Enrichment of White Chocolate with Microencapsulated β-Carotene: Impact on Quality Characteristics and β-Carotene Stability during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. White Chocolate Production
2.3. Development of Encapsulated β-Carotene Structures Using Electrospinning Process, Spray Drying and Freeze Drying
2.3.1. β-Carotene Electrospun Structures
2.3.2. β-Carotene Spray-Dried Structures
2.3.3. β-Carotene Lyophilized Structures
2.4. Incorporation of Encapsulated β-Carotene Structures in White Chocolate
2.5. Thermal Properties
2.6. Rheological Properties
2.7. Hardness
2.8. Color
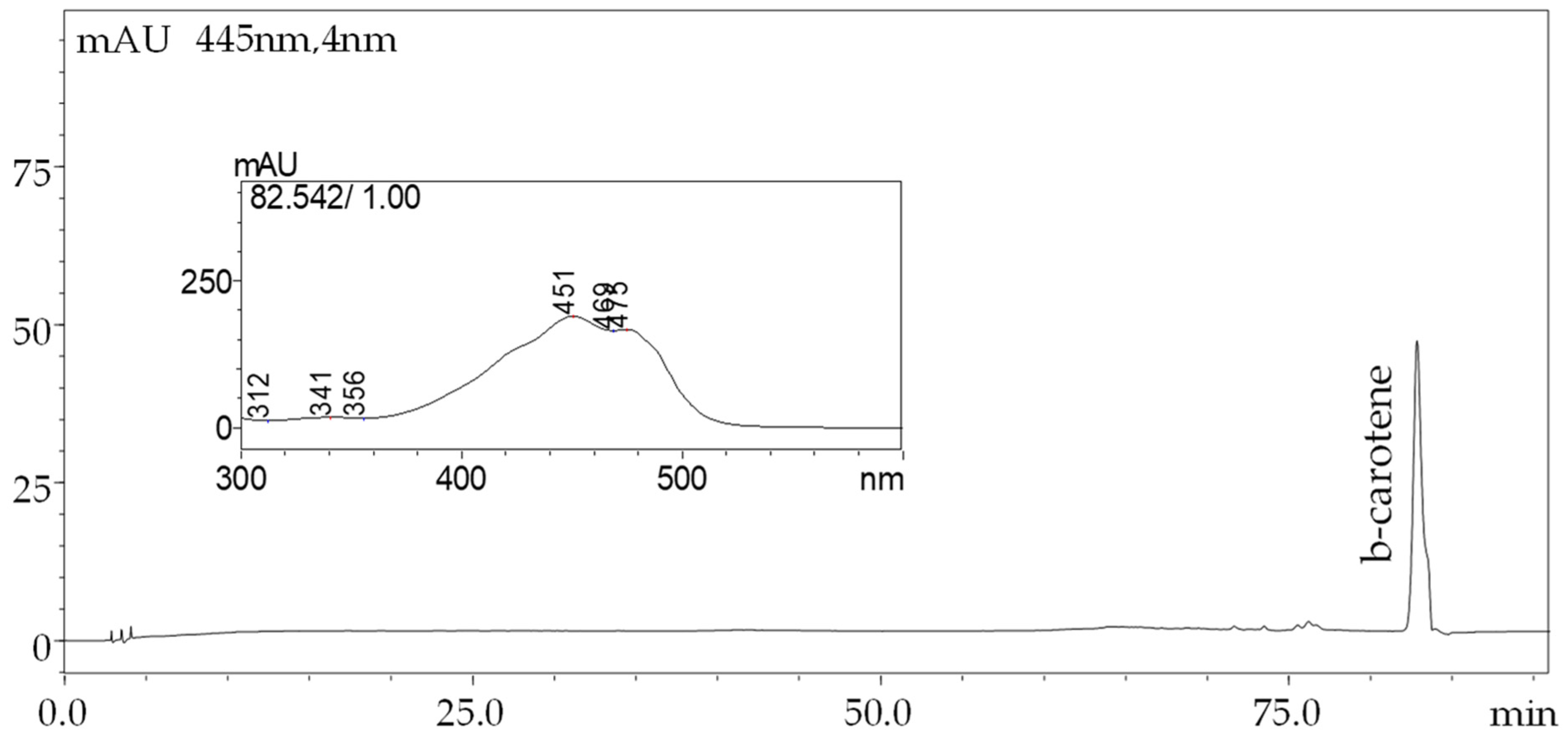
2.9. β-Carotene Content in White Chocolate
2.10. Quality Characteristics of White Chocolates during Storage
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Incorporated β-Carotene on the Quality Characteristics of White Chocolate
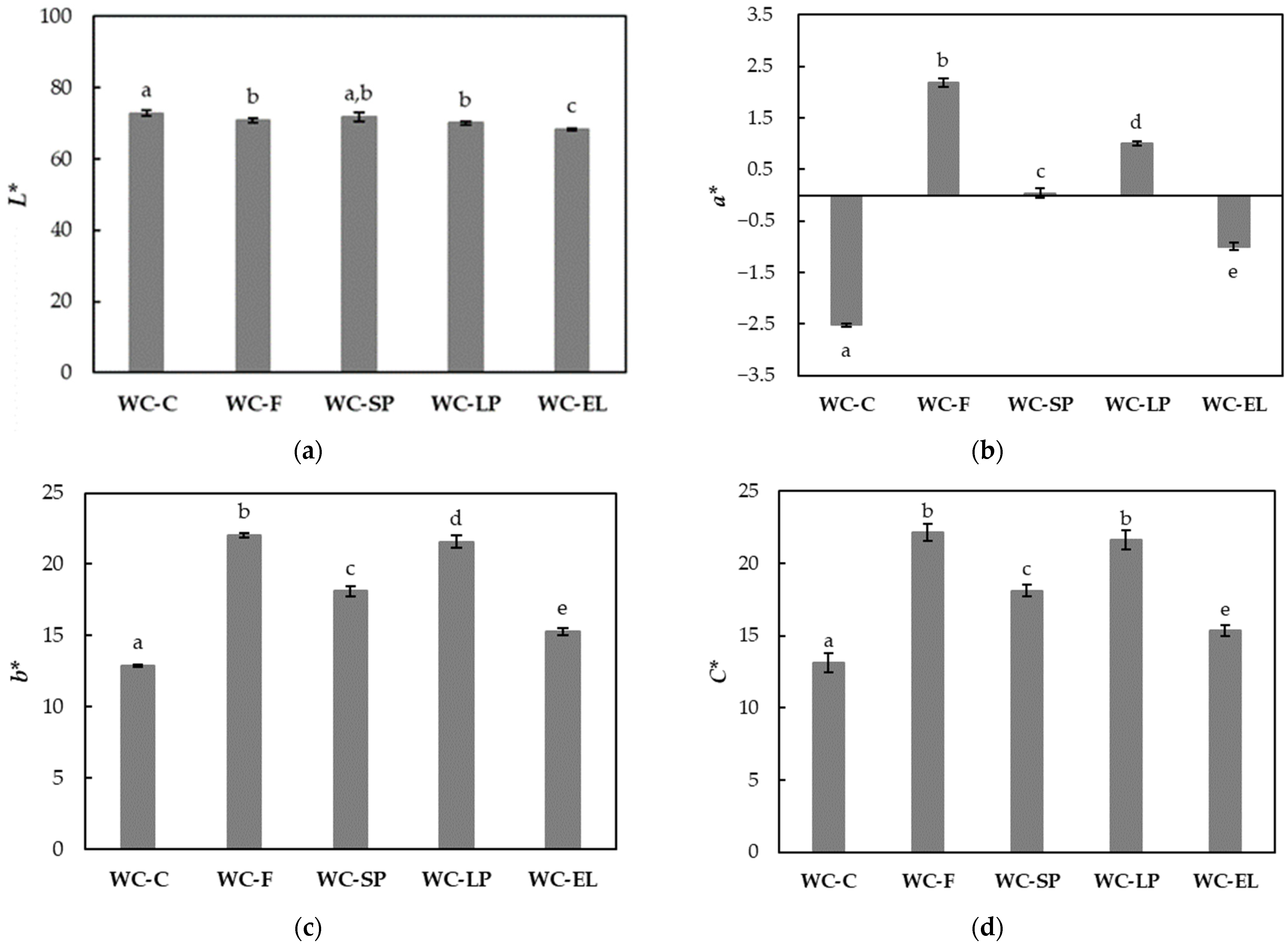
3.1.1. Color
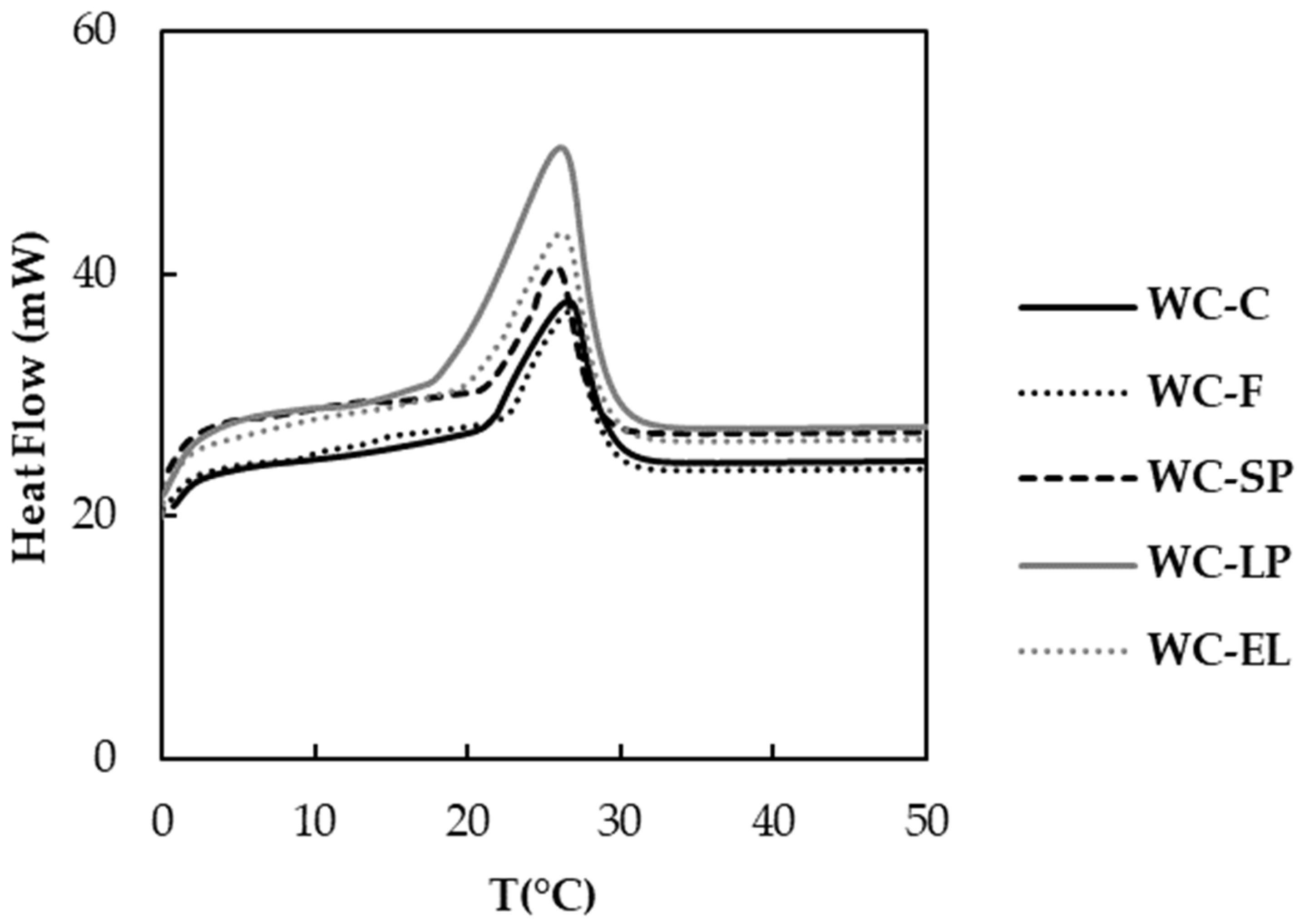
3.1.2. Thermal Properties
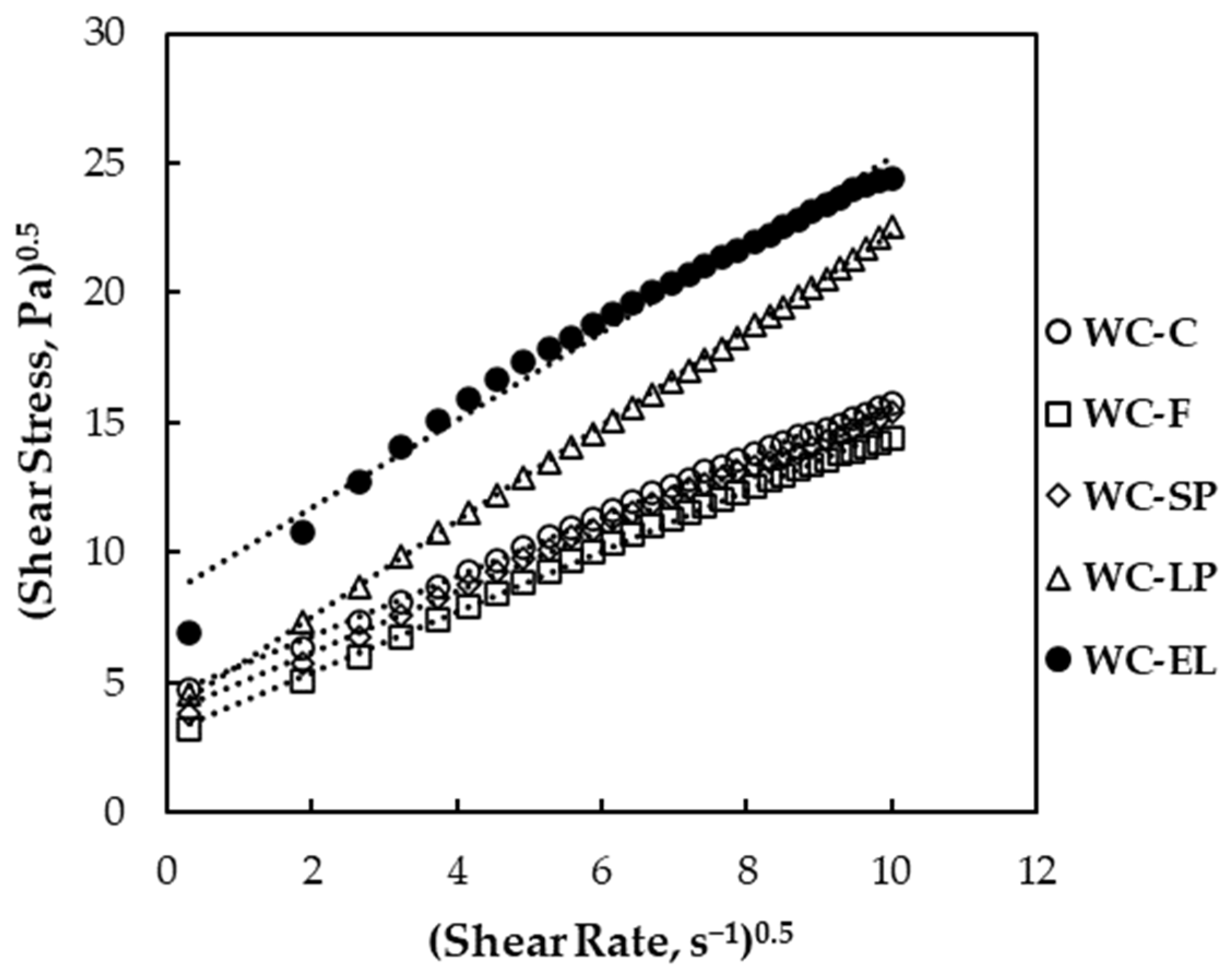
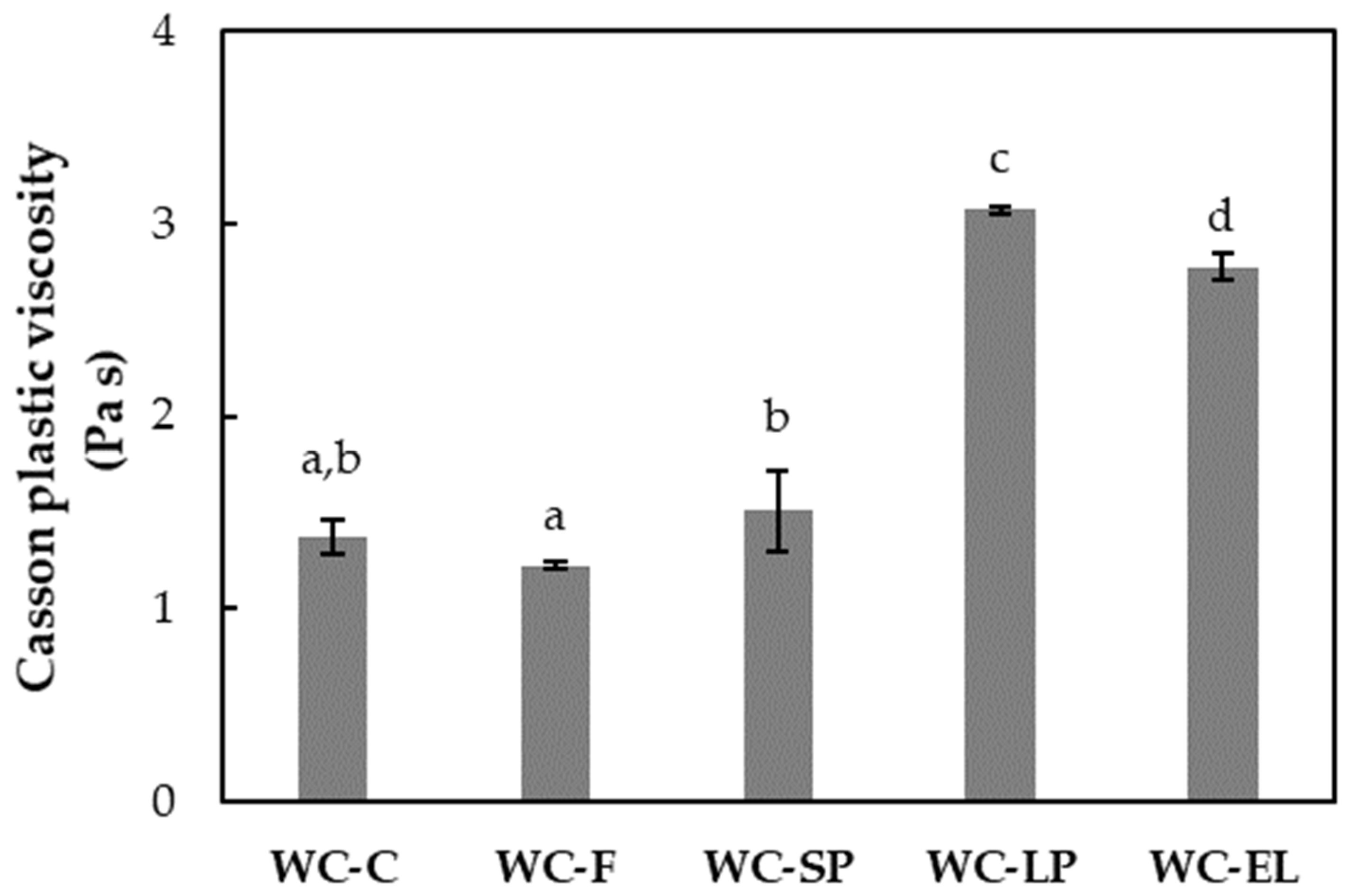
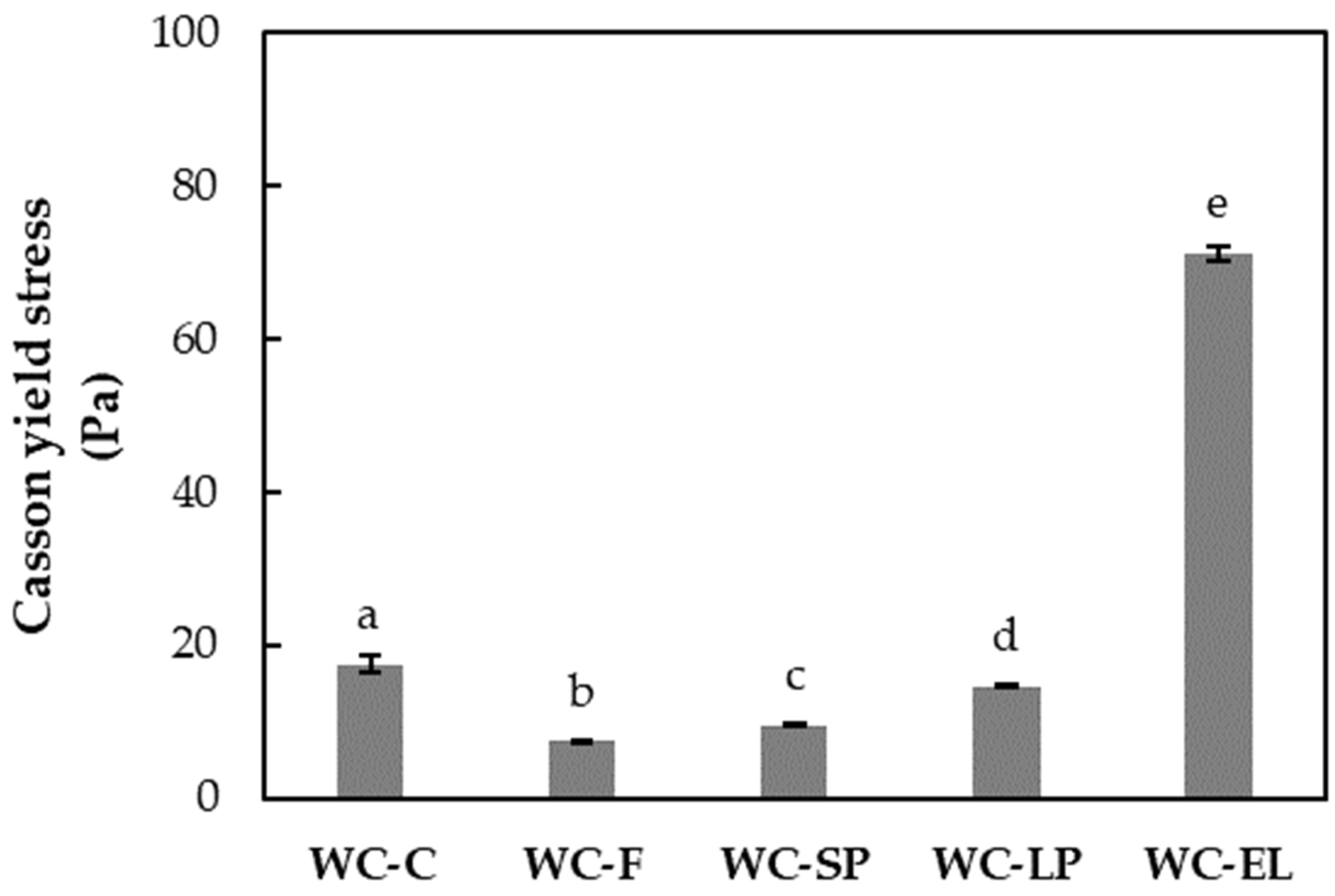
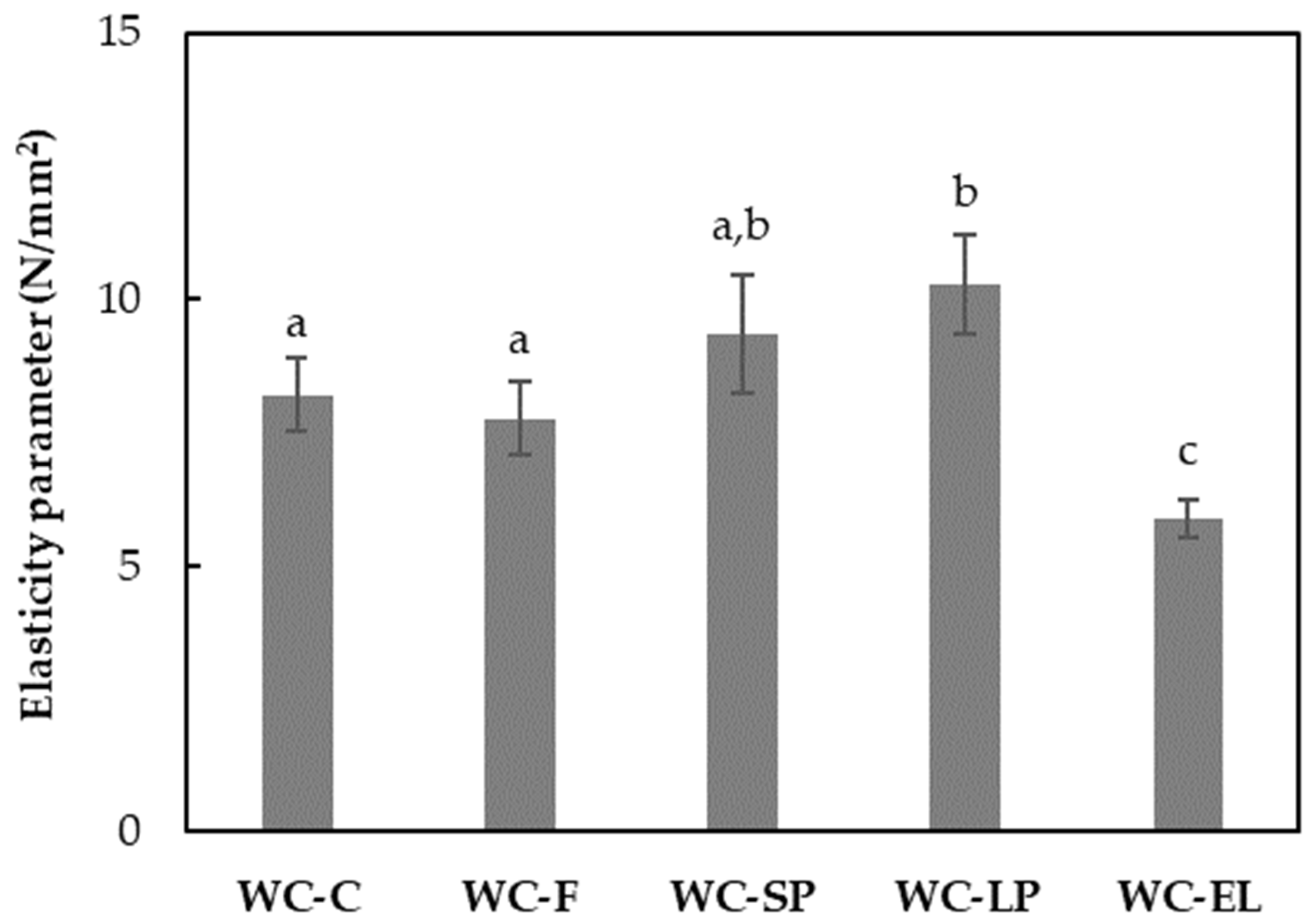
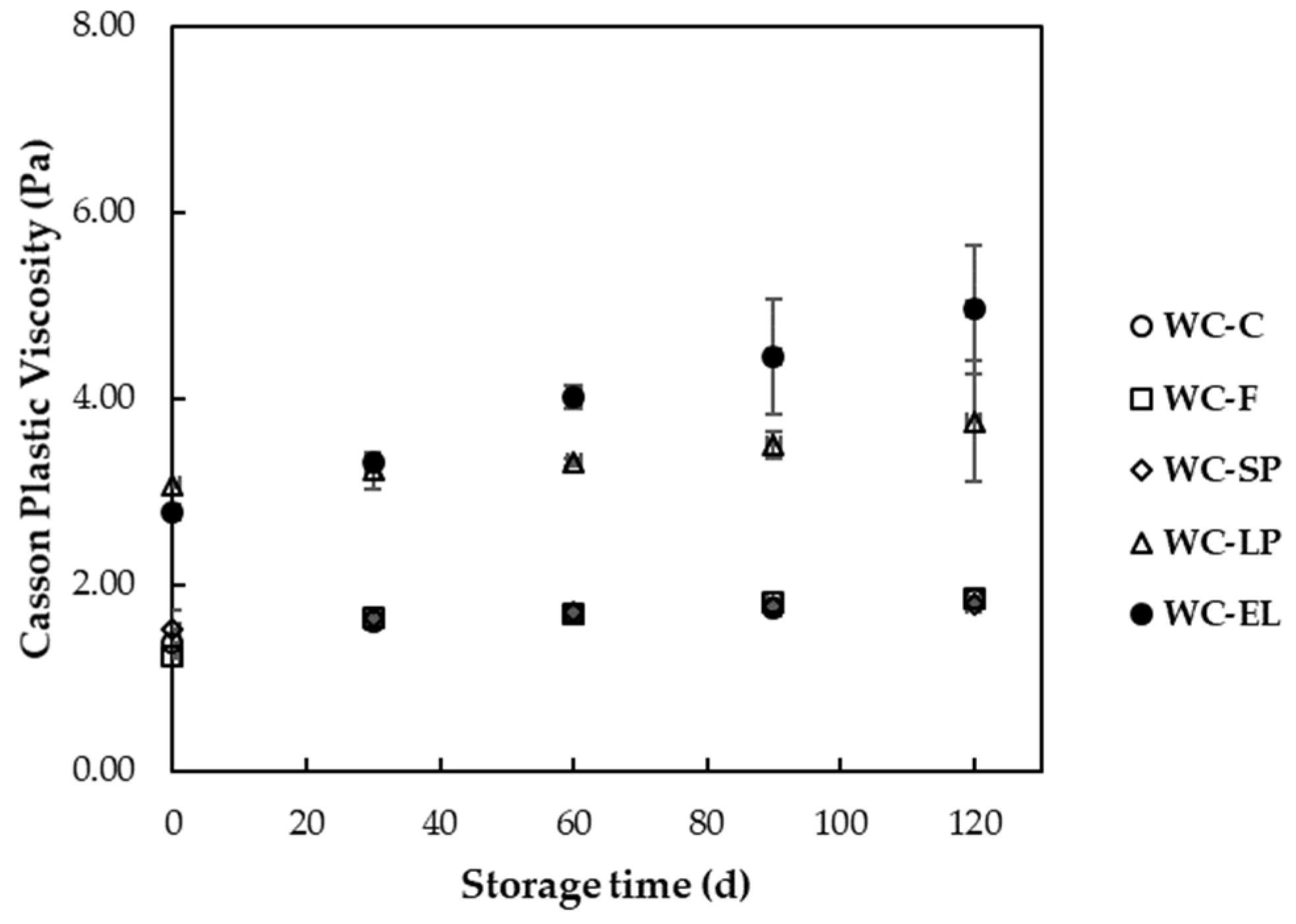
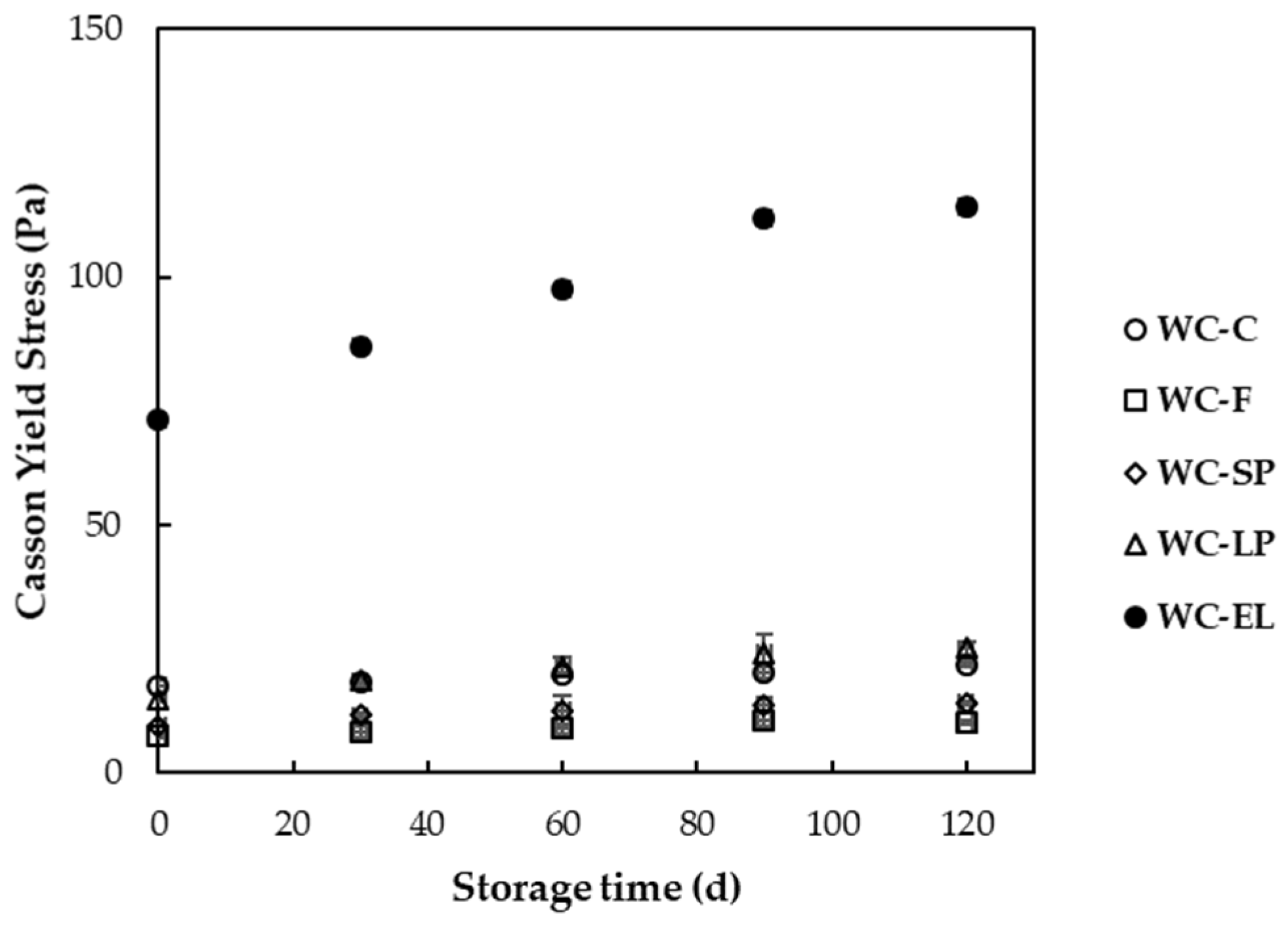
3.1.3. Rheological Properties
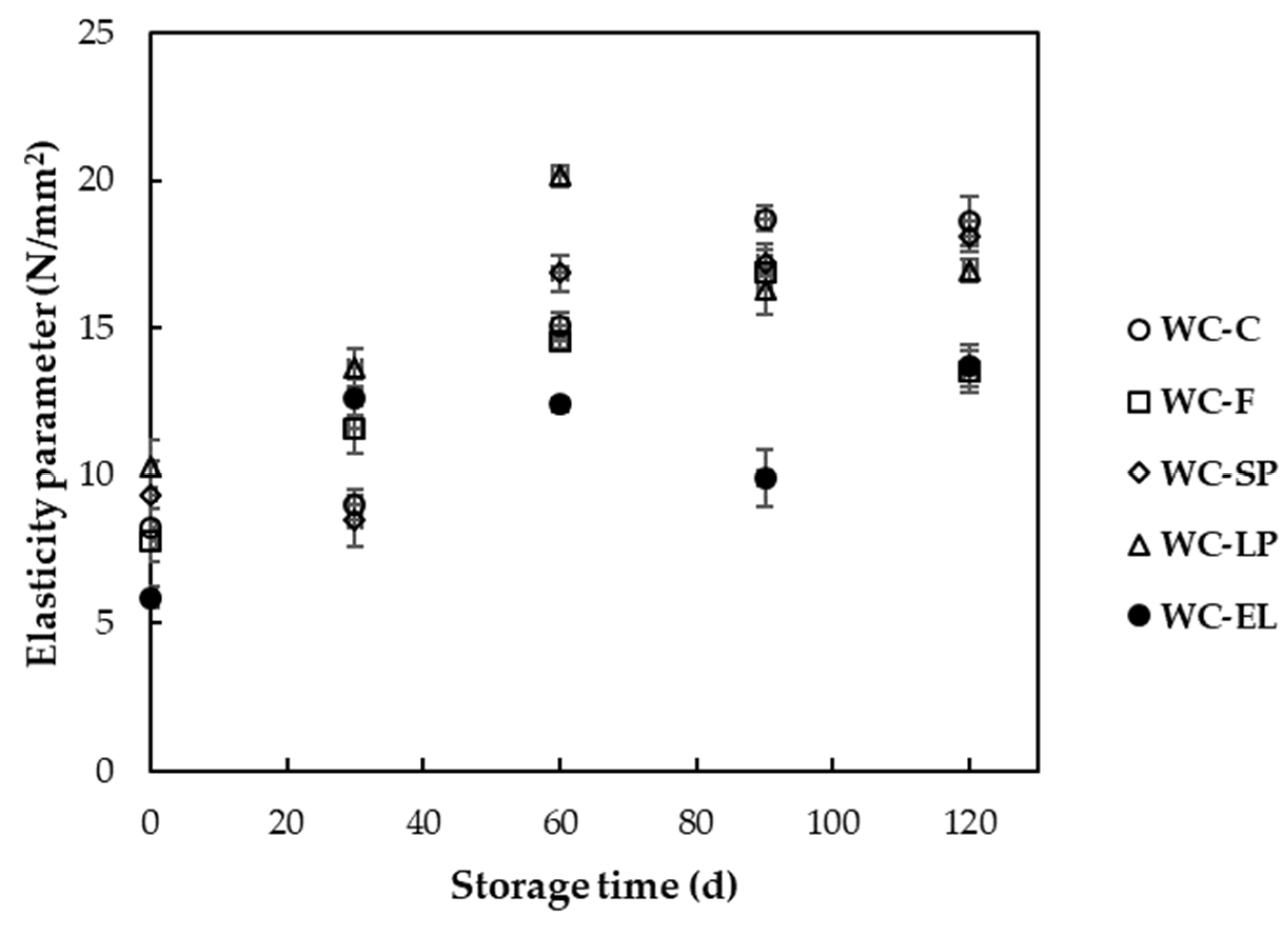
3.1.4. Hardness
3.2. Quality Characteristics of White Chocolates during Storage
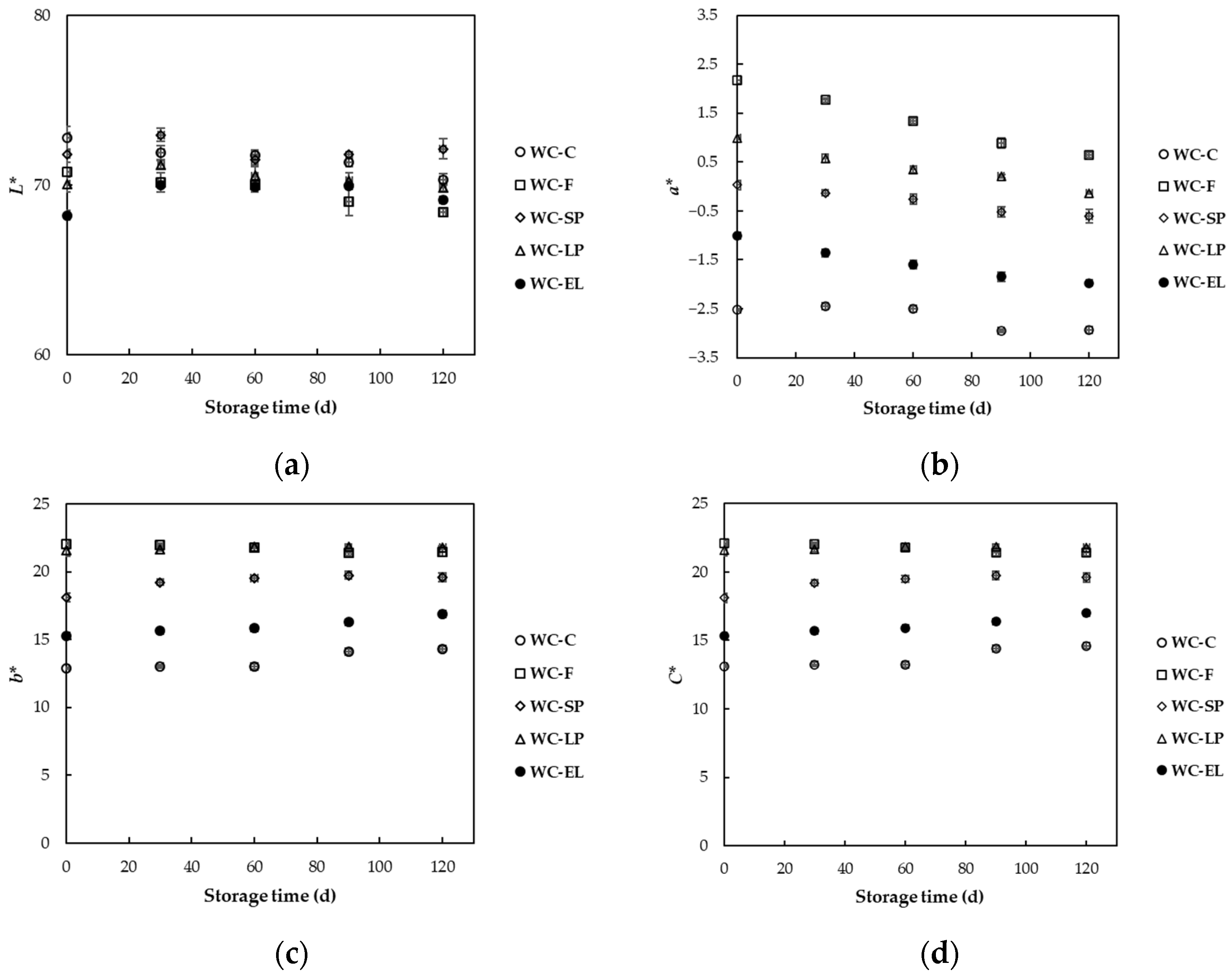
3.2.1. Color
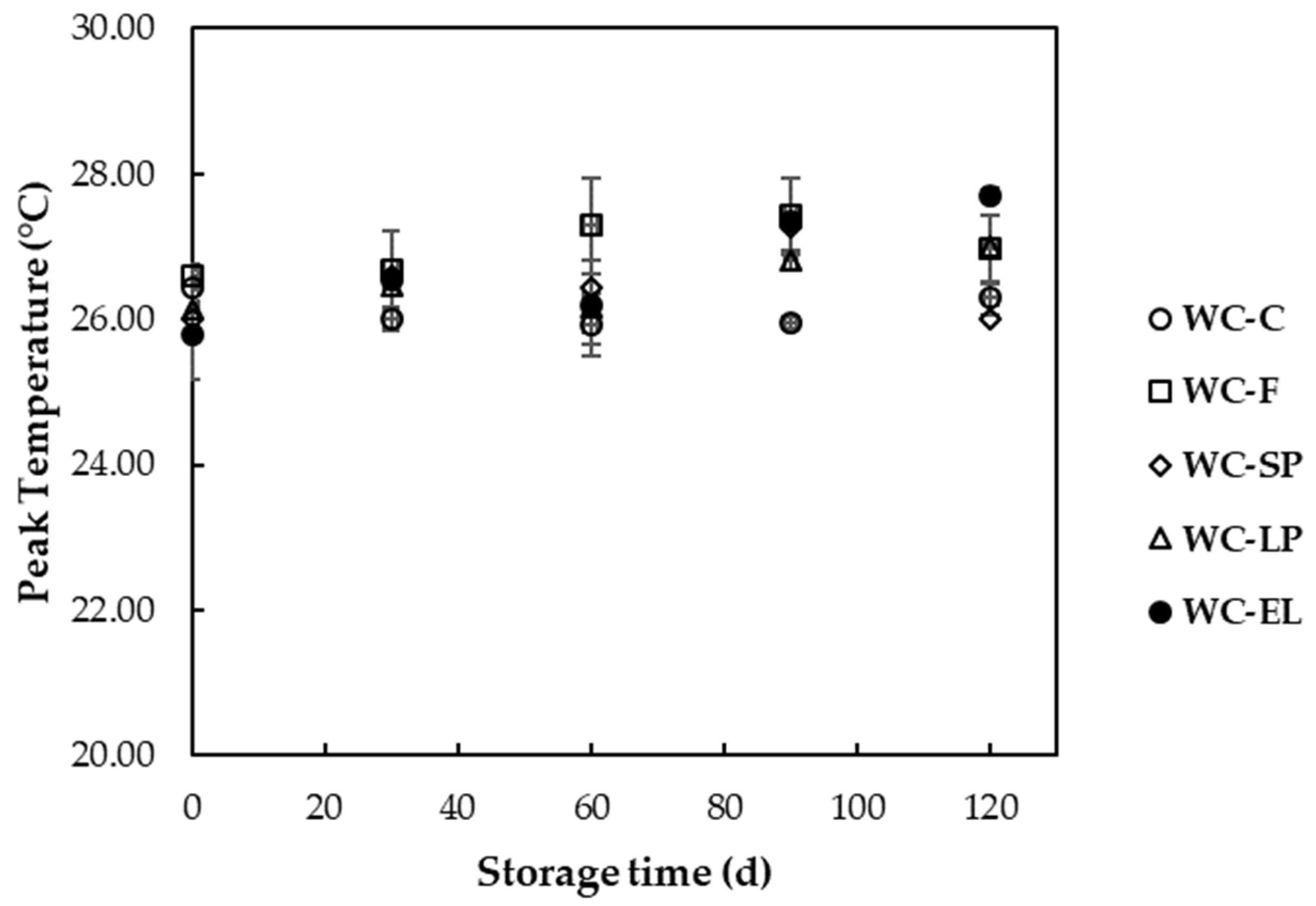
3.2.2. Thermal Properties
3.2.3. Rheological Properties
3.2.4. Hardness
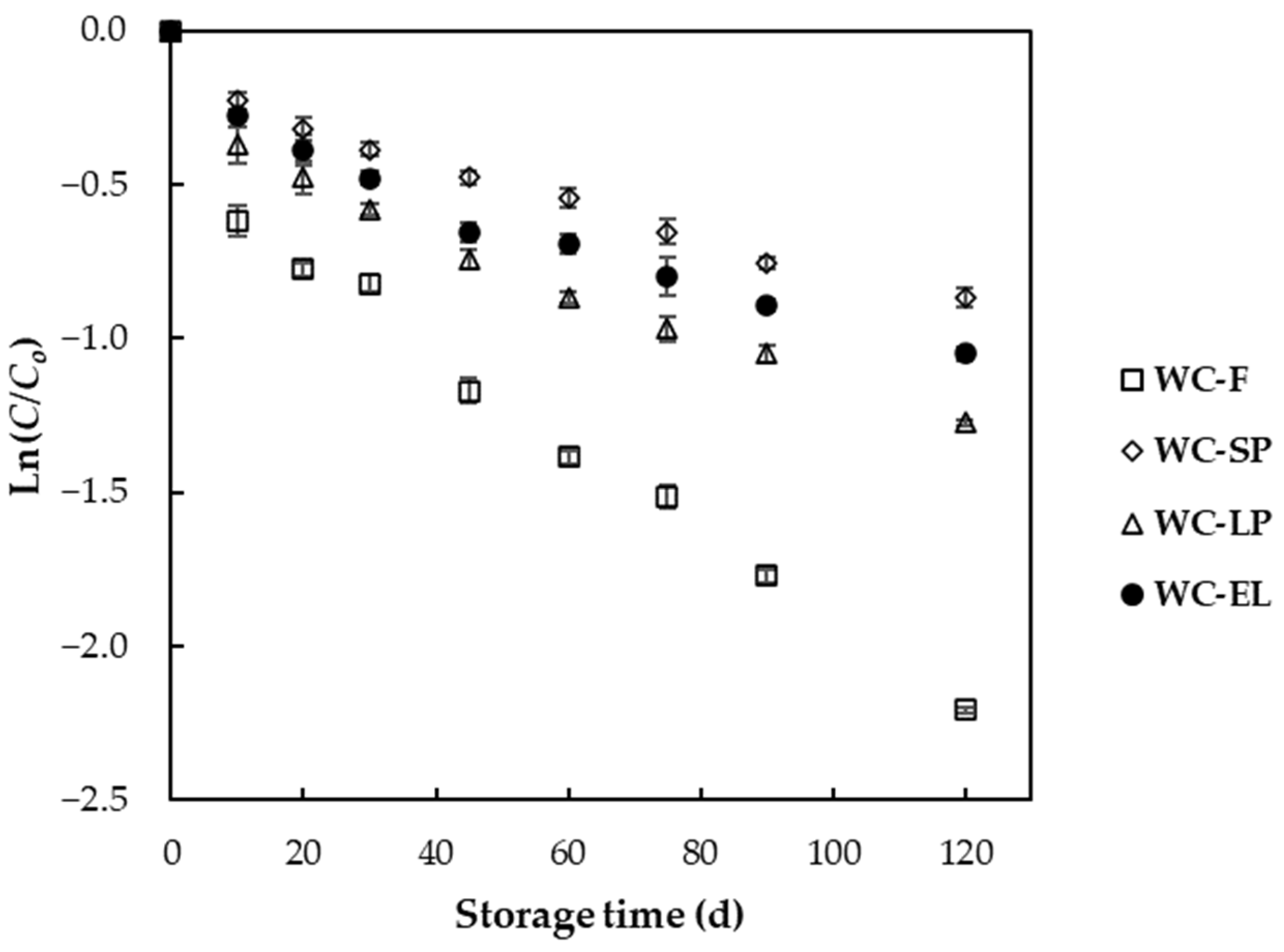
3.2.5. Degradation Kinetics of β-Carotene during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selvasekaran, P.; Chidambaram, R. Advances in formulation for the production of low-fat, fat-free, low-sugar, and sugar-free chocolates: An overview of the past decade. Trends Food Sci. Technol. 2021, 113, 315–334. [Google Scholar] [CrossRef]
