Analysis of Metabolite Differences in Different Tea Liquors Based on Broadly Targeted Metabolomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Tea Liquor
2.3. Sample Extraction
2.4. UPLC-MS/MS
2.5. Qualitative Analysis of Metabolites
2.6. Statistical Analysis
3. Results and Discussion
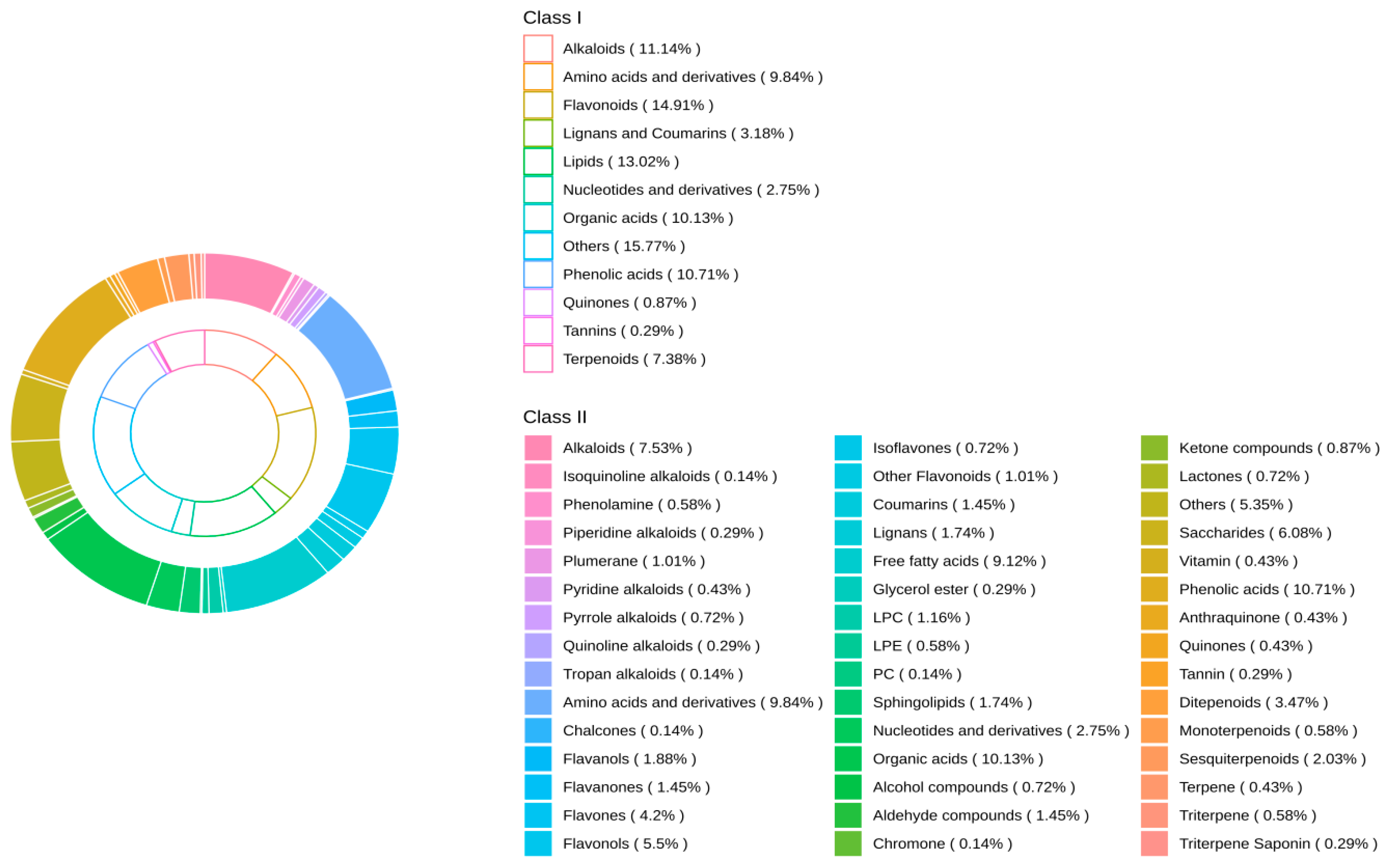
