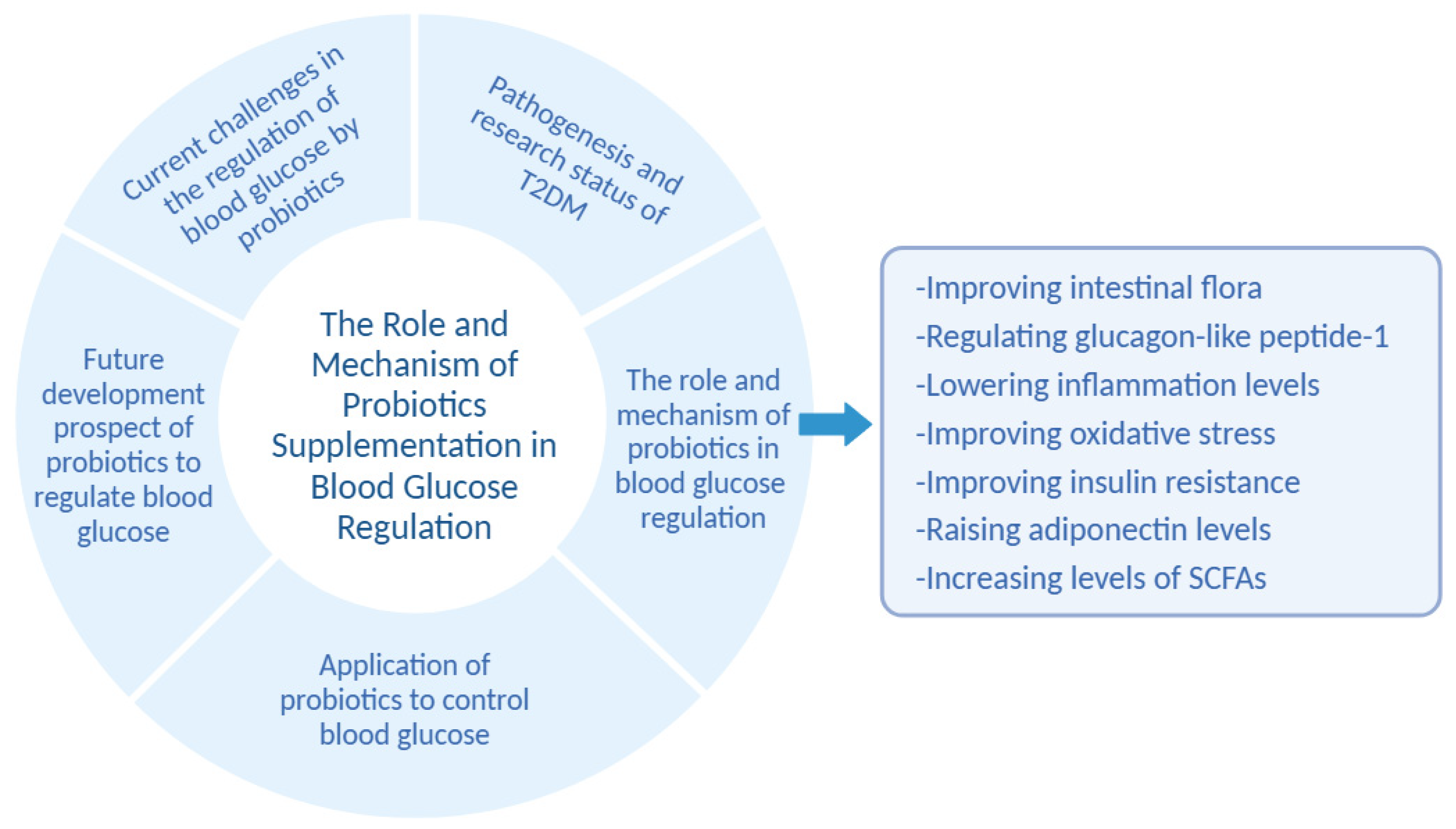
The Role and Mechanism of Probiotics Supplementation in Blood Glucose Regulation: A Review
Abstract
:1. Introduction
2. Methods
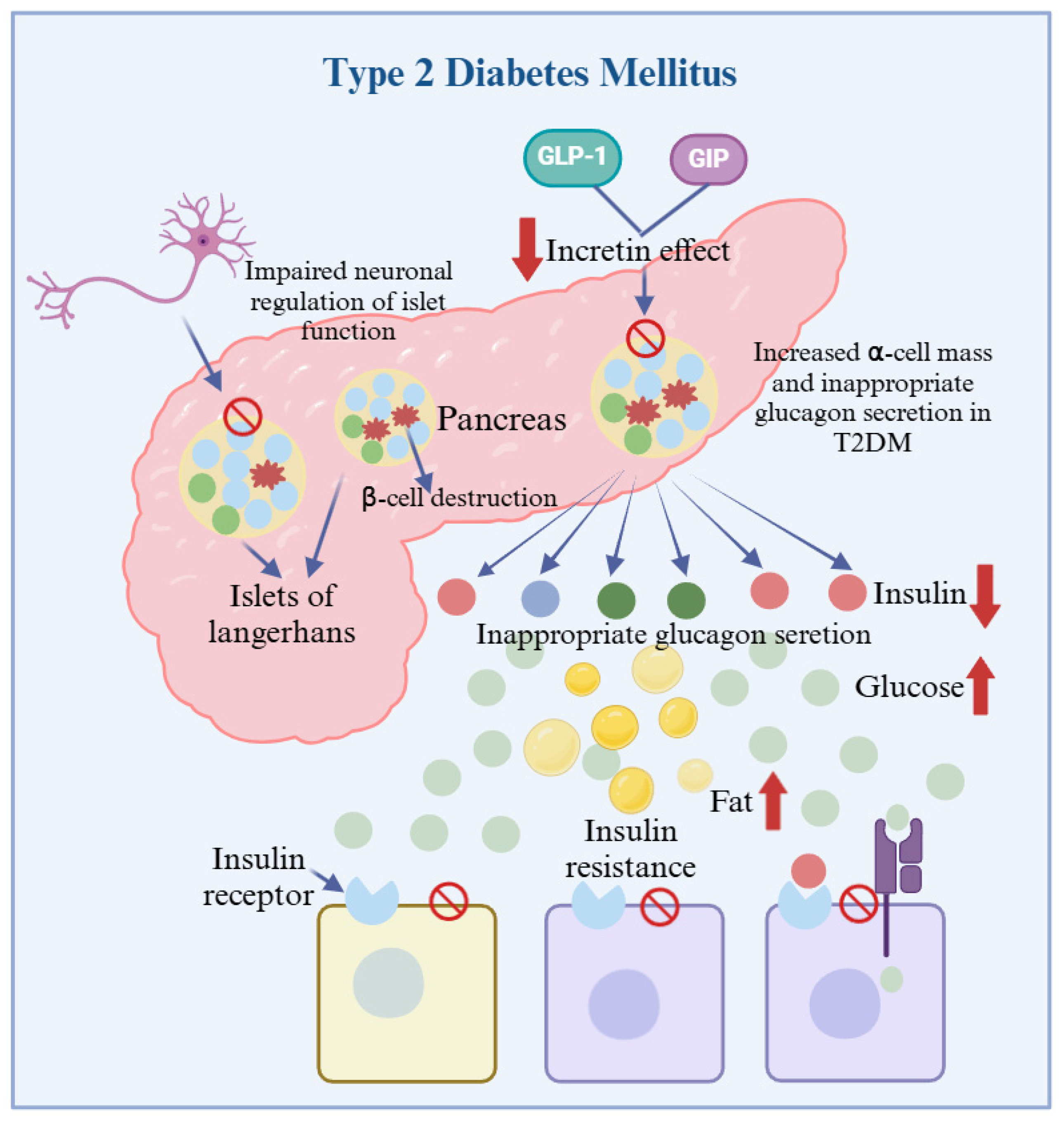
3. Pathogenesis and Research Status of T2DM
4. The Role and Mechanism of Probiotics in Blood Glucose Regulation
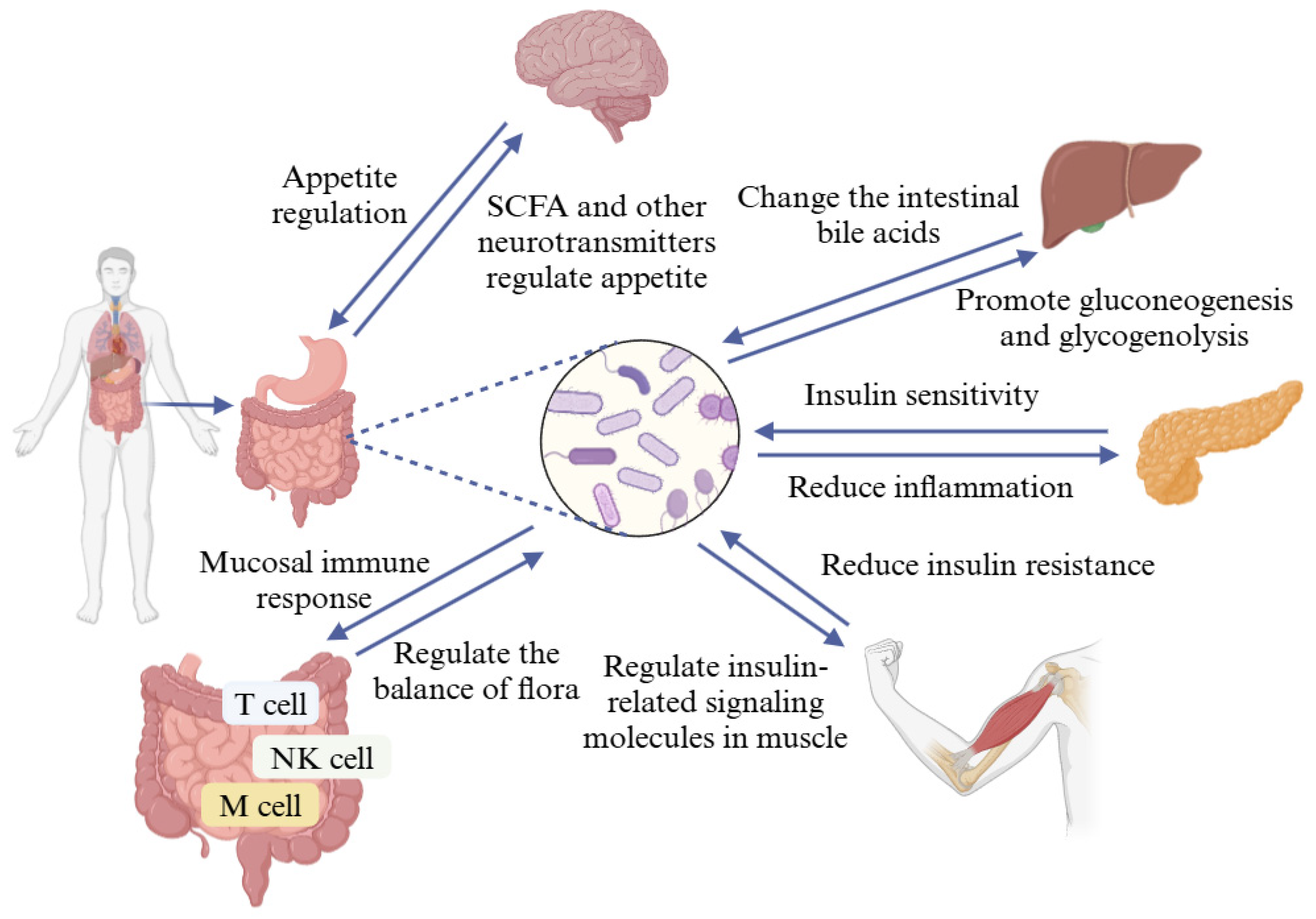
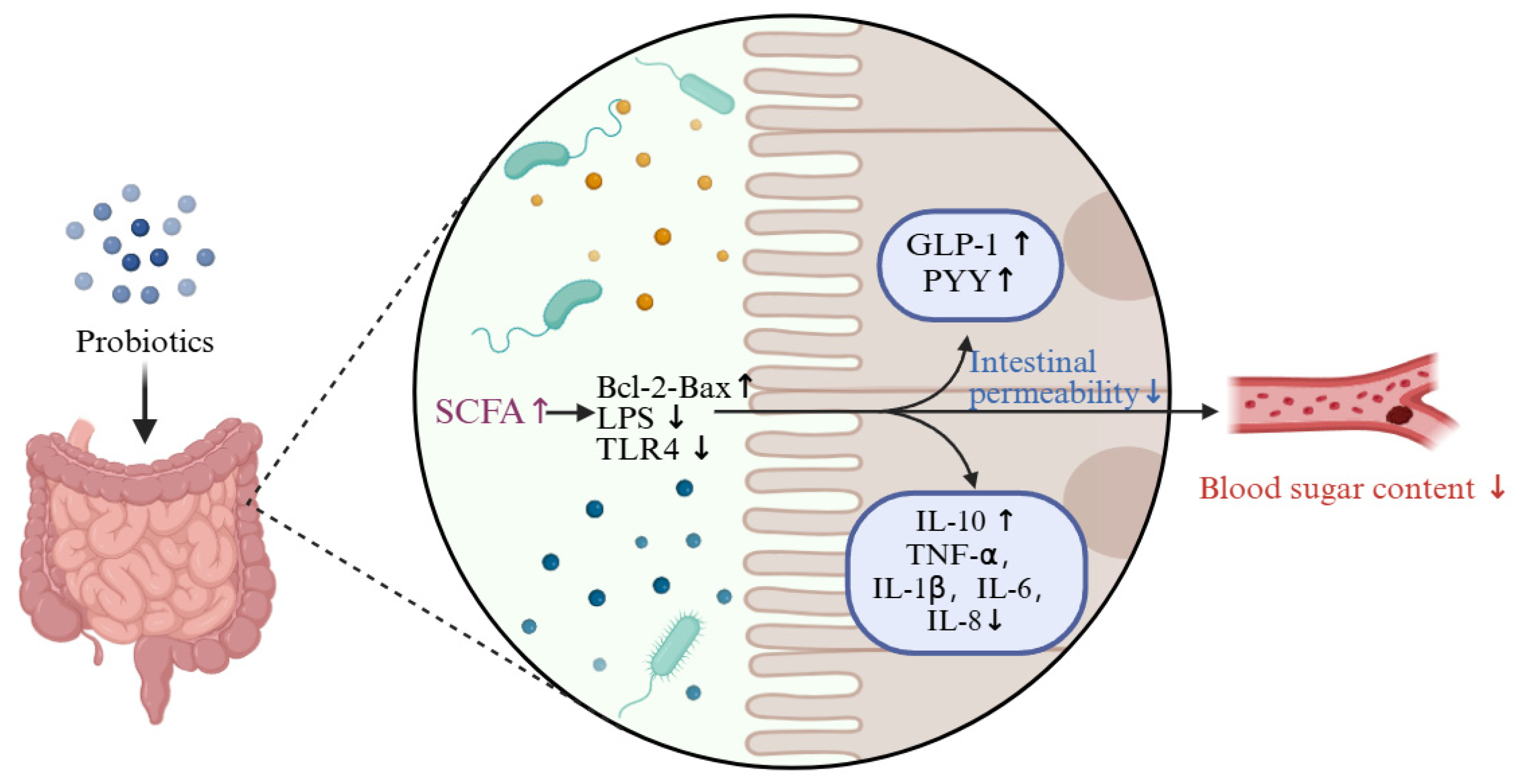
4.1. Probiotics Regulate Blood Glucose by Improving Intestinal Flora
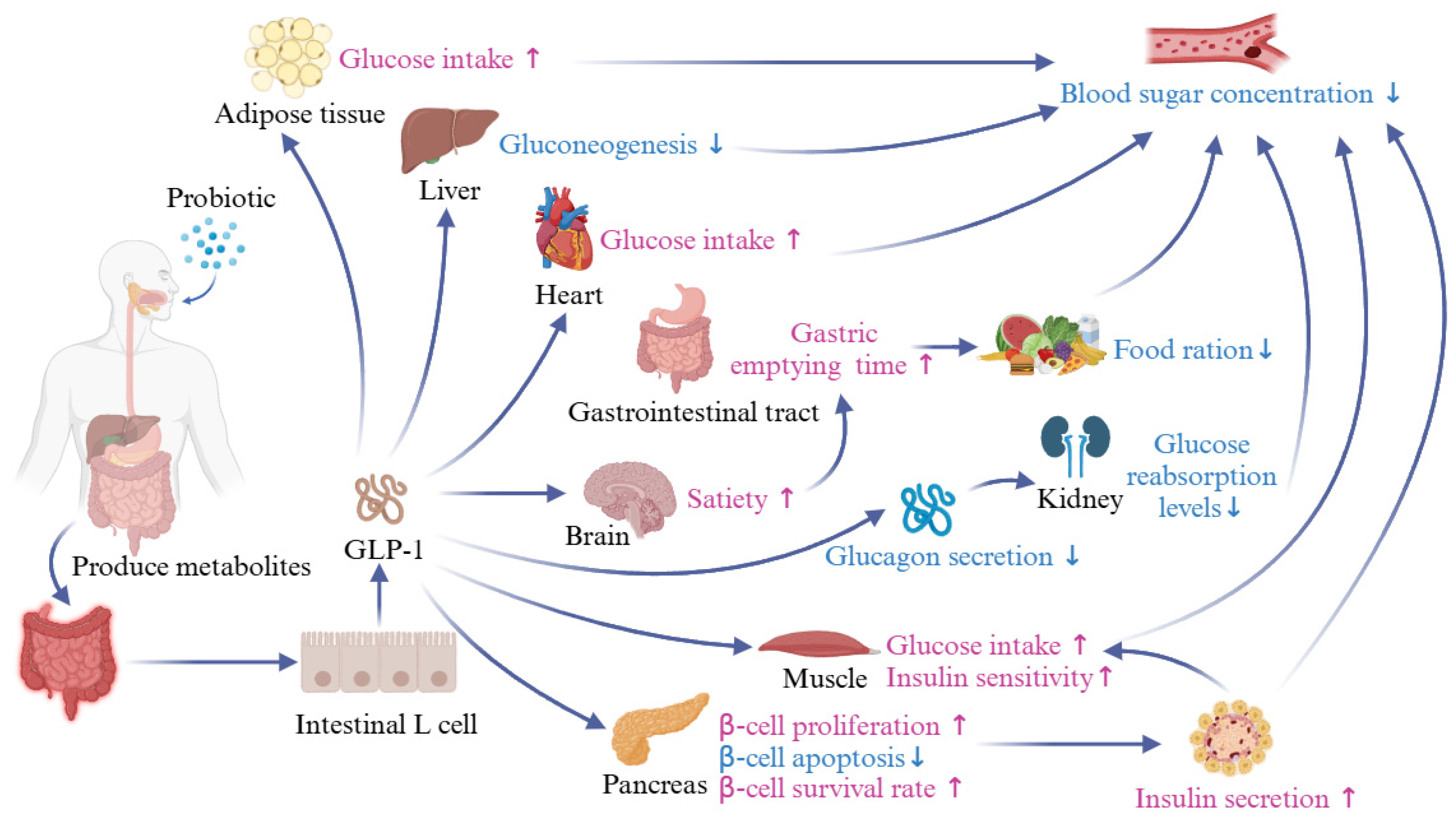
4.2. Probiotics Regulate Blood Glucose by Regulating Glucagon-like Peptide-1
4.3. Probiotics Regulate Blood Glucose by Lowering Inflammation Levels
4.4. Probiotics Regulate Blood Glucose by Improving Oxidative Stress
4.5. Probiotics Regulate Blood Glucose by Improving Insulin Resistance
4.6. Probiotics Regulate Blood Glucose by Raising Adiponectin Levels
4.7. Probiotics Regulate Blood Glucose by Increasing Levels of SCFAs
5. Application of Probiotics to Control Blood Glucose
| Probiotics | Animal Model | Dosage | Duration | Results | Reference |
|---|---|---|---|---|---|
| Lactobacillus plantarum HAC01 | STZ-induced C67BL/6J mice, T2DM | 1 × 109 CFU/mL | 10 weeks | Insulin-positive β-cells area of the islet ↑ FBS, HbA1c, OGTT and HOMA-IR ↓ | Lee et al. [120] |
| Lactiplantibacillus plantarum Y15 | STZ-induced C57BL/6J mice, T2DM | 3 × 108 CFU/mL | 6 weeks | Proinflammatory factors and LPS ↓ SCFA-producing bacteria ↑ Regulated the expression of genes related to inflammation and insulin signaling pathways | Liu et al. [121] |
| Lactobacillus gasseri | Western diet–induced C57BL/6J mice, T2DM | 1 × 109 CFU/mL | 8 weeks | Serum glutathione and bilirubin ↑ Blood glucose, blood lipids ↓ | Rodrigues et al. [122] |
| Lactobacillus plantarum CGMCC 8198 | High fat diet–induced Kunming mice, T2DM | 0.2 mL/10 g | 8 weeks | Harmful bacteria, blood glucose, and blood lipids ↓ Immunity ↑ | Jiang et al. [123] |
| Akkermansia muciniphila | STZ-induced SD rats, T2DM | 1 × 1010 CFU/mL | 4 weeks | HDL-C ↑ Liver glycogen, plasminogen activator inhibitor-1, TNF-α, LPS, malondialdehyde, GLP-1 ↓ | Zhang et al. [124] |
| Lactobacillus reuteri GMNL-263 | STZ-induced Wistar rats, T2DM | 1 × 109 CFU/mL | 4 weeks | Activate the IGF1R cells’ survival pathway ↑ Cells apoptosis via the IGF1R survival pathway in diabetic rats ↓ | Koay et al. [125] |
| Lactobacillus sakei Probio-65 and Lactobacillus plantarum Probio-093 | High fat diet–induced C57BL/6J male mice, T2DM | 0.25 mg/g/day | 8 weeks | α-glucosidase, α-amylase, blood glucose, and body weight ↓ Regulated the intestinal flora | Gulnaz et al. [126] |
| Lactobacillus fermentum TKSN041 | STZ-induced Wistar male rats, T2DM | — | — | Blood glucose, tissue damage; body weight, blood lipids, and inflammation levels ↓ | Zhou et al. [127] |
| Lactobacillus fermentum MCC2759 and Lactobacillus fermentum MCC2760 | STZ-induced Wistar rats, T2DM | 1 × 109 CFU/mL | 12 weeks | OGTT, Insulin, IL-10, ZO-1, GLP-1 ↓ | Archer et al. [128] |
| Probiotics | Sample | Dosage | Duration | Results | Reference |
|---|---|---|---|---|---|
| Bifidobacterium bifidum and Lactobacillus acidophilus | 20 patients with T2DM | 1 × 108 CFU/mL | 2 weeks | HDL-C ↑ Fasting glycemia ↓ | Moroti et al. [129] |
| Lactobacillus paracasei HII01 | 50 patients with T2DM | 50 × 109 CFU/d | 12 weeks | FBS, LPS, TNF-α, IL-6 and hsCRP ↓ | Toejing et al. [14] |
| Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum | 60 patients with TDM | 2 × 109 CFU | 12 weeks | Blood glucose and insulin sensitivity ↓ | Soleimain et al. [130] |
| Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus rhamnosus | 54 patients with T2DM | 1 × 109 CFU/mL | 8 weeks | TGL and HOMA-IR plasma levels ↑ Serum CRP ↓ | Asemi et al. [114] |
| Lactobacillus acidophilus | 136 patients with T2DM | 1 × 108 CFU | 12 weeks | Blood glucose ↓ Activity of antioxidant enzymes ↑ | Mirmiranpour et al. [131] |
| Lactobacillus reuteri DSM 17938 | 46 patients with T2DM | 1 × 1010 CFU/d | 12 weeks | FBS, HbA1c, insulin, TC, TG, LDL-C, CRP ↓ HDL-C ↑ | Mobini et al. [132] |
| Lactobacillus sporogenes | 81 patients with T2DM | 1 × 108 CFU | 8 weeks | Serum insulin levels ↓ | Tajadadi et al. [133] |
6. Future Development Prospect of Probiotics to Regulate Blood Glucose
6.1. Screening of Potential Hypoglycemic Probiotics
6.2. Development of Synthetic Biology
6.3. Next-Generation Probiotics
6.4. Postbiotics and Paraprobiotics
7. Current Challenges in the Regulation of Blood Glucose by Probiotics
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Salkar, M.; Rosenthal, M.; Thakur, T.; Arnold, A. Patient Centered Studies Focusing on Diabetes Self-Management: A Scoping Review. Curr. Diabetes Rev. 2020, 16, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.Y.; Guo, W.J.; Zhu, S.; Gong, G.D.; Chen, M.; Zhong, Z.L.; Guo, J.L.; Zhang, Y.Y. Type 2 diabetes mellitus and the risk of abnormal spermatozoa: A Mendelian randomization study. Front. Endocrinol. 2022, 13, 1035338. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, L.S.; Campbell, N.R.C.; Niebylski, M.L.; Nilsson, P.; Lackland, D.T.; World Hypertension, L. Implementation of World Health Organization Package of Essential Noncommunicable Disease Interventions (WHO PEN) for Primary Health Care in Low-Resource Settings: A Policy Statement From the World Hypertension League. J. Clin. Hypertens. 2016, 18, 5–6. [Google Scholar] [CrossRef]
