Preventing Fungal Spoilage from Raw Materials to Final Product: Innovative Preservation Techniques for Fruit Fillings
Abstract
:1. Introduction
2. Main Fungal Spoilage Issues Associated with Pastry Fruit Fillings
2.1. Matrix Characteristics and Physicochemical Parameters of Fruit Fillings
2.2. Raw Materials (Pre- and Post-Harvest)
2.3. Processing of Raw Materials
2.4. Storage of Final Products
3. Prevention Strategies
3.1. Traditional Techniques
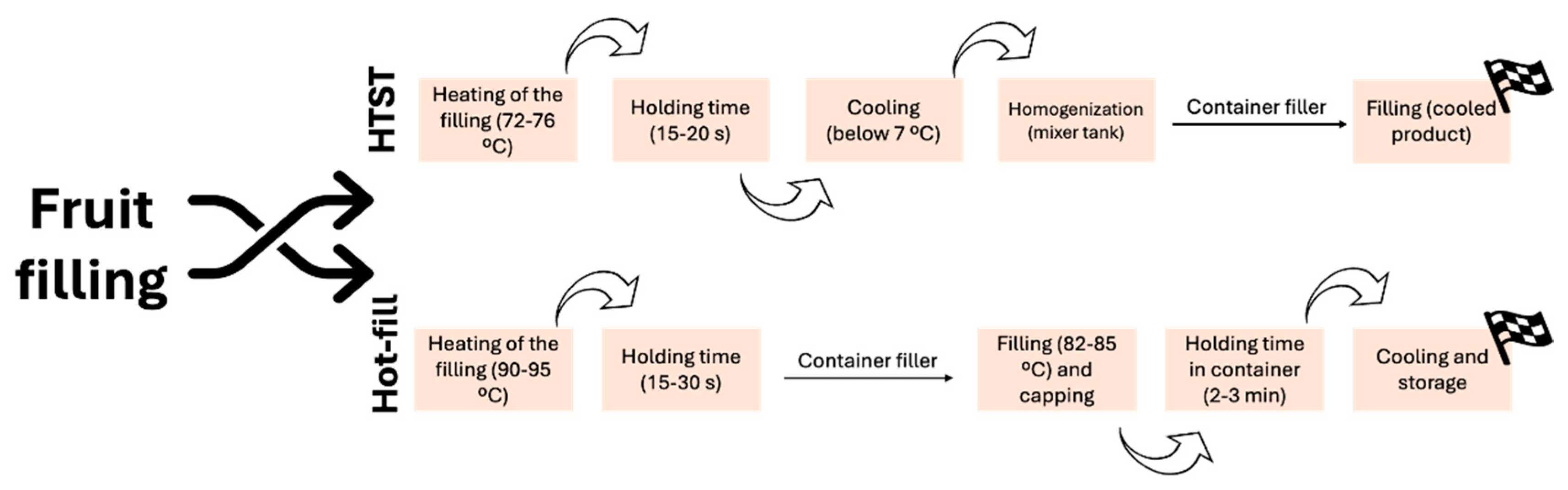
3.1.1. Physical Method—Pasteurization
3.1.2. Chemical Preservatives
3.2. Innovative Techniques
3.2.1. Physical Methods
3.2.2. Chemical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salas, M.L.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal Microbial Agents for Food Biopreservation—A Review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.B.; Churey, J.J.; Worobo, R.W. Association of fungal genera from spoiled processed foods with physicochemical food properties and processing conditions. Food Microbiol. 2019, 83, 211–218. [Google Scholar] [CrossRef]
- Garcia, M.V.; Copetti, M.V. Alternative methods for mould spoilage control in bread and bakery products. Int. Food Res. J. 2019, 26, 737–749. [Google Scholar]
- Snyder, A.B.; Worobo, R.W. Fungal Spoilage in Food Processing. J. Food Prot. 2018, 81, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Saleh, I.; Goktepe, I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef]
- Smith, J.P.; Daifas, D.P.; El-Khoury, W.; Koukoutsis, J.; El-Khoury, A. Shelf Life and Safety Concerns of Bakery Products—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 19–55. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Pickova, D.; Toman, J.; Grosse, Y.; Ostry, V. Hazard characterisation for significant mycotoxins in food. Mycotoxin Res. 2023, 39, 81–93. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A Producing Fungi, Biosynthetic Pathway and Regulatory Mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef]
- Bullerman, L.B. SPOILAGE|Fungi in Food—An Overview. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 5511–5522. [Google Scholar] [CrossRef]
- Degenkolb, T.; Dieckmann, R.; Nielsen, K.F.; Gräfenhan, T.; Theis, C.; Zafari, D.; Chaverri, P.; Ismaiel, A.; Brückner, H.; von Döhren, H.; et al. The Trichoderma brevicompactum clade: A separate lineage with new species, new peptaibiotics, and mycotoxins. Mycol. Prog. 2008, 7, 177–219. [Google Scholar] [CrossRef]
- Liu, Y.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef]
- Ribes, S.; Fuentes, A.; Talens, P.; Barat, J.M. Prevention of fungal spoilage in food products using natural compounds: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2002–2016. [Google Scholar] [CrossRef]
- Institute of Food Technologists. What Is Clean Label? Available online: https://www.ift.org/news-and-publications/blog/2018/november/what-is-clean-label (accessed on 10 October 2023).