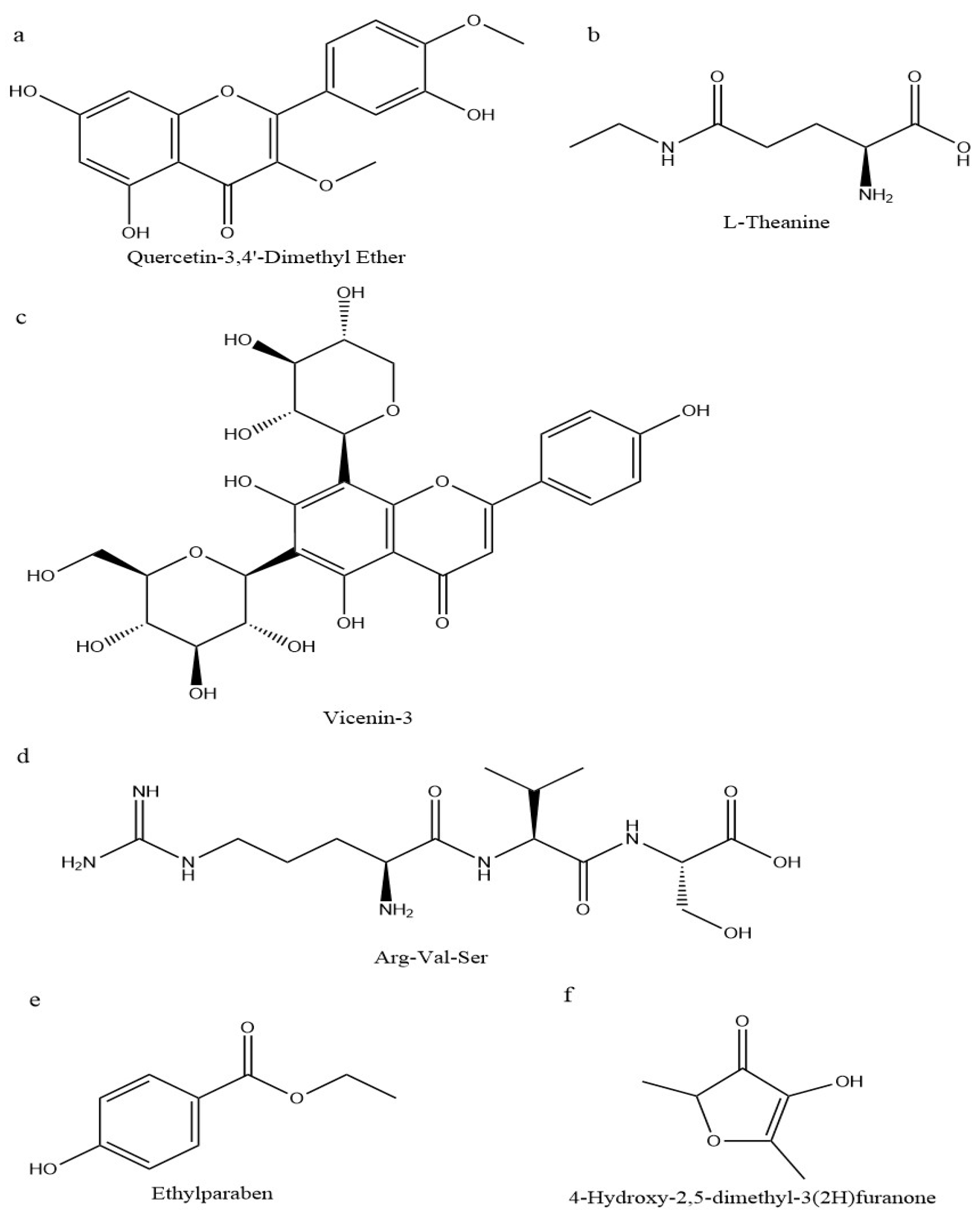
3.1. Overview of Non-Volatile Metabolites in the Four Samples