- Goya, L.; Kongor, J.E.; de Pascual-Teresa, S. From Cocoa to Chocolate: Effect of Processing on Flavanols and Methylxanthines and Their Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 14365. [Google Scholar] [CrossRef] [PubMed]
- Jaćimović, S.; Popović-Djordjević, J.; Sarić, B.; Krstić, A.; Mickovski-Stefanović, V.; Pantelić, N.Đ. Antioxidant Activity and Multi-Elemental Analysis of Dark Chocolate. Foods 2022, 11, 1445. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Comprehensive evaluation of phenolic profile in dark chocolate and dark chocolate enriched with Sakura green tea leaves or turmeric powder. Food Res. Int. 2018, 112, 1–16. [Google Scholar] [CrossRef]
- Konar, N.; Toker, O.S.; Oba, S.; Sagdic, O. Improving functionality of chocolate: A review on probiotic, prebiotic, and/or synbiotic characteristics. Trends Food Sci. Technol. 2016, 49, 35–44. [Google Scholar] [CrossRef]
- Maillard, M.; Landuyt, A. Chocolate: An ideal carrier for probiotics. Agro Food Ind. Hi Tech 2008, 19, 13–15. [Google Scholar]
- Gil-Ramírez, A.; Ruiz-Rodríguez, A.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Effect of ergosterol-enriched extracts obtained from Agaricus bisporus on cholesterol absorption using an in vitro digestion model. J. Funct. Foods 2014, 11, 589–597. [Google Scholar] [CrossRef]
- Poliński, S.; Topka, P.; Tańska, M.; Kowalska, S.; Czaplicki, S.; Szydłowska-Czerniak, A. Impact of Bioactive Compounds of Plant Leaf Powders in White Chocolate Production: Changes in Antioxidant Properties during the Technological Processes. Antioxidants 2022, 11, 752. [Google Scholar] [CrossRef]
- Lončarević, I.; Pajin, B.; Tumbas Šaponjac, V.; Petrović, J.; Vulić, J.; Fišteš, A.; Jovanović, P. Physical, sensorial and bioactive characteristics of white chocolate with encapsulated green tea extract. J. Sci. Food Agric. 2019, 99, 5834–5841. [Google Scholar] [CrossRef]