- Osimani, A.; Garofalo, C.; Harasym, J.; Aquilanti, L. Use of essential oils against foodborne spoilage yeasts: Advantages and drawbacks. Curr. Opin. Food Sci. 2022, 45, 100821. [Google Scholar] [CrossRef]
- Pillai, P.; Ramaswamy, K. Effect of naturally occurring antimicrobials and chemical preservatives on the growth of Aspergillus Parasiticus. J. Food Sci. Technol. 2011, 49, 228–233. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Introduction. In Fungi Food Spoilage, 3rd ed.; Springer: New York, NY, USA, 2009; pp. 1–2. [Google Scholar] [CrossRef]
- Cropotova, J.; Tylewicz, U.; Dellarosa, N.; Laghi, L.; Romani, S.; Dalla Rosa, M. Effect of freezing on microstructure and degree of syneresis in differently formulated fruit fillings. Food Chem. 2016, 195, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Young, N.W.G.; Kappel, G.; Bladt, T. A polyuronan blend giving novel synergistic effects and bake-stable functionality to high soluble solids fruit fillings. Food Hydrocoll. 2003, 17, 407–418. [Google Scholar] [CrossRef]
- Alam, M.; Pant, K.; Singh Brar, D.; Nabi Dar, B.; Nanda, V. Exploring the versatility of diverse hydrocolloids to transform techno-functional, rheological, and nutritional attributes of food fillings. Food Hydrocoll. 2024, 146, 109275. [Google Scholar] [CrossRef]
- Wei, Y.P.; Wang, C.S.; Wu, J.S.B. Flow properties of fruit fillings. Food Res. Int. 2001, 34, 377–381. [Google Scholar] [CrossRef]
- Carcelli, A.; Albertini, A.; Vittadini, E.; Carini, E. A fibre syrup for the sugar reduction in fruit filling for bakery application. Int. J. Gastron. Food Sci. 2022, 28, 100545. [Google Scholar] [CrossRef]
- Agudelo, A.; Varela, P.; Fiszman, S. Fruit fillings development: A multiparametric approach. LWT Food Sci. Technol. 2015, 61, 465–572. [Google Scholar] [CrossRef]
- Agudelo, A.; Varela, P.; Sanz, T.; Fiszman, S.M. Native tapioca starch as a potential thickener for fruit fillings. Evaluation of mixed models containing low-methoxyl pectin. Food Hydrocoll. 2014, 35, 297–304. [Google Scholar] [CrossRef]
- Agudelo, A.; Varela, P.; Sanz, T.; Fiszman, S. Formulating fruit fillings. Freezing and baking stability of a tapioca starch–pectin mixture model. Food Hydrocoll. 2014, 40, 203–213. [Google Scholar] [CrossRef]
- Ashebre, K.M. Pre-Harvest and Post-Harvest Factors Affecting Citrus Fruit and Post-Harvest Treatments. J. Biol. Agric. Healthc. 2015, 5, 19–29. Available online: https://www.iiste.org/Journals/index.php/JBAH/article/view/27566 (accessed on 2 January 2024).
- Botina, A.B.L.; García, M.M.C.; Romero, B.Y. Pre- and post-harvest factors that affect the quality and commercialization of the Tahiti lime. Sci. Hortic. 2019, 257, 108737. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Parmar, A.; Chen, T.; El-Mogy, M.M. Editorial: Advances in pre- and postharvest applications to reduce qualitative and quantitative food loss and waste. Front. Plant Sci. 2023, 14, 1149358. [Google Scholar] [CrossRef]
- Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Plant extracts and other natural compounds as alternatives for post-harvest management of fruit fungal pathogens: A review. Food Biosci. 2021, 41, 100840. [Google Scholar] [CrossRef]
- Hocking, A. FUNGI|Foodborne Fungi. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 68–75. [Google Scholar] [CrossRef]
- Bernardi, A.O.; Garcia, M.V.; Copetti, M.V. Food industry spoilage fungi control through facility sanitization. Curr. Opin. Food Sci. 2019, 29, 28–34. [Google Scholar] [CrossRef]
- Gwinn, K.D.; Leung, M.C.K.; Stephens, A.B.; Punja, Z.K. Fungal and mycotoxin contaminants in cannabis and hemp flowers: Implications for consumer health and directions for further research. Front. Microbiol. 2023, 14, 1278189. [Google Scholar] [CrossRef]
- Olsen, M.; Lindqvist, R.; Bakeeva, A.; Leong, S.L.; Sulyok, M. Distribution of mycotoxins produced by Penicillium spp. inoculated in apple jam and crème fraiche during chilled storage. Int. J. Food Microbiol. 2019, 292, 13–20. [Google Scholar] [CrossRef]
