Identifying Key Markers for Monofloral (Eucalyptus, Rosemary, and Orange Blossom) and Multifloral Honey Differentiation in the Spanish Market by UHPLC-Q-Orbitrap-High-Resolution Mass Spectrometry Fingerprinting and Chemometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Sample Preparation and Extraction
2.3. Honey Fingerprinting by UHPLC-Q-Orbitrap-HRMS Untargeted Analysis
2.4. Processing Honey Omics Data: Designed Untargeted Metabolomics Workflow Using Compound DiscovererTM Software
2.5. Multivariate Data Analysis
2.6. Workflow to Identify Honey Markers
- VIP score cut-off > 1.00;
- Benjamini–Hochberg-adjusted p-values < 0.05;
- FC cut-off > 1.10;
- Two characteristic ions (precursor and at least one fragment ion) with mass accuracy ≤ 5 ppm;
- Fully overlapping chromatographic peaks of the ions at the metabolite retention time (RT ± 0.10 min);
- Study of the potential logical occurrence of the proposed marker in the study matrix based on the relevant published literature and food databases (if available).
3. Results and Discussion
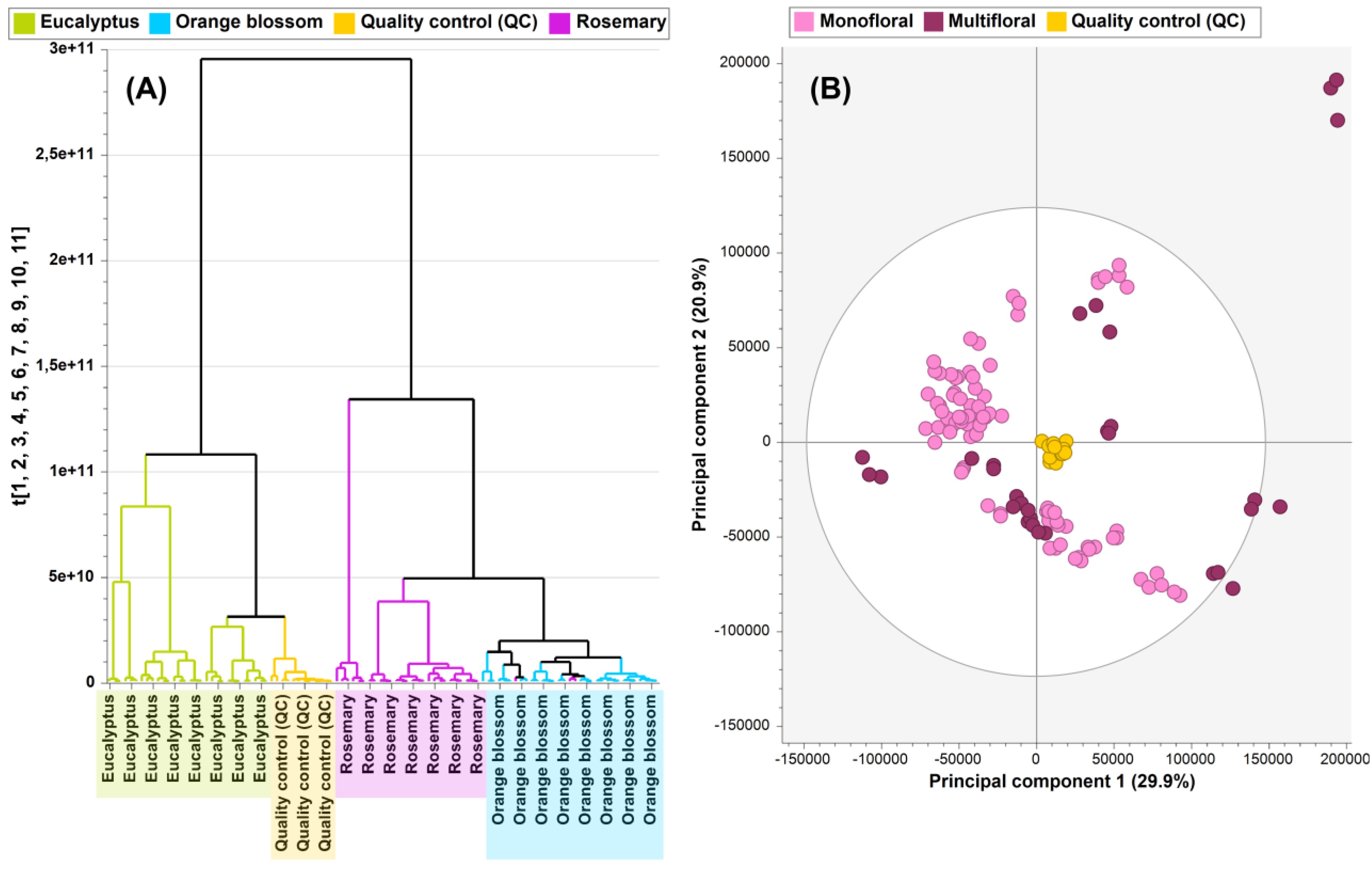
3.1. Unsupervised Multivariate Data Analysis
3.1.1. Overview of Monofloral (Eucalyptus, Rosemary, and Orange Blossom) Honey
3.1.2. Overview of Multifloral vs. Monofloral Honey
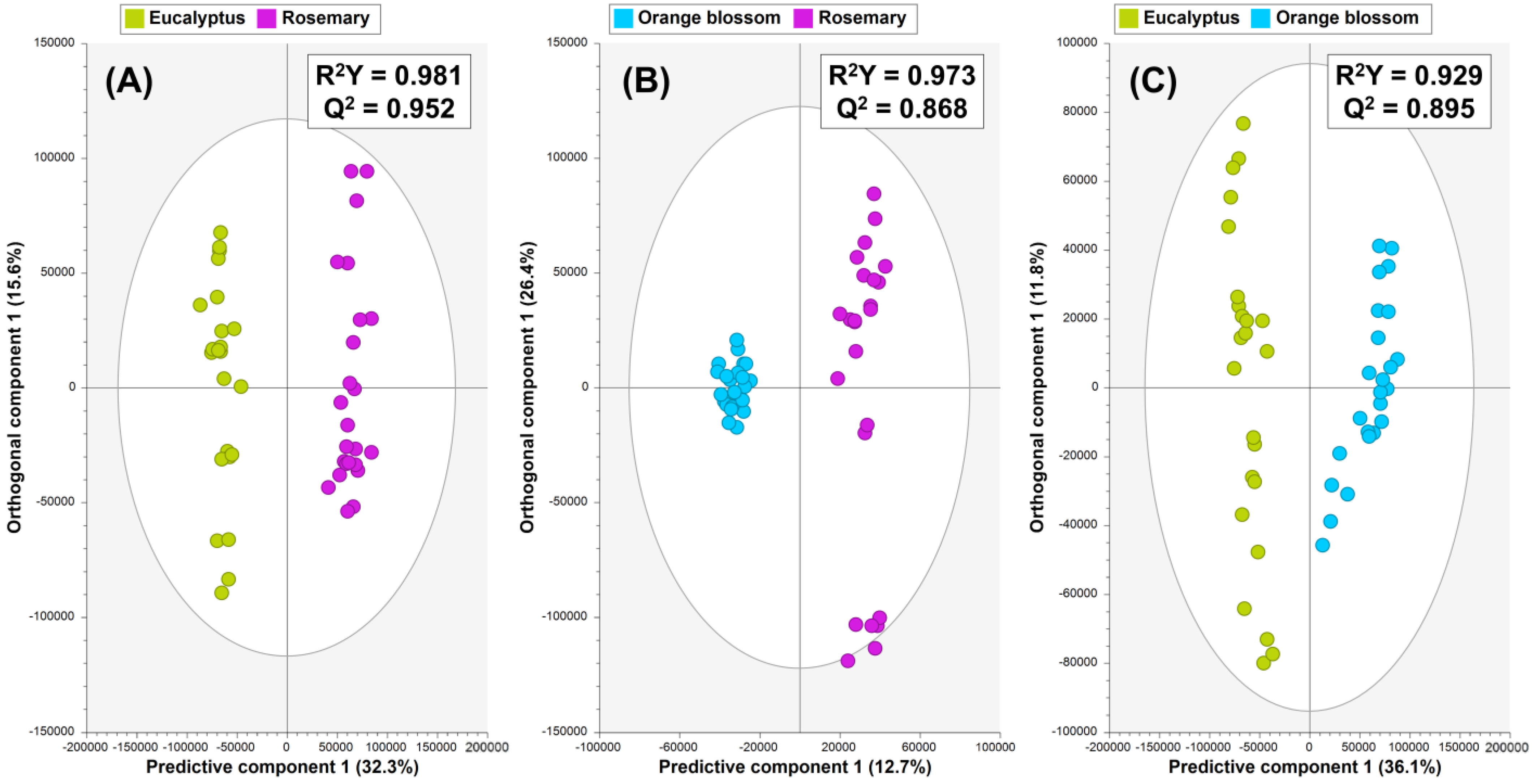
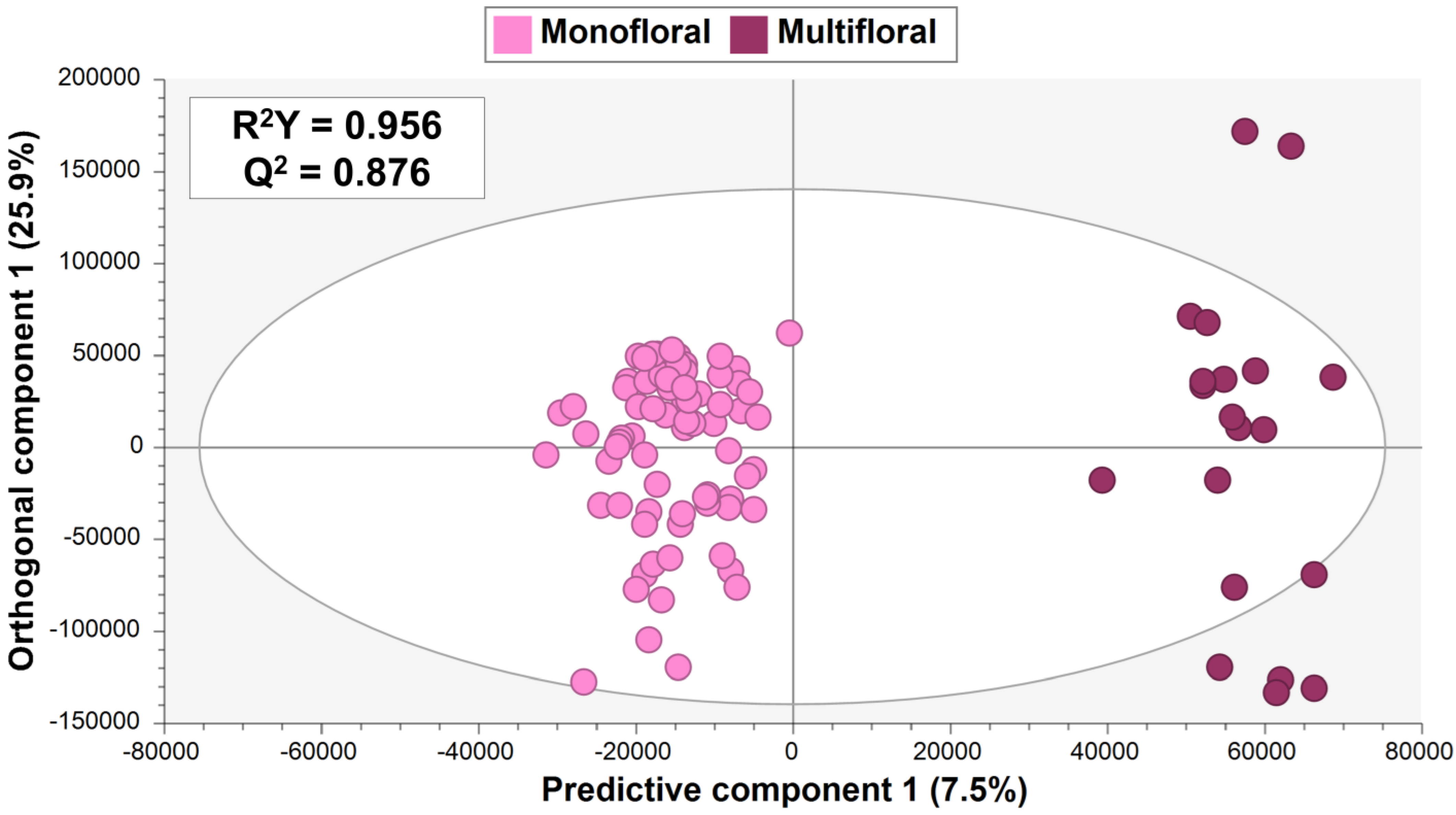
3.2. Supervised Multivariate Data Analysis
3.2.1. Experiment 1: OPLS-DA to Discriminate Monofloral Samples (Eucalyptus, Rosemary, and Orange Blossom Honey)
3.2.2. Experiment 2: OPLS-DA to Discriminate Multifloral and Monofloral Honey
3.3. Identification of Honey Markers
3.3.1. Markers to Discriminate Monofloral Samples (Eucalyptus, Rosemary, and Orange Blossom Honey)
3.3.2. Markers to Discriminate Multifloral vs. Monofloral Honey
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucu, A.-A.; Baci, G.-M.; Moise, A.R.; Dezsi, Ş.; Marc, B.D.; Stângaciu, Ş.; Dezmirean, D.S. Towards a Better Understanding of Nutritional and Therapeutic Effects of Honey and Their Applications in Apitherapy. Appl. Sci. 2021, 11, 4190. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E.; Belay, A.; Heil, J.; Mehl, A.; Borck, H. Effect-Directed Profiling of Monofloral Honeys from Ethiopia by High-Performance Thin-Layer Chromatography and High-Resolution Mass Spectrometry. Molecules 2022, 27, 3541. [Google Scholar] [CrossRef]
- Mohammed, M.E.A. Factors Affecting the Physicochemical Properties and Chemical Composition of Bee’s Honey. Food Rev. Int. 2022, 38, 1330–1341. [Google Scholar] [CrossRef]
- Żak, N.; Wilczyńska, A. The Importance of Testing the Quality and Authenticity of Food Products: The Example of Honey. Foods 2023, 12, 3210. [Google Scholar] [CrossRef]
- Food Authenticity Network. Which Foods Are Most Adulterated? Available online: https://www.foodauthenticity.global/foods-most-reported-as-fraudulent (accessed on 25 July 2024).