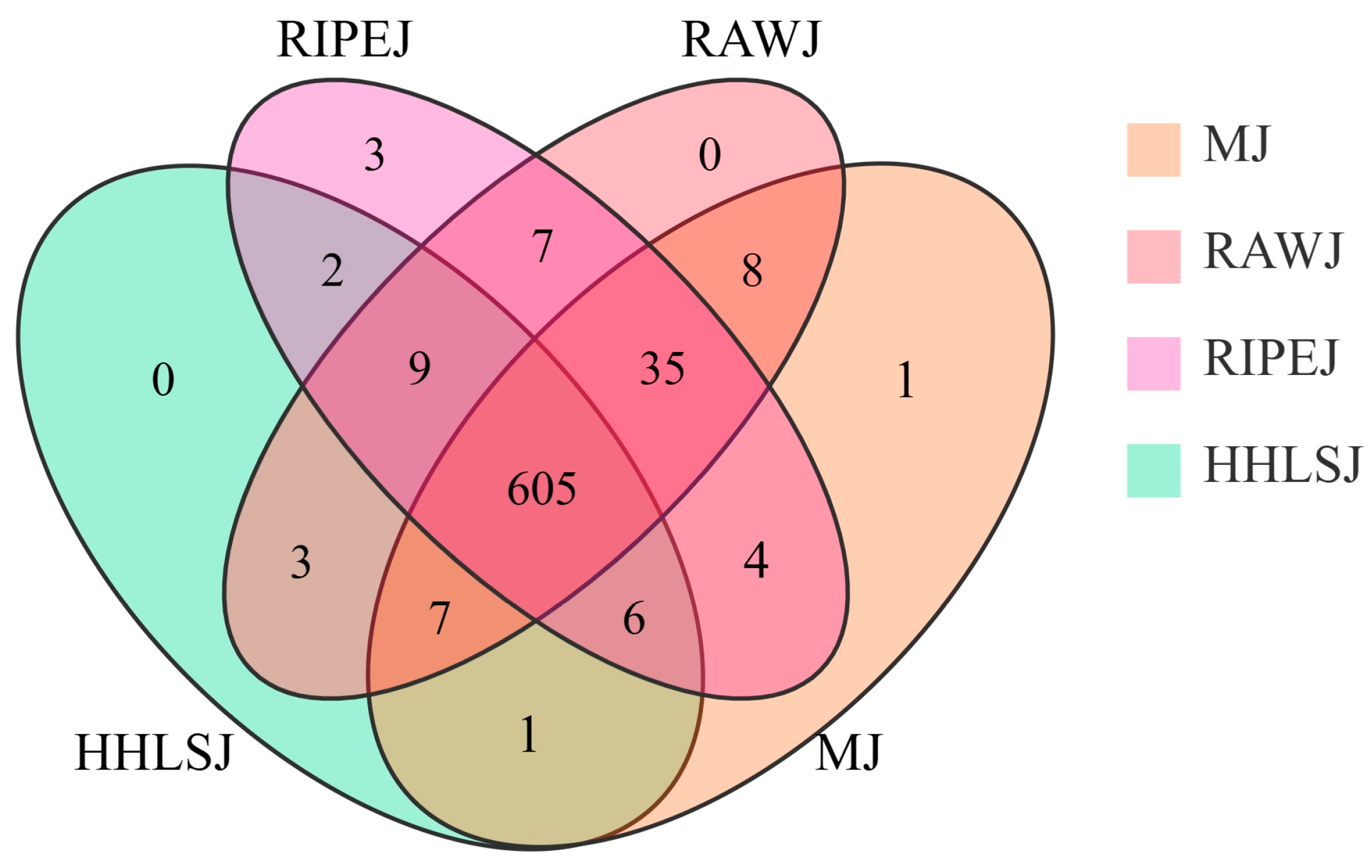
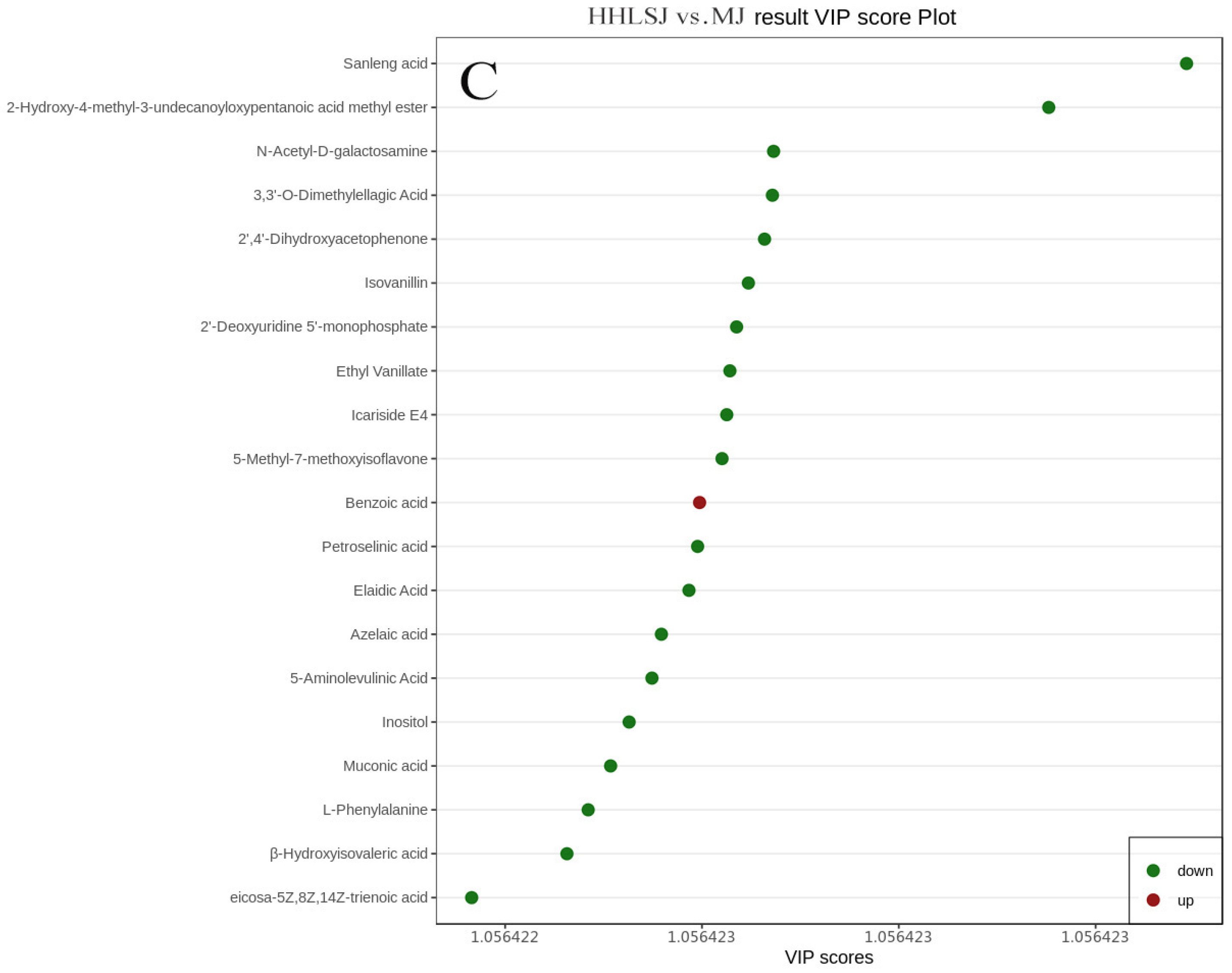
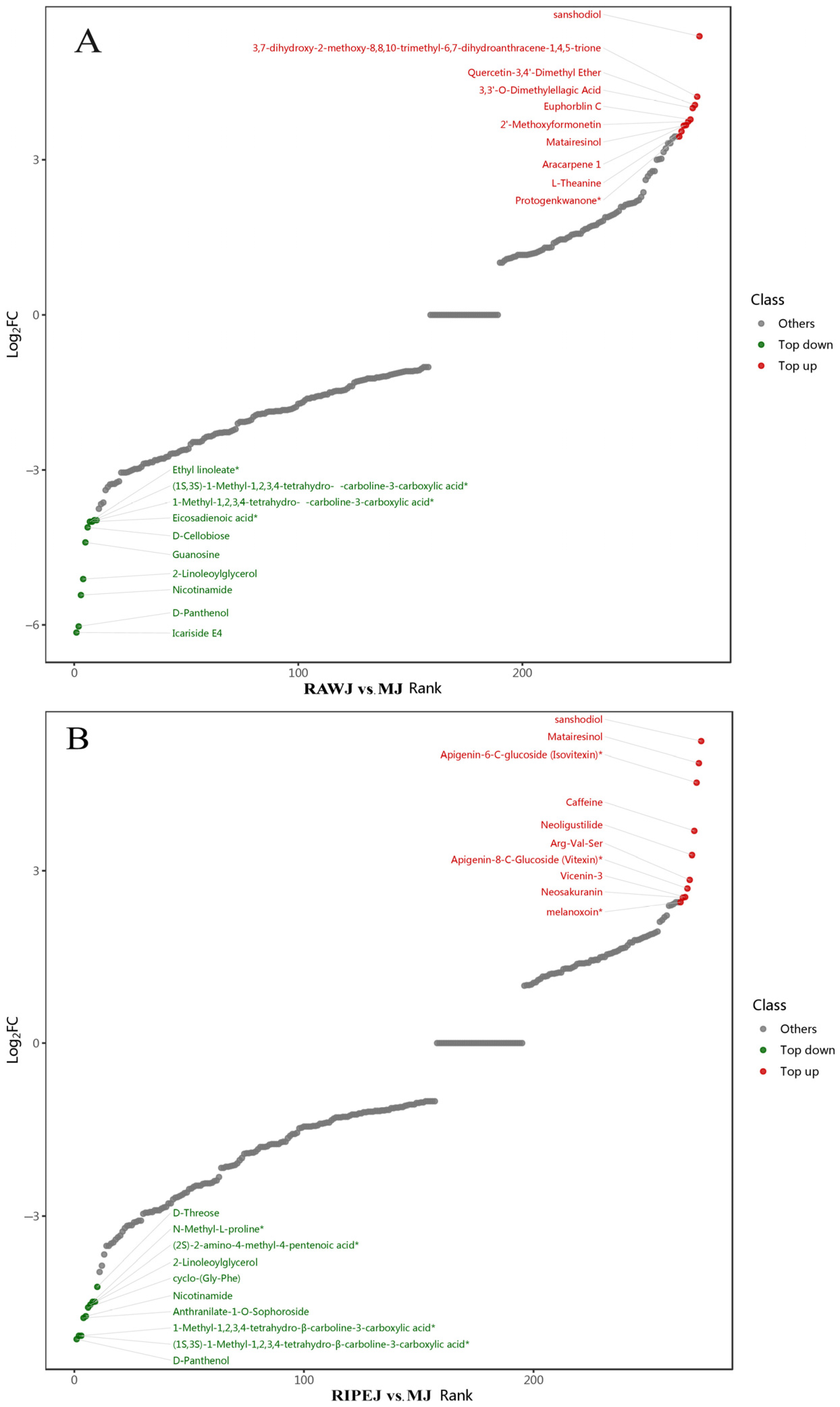