- Snyder, A.; Worobo, R. Risk Mitigation for Immunocompromised Consumers of Mucormycete Spoiled and Fermented Foods: Germane Guidance and Remaining Needs. Microorganisms 2018, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Tournas, V.H.; Heeres, J.; Burgess, L. Moulds and yeasts in fruit salads and fruit juices. Food Microbiol. 2006, 23, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. In vitro evaluation of the antimicrobial activity of eugenol, limonene, and citrus extract against bacteria and yeasts, representative of the spoiling microflora of fruit juices. J. Food Prot. 2010, 73, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Serradilla, M.; Villalobos, M.; Martín, A.; Córdoba, M. Spoilage yeasts: What are the sources of contamination of foods and beverages? Int. J. Food Microbiol. 2018, 286, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Zara, G.; Budroni, M.; Mannazzu, I.; Fancello, F.; Zara, S. Yeast biofilm in food realms: Occurrence and control. World J. Microbiol. Biotechnol. 2020, 36, 134. [Google Scholar] [CrossRef] [PubMed]
- Stratford, M. Food and Beverage Spoilage Yeasts. In Yeasts in Food and Beverages; Springer: Berlin/Heidelberg, Germany, 2006; pp. 335–379. [Google Scholar] [CrossRef]
- Pfliegler, W.P.; Pócsi, I.; Győri, Z.; Pusztahelyi, T. The Aspergilli and Their Mycotoxins: Metabolic Interactions With Plants and the Soil Biota. Front. Microbiol. 2020, 10, 488850. [Google Scholar] [CrossRef]
- Luciano-Rosario, D.; Keller, N.P.; Jurick, W.M.; Wayne Jurick, C.M. Penicillium expansum: Biology, omics, and management tools for a global postharvest pathogen causing blue mould of pome fruit. Mol. Plant Pathol. 2020, 21, 1391–1404. [Google Scholar] [CrossRef]
- Yu, L.; Qiao, N.; Zhao, J.; Zhang, H.; Tian, F.; Zhai, Q.; Chen, W. Postharvest control of Penicillium expansum in fruits: A review. Food Biosci. 2020, 36, 100633. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Y.; Hu, C.; Liu, G.; Li, Y.; Wang, S. Identification, pathogenic mechanism and control of Rhizopus oryzae causing postharvest fruit rot in pumpkin. Postharvest Biol. Technol. 2023, 204, 112460. [Google Scholar] [CrossRef]
- Mahmood, T.; Moosa, A.; Khan, A.U.R.; Maqsood, A.; Kiani, F.; Abbas, G.; Alyas, K.; Khalid, B. Using Essential Oils to Protect Peaches from Post-Harvest Rot Caused by Rhizopus Species. Plant Prot. 2023, 7, 217–223. [Google Scholar] [CrossRef]
- Al-Hindi, R.R.; Al-Najada, A.R.; Mohamed, S.A. Isolation and identification of some fruit spoilage fungi: Screening of plant cell wall degrading enzymes. Afr. J. Microbiol. Res. 2011, 5, 443–448. [Google Scholar]
- Alabid, I.; Glaeser, S.P.; Kogel, K.H. Endofungal Bacteria Increase Fitness of their Host Fungi and Impact their Association with Crop Plants. Curr. Issues Mol. Biol. 2018, 30, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Birol, D.; Gunyar, O.A. Investigation of presence of endofungal bacteria in Rhizopus spp. isolated from the different food samples. Arch. Microbiol. 2021, 203, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Agyare, S.; Magan, N.; Xu, X. Conditions for infection of strawberry fruit by M. piriformis and Rhizopus spp. Eur. J. Plant Pathol. 2020, 157, 65–75. [Google Scholar] [CrossRef]
- Ahmad, T.; Xing, F.; Nie, C.; Cao, C.; Xiao, Y.; Yu, X.; Moosa, A.; Liu, Y. Biocontrol potential of lipopeptides produced by the novel Bacillus subtilis strain Y17B against postharvest Alternaria fruit rot of cherry. Front. Microbiol. 2023, 14, 1150217. [Google Scholar] [CrossRef]
- Al-Maawalia, S.S.; Al-Sadia, A.M.; Alsheriqia, S.A.K.; Al-Sabahi, J.N.; Velazhahan, R. The potential of antagonistic yeasts and bacteria from tomato phyllosphere and fructoplane in the control of Alternaria fruit rot of tomato. All Life 2021, 14, 34–48. [Google Scholar] [CrossRef]
- Gur, L.; Reuveni, M.; Cohen, Y. β-Aminobutyric Acid Induced Resistance against Alternaria Fruit Rot in Apple Fruits. J. Fungi 2021, 7, 564. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Xiao, C.L. Phylogenetic, Morphological, and Pathogenic Characterization of Alternaria Species Associated with Fruit Rot of Blueberry in California. Phytopathology 2015, 105, 1555–1567. [Google Scholar] [CrossRef]
- Chu, F.S. MYCOTOXINS|Toxicology. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2003; pp. 4096–4108. [Google Scholar]