- Lončarević, I.; Pajin, B.; Fišteš, A.; Tumbas Šaponjac, V.; Petrović, J.; Jovanović, P.; Vulić, J.; Zarić, D. Enrichment of white chocolate with blackberry juice encapsulate: Impact on physical properties, sensory characteristics and polyphenol content. LWT 2018, 92, 458–464. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Komes, D.; Benković, M.; Karlović, S.; Hečimović, I.; Ježek, D.; Bauman, I. Innovative formulations of chocolates enriched with plant polyphenols from Rubus idaeus L. leaves and characterization of their physical, bioactive and sensory properties. Food Res. Int. 2012, 48, 820–830. [Google Scholar] [CrossRef]
- Toker, O.S.; Konar, N.; Pirouzian, H.R.; Oba, S.; Polat, D.G.; Palabiyik, İ.; Poyrazoglu, E.S.; Sagdic, O. Developing functional white chocolate by incorporating different forms of EPA and DHA-Effects on product quality. LWT 2018, 87, 177–185. [Google Scholar] [CrossRef]
- Genc Polat, D.; Durmaz, Y.; Konar, N.; Toker, O.S.; Palabiyik, I.; Tasan, M. Using encapsulated Nannochloropsis oculata in white chocolate as coloring agent. J. Appl. Phycol. 2020, 32, 3077–3088. [Google Scholar] [CrossRef]
- Eun, J.-B.; Maruf, A.; Das, P.R.; Nam, S.-H. A review of encapsulation of carotenoids using spray drying and freeze drying. Crit. Rev. Food Sci. Nutr. 2020, 60, 3547–3572. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food Colour Additives: A Synoptical Overview on Their Chemical Properties, Applications in Food Products, and Health Side Effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef]
- Santos, P.D.d.F.; Rubio, F.T.V.; da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich materials: A review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Hamdi, S. Kinetic Degradation and Storage Stability of β-Carotene Encapsulated by Freeze-Drying Using Almond Gum and Gum Arabic as Wall Materials. J. Food Process. Preserv. 2015, 39, 896–906. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D.; Stathopoulos, C.E. A storage study of encapsulated gac (Momordica cochinchinensis) oil powder and its fortification into foods. Food Bioprod. Process. 2015, 96, 113–125. [Google Scholar] [CrossRef]