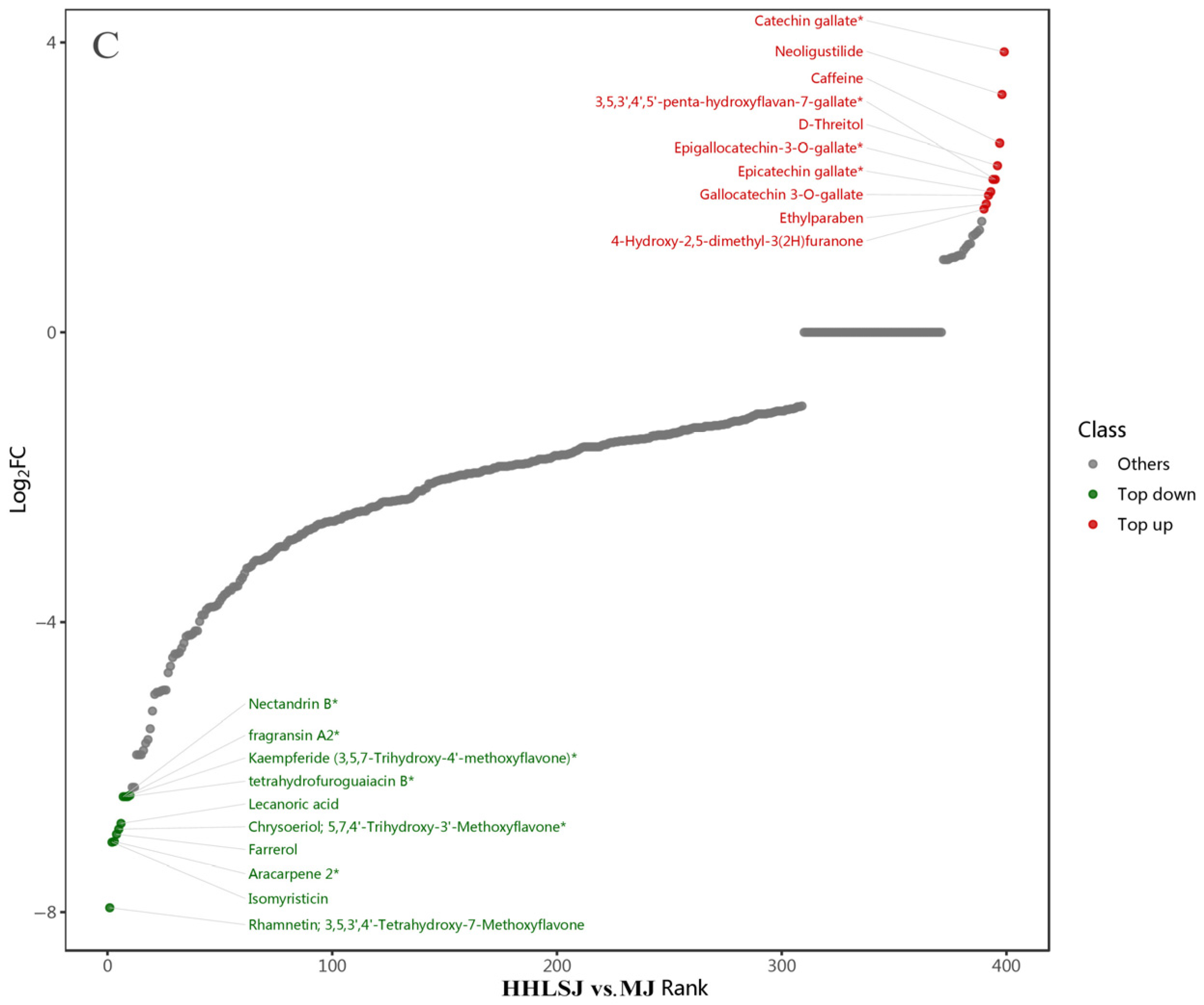
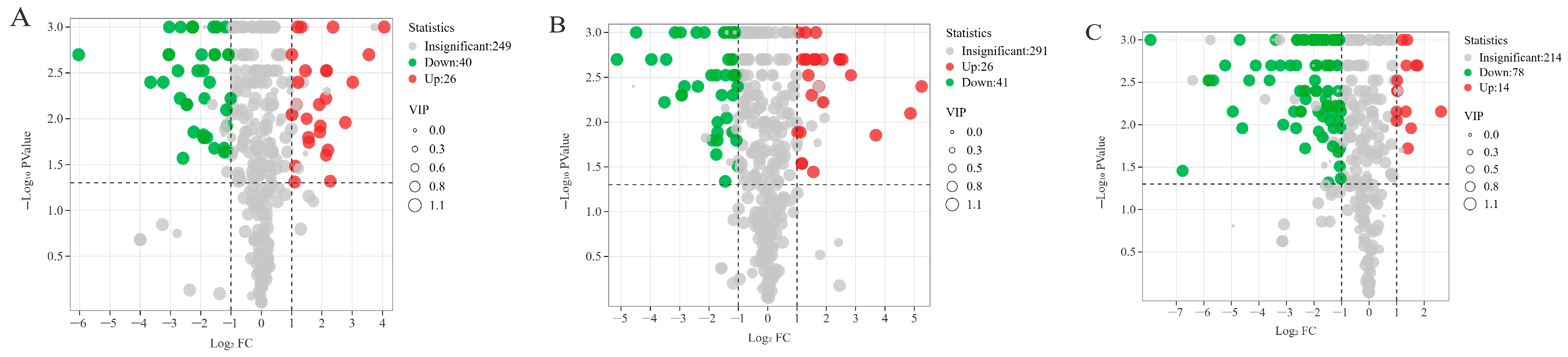