- Krisch, J.; Chandrasekaran, M.; Kadaikunnan, S.; Alharbi, N.S.; Vágvölgyi, C. Latest about Spoilage by Yeasts: Focus on the Deterioration of Beverages and Other Plant-Derived Products. J. Food Prot. 2016, 79, 825–829. [Google Scholar] [CrossRef]
- Cai, S.; Snyder, A.B. Machinery Mold (Galactomyces geotrichum) Survival following Thermal and Hydrostatic Pressure Processing. J. Food Prot. 2019, 82, 1034–1038. [Google Scholar] [CrossRef]
- Deak, T.; Beuchat, L.R. Yeasts Associated with Fruit Juice Concentrates. J. Food Prot. 1993, 56, 777–782. [Google Scholar] [CrossRef]
- Mohapatra, D.; Kumar, S.; Kotwaliwale, N.; Singh, K.K. Critical factors responsible for fungi growth in stored food grains and non-chemical approaches for their control. Ind. Crops Prod. 2017, 108, 162–182. [Google Scholar] [CrossRef]
- Mousa, W.; Ghazali, F.M.; Jinap, S.; Ghazali, H.M.; Radu, S.; Salama, A.E.-R. Temperature, water activity and gas composition effects on the growth and aflatoxin production by Aspergillus flavus on paddy. J. Stored Prod. Res. 2016, 67, 49–55. [Google Scholar] [CrossRef]
- Robertson, G.L. History of Food Packaging. Ref. Modul. Food Sci. 2019. preview. [Google Scholar] [CrossRef]
- Otoni, C.G.; Espitia, P.J.P.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Ooraikul, B. Modified Atmosphere Packaging of Bakery Products. In Modified Atmosphere Packaging of Food; Ooraikul, B., Stiles, E., Eds.; Ellis Horwood: New York, NY, USA, 1991; pp. 49–117. [Google Scholar]
- Yahia, E.M. Chapter 1—Introduction. In Postharvest Technol Perish Hortic Commod; Yahia, E.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–41. [Google Scholar] [CrossRef]
- DRYAIR. Humidity Control Design. 2024. Available online: https://www.dry-air.co.uk/knowledge-base/humidity-control-design (accessed on 12 January 2024).
- Roy, S.; Anantheswaran, R.C.; Beelman, R.B. Modified Atmosphere and Modified Humidity Packagingof Fresh Mushrooms. J. Food Sci. 1996, 61, 391–397. [Google Scholar]
- Caleb, O.J.; Ilte, K.; Fröhling, A.; Geyer, M.; Mahajan, P.V. Integrated modified atmosphere and humidity package design for minimally processed Broccoli (Brassica oleracea L. var. italica). Postharvest Biol. Technol. 2016, 121, 87–100. [Google Scholar] [CrossRef]
- Subramaniam, P. The stability and shelf life of confectionery products. In Food and Beverage Stability and Shelf Life; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing Limited: Thorston, UK, 2011; pp. 716–742. [Google Scholar] [CrossRef]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, Applications, and Comparison of Thermal (Pasteurization, Sterilization, and Aseptic Packaging) against Non-Thermal (Ultrasounds, UV Radiation, Ozonation, High Hydrostatic Pressure) Technologies in Food Processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Therdtatha, P.; Tandumrongpong, C.; Pilasombut, K.; Matsusaki, H.; Keawsompong, S.; Nitisinprasert, S. Characterization of antimicrobial substance from Lactobacillus salivarius KL-D4 and its application as biopreservative for creamy filling. Springerplus 2016, 5, 1060. [Google Scholar] [CrossRef]
- Food and Drug Administration (Department of Health and Human Services). ECFR: 21 CFR 120—Hazard Analysis and Critical Control Point (HACCP) Systems. Available online: https://www.ecfr.gov/current/title-21/part-120/section-120.1 (accessed on 25 January 2024).
- Rodriguez-Mateos, A.; Cifuentes-Gomez, T.; George, T.W.; Spencer, J.P.E. Impact of Cooking, Proving, and Baking on the (Poly)phenol Content of Wild Blueberry. J. Agric. Food Chem. 2014, 62, 3979–3986. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Yu, L.; Chen, P. Retention of polyphenols in blueberries (Vaccinium corymbosum) after different cooking methods, using UHPLC–DAD–MS based metabolomics. J. Food Compos. Anal. 2017, 56, 55–66. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Silva, C.L.M. Quality optimization of hot filled pasteurized fruit purees: Container characteristics and filling temperatures. J. Food Eng. 1997, 32, 351–364. [Google Scholar] [CrossRef]
- Hariyadi, P. Hot-Fill Processing of Beverages. Foodreview Int. 2013, 1, 46–49. Available online: https://www.researchgate.net/publication/259255039 (accessed on 25 July 2024).