- Iqbal, J.; Wu, H.X.; Hu, N.; Zhou, Y.H.; Li, L.; Xiao, F.; Wang, T.; Jiang, H.L.; Xu, S.N.; Huang, B.L.; et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus-a systematic review and meta-analysis of randomized control trials. Obes. Rev. 2022, 23, e13435. [Google Scholar] [CrossRef] [PubMed]
- Amjad, S.; Jafri, A.; Sharma, A.K.; Serajuddin, M. A novel strategy of nanotized herbal drugs and their delivery in the treatment of diabetes: Present status and future prospects. J. Herb. Med. 2019, 17–18, 100279. [Google Scholar] [CrossRef]
- Shen, X.Y.; Xie, A.J.; Li, Z.J.; Jiang, C.X.; Wu, J.Q.; Li, M.H.; Yue, X.Q. Research Progress for Probiotics Regulating Intestinal Flora to Improve Functional Dyspepsia: A Review. Foods 2024, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.J.; Zhao, S.S.; Liu, Z.F.; Yue, X.Q.; Shao, J.H.; Li, M.H.; Li, Z.W. Polysaccharides, proteins, and their complex as microencapsulation carriers for delivery of probiotics: A review on carrier types and encapsulation techniques. Int. J. Biol. Macromol. 2023, 242, 124784. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, J.C.; Martínez-Tapia, A.; Lazcano-Hernández, G.; García-Pérez, B.E.; Castrejón-Jiménez, N.S. Bacteriocins from Lactic Acid Bacteria. A Powerful Alternative as Antimicrobials, Probiotics, and Immunomodulators in Veterinary Medicine. Animals 2021, 11, 979. [Google Scholar] [CrossRef]
- Peng, X.Q.; Ed-Dra, A.; Song, Y.; Elbediwi, M.; Nambiar, R.B.; Zhou, X.; Yue, M. Lacticaseibacillus rhamnosus alleviates intestinal inflammation and promotes microbiota-mediated protection against Salmonella fatal infections. Front. Immunol. 2022, 13, 973224. [Google Scholar] [CrossRef]
- Smith, D.; Jheeta, S.; Fuentes, H.V.; Palacios-Pérez, M. Feeding Our Microbiota: Stimulation of the Immune/Semiochemical System and the Potential Amelioration of Non-Communicable Diseases. Life 2022, 12, 1197. [Google Scholar] [CrossRef]
- Yang, Y.; Song, X.; Wang, G.Q.; Xia, Y.J.; Xiong, Z.Q.; Ai, L.Z. Understanding Ligilactobacillus salivarius from Probiotic Properties to Omics Technology: A Review. Foods 2024, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Yabut, J.M.; Choo, J.M.; Page, A.J.; Sun, E.W.; Jessup, C.F.; Wesselingh, S.L.; Khan, W.I.; Rogers, G.B.; Steinberg, G.R.; et al. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc. Natl. Acad. Sci. USA 2019, 116, 19802–19804. [Google Scholar] [CrossRef] [PubMed]
- Toejing, P.; Khampithum, N.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Influence of Lactobacillus paracasei HII01 Supplementation on Glycemia and Inflammatory Biomarkers in Type 2 Diabetes: A Randomized Clinical Trial. Foods 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.X.; Chen, H.R.; Li, X.; Li, D.; Sun, Y.; Yang, L.; Ma, Y.; Chan, E.C.Y. Lactobacillus paracasei IMC 502 ameliorates type 2 diabetes by mediating gut microbiota-SCFA-hormone/inflammation pathway in mice. J. Sci. Food Agric. 2023, 103, 2949–2959. [Google Scholar] [CrossRef]
- Colston, J.M.; Taniuchi, M.; Ahmed, T.; Ferdousi, T.; Kabir, F.; Mduma, E.; Nshama, R.; Iqbal, N.T.; Haque, R.; Ahmed, T.; et al. Intestinal Colonization with Bifidobacterium longum Subspecies Is Associated with Length at Birth, Exclusive Breastfeeding, and Decreased Risk of Enteric Virus Infections, but Not with Histo-Blood Group Antigens, Oral Vaccine Response or Later Growth in Three Birth Cohorts. Front. Pediatr. 2022, 10, 804798. [Google Scholar]
- Chichlowski, M.; Shah, N.; Wampler, J.L.; Wu, S.S.; Vanderhoof, J.A. Bifidobacterium longum Subspecies infantis (B. infantis) in Pediatric Nutrition: Current State of Knowledge. Nutrients 2020, 12, 1581. [Google Scholar] [CrossRef]
- Feng, X.B.; Su, Y.; Jiang, J.; Li, N.; Ding, W.W.; Wang, Z.M.; Hu, X.H.; Zhu, W.Y.; Li, J.S. Changes in Fecal and Colonic Mucosal Microbiota of Patients with Refractory Constipation after a Subtotal Colectomy. Am. Surg. 2015, 81, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.C.; Lee, C.L.; Chai, C.Y.; Chen, W.T.; Lu, Y.C.; Wu, C.S. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr. Metab. 2013, 10, 35. [Google Scholar] [CrossRef]
- Huang, H.Y.; Korivi, M.; Tsai, C.H.; Yang, J.H.; Tsai, Y.C. Supplementation of Lactobacillus plantarum K68 and Fruit-Vegetable Ferment along with High Fat-Fructose Diet Attenuates Metabolic Syndrome in Rats with Insulin Resistance. Evid.-Based Complement. Altern. Med. 2013, 2013, 943020. [Google Scholar] [CrossRef]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.G.; Moller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.H.; Stehle, P.; Pacini, G.; et al. Intake of Lactobacillus reuteri Improves Incretin and Insulin Secretion in Glucose-Tolerant Humans: A Proof of Concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Ozaki, M.; Tamura, A.; Yamada, N.; Ishida, T.; Hosoda, M.; Hosono, A. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2003, 67, 1421–1424. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef]
- Meucci, A.; Zago, M.; Rossetti, L.; Fornasari, M.E.; Bonvini, B.; Tidona, F.; Povolo, M.; Contarini, G.; Carminati, D.; Giraffa, G. Lactococcus hircilactis sp nov and Lactococcus laudensis sp nov., isolated from milk. Int. J. Syst. Evol. Microbiol. 2015, 65, 2091–2096. [Google Scholar] [CrossRef]