- Coronel-Aguilera, C.P.; San Martín-González, M.F. Encapsulation of spray dried β-carotene emulsion by fluidized bed coating technology. LWT-Food Sci. Technol. 2015, 62, 187–193. [Google Scholar] [CrossRef]
- Drosou, C.G.; Krokida, M.K.; Biliaderis, C.G. Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: A comparative assessment of food-related applications. Dry. Technol. 2017, 35, 139–162. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M. A Comparative Study of Encapsulation of β-Carotene via Spray-Drying and Freeze-Drying Techniques Using Pullulan and Whey Protein Isolate as Wall Material. Foods 2024, 13, 1933. [Google Scholar] [CrossRef] [PubMed]
- Laina, K.T.; Drosou, C.; Giannenas, I.; Krokida, M. Formulation of novel functional extrudates containing natural bioactive compounds. Dry. Technol. 2024, 1–13. [Google Scholar] [CrossRef]
- Laina, K.T.; Drosou, C.; Krokida, M. Evaluation of functional extrudates enriched with essential oils for enhanced stability. Food Bioprod. Process. 2024, 147, 264–276. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Composite pullulan-whey protein nanofibers made by electrospinning: Impact of process parameters on fiber morphology and physical properties. Food Hydrocoll. 2018, 77, 726–735. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Encapsulation of β-carotene into food-grade nanofibers via coaxial electrospinning of hydrocolloids: Enhancement of oxidative stability and photoprotection. Food Hydrocoll. 2022, 133, 107949. [Google Scholar] [CrossRef]
- Stramarkou, M.; Oikonomopoulou, V.; Panagiotopoulou, M.; Papadaki, S.; Krokida, M. Sustainable Valorisation of Peach and Apricot Waste Using Green Extraction Technique with Conventional and Deep Eutectic Solvents. Resources 2023, 12, 72. [Google Scholar] [CrossRef]
- Aidoo, R.P.; Afoakwa, E.O.; Dewettinck, K. Rheological properties, melting behaviours and physical quality characteristics of sugar-free chocolates processed using inulin/polydextrose bulking mixtures sweetened with stevia and thaumatin extracts. LWT-Food Sci. Technol. 2015, 62, 592–597. [Google Scholar] [CrossRef]
