, M.; Guo, J.; Wang, X.; Guo, L. Acidic hydrolysate fingerprints based on HILIC-ELSD/MS combined with multivariate analysis for investigating the quality of Ganoderma lucidum polysaccharides. Int. J. Biol. Macromol. 2020, 163, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.; O’Connor, N.; Jose, D.; Barrett, A.; Regan, F. Selection and optimization of protein and carbohydrate assays for the characterization of marine biofouling. Anal. Methods 2020, 12, 2228–2236. [Google Scholar] [CrossRef]
- Li, L.-F.; Zhang, Q.-W.; Han, Q.-B. Recent advances in qualitative and quantitative analysis of polysaccharides in natural medicines: A critical review. J. Pharm. Biomed. Anal. 2022, 220, 115016. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, Y.; Liu, H.; Li, R. Determination of Ganoderma lucidum Polysaccharide by Reversed-phase High Performance Liquid Chromatography. J. Agric. Sci. Technol. 2009, 11, 65–67. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, S.; Feng, N.; Liu, Y.; Wang, J.; Tang, Q.; Han, W.; Zhang, J. Directional Harvest of Ganoderma lucidum ‘Hunong No. 1’ for High Triterpenoids and Water Soluble Polysaccharides. Acta Edulis Fungi 2023, 30, 113–122. [Google Scholar] [CrossRef]
- Goodwin, N.L.; Choong, J.J.; Hwang, S.; Pitts, K.; Bloom, L.; Islam, A.; Zhang, Y.Y.; Szelenyi, E.R.; Tong, X.; Newman, E.L.; et al. Simple Behavioral Analysis (SimBA) as a platform for explainable machine learning in behavioral neuroscience. Nat. Neurosci. 2024, 27, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Widman, A.J.; Shah, M.; Frydendahl, A.; Halmos, D.; Khamnei, C.C.; Øgaard, N.; Rajagopalan, S.; Arora, A.; Deshpande, A.; Hooper, W.F.; et al. Ultrasensitive plasma-based monitoring of tumor burden using machine-learning-guided signal enrichment. Nat. Med. 2024, 30, 1655–1666. [Google Scholar] [CrossRef]
- Puszkarska, A.M.; Taddese, B.; Revell, J.; Davies, G.; Field, J.; Hornigold, D.C.; Buchanan, A.; Vaughan, T.J.; Colwell, L.J. Machine learning designs new GCGR/GLP-1R dual agonists with enhanced biological potency. Nat. Chem. 2024. [Google Scholar] [CrossRef]
- Li, B.; Raji, I.O.; Gordon, A.G.R.; Sun, L.; Raimondo, T.M.; Oladimeji, F.A.; Jiang, A.Y.; Varley, A.; Langer, R.S.; Anderson, D.G. Accelerating ionizable lipid discovery for mRNA delivery using machine learning and combinatorial chemistry. Nat. Mater. 2024, 23, 1002–1008. [Google Scholar] [CrossRef]
- Mathis, N.; Allam, A.; Tálas, A.; Kissling, L.; Benvenuto, E.; Schmidheini, L.; Schep, R.; Damodharan, T.; Balázs, Z.; Janjuha, S.; et al. Machine learning prediction of prime editing efficiency across diverse chromatin contexts. Nat. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Zeng, X.; Cao, R.; Xi, Y.; Li, X.; Yu, M.; Zhao, J.; Cheng, J.; Li, J. Food flavor analysis 4.0: A cross-domain application of machine learning. Trends Food Sci. Technol. 2023, 138, 116–125. [Google Scholar] [CrossRef]
- Ji, H.; Pu, D.; Yan, W.; Zhang, Q.; Zuo, M.; Zhang, Y. Recent advances and application of machine learning in food flavor prediction and regulation. Trends Food Sci. Technol. 2023, 138, 738–751. [Google Scholar] [CrossRef]
- Liu, Y.; Long, Y.; Liu, H.; Lan, Y.; Long, T.; Kuang, R.; Wang, Y.; Zhao, J. Polysaccharide prediction in Ganoderma lucidum fruiting body by hyperspectral imaging. Food Chem. X 2022, 13, 100199. [Google Scholar] [CrossRef]
- Ma, Y.; He, H.; Wu, J.; Wang, C.; Chao, K.; Huang, Q. Assessment of Polysaccharides from Mycelia of genus Ganoderma by Mid-Infrared and Near-Infrared Spectroscopy. Sci. Rep. 2018, 8, 10. [Google Scholar] [CrossRef]
- Ni, H.; Fu, W.; Wei, J.; Zhang, Y.; Chen, D.; Tong, J.; Chen, Y.; Liu, X.; Luo, Y.; Xu, T. Non-destructive detection of polysaccharides and moisture in Ganoderma lucidum using near-infrared spectroscopy and machine learning algorithm. LWT 2023, 184, 115001. [Google Scholar] [CrossRef]