- Kang, M.J.; Kim, K.-R.; Kim, K.; Morrill, A.G.; Jung, C.; Sun, S.; Lee, D.-H.; Suh, J.H.; Sung, J. Metabolomic Analysis Reveals Linkage between Chemical Composition and Sensory Quality of Different Floral Honey Samples. Food Res. Int. 2023, 173, 113454. [Google Scholar] [CrossRef] [PubMed]
- Castell, A.; Arroyo-Manzanares, N.; Guerrero-Núñez, Y.; Campillo, N.; Viñas, P. Headspace with Gas Chromatography-Mass Spectrometry for the Use of Volatile Organic Compound Profile in Botanical Origin Authentication of Honey. Molecules 2023, 28, 4297. [Google Scholar] [CrossRef]
- García-Seval, V.; Saurina, J.; Sentellas, S.; Núñez, O. Off-Line SPE LC-LRMS Polyphenolic Fingerprinting and Chemometrics to Classify and Authenticate Spanish Honey. Molecules 2022, 27, 7812. [Google Scholar] [CrossRef]
- Yong, C.-H.; Muhammad, S.A.; Aziz, F.A.; Nasir, F.I.; Mustafa, M.Z.; Ibrahim, B.; Kelly, S.D.; Cannavan, A.; Seow, E.-K. Detecting Adulteration of Stingless Bee Honey Using Untargeted 1H NMR Metabolomics with Chemometrics. Food Chem. 2022, 368, 130808. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, Y.; Wu, H.; Dong, J.; Feng, J. Origin Identification and Quantitative Analysis of Honeys by Nuclear Magnetic Resonance and Chemometric Techniques. Food Anal. Methods 2016, 9, 1470–1479. [Google Scholar] [CrossRef]
- Mara, A.; Migliorini, M.; Ciulu, M.; Chignola, R.; Egido, C.; Núñez, O.; Sentellas, S.; Saurina, J.; Caredda, M.; Deroma, M.A.; et al. Elemental Fingerprinting Combined with Machine Learning Techniques as a Powerful Tool for Geographical Discrimination of Honeys from Nearby Regions. Foods 2024, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Drivelos, S.A.; Danezis, G.P.; Halagarda, M.; Popek, S.; Georgiou, C.A. Geographical Origin and Botanical Type Honey Authentication through Elemental Metabolomics via Chemometrics. Food Chem. 2021, 338, 127936. [Google Scholar] [CrossRef] [PubMed]
- Chaji, S.; Ajal, E.A.; Olmo-García, L.; Serrano-García, I.; Carrasco-Pancorbo, A.; Bajoub, A. Metabolomic Approaches Applied to Food Authentication: From Data Acquisition to Biomarkers Discovery. In Food Authentication and Traceability; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 331–378. [Google Scholar]
- Picó, Y. Mass Spectrometry in Food Quality and Safety: An Overview of the Current Status. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 68, pp. 3–76. [Google Scholar]