- Rodriguez Furlán, L.T.; Baracco, Y.; Lecot, J.; Zaritzky, N.; Campderrós, M.E. Influence of hydrogenated oil as cocoa butter replacers in the development of sugar-free compound chocolates: Use of inulin as stabilizing agent. Food Chem. 2017, 217, 637–647. [Google Scholar] [CrossRef]
- Konar, N.; Palabiyik, I.; Toker, O.S.; Polat, D.G.; Kelleci, E.; Pirouzian, H.R.; Akcicek, A.; Sagdic, O. Conventional and sugar-free probiotic white chocolate: Effect of inulin DP on various quality properties and viability of probiotics. J. Funct. Foods 2018, 43, 206–213. [Google Scholar] [CrossRef]
- Didar, Z. Characterization of White Chocolate Enriched with Free or Encapsulated Pomegranate Extract. J. Nutr. Fasting Health 2020, 8, 302–309. [Google Scholar] [CrossRef]
- Glicerina, V.; Romani, S. Chapter 18-Advances in Yield Stress Measurements for Chocolate. In Advances in Food Rheology and Its Applications; Ahmed, J., Ptaszek, P., Basu, S., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 459–481. [Google Scholar] [CrossRef]
- Zarić, D.B.; Bulatović, M.L.; Rakin, M.B.; Krunić, T.Ž.; Lončarević, I.S.; Pajin, B.S. Functional, rheological and sensory properties of probiotic milk chocolate produced in a ball mill. RSC Adv. 2016, 6, 13934–13941. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Vieira, J. Characterization of melting properties in dark chocolates from varying particle size distribution and composition using differential scanning calorimetry. Food Res. Int. 2008, 41, 751–757. [Google Scholar] [CrossRef]
- Shah, A.B.; Jones, G.P.; Vasiljevic, T. Sucrose-free chocolate sweetened with Stevia rebaudiana extract and containing different bulking agents–effects on physicochemical and sensory properties. Int. J. Food Sci. Technol. 2010, 45, 1426–1435. [Google Scholar] [CrossRef]