- Firmani, S.E.; Maples, H.D.; Balamohan, A. Lactococcus Species Central Line-Associated Bloodstream Infection in Pediatrics: A Case Series. Front. Med. 2022, 9, 802493. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Guo, Q.B.; Zhang, H.; Wu, Y.; Hang, X.M.; Ai, L.Z. Exopolysaccharide produced by Streptococcus thermophiles S-3: Molecular, partial structural and rheological properties. Carbohydr. Polym. 2018, 194, 132–138. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, M.S.; Jung, J.Y.; Jeon, C.O. Leuconostoc miyukkimchii sp nov., isolated from brown algae (Undaria pinnatifida) kimchi. Int. J. Syst. Evol. Microbiol. 2012, 62, 1098–1103. [Google Scholar] [CrossRef]
- Olsen, K.N.; Brockmann, E.; Molin, S. Quantification of Leuconostoc populations in mixed dairy starter cultures using fluorescence in situ hybridization. J. Appl. Microbiol. 2007, 103, 855–863. [Google Scholar] [CrossRef]
- Yue, X.Q.; Li, M.H.; Liu, Y.M.; Zhang, X.M.; Zheng, Y. Microbial diversity and function of soybean paste in East Asia: What we know and what we don’t. Curr. Opin. Food Sci. 2021, 37, 145–152. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.M.; Liu, W.Y.; Zhao, J.X.; Zhang, H.; Zhai, Q.X.; Chen, W. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Alexandraki, V.; Georgalaki, M.; Anastasiou, R.; Manolopoulou, E.; Tsakalidou, E.; Papadimitriou, K. Production of probiotic Feta cheese using Propionibacterium freudenreichii subsp shermanii as adjunct. Int. Dairy J. 2017, 66, 135–139. [Google Scholar] [CrossRef]
- Reiche, A.; Sivell, J.L.; Kirkwood, K.M. Electricity generation by Propionibacterium freudenreichii in a mediatorless microbial fuel cell. Biotechnol. Lett. 2016, 38, 51–55. [Google Scholar] [CrossRef]
- Lara, Z.B.; Amoranto, M.B.C.; Elegado, F.B.; Dalmacio, L.M.M.; Balolong, M.P. Draft genome sequence of Pediococcus acidilactici 3G3 isolated from Philippine fermented pork. Microbiol. Resour. Announc. 2024, 13, e0129923. [Google Scholar] [CrossRef] [PubMed]
- Zommiti, M.; Boukerb, A.M.; Feuilloley, M.G.J.; Ferchichi, M.; Connil, N. Draft Genome Sequence of Pediococcus pentosaceus MZF16, a Bacteriocinogenic Probiotic Strain Isolated from Dried Ossban in Tunisia. Microbiol. Resour. Announc. 2019, 8, 19. [Google Scholar] [CrossRef]
- Xie, A.J.; Dong, Y.S.; Liu, Z.F.; Li, Z.W.; Shao, J.H.; Li, M.H.; Yue, X.Q. A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies. Foods 2023, 12, 3952. [Google Scholar] [CrossRef]
- Suzuki, K.; Ohsumi, Y. Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett. 2007, 581, 2156–2161. [Google Scholar] [CrossRef]
- Borren, E.; Tian, B. The Important Contribution of Non-Saccharomyces Yeasts to the Aroma Complexity of Wine: A Review. Foods 2021, 10, 13. [Google Scholar] [CrossRef]
- Yarimizu, T.; Nonklang, S.; Nakamura, J.; Tokuda, S.; Nakagawa, T.; Lorreungsil, S.; Sutthikhumpha, S.; Pukahuta, C.; Kitagawa, T.; Nakamura, M.; et al. Identification of auxotrophic mutants of the yeast Kluyveromyces marxianus by non-homologous end joining-mediated integrative transformation with genes from Saccharomyces cerevisiae. Yeast 2013, 30, 485–500. [Google Scholar] [CrossRef]
- Stepanovic, S.; Dakic, I.; Hauschild, T.; Vukovic, D.; Morrison, D.; Jezek, P.; Cirkovic, I.; Petras, P. Supplementary biochemical tests useful for the differentiation of oxidase positive staphylococci. Syst. Appl. Microbiol. 2007, 30, 316–318. [Google Scholar] [CrossRef]
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent Developments in Methicillin-Resistant Staphylococcus aureus (MRSA) Treatment: A Review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef]
- Vernozy-Rozand, C.; Mazuy, C.; Meugnier, H.; Bes, M.; Lasne, Y.; Fiedler, F.; Etienne, J.; Freney, J. Staphylococcus fleurettii sp. nov., isolated from goat’s milk cheeses. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 4, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Chai, Z.; Hutabarat, R.P.; Beta, T.; Feng, J.; Ma, K.Y.; Li, D.J.; Huang, W.Y. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biol. 2021, 46, 102100. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes, J.D.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Jeon, D.; Kim, S.; Kim, M.A.; Chong, Y.P.; Shim, T.S.; Jung, C.H.; Kim, Y.J.; Jo, K.W. Type 2 Diabetes Mellitus- and Complication-Related Risk of Nontuberculous Mycobacterial Disease in a South Korean Cohort. Microbiol. Spectr. 2023, 11, 4511–4522. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Mandal, S.; Tomar, S.K.; Anand, S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur. J. Nutr. 2015, 54, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, K.Y.; Wang, R.N.; Han, W.H.; Kuny, S.; Zelmanovitz, P.H.; Sauvé, Y.; Chan, C.B. β-Cell compensation concomitant with adaptive endoplasmic reticulum stress and β-cell neogenesis in a diet-induced type 2 diabetes model. Appl. Physiol. Nutr. Metab. 2019, 44, 1355–1366. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.P.; Pan, A.; Hu, Y.; Chen, S.Y.; Qian, F.; Rimm, E.B.; Manson, J.E.; Stampfer, M.J.; Giatsidis, G.; et al. Adherence to a Healthy Lifestyle in Association with Microvascular Complications Among Adults with Type 2 Diabetes. JAMA Netw. Open 2023, 6, 52239. [Google Scholar] [CrossRef]
- Ikeda, S.; Shinohara, K.; Enzan, N.; Matsushima, S.; Tohyama, T.; Funakoshi, K.; Kishimoto, J.; Itoh, H.; Komuro, I.; Tsutsui, H. A higher resting heart rate is associated with cardiovascular event risk in patients with type 2 diabetes mellitus without known cardiovascular disease. Hypertens. Res. 2023, 46, 1090–1099. [Google Scholar] [CrossRef]