- Koulis, G.A.; Tsagkaris, A.S.; Aalizadeh, R.; Dasenaki, M.E.; Panagopoulou, E.I.; Drivelos, S.; Halagarda, M.; Georgiou, C.A.; Proestos, C.; Thomaidis, N.S. Honey Phenolic Compound Profiling and Authenticity Assessment Using HRMS Targeted and Untargeted Metabolomics. Molecules 2021, 26, 2769. [Google Scholar] [CrossRef] [PubMed]
- García-Seval, V.; Saurina, J.; Sentellas, S.; Núñez, O. Characterization and Classification of Spanish Honey by Non-Targeted LC–HRMS (Orbitrap) Fingerprinting and Multivariate Chemometric Methods. Molecules 2022, 27, 8357. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Baira, E.; Iosifidou, S.; Manea-Karga, E.; Tsipi, D.; Gounari, S.; Theologidis, I.; Barmpouni, T.; Danieli, P.P.; Lazzari, F.; et al. Fingerprinting Chemical Markers in the Mediterranean Orange Blossom Honey: UHPLC-HRMS Metabolomics Study Integrating Melissopalynological Analysis, GC-MS and HPLC-PDA-ESI/MS. Molecules 2023, 28, 3967. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Yang, S.; Zhang, W.; Zhang, J.; Zhao, W.; Chen, L.; Wen, Y.; Zhang, Y.; Lu, K.; et al. Strategy for Comparative Untargeted Metabolomics Reveals Honey Markers of Different Floral and Geographic Origins Using Ultrahigh-Performance Liquid Chromatography-Hybrid Quadrupole-Orbitrap Mass Spectrometry. J. Chromatogr. A 2017, 1499, 78–89. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Chingin, K. Applications of Direct Analysis in Real Time Mass Spectrometry in Food Analysis: A Review. Rapid Commun. Mass Spectrom. 2021, 35, e9013. [Google Scholar] [CrossRef]
- Lippolis, V.; De Angelis, E.; Fiorino, G.M.; Di Gioia, A.; Arlorio, M.; Logrieco, A.F.; Monaci, L. Geographical Origin Discrimination of Monofloral Honeys by Direct Analysis in Real Time Ionization-High Resolution Mass Spectrometry (DART-HRMS). Foods 2020, 9, 1205. [Google Scholar] [CrossRef]
- Spiteri, M.; Dubin, E.; Cotton, J.; Poirel, M.; Corman, B.; Jamin, E.; Lees, M.; Rutledge, D. Data Fusion between High Resolution 1H-NMR and Mass Spectrometry: A Synergetic Approach to Honey Botanical Origin Characterization. Anal. Bioanal. Chem. 2016, 408, 4389–4401. [Google Scholar] [CrossRef]