- Aidoo, R.P.; Depypere, F.; Afoakwa, E.O.; Dewettinck, K. Industrial manufacture of sugar-free chocolates–Applicability of alternative sweeteners and carbohydrate polymers as raw materials in product development. Trends Food Sci. Technol. 2013, 32, 84–96. [Google Scholar] [CrossRef]
- Konar, N.; Özhan, B.; Artık, N.; Dalabasmaz, S.; Poyrazoglu, E.S. Rheological and physical properties of Inulin-containing milk chocolate prepared at different process conditions. CyTA-J. Food 2014, 12, 55–64. [Google Scholar] [CrossRef]
- Rocha, G.A.; Fávaro-Trindade, C.S.; Grosso, C.R.F. Microencapsulation of lycopene by spray drying: Characterization, stability and application of microcapsules. Food Bioprod. Process. 2012, 90, 37–42. [Google Scholar] [CrossRef]
- Gomes, L.M.M.; Petito, N.; Costa, V.G.; Falcão, D.Q.; de Lima Araújo, K.G. Inclusion complexes of red bell pepper pigments with β-cyclodextrin: Preparation, characterisation and application as natural colorant in yogurt. Food Chem. 2014, 148, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.T.; Aryana, K.J.; Losso, J.N. Storage Stability of Lutein During Ripening of Cheddar Cheese. J. Dairy Sci. 2005, 88, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, L.M.; Lee, S.-Y.; Engeseth, N.J. Impact of Storage on Dark Chocolate: Texture and Polymorphic Changes. J. Food Sci. 2011, 76, C142–C153. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Furlán, L.T.; Baracco, Y.; Lecot, J.; Zaritzky, N.; Campderrós, M.E. Effect of sweetener combination and storage temperature on physicochemical properties of sucrose free white chocolate. Food Chem. 2017, 229, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Ligęza, E.; Marzec, A.; Górska, A.; Wirkowska-Wojdyła, M.; Bryś, J.; Rejch, A.; Czarkowska, K. A comparative study of thermal and textural properties of milk, white and dark chocolates. Thermochim. Acta 2019, 671, 60–69. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Vieira, J. Fat bloom development and structure-appearance relationships during storage of under-tempered dark chocolates. J. Food Eng. 2009, 91, 571–581. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Joshi, Y.M. Effect of thermal and mechanical rejuvenation on the rheological behavior of chocolate. Phys. Fluids 2022, 34, 037111. [Google Scholar] [CrossRef]