- Pieber, T.R.; Bajaj, H.S.; Heller, S.R.; Jia, T.; Khunti, K.; Klonoff, D.C.; Ladelund, S.; Leiter, L.A.; Wagner, L.; Philis-Tsimikas, A. Impact of kidney function on the safety and efficacy of insulin degludec versus insulin glargine U300 in people with type 2 diabetes: A post hoc analysis of the CONCLUDE trial. Diabetes Obes. Metab. 2022, 24, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.D.; Liang, F.X.; Tian, H.R.; Luo, D.; Wang, Y.Y.; Yang, S.R. Mechanisms of gut microbiota-immune-host interaction on glucose regulation in type 2 diabetes. Front. Microbiol. 2023, 14, 1121695. [Google Scholar] [CrossRef]
- Pintaric, M.; Langerholc, T. Probiotic Mechanisms Affecting Glucose Homeostasis: A Scoping Review. Life 2022, 12, 1187. [Google Scholar] [CrossRef]
- Liu, D.L.; Zhang, S.B.; Li, S.J.; Zhang, Q.; Cai, Y.; Li, P.; Li, H.; Shen, B.C.; Liao, Q.F.; Hong, Y.J.; et al. Indoleacrylic acid produced by Parabacteroides distasonis alleviates type 2 diabetes via activation of AhR to repair intestinal barrier. BMC Biol. 2023, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Shahali, A.; Soltani, R.; Akbari, V. Probiotic Lactobacillus and the potential risk of spreading antibiotic resistance: A systematic review. Res. Pharm. Sci. 2023, 18, 468–477. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Backer, J.L.; Churchill, T.A.; Obermeier, F.; Krause, D.O.; Madsen, K.L. Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect. Immun. 2007, 75, 2572–2579. [Google Scholar] [CrossRef]
- Ahmadi, S.; Wang, S.H.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.W.; Wang, Z.; Kavanagh, K.; et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 2020, 5, 132055. [Google Scholar] [CrossRef]
- Rebolledo, M.; Rojas, E.; Salgado, F. Effect of Two Probiotics Containing Lactobacillus casei rhamnosus and Lactobacillus johnsonii variety on the in vitro Growth of Streptococcus mutans. Int. J. Odontostomatol. 2013, 7, 415–419. [Google Scholar] [CrossRef]
- Miraghajani, M.; Dehsoukhteh, S.S.; Rafie, N.; Hamedani, S.G.; Sabihi, S.; Ghiasvand, R. Potential mechanisms linking probiotics to diabetes: A narrative review of the literature. Sao Paulo Med. J. 2017, 135, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Gabanyi, I.; Lepousez, G.; Wheeler, R.; Vieites-Prado, A.; Nissant, A.; Wagner, S.; Moigneu, C.; Dulauroy, S.; Hicham, S.; Polomack, B.; et al. Bacterial sensing via neuronal Nod2 regulates appetite and body temperature. Science 2022, 376, eabj3986. [Google Scholar] [CrossRef]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and Potentially Probiotic Yeasts-Characteristics and Food Application. Foods 2021, 10, 1306. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Cani, P.D.; Everard, A.; Neyrinck, A.M.; Bindels, L.B. Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia 2015, 58, 2206–2217. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Nakatsu, Y.; Kushiyama, A.; Yamamotoya, T.; Matsunaga, Y.; Inoue, M.K.; Fujishiro, M.; Sakoda, H.; Ohno, H.; Yoneda, M.; et al. Gut Microbiota as a Therapeutic Target for Metabolic Disorders. Curr. Med. Chem. 2018, 25, 984–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Dilidaxi, D.; Wu, Y.C.; Sailike, J.; Sun, X.; Nabi, X.H. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Ahn, Y.T.; Park, S.H.; Huh, C.S.; Yoo, S.R.; Yu, R.; Sung, M.K.; McGregor, R.A.; Choi, M.S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in Diet-Induced Obese Mice Is Associated with Gut Microbial Changes and Reduction in Obesity. PLoS ONE 2013, 8, e59470. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial Metabolic Effects of a Probiotic via Butyrate-induced GLP-1 Hormone Secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Wang, X.L.; Ye, F.; Li, J.; Zhu, L.Y.; Feng, G.; Chang, X.Y.; Sun, K. Impaired secretion of glucagon-like peptide 1 during oral glucose tolerance test in patients with newly diagnosed type 2 diabetes mellitus. Saudi Med. J. 2016, 37, 48–54. [Google Scholar] [CrossRef]
- Sun, X.L.; Zhang, Z.Y.; Liu, M.Y.; Zhang, P.; Nie, L.Q.; Liu, Y.Q.; Chen, Y.; Xu, F.J.; Liu, Z.H.; Zeng, Y.L. Small-molecule albumin ligand modification to enhance the anti-diabetic ability of GLP-1 derivatives. Biomed. Pharmacother. 2022, 148, 112722. [Google Scholar] [CrossRef]
- Babu, S.N.; Govindarajan, S.; Vijayalakshmi, M.A.; Noor, A. Role of zonulin and GLP-1/DPP-IV in alleviation of diabetes mellitus by peptide/polypeptide fraction of Aloe vera in streptozotocin- induced diabetic wistar rats. J. Ethnopharmacol. 2021, 272, 113949. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Mudgil, P.; Palakkott, A.; Iratni, R.; Gan, C.Y.; Maqsood, S.; Ayoub, M.A. Molecular basis of the anti-diabetic properties of camel milk through profiling of its bioactive peptides on dipeptidyl peptidase IV (DPP-IV) and insulin receptor activity. J. Dairy Sci. 2021, 104, 61–77. [Google Scholar] [CrossRef]
- Xiao, J.B.; Högger, P. Dietary Polyphenols and Type 2 Diabetes: Current Insights and Future Perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef]
- Stefanou, M.I.; Theodorou, A.; Malhotra, K.; de Sousa, D.A.; Katan, M.; Palaiodimou, L.; Katsanos, A.H.; Koutroulou, I.; Lambadiari, V.; Lemmens, R.; et al. Risk of major adverse cardiovascular events and stroke associated with treatment with GLP-1 or the dual GIP/GLP-1 receptor agonist tirzepatide for type 2 diabetes: A systematic review and meta-analysis. Eur. Stroke J. 2024, 9, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Pegah, A.; Abbasi-Oshaghi, E.; Khodadadi, I.; Mirzaei, F.; Tayebinai, H. Probiotic and resveratrol normalize GLP-1 levels and oxidative stress in the intestine of diabetic rats. Metab. Open 2021, 10, 100093. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Q.; Liu, Q.B.; Cao, J.; Xu, Y.S.; Pei, Z.S.; Fan, H.F.; Yuan, Y.Q.; Shen, X.R.; Li, C. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto-Kakizaki rats. Food Chem. Toxicol. 2020, 135, 110886. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Berlett, B.S. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab. Rev. 1998, 30, 225–243. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Zhang, P.P.; Zhu, M.L.; Li, L.; Mao, X.M.; Sha, X.T.; Li, L.L. Kazak faecal microbiota transplantation induces short-chain fatty acids that promote glucagon-like peptide-1 secretion by regulating gut microbiota in db/db mice. Pharm. Biol. 2021, 59, 1077–1087. [Google Scholar] [CrossRef]