- Sadygov, R.G.; Martin Maroto, F.; Hühmer, A.F.R. ChromAlign: A Two-Step Algorithmic Procedure for Time Alignment of Three-Dimensional LC−MS Chromatographic Surfaces. Anal. Chem. 2006, 78, 8207–8217. [Google Scholar] [CrossRef]
- Wang, F.; Allen, D.; Tian, S.; Oler, E.; Gautam, V.; Greiner, R.; Metz, T.; Wishart, D. CFM-ID 4.0–a Web Server for Accurate MS-Based Metabolite Identification. Nucleic Acids Res. 2022, 50, W165–W174. [Google Scholar] [CrossRef] [PubMed]
- Ruttkies, C.; Schymanski, E.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag Relaunched: Incorporating Strategies beyond in Silico Fragmentation. J. Cheminform. 2016, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The Role of Reporting Standards for Metabolite Annotation and Identification in Metabolomic Studies. Gigascience 2013, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Eriksson, L.; Jaworska, J.; Worth, A.P.; Cronin, M.T.D.; McDowell, R.M.; Gramatica, P. Methods for Reliability and Uncertainty Assessment and for Applicability Evaluations of Classification- and Regression-Based QSARs. Environ. Health Perspect. 2003, 111, 1361–1375. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Tarapoulouzi, M.; Mironescu, M.; Drouza, C.; Mironescu, I.D.; Agriopoulou, S. Insight into the Recent Application of Chemometrics in Quality Analysis and Characterization of Bee Honey during Processing and Storage. Foods 2023, 12, 473. [Google Scholar] [CrossRef]
- Terrab, A.; Díez, M.J.; Heredia, F.J. Characterisation of Moroccan Unifloral Honeys by Their Physicochemical Characteristics. Food Chem. 2002, 79, 373–379. [Google Scholar] [CrossRef]
- Peixoto, J.A.B.; Álvarez-Rivera, G.; Alves, R.C.; Costa, A.S.G.; Machado, S.; Cifuentes, A.; Ibáñez, E.; Oliveira, M.B.P.P. Comprehensive Phenolic and Free Amino Acid Analysis of Rosemary Infusions: Influence on the Antioxidant Potential. Antioxidants 2021, 10, 500. [Google Scholar] [CrossRef]
- Popova, M.; Gerginova, D.; Trusheva, B.; Simova, S.; Tamfu, A.N.; Ceylan, O.; Clark, K.; Bankova, V. A Preliminary Study of Chemical Profiles of Honey, Cerumen, and Propolis of the African Stingless Bee Meliponula Ferruginea. Foods 2021, 10, 997. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, M.; Yuan, Y.; Mu, G.; Xu, H.; Zhao, T.; Wang, Y.; Xue, X. Chaste Honey in Long Term-Storage: Occurrence and Accumulation of Maillard Reaction Products, and Safety Assessment. Food Chem. 2023, 424, 136457. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Ma, M.; Wang, Z.; Hayat, K.; Zhang, X.; Ho, C.-T. Temperature-Dependent Catalysis of Glycylglycine on Its Amadori Compound Degradation to Deoxyosone. J. Agric. Food Chem. 2022, 70, 8409–8416. [Google Scholar] [CrossRef]