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Anyasi, T.A.; Jideani, A.I.O.; Edokpayi, J.N.; Anokwuru, C.P. Application of organic acids in food preservation. In Organic Acids: Characteristics, Properties and Synthesis; Vargas, C., Ed.; Nova Science Publishers: New York, NY, USA, 2017; pp. 1–45. [Google Scholar]
- Dehghan, P.; Mohammadi, A.; Mohammadzadeh-Aghdash, H.; Ezzati Nazhad Dolatabadi, J. Pharmacokinetic and toxicological aspects of potassium sorbate food additive and its constituents. Trends Food Sci. Technol. 2018, 80, 123–130. [Google Scholar] [CrossRef]
- Mohammadzadeh-Aghdash, H.; Sohrabi, Y.; Mohammadi, A.; Shanehbandi, D.; Dehghan, P.; Dolatabadi, J.E.N. Safety assessment of sodium acetate, sodium diacetate and potassium sorbate food additives. Food Chem. 2018, 257, 211–215. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Javadi, M.; Nassiri-Asl, M. An Overview on the Effects of Sodium Benzoate as a Preservative in Food Products. Biotechnol. Health Sci. 2016, 3, 35084. [Google Scholar] [CrossRef]
- Kagliwal, L.; Jadhav, S.; Singhal, R.; Kulkarni, P. PRESERVATIVES|Permitted Preservatives—Propionic Acid. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 99–101. [Google Scholar] [CrossRef]
- EFSA Panel on Food additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of propionic acid (E 280), sodium propionate (E 281), calcium propionate (E 282) and potassium propionate (E 283) as food additives. EFSA J. 2014, 12, 3779. [Google Scholar] [CrossRef]
- Brock, M.; Buckel, W. On the mechanism of action of the antifungal agent propionate. Eur. J. Biochem. 2004, 271, 3227–3241. [Google Scholar] [CrossRef]
- Mollapour, M.; Piper, P.W. The ZbYME2 gene from the food spoilage yeast Zygosaccharomyces bailii confers not only YME2 functions in Saccharomyces cerevisiae, but also the capacity for catabolism of sorbate and benzoate, two major weak organic acid preservatives. Mol. Microbiol. 2001, 42, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.J.; Rodrigues, F.; Coôrte-Real, M.; Leão, C. Mechanisms underlying the transport and intracellular metabolism of acetic acid in the presence of glucose in the yeast Zygosaccharomyces bailii. Microbiology 1998, 144, 665–670. [Google Scholar] [CrossRef]
- Stratford, M.; Plumridge, A.; Archer, D.B. Decarboxylation of Sorbic Acid by Spoilage Yeasts Is Associated with the PAD1 Gene. Appl. Environ. Microbiol. 2007, 73, 6534–6542. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Sodium Benzoate—Harmfulness and Potential Use in Therapies for Disorders Related to the Nervous System: A Review. Nutrients 2022, 14, 1497. [Google Scholar] [CrossRef] [PubMed]
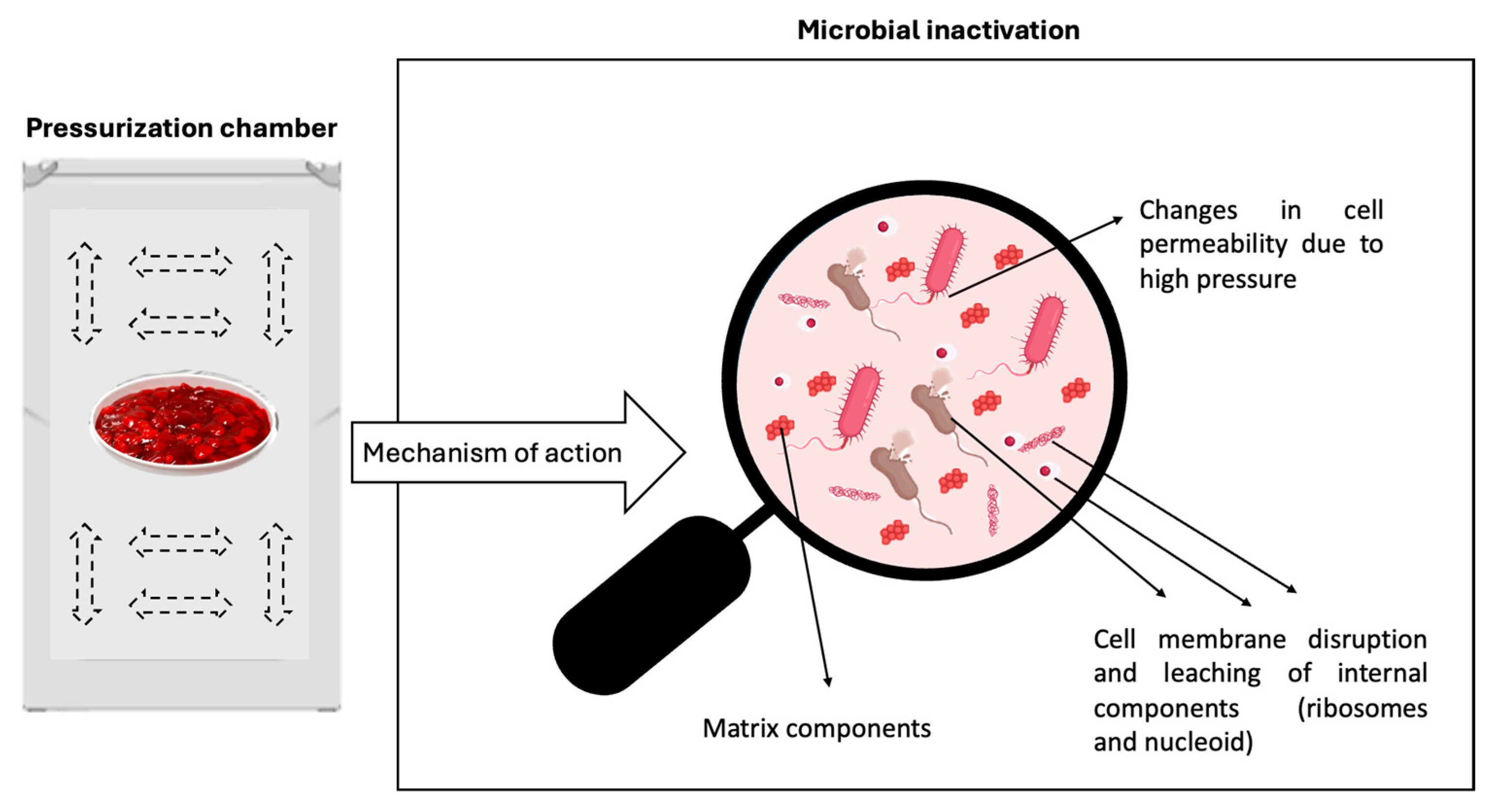
- Huang, H.-W.; Wu, S.; Lu, J.; Shyu, Y.; Wang, C. Current status and future trends of high-pressure processing in food industry. Food Control. 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Q.; Denoya, G.I.; Yang, C.; Barba, F.J.; Yu, H.; Chen, X. High Hydrostatic Pressure-Based Combination Strategies for Microbial Inactivation of Food Products: The Cases of Emerging Combination Patterns. Front. Nutr. 2022, 9, 878904. [Google Scholar] [CrossRef] [PubMed]
- Nema, P.K.; Sehrawat, R.; Ravichandran, C.; Kaur, B.P.; Kumar, A.; Tarafdar, A. Inactivating Food Microbes by High-Pressure Processing and Combined Nonthermal and Thermal Treatment: A Review. J. Food Qual. 2022, 2022, 5797843. [Google Scholar] [CrossRef]
- García-Parra, J.; González-Cebrino, F.; Cava, R.; Ramírez, R. Effect of a different high pressure thermal processing compared to a traditional thermal treatment on a red flesh and peel plum purée. Innov. Food Sci. Emerg. Technol. 2014, 26, 26–33. [Google Scholar] [CrossRef]