- Kobyliak, N.; Falalyeyeva, T.; Mykhalchyshyn, G.; Kyriienko, D.; Komissarenko, I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab. Syndr. 2018, 12, 617–624. [Google Scholar] [CrossRef]
- Cimini, F.A.; D’Eliseo, D.; Barchetta, I.; Bertoccini, L.; Velotti, F.; Cavallo, M.G. Increased circulating granzyme B in type 2 diabetes patients with low-grade systemic inflammation. Cytokine 2019, 115, 104–108. [Google Scholar] [CrossRef]
- Fadaei, R.; Bagheri, N.; Heidarian, E.; Nouri, A.; Hesari, Z.; Moradi, N.; Ahmadi, A.; Ahmadi, R. Serum levels of IL-32 in patients with type 2 diabetes mellitus and its relationship with TNF-α and IL-6. Cytokine 2020, 125, 154832. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell. Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Guo, Y.Z.; Jiang, C.X.; Xie, A.J.; Yue, X.Q.; Li, M.H. A review of casein phosphopeptides: From enrichment identification to biological properties. Food Biosci. 2024, 59, 104217. [Google Scholar] [CrossRef]
- Naseri, K.; Saadati, S.; Ghaemi, F.; Ashtary-Larky, D.; Asbaghi, O.; Sadeghi, A.; Afrisham, R.; de Courten, B. The effects of probiotic and synbiotic supplementation on inflammation, oxidative stress, and circulating adiponectin and leptin concentration in subjects with prediabetes and type 2 diabetes mellitus: A GRADE-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Eur. J. Nutr. 2023, 62, 543–561. [Google Scholar]
- You, S.Y.; Ma, Y.C.; Yan, B.W.; Pei, W.H.; Wu, Q.M.; Ding, C.; Huang, C.X. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. Embo Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Wang, L.H.; Cheng, S.S.; Zhang, Y.; Yang, M.; Fang, R.X.; Li, H.X.; Man, C.X.; Jiang, Y.J. A Potential Synbiotic Strategy for the Prevention of Type 2 Diabetes: Lactobacillus paracasei JY062 and Exopolysaccharide Isolated from Lactobacillus plantarum JY039. Nutrients 2022, 14, 377. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.D.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.M.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Lenzen, S. Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic β-cells. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 1929–1942. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, L.L.; Tian, P.J.; Jin, X.; Zhao, J.X.; Zhang, H.; Wang, G.; Zhu, M.M. The Effect of Probiotic Supplementation on Glucolipid Metabolism in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3240. [Google Scholar] [CrossRef]
- Shen, X.Y.; Yue, J.Z.; Fu, J.; Guo, Y.Z.; Yang, H.Y.; Liu, Q.M.; Xu, N.; Yue, X.Q.; Li, M.H. Nutritional evaluation of almond protein-whey protein double system and its effect on lipid metabolism in HepG2 cells. Food Biosci. 2024, 61, 104670. [Google Scholar] [CrossRef]
- Jadzinsky, M.; Pfuetzner, A.; Paz-Pacheco, F.; Xu, Z.; Allen, F.; Chen, R. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: A randomized controlled trial. Diabetes Obes. Metab. 2009, 11, 611–622, Erratum in Commun. Math. Phys. 2010, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Wu, Y.C.; Fei, X.Q. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina 2016, 52, 28–34. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P.R. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 2007, 23, 62–68. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P.R. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J. Dairy Res. 2008, 75, 189–195. [Google Scholar] [CrossRef]
- Wu, H.J.; Shang, H.; Wu, J. Effect of ezetimibe on glycemic control: A systematic review and meta-analysis of randomized controlled trials. Endocrine 2018, 60, 229–239. [Google Scholar] [CrossRef]
- Siebler, J.; Galle, P.R.; Weber, M.M. The gut-liver-axis: Endotoxemia, inflammation, insulin resistance and NASH. J. Hepatol. 2008, 48, 1032–1034. [Google Scholar] [CrossRef]
- Nie, Z.H.; Xu, L.M.; Li, C.Y.; Tian, T.; Xie, P.P.; Chen, X.; Li, B.J. Association of endothelial progenitor cells and peptic ulcer treatment in patients with type 2 diabetes mellitus. Exp. Ther. Med. 2016, 11, 1581–1586. [Google Scholar] [CrossRef]
- Malenica, M.; Prnjavorac, B.; Causevic, A.; Dujic, T.; Bego, T.; Semiz, S. Use of Databases for Early Recognition of Risk of Diabetic Complication by Analysis of Liver Enzymes in Type 2 Diabetes Mellitus. Acta Inform. Medica 2016, 24, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Roelofsen, H.; Priebe, M.G.; Vonk, R.J. The interaction of short-chain fatty acids with adipose tissue: Relevance for prevention of type 2 diabetes. Benef. Microbes 2010, 1, 433–437. [Google Scholar] [CrossRef]
- Hong, R.; Xie, A.; Jiang, C.; Guo, Y.; Zhang, Y.; Chen, J.; Shen, X.; Li, M.; Yue, X. A review of the biological activities of lactoferrin: Mechanisms and potential applications. Food Funct. 2024, 15, 8182–8199. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Lachmandas, E.; van den Heuvel, C.; Damen, M.; Cleophas, M.C.P.; Netea, M.G.; van Crevel, R. Diabetes Mellitus and Increased Tuberculosis Susceptibility: The Role of Short-Chain Fatty Acids. J. Diabetes Res. 2016, 2016, 6014631. [Google Scholar] [CrossRef]