- Inoue, Y.; Katsumata, T.; Watanabe, H.; Hayase, F. Mechanisms of D-Amino Acid Formation during Maturation of Sweet Rice Wine (Mirin). Food Sci. Technol. Res. 2016, 22, 679–686. [Google Scholar] [CrossRef][Green Version]
- Moreira, R.F.A.; De Maria, C.A.B.; Pietroluongo, M.; Trugo, L.C. Chemical Changes in the Non-Volatile Fraction of Brazilian Honeys during Storage under Tropical Conditions. Food Chem. 2007, 104, 1236–1241. [Google Scholar] [CrossRef]
- Tedesco, R.; Scalabrin, E.; Malagnini, V.; Strojnik, L.; Ogrinc, N.; Capodaglio, G. Characterization of Botanical Origin of Italian Honey by Carbohydrate Composition and Volatile Organic Compounds (VOCs). Foods 2022, 11, 2441. [Google Scholar] [CrossRef]
- Carabetta, S.; Di Sanzo, R.; Fuda, S.; Muscolo, A.; Russo, M. A Predictive Model to Correlate Amino Acids and Aromatic Compounds in Calabrian Honeys. Foods 2023, 12, 3284. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Z.; Li, X.; Guan, Z.; Huang, X.; Zu, T.; Jia, G.; Zhu, F.; Li, J.; Zhang, J. Simultaneous Determination of Trigonelline and Caffeine and Its Application in the Identification of Chinese Citrus, Coffee and Rape Honey. J. Food Meas. Charact. 2024, 18, 962–979. [Google Scholar] [CrossRef]
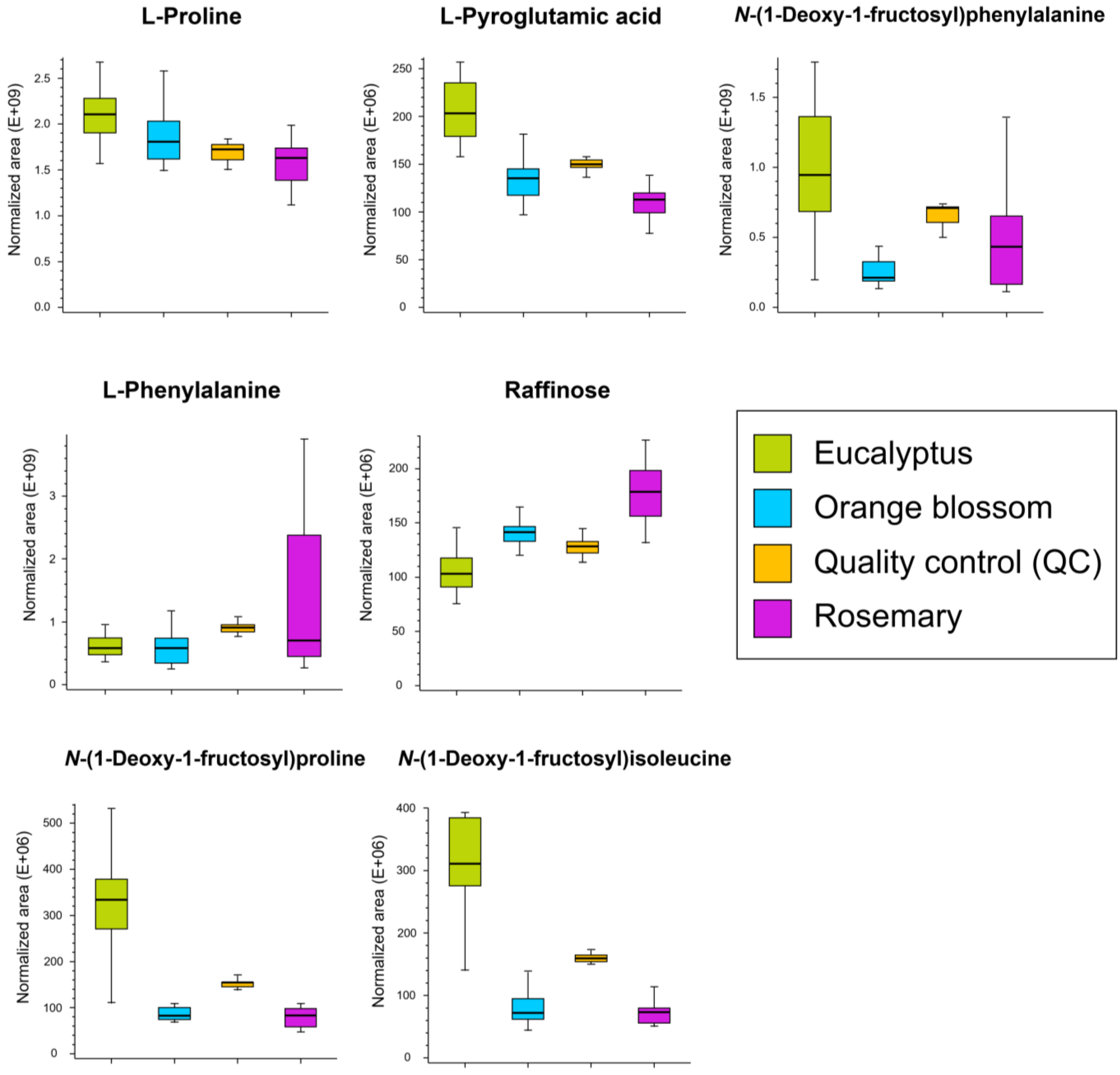
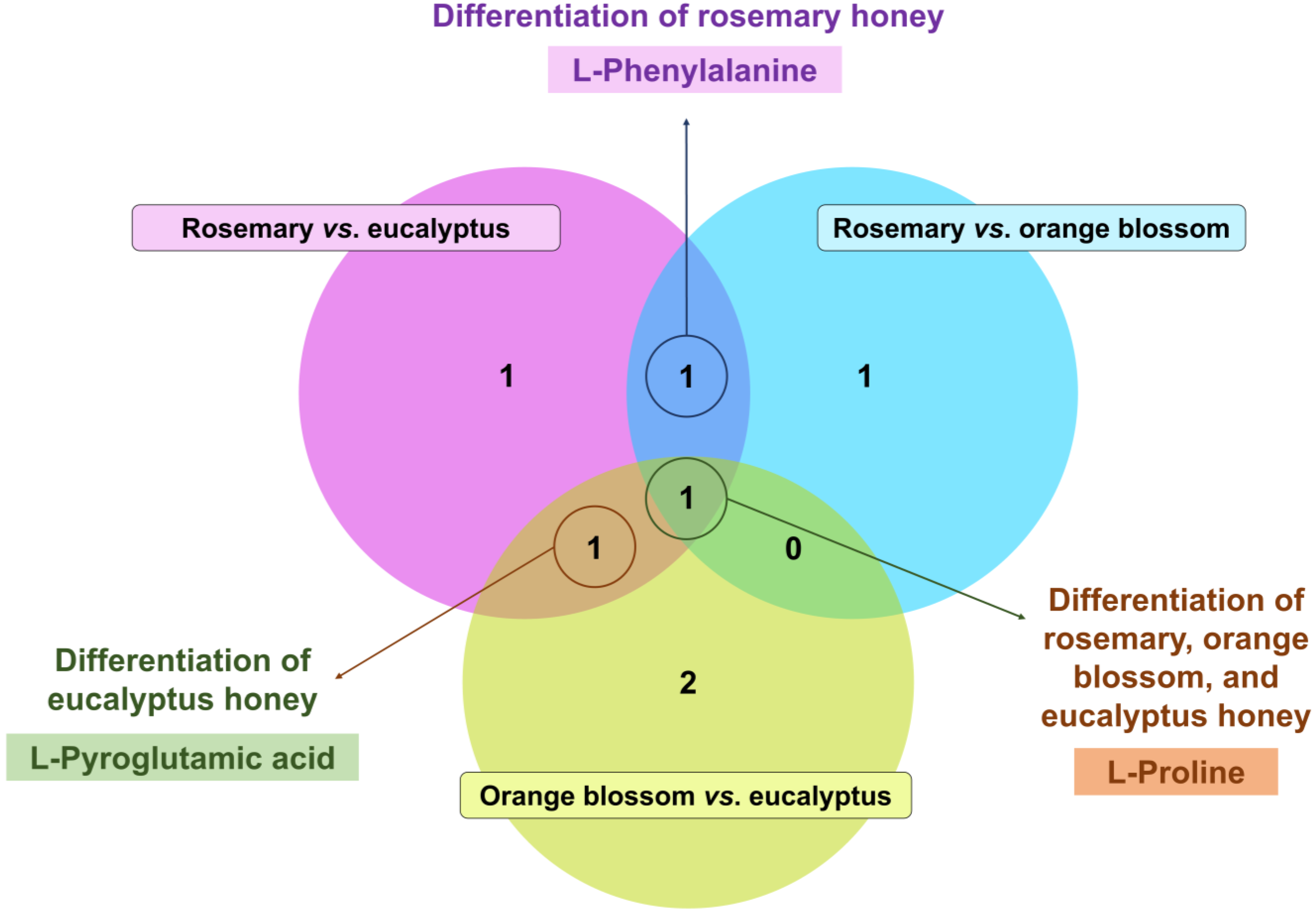
| No. | RT (min) | Marker Name | Molecular Formula | VIP Score | p-Value 1 | Log2(FC) 2 |
|---|---|---|---|---|---|---|
| Rosemary vs. eucalyptus honey | ||||||
| 1 | 1.039 | L-proline | C5H9NO2 | 2.39 ± 1.06 | 1.2 × 10−8 | −0.37 |
| 2 | 1.083 | L-pyroglutamic acid | C5H7NO3 | 1.17 ± 0.74 | 8.9 × 10−9 | −0.85 |
| 3 | 1.133 | N-(1-deoxy-1-fructosyl)phenylalanine | C15H21NO7 | 2.45 ± 0.71 | 2.9 × 10−3 | −1.12 |
| 4 | 1.207 | L-phenylalanine | C9H12NO2 | 3.50 ± 1.88 | 2.9 × 10−3 | 0.27 |
| Rosemary vs. orange blossom honey | ||||||
| 5 | 1.039 | L-proline | C5H9NO2 | 2.25 ± 1.36 | 2.8 × 10−2 | −0.15 |
| 6 | 1.062 | Raffinose | C18H32016 | 1.05 ± 0.55 | 9.4 × 10−7 | 0.34 |
| 7 | 1.207 | L-phenylalanine | C9H12NO2 | 5.55 ± 2.08 | 5.0 × 10−3 | 0.27 |