- Palou, E.; López-Malo, A.; Barbosa-Cánovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase Activity and Color of Blanched and High Hydrostatic Pressure Treated Banana Puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Sulaiman, A.; Soo, M.J.; Yoon, M.M.L.; Farid, M.; Silva, F.V.M. Modeling the polyphenoloxidase inactivation kinetics in pear, apple and strawberry purees after High Pressure Processing. J. Food Eng. 2015, 147, 89–94. [Google Scholar] [CrossRef]
- Marszałek, K.; Mitek, M.; Skąpska, S. The effect of thermal pasteurization and high pressure processing at cold and mild temperatures on the chemical composition, microbial and enzyme activity in strawberry purée. Innov. Food Sci. Emerg. Technol. 2015, 27, 48–56. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. Inactivation of Byssochlamys nivea ascospores in strawberry puree by high pressure, power ultrasound and thermal processing. Int. J. Food Microbiol. 2015, 214, 129–136. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Góngora-Nieto, M.M.; Pothakamury, U.R.; Swanson, B. Biological Principles for Microbial Inactivation in Electric Fields. In Preservation of Foods with Pulsed Electric Fields; Barbosa-Cánovas, G.V., Góngora-Nieto, M.M., Pothakamury, U.R., Swanson, B., Eds.; Elsevier: San Diego, CA, USA, 1999; pp. 47–75. [Google Scholar] [CrossRef]
- Tsong, T.Y. Electroporation of cell membranes. Biophys. J. 1991, 60, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, R.N.; Balthazar, C.F.; Margalho, L.P.; Freitas, M.Q.; Sant’Ana, A.S.; Cruz, A.G. Pulsed electric field-based technology for microbial inactivation in milk and dairy products. Curr. Opin. Food Sci. 2023, 54, 101087. [Google Scholar] [CrossRef]
- Kantala, C.; Supasin, S.; Intra, P.; Rattanadecho, P. Evaluation of Pulsed Electric Field and Conventional Thermal Processing for Microbial Inactivation in Thai Orange Juice. Foods 2022, 11, 1102. [Google Scholar] [CrossRef]
- Walkling-Ribeiro, M.; Noci, F.; Cronin, D.A.; Lyng, J.G.; Morgan, D.J. Shelf life and sensory attributes of a fruit smoothie-type beverage processed with moderate heat and pulsed electric fields. LWT Food Sci. Technol. 2010, 43, 1067–1073. [Google Scholar] [CrossRef]
- Wan, J. Non-thermal Treatment of Milk: Pulsed Electric Field Technology. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 717–723. [Google Scholar] [CrossRef]
- Yogesh, K. Pulsed electric field processing of egg products: A review. J. Food Sci. Technol. 2016, 53, 934–945. [Google Scholar] [CrossRef]
- Geveke, D.J.; Aubuchon, I.; Zhang, H.Q.; Boyd, G.; Sites, J.E.; Bigley, A.B.W. Validation of a pulsed electric field process to pasteurize strawberry purée. J. Food Eng. 2015, 166, 384–389. [Google Scholar] [CrossRef]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of High Hydrostatic Pressure and Pulsed Electric Fields for Energy Efficient and Environmentally Friendly Food Processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- Pataro, G.; Ferrari, G. Limitations of pulsed electric field utilization in food industry. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–310. [Google Scholar] [CrossRef]
- Baumann, A.R.; Martin, S.E.; Feng, H. Power Ultrasound Treatment of Listeria monocytogenes in Apple Cider. J. Food Prot. 2005, 68, 2333–2340. [Google Scholar] [CrossRef]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.-U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- O’Donnell, C.P.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010, 21, 358–367. [Google Scholar] [CrossRef]
- Dolas, R.; Saravanan, C.; Kaur, B.P. Emergence and era of ultrasonic’s in fruit juice preservation: A review. Ultrason. Sonochemistry 2019, 58, 104609. [Google Scholar] [CrossRef]