- Hur, K.Y.; Lee, M.S. Gut Microbiota and Metabolic Disorders. Diabetes Metab. J. 2015, 39, 198–203. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Al-Salami, H.; Butt, G.; Fawcett, J.P.; Tucker, I.G.; Golocorbin-Kon, S.; Mikov, M. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur. J. Drug Metab. Pharmacokinet. 2008, 33, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M. Obesity, Diabetes, and Gut Microbiota The hygiene hypothesis expanded? Diabetes Care 2010, 33, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of Multispecies Probiotic Supplements on Metabolic Profiles, hs-CRP, and Oxidative Stress in Patients with Type 2 Diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Eslamparast, T.; Zamani, F.; Hekmatdoost, A.; Sharafkhah, M.; Eghtesad, S.; Malekzadeh, R.; Poustchi, H. Effects of synbiotic supplementation on insulin resistance in subjects with the metabolic syndrome: A randomised, double-blind, placebo-controlled pilot study. Br. J. Nutr. 2014, 112, 438–445. [Google Scholar] [CrossRef]
- Xiong, T.; Song, S.H.; Huang, X.H.; Feng, C.; Liu, G.Q.; Huang, J.Q.; Xie, M.Y. Screening and Identification of Functional Lactobacillus Specific for Vegetable Fermentation. J. Food Sci. 2013, 78, M84–M89. [Google Scholar] [CrossRef]
- Johansson, S.; Diehl, B.; Christakopoulos, P.; Austin, S.; Vafiadi, C. Oligosaccharide Synthesis in Fruit Juice Concentrates Using a Glucansucrase From Lactobacillus reuteri 180. Food Bioprod. Process. 2016, 98, 201–209. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, X.Z.; Xu, Y.H.; Xie, Q.L.; Zhu, M.Z.; Zhang, H.S.; Zhao, Z.F.; Hao, J.Y.; Li, H.Q.; Du, J.R.; et al. Effects of Coix Seed Extract, Bifidobacterium BPL1, and Their Combination on the Glycolipid Metabolism in Obese Mice. Front. Nutr. 2022, 9, 939423. [Google Scholar] [CrossRef]
- Jang, S.H.; Park, J.; Kim, S.H.; Choi, K.M.; Ko, E.S.; Cha, J.D.; Lee, Y.R.; Jang, H.; Jang, Y.S. Red ginseng powder fermented with probiotics exerts antidiabetic effects in the streptozotocin-induced mouse diabetes model. Pharm. Biol. 2017, 55, 317–323. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, D.; Park, G.S.; Ko, S.H.; Park, J.; Lee, Y.K.; Kang, J. Lactobacillus plantarum HAC01 ameliorates type 2 diabetes in high-fat diet and streptozotocin-induced diabetic mice in association with modulating the gut microbiota. Food Funct. 2021, 12, 6363–6373. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, S.J.; Cui, J.L.; Guo, T.T.; Zhang, J.T. Lactiplantibacillus plantarum Y15 alleviate type 2 diabetes in mice via modulating gut microbiota and regulating NF-κB and insulin signaling pathway. Braz. J. Microbiol. 2022, 53, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.R.; Gurung, M.; Li, Z.P.; García-Jaramillo, M.; Greer, R.; Gaulke, C.; Bauchinger, F.; You, H.; Pederson, J.W.; Vasquez-Perez, S.; et al. Transkingdom interactions between Lactobacilli and hepatic mitochondria attenuate western diet-induced diabetes. Nat. Commun. 2021, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.R.; Cai, M.M.; Shen, B.Y.; Wang, Q.; Zhang, T.C.; Zhou, X. Synbiotics and Gut Microbiota: New Perspectives in the Treatment of Type 2 Diabetes Mellitus. Foods 2022, 11, 2438. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Q.Q.; Liu, M.N.; Zhang, X.L.; He, F.; Wang, G.Q. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog. Dis. 2018, 76, fty028. [Google Scholar] [CrossRef]
- Koay, K.P.; Tsai, B.C.K.; Kuo, C.H.; Kuo, W.W.; Luk, H.N.; Day, C.H.; Chen, R.J.; Chen, M.Y.C.; Padma, V.V.; Huang, C.Y. Hyperglycemia-Induced Cardiac Damage Is Alleviated by Heat-Inactivated Lactobacillus reuteri GMNL-263 via Activation of the IGF1R Survival Pathway. Probiotics Antimicrob. Proteins 2021, 13, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Gulnaz, A.; Nadeem, J.; Han, J.H.; Lew, L.C.; Son, J.D.; Park, Y.H.; Rather, I.A.; Hor, Y.Y. Lactobacillus Sps in Reducing the Risk of Diabetes in High-Fat Diet-Induced Diabetic Mice by Modulating the Gut Microbiome and Inhibiting Key Digestive Enzymes Associated with Diabetes. Biology 2021, 10, 348. [Google Scholar] [CrossRef]
- Zhou, X.R.; Shang, G.S.; Tan, Q.; He, Q.; Tan, X.Y.; Park, K.Y.; Zhao, X. Effect of Lactobacillus fermentum TKSN041 on improving streptozotocin-induced type 2 diabetes in rats. Food Funct. 2021, 12, 7938–7953. [Google Scholar] [CrossRef]
- Archer, A.C.; Muthukumar, S.P.; Halami, P.M. Lactobacillus fermentum MCC2759 and MCC2760 Alleviate Inflammation and Intestinal Function in High-Fat Diet-Fed and Streptozotocin-Induced Diabetic Rats. Probiotics Antimicrob. Proteins 2021, 13, 1068, Erratum in Probiotics Antimicrob. Proteins 2023, 15, 1078. [Google Scholar] [CrossRef]
- Moroti, C.; Magri, L.F.S.; Costa, M.D.; Cavallini, D.C.U.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29. [Google Scholar] [CrossRef]
- Soleimani, A.; Motamedzadeh, A.; Mojarrad, M.Z.; Bahmani, F.; Amirani, E.; Ostadmohammadi, V.; Tajabadi-Ebrahimi, M.; Asemi, Z. The Effects of Synbiotic Supplementation on Metabolic Status in Diabetic Patients Undergoing Hemodialysis: A Randomized, Double-Blinded, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1248–1256. [Google Scholar] [CrossRef]