- Vásquez, C.; Henríquez, G.; López, J.V.; Penott-Chang, E.K.; Sandoval, A.J.; Müller, A.J. The effect of composition on the rheological behavior of commercial chocolates. LWT 2019, 111, 744–750. [Google Scholar] [CrossRef]
- Silva, M.; Tulini, F.; Marinho, J.; Mazzocato, M.; Martinis, E.; Luccas, V.; Favaro-Trindade, C. Semisweet chocolate as a vehicle for the probiotics Lactobacillus acidophilus LA3 and Bifidobacterium animalis subsp. lactis BLC1: Evaluation of chocolate stability and probiotic survival under in vitro simulated gastrointestinal conditions. LWT-Food Sci. Technol. 2016, 75, 640–647. [Google Scholar] [CrossRef]
- Nafingah, R.; Kurniasari, J.; Cahyani, A.; Harmayani, E.; Saputro, A.D. Investigating the impact of Palm Sap Sugar proportion and fat content on heat stability of Milk Chocolate. IOP Conf. Ser. Earth Environ. Sci. 2019, 355, 012043. [Google Scholar] [CrossRef]
- Liang, R.; Huang, Q.; Ma, J.; Shoemaker, C.F.; Zhong, F. Effect of relative humidity on the store stability of spray-dried beta-carotene nanoemulsions. Food Hydrocoll. 2013, 33, 225–233. [Google Scholar] [CrossRef]
- Demiray, E.; Tulek, Y. Degradation kinetics of β-carotene in carrot slices during convective drying. Int. J. Food Prop. 2017, 20, 151–156. [Google Scholar] [CrossRef]
- Laina, K.T.; Drosou, C.; Krokida, M. Comparative assessment of encapsulated essential oils through the innovative electrohydrodynamic processing and the conventional spray drying, and freeze-drying techniques. Innov. Food Sci. Emerg. Technol. 2024, 95, 103720. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Indrani, D.; Jena, B.S.; Anandharamakrishnan, C. Freeze drying technique for microencapsulation of Garcinia fruit extract and its effect on bread quality. J. Food Eng. 2013, 117, 513–520. [Google Scholar] [CrossRef]

| WC-C | WC-F | WC-SP | WC-LP | WC-EL | |