- Onyeaka, H.; Miri, T.; Hart, A.; Anumudu, C.; Nwabor, O.F. Application of Ultrasound Technology in Food Processing with emphasis on bacterial spores. FOOD Rev. Int. 2023, 39, 3663–3675. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Singh Sharanagat, V. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochemistry 2020, 70, 105293. [Google Scholar] [CrossRef]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef]
- Teixeira, A.; Sánchez-Hernández, E.; Noversa, J.; Cunha, A.; Cortez, I.; Marques, G.; Martín-Ramos, P.; Oliveira, R. Antifungal Activity of Plant Waste Extracts against Phytopathogenic Fungi: Allium sativum Peels Extract as a Promising Product Targeting the Fungal Plasma Membrane and Cell Wall. Horticulturae 2023, 9, 136. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Mancini, E.; Camele, I.; De Martino, L.; De Feo, V. In vivo antifungal activity of two essential oils from Mediterranean plants against postharvest brown rot disease of peach fruit. Ind. Crops Prod. 2015, 66, 11–15. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Pérez-Pérez, J.C.; Varillas-Torres, J.M.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Munguía-Pérez, R.; Cid-Pérez, T.S.; Avila-Sosa, R. Starch Edible Films/Coatings Added with Carvacrol and Thymol: In Vitro and In Vivo Evaluation against Colletotrichum gloeosporioides. Foods 2021, 10, 175. [Google Scholar] [CrossRef]
- Ribes, S.; Fuentes, A.; Talens, P.; Barat, J.M. Use of oil-in-water emulsions to control fungal deterioration of strawberry jams. Food Chem. 2016, 211, 92–99. [Google Scholar] [CrossRef]
- Baghi, F.; Gharsallaoui, A.; Dumas, E.; Ghnimi, S. Advancements in Biodegradable Active Films for Food Packaging: Effects of Nano/Microcapsule Incorporation. Foods 2022, 11, 760. [Google Scholar] [CrossRef]
- Targino de Souza Pedrosa, G.; Pimentel, T.C.; Gavahian, M.; Lucena de Medeiros, L.; Pagán, R.; Magnani, M. The combined effect of essential oils and emerging technologies on food safety and quality. LWT 2021, 147, 111593. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Shelef, L.A.; Jyothi, E.K.; Bulgarellii, M.A. Growth of Enteropathogenic and Spoilage Bacteria in Sage-Containing Broth and Foods. J. Food Sci. 1984, 49, 737–740. [Google Scholar] [CrossRef]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A natural preservative for food applications—A review. Food Sci. Biotechnol. 2021, 30, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.; Mahony, J.; van Sinderen, D. Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci. Technol. 2013, 33, 93–109. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- van Heel, A.J.; de Jong, A.; Montalbán-López, M.; Kok, J.; Kuipers, O.P. BAGEL3, automated identification of genes encoding bacteriocins and (non-)bactericidal posttranslationally modified peptides. Nucleic Acids Res. 2013, 41, W448–W453. [Google Scholar] [CrossRef]
- Liu, J.; Huang, R.; Song, Q.; Xiong, H.; Ma, J.; Xia, R.; Qiao, J. Combinational Antibacterial Activity of Nisin and 3-Phenyllactic Acid and Their Co-production by Engineered Lactococcus lactis. Front. Bioeng. Biotechnol. 2021, 9, 612105. [Google Scholar] [CrossRef]
- Hondrodimou, O.; Kourkoutas, Y.; Panagou, E.Z. Efficacy of natamycin to control fungal growth in natural black olive fermentation. Food Microbiol. 2011, 28, 621–627. [Google Scholar] [CrossRef]
- Arrarte, E.; Garmendia, G.; Wisniewski, M.; Vero, S. Biocontrol activity of Debaryomyces hansenii against blue mold on apple and pear during cold storage. Agrociencia Urug. 2022, 25, NE2. [Google Scholar] [CrossRef]
- Wei, Y.; Mao, S.; Tu, K. Effect of preharvest spraying Cryptococcus laurentii on postharvest decay and quality of strawberry. Biol. Control. 2014, 73, 68–74. [Google Scholar] [CrossRef]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]