- Mirmiranpour, H.; Huseini, H.F.; Derakhshanian, H.; Khodaii, Z.; Tavakoli-Far, B. Effects of probiotic, cinnamon, and synbiotic supplementation on glycemic control and antioxidant status in people with type 2 diabetes; a randomized, double-blind, placebo-controlled study. J. Diabetes Metab. Disord. 2020, 19, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mobini, R.; Tremaroli, V.; Stahlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Perkins, R.; Perkins, R.; Bäackhed, F.; et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Tajadadi-Ebrahimi, M.; Bahmani, F.; Shakeri, H.; Hadaegh, H.; Hijijafari, M.; Abedi, F.; Asemi, Z. Effects of Daily Consumption of Synbiotic Bread on Insulin Metabolism and Serum High-Sensitivity C-Reactive Protein among Diabetic Patients: A Double-Blind, Randomized, Controlled Clinical Trial. Ann. Nutr. Metab. 2014, 65, 34–41. [Google Scholar] [CrossRef]
- Kozawa, T.; Aoyagi, H. Novel method for screening probiotic candidates tolerant to human gastrointestinal stress. J. Microbiol. Methods 2024, 222, 106945. [Google Scholar] [CrossRef] [PubMed]
- Ramlucken, U.; Roets, Y.; Ramchuran, S.O.; Moonsamy, G.; van Rensburg, C.J.; Thantsha, M.S.; Lalloo, R. Isolation, selection and evaluation of Bacillus spp. as potential multi-mode probiotics for poultry. J. Gen. Appl. Microbiol. 2020, 66, 228–238. [Google Scholar] [CrossRef]
- Wang, J.B.; Yu, L.Y.; Zeng, X.; Zheng, J.W.; Wang, B.; Pan, L. Screening of probiotics with efficient α-glucosidase inhibitory ability and study on the structure and function of its extracellular polysaccharide. Food Biosci. 2022, 45, 101452. [Google Scholar] [CrossRef]
- Oberhardt, M.A.; Zarecki, R.; Gronow, S.; Lang, E.; Klenk, H.P.; Gophna, U.; Ruppin, E. Harnessing the landscape of microbial culture media to predict new organism-media pairings. Nat. Commun. 2015, 6, 8493. [Google Scholar] [CrossRef]
- Oliveira, D.; Vidal, L.; Ares, G.; Walter, E.H.M.; Rosenthal, A.; Deliza, R. Sensory, microbiological and physicochemical screening of probiotic cultures for the development of non-fermented probiotic milk. Lwt-Food Sci. Technol. 2017, 79, 234–241. [Google Scholar] [CrossRef]
- Xie, W.W.; Wu, Y.; Tian, Y.; Li, S.Y.; Zhang, H.Y.; Liu, J.T. Screening for protential new probiotic based on probiotic proper ties and cholesterol degradation activity from Douchi. Asia-Pac. J. Clin. Oncol. 2022, 18, 80. [Google Scholar]
- Bijukumar, G.; Somvanshi, P.R. Reverse Engineering in Biotechnology: The Role of Genetic Engineering in Synthetic Biology. Methods Mol. Biol. 2024, 2719, 307–324. [Google Scholar]
- Duan, F.F.; Liu, J.H.; March, J.C. Engineered Commensal Bacteria Reprogram Intestinal Cells Into Glucose-Responsive Insulin-Secreting Cells for the Treatment of Diabetes. Diabetes 2015, 64, 1794–1803. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ma, N.; Ling, W.; Pang, G.J.; Sun, T.; Liu, J.; Pan, H.Z.; Cui, M.H.; Han, C.L.; Yang, C.; et al. A micro-nano optogenetic system based on probiotics for in situ host metabolism regulation. Nano Res. 2023, 16, 2829–2839. [Google Scholar] [CrossRef]
- Sampson, K.; Sorenson, C.; Adamala, K.P. Preparing for the future of precision medicine: Synthetic cell drug regulation. Synth. Biol. 2024, 9, ysae004. [Google Scholar] [CrossRef] [PubMed]
- Komera, I.; Chen, X.L.; Liu, L.M.; Gao, C. Microbial Synthetic Epigenetic Tools Design and Applications. Acs Synth. Biol. 2024, 13, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Dwibedi, C.; Sundh, D.; Pradhan, M.; Kraft, J.D.; Caesar, R.; Tremaroli, V.; Lorentzon, M.; Backhed, F. Synergy and oxygen adaptation for development of next-generation probiotics. Nature 2023, 620, 7973. [Google Scholar] [CrossRef] [PubMed]
- Torp, A.M.; Bahl, M.I.; Boisen, A.; Licht, T.R. Optimizing oral delivery of next generation probiotics. Trends Food Sci. Technol. 2022, 119, 101–109. [Google Scholar] [CrossRef]
- Kobyliak, N.; Falalyeyeva, T.; Kyriachenko, Y.; Tseyslyer, Y.; Kovalchuk, O.; Hadiliia, O.; Eslami, M.; Yousefi, B.; Abenavoli, L.; Fagoonee, S.; et al. Akkermansia muciniphila as a novel powerful bacterial player in the treatment of metabolic disorders. Minerva Endocrinol. 2022, 47, 242–252. [Google Scholar] [CrossRef]
- Jian, H.F.; Liu, Y.T.; Wang, X.M.; Dong, X.Y.; Zou, X.T. Akkermansia muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: A Role Mediated by Gut-Liver-Brain Axes? Int. J. Mol. Sci. 2023, 24, 3900. [Google Scholar] [CrossRef]
- Udayappan, S.; Manneras-Holm, L.; Chaplin-Scott, A.; Belzer, C.; Herrema, H.; Dallinga-Thie, G.M.; Duncan, S.H.; Stroes, E.S.G.; Groen, A.K.; Flint, H.J.; et al. Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes 2016, 2, 16009. [Google Scholar] [CrossRef]
- del Pulgar, E.M.G.; Benítez-Páez, A.; Sanz, Y. Safety Assessment of Bacteroides Uniformis CECT 7771, a Symbiont of the Gut Microbiota in Infants. Nutrients 2020, 12, 551. [Google Scholar] [CrossRef]
- Wang, T.Y.; Zhang, X.Q.; Chen, A.L.; Zhang, J.; Lv, B.H.; Ma, M.H.; Lian, J.; Wu, Y.X.; Zhou, Y.T.; Ma, C.C.; et al. A comparative study of microbial community and functions of type 2 diabetes mellitus patients with obesity and healthy people. Appl. Microbiol. Biotechnol. 2020, 104, 7143–7153. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.-F.; Li, X.; Hou, Y.-Y.; Fan, Y.-R.; Liu, W.-H.; Xu, G.-X. Bifidobacterium longum as an oral delivery system of endostatin for gene therapy on solid liver cancer. Cancer Gene Ther. 2005, 12, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, X.; Kou, L.; Hu, B.; Zhu, L.P.; Fan, Y.R.; Wu, Z.W.; Wang, J.J.; Xu, G.X. Selenium-Bifidobacterium longum as a delivery system of endostatin for inhibition of pathogenic bacteria and selective regression of solid tumor. Exp. Ther. Med. 2010, 1, 129–135. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.J.; Xie, W.; Su, Z.A.; Qin, G.M.; Yu, C.H.N. Postbiotics: Emerging therapeutic approach in diabetic retinopathy. Front. Microbiol. 2024, 15, 1359949. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Mishra, S.P.; Wang, B.; Jian, S.; Ding, J.Z.; Rejeski, J.; Furdui, C.M.; Kitzman, D.W.; Taraphder, S.; Brechot, C.; Kumar, A.; et al. A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut 2023, 72, 1848–1865. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed]