|---|---|---|---|---|---|
| β-carotene concentration in white chocolate | 1.0 mg/100 g white chocolate | ||||
| Mass of encapsulated β-carotene structures /total mass of white chocolate fortified with encapsulated structures (% (w/w)) | 0 | 0 | 0.67 | 7.50 | 2.67 |
| Onset temperature (°C) | 21.79 ± 0.77 a | 23.05 ± 0.32 b | 23.58 ± 0.77 b | 17.24 ± 0.15 c | 20.77 ± 0.24 d |
| End temperature (°C) | 31.08 ± 0.05 a,c | 31.31 ± 0.10 a | 30.25 ± 0.14 b | 31.32 ± 0.05 a | 30.63 ± 0.72 b,c |
| Peak temperature (°C) | 26.43 ± 0.32 a | 26.60 ± 0.08 a | 26.02 ± 0.04 a,b | 26.15 ± 0.03 a,b | 25.79 ± 0.61 b |
| Enthalpy required for melting of chocolate (ΔH, J/g) | 12.42 ± 1.18 a | 9.68 ± 1.55 b | 8.29 ± 0.58 b | 30.88 ± 1.94 c | 16.00 ± 0.53 d |
| Sample | k·10−3 ± sk·10−3 (d−1) | t1/2 (d) | R2 |
|---|---|---|---|
| WC-F | 16.4 ± 1.6 a | 61.95 | 0.95 |
| WC-SP | 6.6 ± 0.6 d | 126.04 | 0.95 |
| WC-LP | 9.4 ± 0.2 b | 98.33 | 0.93 |
| WC-EL | 8.0 ± 0.4 c | 109.51 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drosou, C.; Krokida, M. Enrichment of White Chocolate with Microencapsulated β-Carotene: Impact on Quality Characteristics and β-Carotene Stability during Storage. Foods 2024, 13, 2699. https://doi.org/10.3390/foods13172699
Drosou C, Krokida M. Enrichment of White Chocolate with Microencapsulated β-Carotene: Impact on Quality Characteristics and β-Carotene Stability during Storage. Foods. 2024; 13(17):2699. https://doi.org/10.3390/foods13172699
Chicago/Turabian StyleDrosou, Christina, and Magdalini Krokida. 2024. "Enrichment of White Chocolate with Microencapsulated β-Carotene: Impact on Quality Characteristics and β-Carotene Stability during Storage" Foods 13, no. 17: 2699. https://doi.org/10.3390/foods13172699
APA StyleDrosou, C., & Krokida, M. (2024). Enrichment of White Chocolate with Microencapsulated β-Carotene: Impact on Quality Characteristics and β-Carotene Stability during Storage. Foods, 13(17), 2699. https://doi.org/10.3390/foods13172699