- Pandeirada, C.O.; Achterweust, M.; Janssen, H.G.; Westphal, Y.; Schols, H.A. Periodate oxidation of plant polysaccharides provides polysaccharide-specific oligosaccharides. Carbohydr. Polym. 2022, 291, 119540. [Google Scholar] [CrossRef]
- Yao, H.Y.; Wang, J.Q.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, W.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Monosaccharide composition analysis of polysaccharides from natural sources: Hydrolysis condition and detection method development. Food Hydrocoll. 2021, 116, 106641. [Google Scholar] [CrossRef]
- Nagar, S.; Lakhera, A.K.; Kumar, V. Upgrading Methylation Method for Structural Studies of Polysaccharides: Case Analysis of a Bioactive Polysaccharide from Acacia tortilis. J. Biol. Act. Prod. Nat. 2020, 10, 70–85. [Google Scholar] [CrossRef]
- Zheng, Y.; Yan, J.; Cao, C.; Liu, Y.; Yu, D.; Liang, X. Application of chromatography in purification and structural analysis of natural polysaccharides: A review. J. Sep. Sci. 2023, 46, e2300368. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Wu, D.-T.; Deng, Y.; Leong, F.; Zhao, J.; Zhang, W.-J.; Li, S.-P. Qualitation and quantification of specific polysaccharides from Panax species using GC–MS, saccharide mapping and HPSEC-RID-MALLS. Carbohydr. Polym. 2016, 153, 47–54. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Ren, H.; Pang, M.; Sui, X.; Du, X. Extraction, Purification, Structural Characterization and Antioxidant Activity of Polysaccharides from the Fruiting Body of Guanxian Ganoderma lucidum. Sci. Technol. Food Ind. 2023, 44, 81–89. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Zhao, D. Structural analysis of biomacromolecules using circular dichroism spectroscopy. In Advanced Spectroscopic Methods to Study Biomolecular Structure and Dynamics; Academic Press: San Diego, CA, USA, 2023; pp. 77–103. [Google Scholar]
- Fu, Y.-L.; Shi, L. Methods of study on conformation of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2024, 263, 130275. [Google Scholar] [CrossRef] [PubMed]
- Kuttel, M.M.; Ståhle, J.; Widmalm, G. CarbBuilder: Software for building molecular models of complex oligo- and polysaccharide structures. J. Comput. Chem. 2016, 37, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Liu, Y.; Yang, Y.; Wang, R.; Li, T. Advances in sulfonated modification and bioactivity of polysaccharides. Int. J. Biol. Macromol. 2023, 253, 126400. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Zhao, X.; Tang, Q.; Dernedde, J.; Zhang, J.; Fan, H. Anti-inflammatory properties of GLPss58, a sulfated polysaccharide from Ganoderma lucidum. Int. J. Biol. Macromol. 2018, 107, 486–493. [Google Scholar] [CrossRef]
- Zheng, D.; Zhao, J.; Li, Y.; Zhu, L.; Jin, M.; Wang, L.; Liu, J.; Lei, J.; Li, Z. Self-Assembled pH-Sensitive Nanoparticles Based on Ganoderma lucidum Polysaccharide–Methotrexate Conjugates for the Co-delivery of Anti-tumor Drugs. ACS Biomater. Sci. Eng. 2021, 7, 3764–3773. [Google Scholar] [CrossRef]
- Zhang, S.; Pang, G.; Chen, C.; Qin, J.; Yu, H.; Liu, Y.; Zhang, X.; Song, Z.; Zhao, J.; Wang, F.; et al. Effective cancer immunotherapy by Ganoderma lucidum polysaccharide-gold nanocomposites through dendritic cell activation and memory T cell response. Carbohydr. Polym. 2019, 205, 192–202. [Google Scholar] [CrossRef]
- Chen, S.K.; Wang, X.; Guo, Y.Q.; Song, X.X.; Yin, J.Y.; Nie, S.P. Exploring the partial degradation of polysaccharides: Structure, mechanism, bioactivities, and perspectives. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4831–4870. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu, X.; Wang, L.; Wang, X.; Li, Z.; Gao, M.; Yang, Y.; Wang, Q.; et al. Effect of monosaccharide composition and proportion on the bioactivity of polysaccharides: A review. Int. J. Biol. Macromol. 2024, 254, 127955. [Google Scholar] [CrossRef]