| Orange blossom vs. eucalyptus honey | ||||||
| 8 | 1.039 | L-proline | C5H9NO2 | 1.36 ± 0.66 | 3.7 × 10−3 | −0.22 |
| 9 | 1.040 | N-(1-deoxy-1-fructosyl)proline | C11H19NO7 | 1.97 ± 0.30 | 8.9 × 10−9 | −2.02 |
| 10 | 1.083 | L-pyroglutamic acid | C5H7NO3 | 1.19 ± 0.80 | 1.8 × 10−7 | −0.59 |
| 11 | 1.089 | N-(1-deoxy-1-fructosyl)isoleucine | C12H23NO7 | 1.95 ± 0.52 | 8.7 × 10−9 | −2.10 |
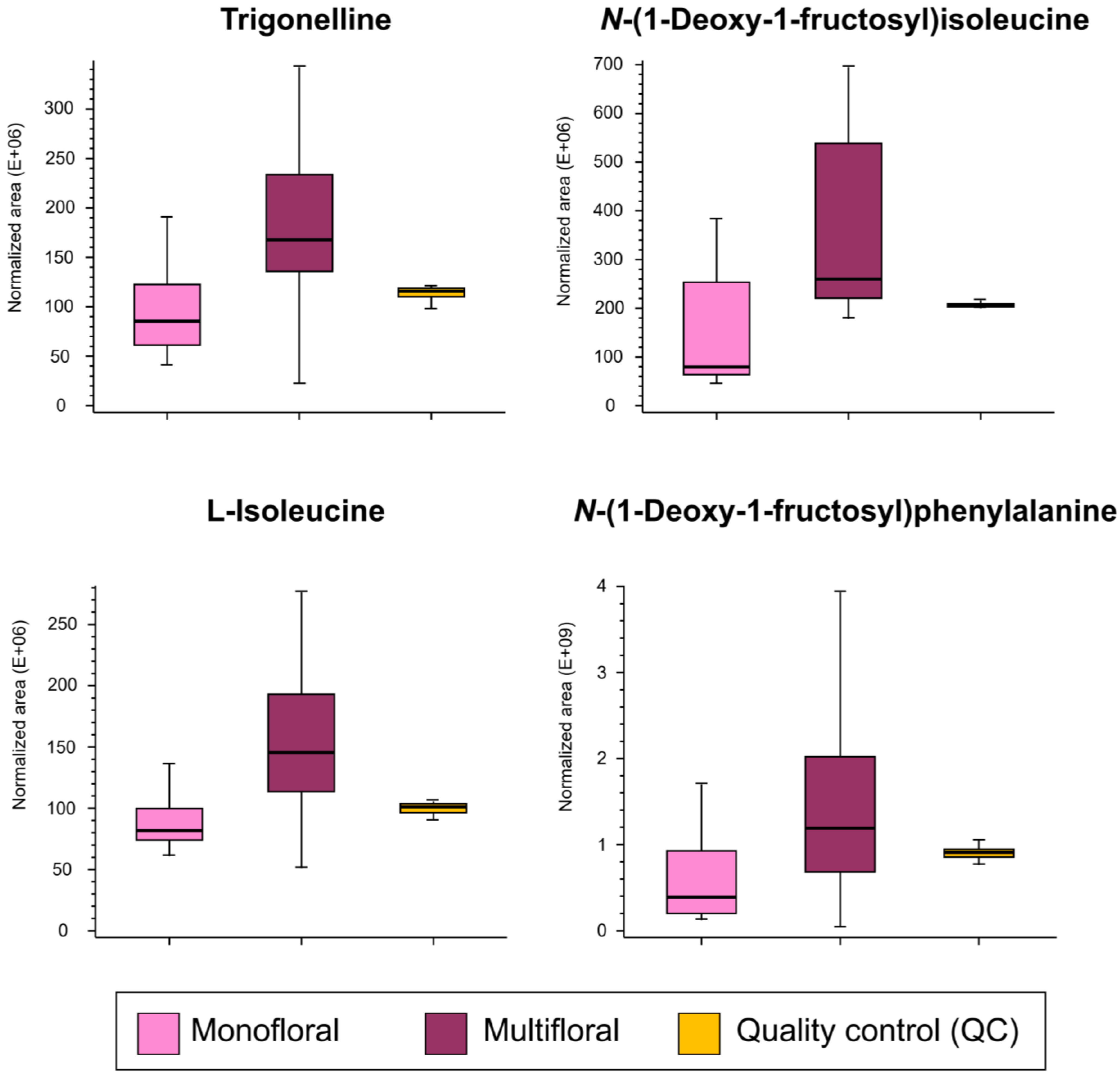
| Multifloral vs. monofloral honey | ||||||
| 12 | 1.035 | Trigonelline | C7H7NO2 | 1.45 ± 0.34 | 1.4 × 10−3 | 0.97 |
| 13 | 1.089 | N-(1-deoxy-1-fructosyl)isoleucine | C12H23NO7 | 2.49 ± 1.40 | 6.3 × 10−5 | 1.70 |
| 14 | 1.102 | L-isoleucine | C6H13NO2 | 1.42 ± 0.57 | 3.9 × 10−4 | 0.83 |
| 15 | 1.133 | N-(1-deoxy-1-fructosyl)phenylalanine | C15H21NO7 | 4.88 ± 1.12 | 7.3 × 10−3 | 1.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Pérez, A.; Navarro-Herrera, A.M.; Garrido Frenich, A. Identifying Key Markers for Monofloral (Eucalyptus, Rosemary, and Orange Blossom) and Multifloral Honey Differentiation in the Spanish Market by UHPLC-Q-Orbitrap-High-Resolution Mass Spectrometry Fingerprinting and Chemometrics. Foods 2024, 13, 2755. https://doi.org/10.3390/foods13172755
Rivera-Pérez A, Navarro-Herrera AM, Garrido Frenich A. Identifying Key Markers for Monofloral (Eucalyptus, Rosemary, and Orange Blossom) and Multifloral Honey Differentiation in the Spanish Market by UHPLC-Q-Orbitrap-High-Resolution Mass Spectrometry Fingerprinting and Chemometrics. Foods. 2024; 13(17):2755. https://doi.org/10.3390/foods13172755
Chicago/Turabian StyleRivera-Pérez, Araceli, Alba María Navarro-Herrera, and Antonia Garrido Frenich. 2024. "Identifying Key Markers for Monofloral (Eucalyptus, Rosemary, and Orange Blossom) and Multifloral Honey Differentiation in the Spanish Market by UHPLC-Q-Orbitrap-High-Resolution Mass Spectrometry Fingerprinting and Chemometrics" Foods 13, no. 17: 2755. https://doi.org/10.3390/foods13172755
APA StyleRivera-Pérez, A., Navarro-Herrera, A. M., & Garrido Frenich, A. (2024). Identifying Key Markers for Monofloral (Eucalyptus, Rosemary, and Orange Blossom) and Multifloral Honey Differentiation in the Spanish Market by UHPLC-Q-Orbitrap-High-Resolution Mass Spectrometry Fingerprinting and Chemometrics. Foods, 13(17), 2755. https://doi.org/10.3390/foods13172755