| Product Type | Spoilage Microorganisms | Contamination Source | Spoilage | Mycotoxin Production Potential | References |
|---|---|---|---|---|---|
| Shelf-stable, hot-filled products | Aspergillus | Raw material storage and air of the food processing facility | Visible deposits of black conidia on surface | Yes | [4,31,32,33] |
| Penicillium | Small patches of growth that may be hard to visualize | Yes | [4,33,34] | ||
| Cladosporium | Product discoloration | Yes | [11,33] | ||
| Stored fresh fruit and processed foods with low-to-medium water activity | Mucor | Raw material | Aerial hyphae of mucoralean fungi visible on product surface; product fermentation with gas production | Yes | [4,33,35] |
| Rhizopus | Yes | ||||
| Stored fresh fruit, processed fruit products | Botrytis | Raw material; air of the food processing facility | Gel formation | No | [18,36] |
| Product Type | Spoilage Microorganisms | Contamination Source | Spoilage | References |
|---|---|---|---|---|
| Processed fruit products | Pichia | Raw material | Off-flavor and gas production due to fermentation | [16,37] |
| High-sugar-content products | Zygosaccharomyces | Raw material | Visible growth on the surface, fermentation, off-flavor, and off-odors | [38,39,40] |
| Processed fruit products | Saccharomyces | Raw material | Production of ethanol and film formation on surface | [37,38,39] |
| Processed fruit products | Candida | Raw material, lack of effective hygiene protocols | Film formation on surface | [4,37,39] |
| Processed fruit products and products stored at low temperatures | Rhodotorula | Airflow, vectors, processing facility equipment | Visible red colonies on product surface without fermentation | [37,38,39,40] |
| Preservative | Mechanism of Action | Uses | Reference |
|---|---|---|---|
| Organic acids | Decreasing bacterial intracellular pH value | Used as emulsifiers, stabilizers, preservatives, flavour enhancers, and firming agents | [76] |
| Potassium sorbate | Modifications to the structure and functionality of the bacterial cell membrane, as well as suppression of metabolic activity and transport functions | Bacteriostatic and fungistatic agents in a variety of processed food | [77] |
| Sodium diacetate | Decreasing bacterial intracellular pH value | Used as a flavouring agent/adjuvant for control of pH and as an antimicrobial preservative | [78] |
| Sodium benzoate | Decreasing bacterial intracellular pH value and inhibition of anaerobic fermentation of glucose | Antimicrobial preservative | [79] |
| Calcium propionate | Inhibition of enzymes necessary for metabolism and decreasing bacterial intracellular pH value | Antimicrobial preservative | [80,81] |
| Sodium propionate | Inhibition of enzymes necessary for metabolism and decreasing bacterial intracellular pH value | Antimicrobial preservative | [81,82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento de Carvalho, T.; Silva, B.N.; Tomé, E.; Teixeira, P. Preventing Fungal Spoilage from Raw Materials to Final Product: Innovative Preservation Techniques for Fruit Fillings. Foods 2024, 13, 2669. https://doi.org/10.3390/foods13172669
Bento de Carvalho T, Silva BN, Tomé E, Teixeira P. Preventing Fungal Spoilage from Raw Materials to Final Product: Innovative Preservation Techniques for Fruit Fillings. Foods. 2024; 13(17):2669. https://doi.org/10.3390/foods13172669
Chicago/Turabian StyleBento de Carvalho, Teresa, Beatriz Nunes Silva, Elisabetta Tomé, and Paula Teixeira. 2024. "Preventing Fungal Spoilage from Raw Materials to Final Product: Innovative Preservation Techniques for Fruit Fillings" Foods 13, no. 17: 2669. https://doi.org/10.3390/foods13172669