- Liu, S.-R.; Ke, B.-R.; Zhang, W.-R.; Liu, X.-R.; Wu, X.-P. Breeding of new Ganoderma lucidum strains simultaneously rich in polysaccharides and triterpenes by mating basidiospore-derived monokaryons of two commercial cultivars. Sci. Hortic. 2017, 216, 58–65. [Google Scholar] [CrossRef]
- Tang, C.; Tan, Y.; Zhang, J.; Zhou, S.; Honda, Y.; Zhang, H. A Novel Strain Breeding of Ganoderma lucidum UV119 (Agaricomycetes) with High Spores Yield and Strong Resistant Ability to Other Microbes’ Invasions. Foods 2023, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, M.; Zhao, L.; Chen, L.; Ding, Z. Novel Insights into the Mechanism Underlying High Polysaccharide Yield in Submerged Culture of Ganoderma lucidum Revealed by Transcriptome and Proteome Analyses. Microorganisms 2023, 11, 772. [Google Scholar] [CrossRef] [PubMed]
| Method | Technical Principle | Advantage and Disadvantage | References |
|---|---|---|---|
| Water extraction | Polysaccharides are soluble in water but insoluble in organic solvents | Advantage: Simple and safe, low-cost, will not cause polysaccharide degradation | [40] |
| Disadvantage: Time-consuming, low extraction rate | |||
| Alkali extraction | Under alkaline conditions, the fibers of GL decompose and accelerate the release of polysaccharides | Advantage: Efficient | [41] |
| Disadvantage: Environmental pollution | |||
| Ultrasonic-assisted extraction | Cavitation and mechanical effects | Advantage: Simple, high extraction rate, no material loss | [26,42,43] |
| Disadvantage: Will destroy secondary and tertiary structures | |||
| Microwave-assisted extraction | Using heat to rupture the cell wall | Advantage: Simple, efficient, no pollution | [31,44] |
| Disadvantage: Excessive time can lead to degradation | |||
| Enzyme extraction | Macromolecular substances are separated from GLP by enzymes, usually using complex enzymes | Advantage: Mild, efficient, high biological activity | [45] |
| Disadvantage: Extraction efficiency is affected by enzyme activity, high cost | |||
| Squeeze blasting | Set mixing, stirring, crushing, heating, blasting, sterilization, and molding as one of the high-tech methods | Advantage: High efficiency, low cost | [46,47] |
| Disadvantage: The experimental parameters are difficult to control | |||
| Fermentation extraction | Microorganisms produce active enzymes during fermentation | Advantage: High utilization of raw materials, mild | [36] |
| DESs | Triple hydrogen bond interaction and a high binding energy | Advantage: Commendable cyclic stability, high recovery rate, efficient | [37] |
| CPTE | Continuous fresh solvents enhance the concentration gradient and increase the mass transfer rate | Advantage: Fast, efficient | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Tan, P.; Lin, H.; Zhang, D.; Chen, X.; Pang, J.; Mu, R. A Review of Ganoderma lucidum Polysaccharide: Preparations, Structures, Physicochemical Properties and Application. Foods 2024, 13, 2665. https://doi.org/10.3390/foods13172665
Zhong Y, Tan P, Lin H, Zhang D, Chen X, Pang J, Mu R. A Review of Ganoderma lucidum Polysaccharide: Preparations, Structures, Physicochemical Properties and Application. Foods. 2024; 13(17):2665. https://doi.org/10.3390/foods13172665
Chicago/Turabian StyleZhong, Yuanbo, Pingping Tan, Huanglong Lin, Di Zhang, Xianrui Chen, Jie Pang, and Ruojun Mu. 2024. "A Review of Ganoderma lucidum Polysaccharide: Preparations, Structures, Physicochemical Properties and Application" Foods 13, no. 17: 2665. https://doi.org/10.3390/foods13172665
APA StyleZhong, Y., Tan, P., Lin, H., Zhang, D., Chen, X., Pang, J., & Mu, R. (2024). A Review of Ganoderma lucidum Polysaccharide: Preparations, Structures, Physicochemical Properties and Application. Foods, 13(17), 2665. https://doi.org/10.3390/foods13172665