- Baral, K.C.; Bajracharya, R.; Lee, S.H.; Han, H.K. Advancements in the Pharmaceutical Applications of Probiotics: Dosage Forms and Formulation Technology. Int. J. Nanomed. 2021, 16, 7535–7556. [Google Scholar] [CrossRef]
- Koczarski, M. The Effect of Probiotic Supplementation on Glycemic Control in Women with Gestational Diabetes Mellitus A Systematic Review. Top. Clin. Nutr. 2020, 35, 270–276. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rodiles, A.; Hatef, A.; Picchietti, S.; Cossignani, L.; Merrifield, D.L.; Unniappan, S.; Carnevali, O. Influence of Probiotics Administration on Gut Microbiota Core: A Review on the Effects on Appetite Control, Glucose, and Lipid Metabolism. J. Clin. Gastroenterol. 2018, 52, S50–S56. [Google Scholar] [CrossRef]
- Wu, L.; Gao, Y.; Su, Y.; Li, J.; Ren, W.C.; Wang, Q.H.; Kuang, H.X. Probiotics with anti-type 2 diabetes mellitus properties: Targets of polysaccharides from traditional Chinese medicine. Chin. J. Nat. Med. 2022, 20, 641–655. [Google Scholar] [CrossRef] [PubMed]

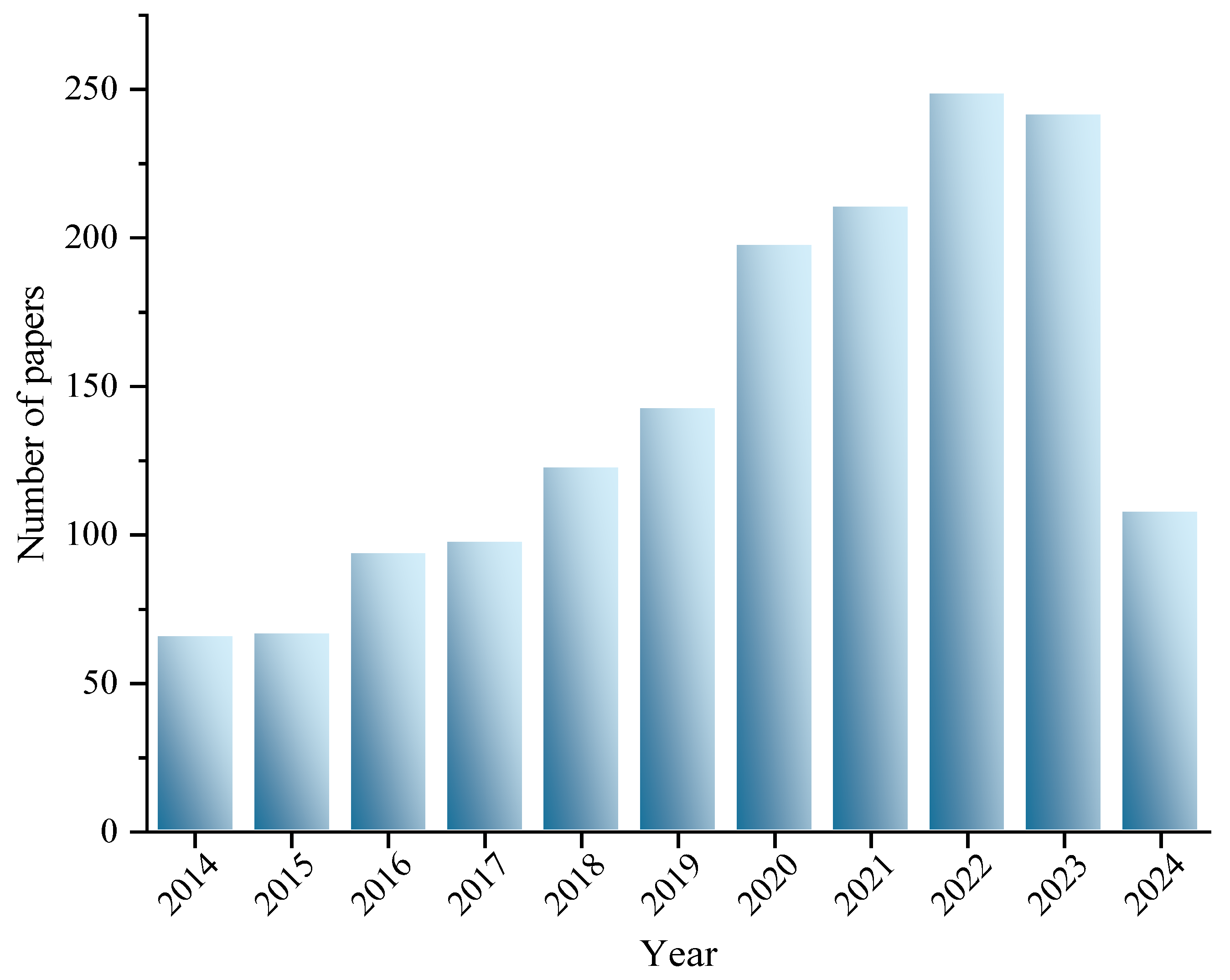
| Bacterial Genus | Probiotics | Main Functions | Reference |
|---|---|---|---|
| Bifidobacterium | Bifidobacterium infantis | Prevent constipation, regulate blood glucose, inhibit intestinal pathogens, regulate intestinal balance, reduce cholesterol, promote the digestion and absorption of nutrients, delay aging, and enhance the body’s immune activity. | [16,17,18] |
| Bifidobacterium longum | |||
| Bifidobacterium bifidum | |||
| Bifidobacterium breve | |||
| Bifidobacterium animalis (Bifidobacterium lactis) | |||
| Bifidobacterium adolescentis | |||
| Lactobacillus | Lactobacillus fermentum | Prevent diarrhea and intestinal infections, relieve inflammatory intestinal diseases, regulate blood glucose, improve insulin resistance, increase SCFA levels, inhibit the growth of pathogenic bacteria, and reduce cholesterol levels. | [19,20,21,22,23,24] |
| Lactobacillus casei | |||
| Lactobacillus plantarum | |||
| Lactobacillus rhamnosus | |||
| Lactobacillus reuteri | |||
| Lactobacillus paracasei | |||
| Lactobacillus acidophilus | |||
| Lactobacillus crispatus | |||
| Lactobacillus bulgaricus | |||
| Lactobacillus gasseri | |||
| Lactobacillus helveticus | |||
| Lactobacillus johnsonii | |||
| Lactobacillus salivarius | |||
| Lactobacillus sakei | |||
| Lactococcus | Lactococcus Lactis subsp. Lactis | Regulate immunity and produce antimicrobial substances. | [25,26] |
| Lactococcus Lactis subsp. Cremoris | |||
| Lactococcus Lactis subsp. Diacetylactis | |||
| Streptococcus | Streptococcus thermophiles | Regulate immunity and improve intestinal microenvironment. | [27] |
| Leuconostoc | Leuconostoc mesenteroides subsp. Mesenteroides | Regulate immunity, inhibit harmful bacteria, and improve intestinal microenvironment. | [28,29,30] |
| Bacillus | Bacillus coagulans | Relieve and treat diarrhea, constipation, and indigestion. | [31] |
| Propionibacterium | Propionibacterium freudenreichii subsp. Shermanii | Regulate immunity, promote intestinal flora balance, and anti-inflammatory. | [32,33] |
| Propionibacterium acidpropionici | |||
| Pediococcus | Pediococcus acidilactici | Enhance immunity, promote intestinal flora balance, anti-inflammatory, and inhibit pathogenic bacteria. | [34,35,36] |
| Pediococcus pentosaceus | |||
| Saccharomyces | Kluyveromyces marxianus | Enhance immunity, anti-inflammatory, and inhibit the growth of pathogenic bacteria. | [37,38,39] |
| Saccharomyces cerevisiae | |||
| Saccharomyces boulardii | |||
| Staphylococcus | Staphylococcus fleurettii | As a starter and enrich the flavor of the product. | [40,41,42] |
| Staphylococcus hominis | |||
| Staphylococcus aureus | |||
| Staphylococcus carnosus | |||
| Staphylococcus vitulinus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Ma, C.; Yang, Y.; Liu, X.; Wang, B.; Wang, Y.; Zhang, G.; Bian, X.; Zhang, N. The Role and Mechanism of Probiotics Supplementation in Blood Glucose Regulation: A Review. Foods 2024, 13, 2719. https://doi.org/10.3390/foods13172719
Shen X, Ma C, Yang Y, Liu X, Wang B, Wang Y, Zhang G, Bian X, Zhang N. The Role and Mechanism of Probiotics Supplementation in Blood Glucose Regulation: A Review. Foods. 2024; 13(17):2719. https://doi.org/10.3390/foods13172719
Chicago/Turabian StyleShen, Xinyu, Chunmin Ma, Yang Yang, Xiaofei Liu, Bing Wang, Yan Wang, Guang Zhang, Xin Bian, and Na Zhang. 2024. "The Role and Mechanism of Probiotics Supplementation in Blood Glucose Regulation: A Review" Foods 13, no. 17: 2719. https://doi.org/10.3390/foods13172719
APA StyleShen, X., Ma, C., Yang, Y., Liu, X., Wang, B., Wang, Y., Zhang, G., Bian, X., & Zhang, N. (2024). The Role and Mechanism of Probiotics Supplementation in Blood Glucose Regulation: A Review. Foods, 13(17), 2719. https://doi.org/10.3390/foods13172719