3.2. Screening of Differential Metabolites
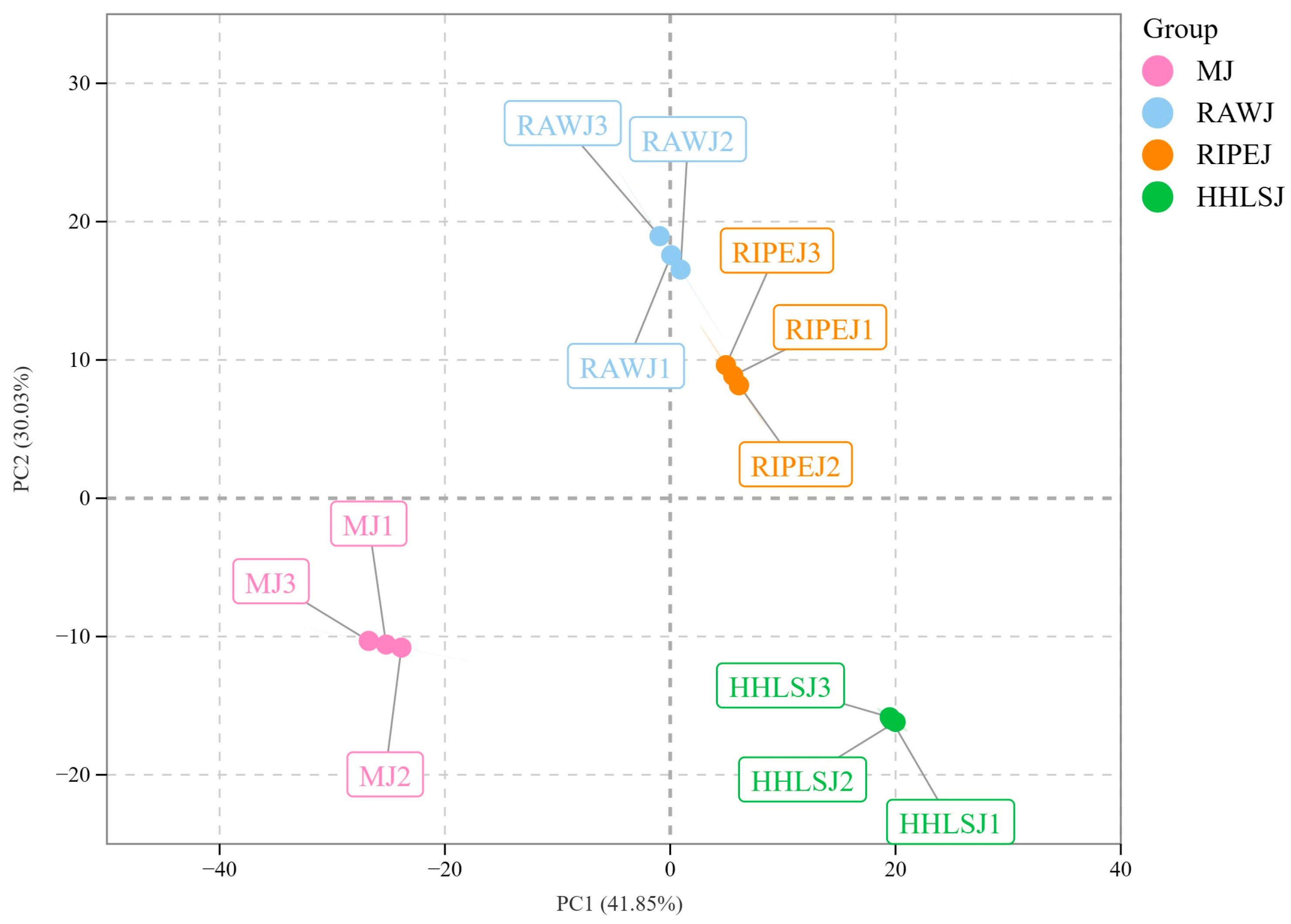
3.2.1. Data Quality Assessment
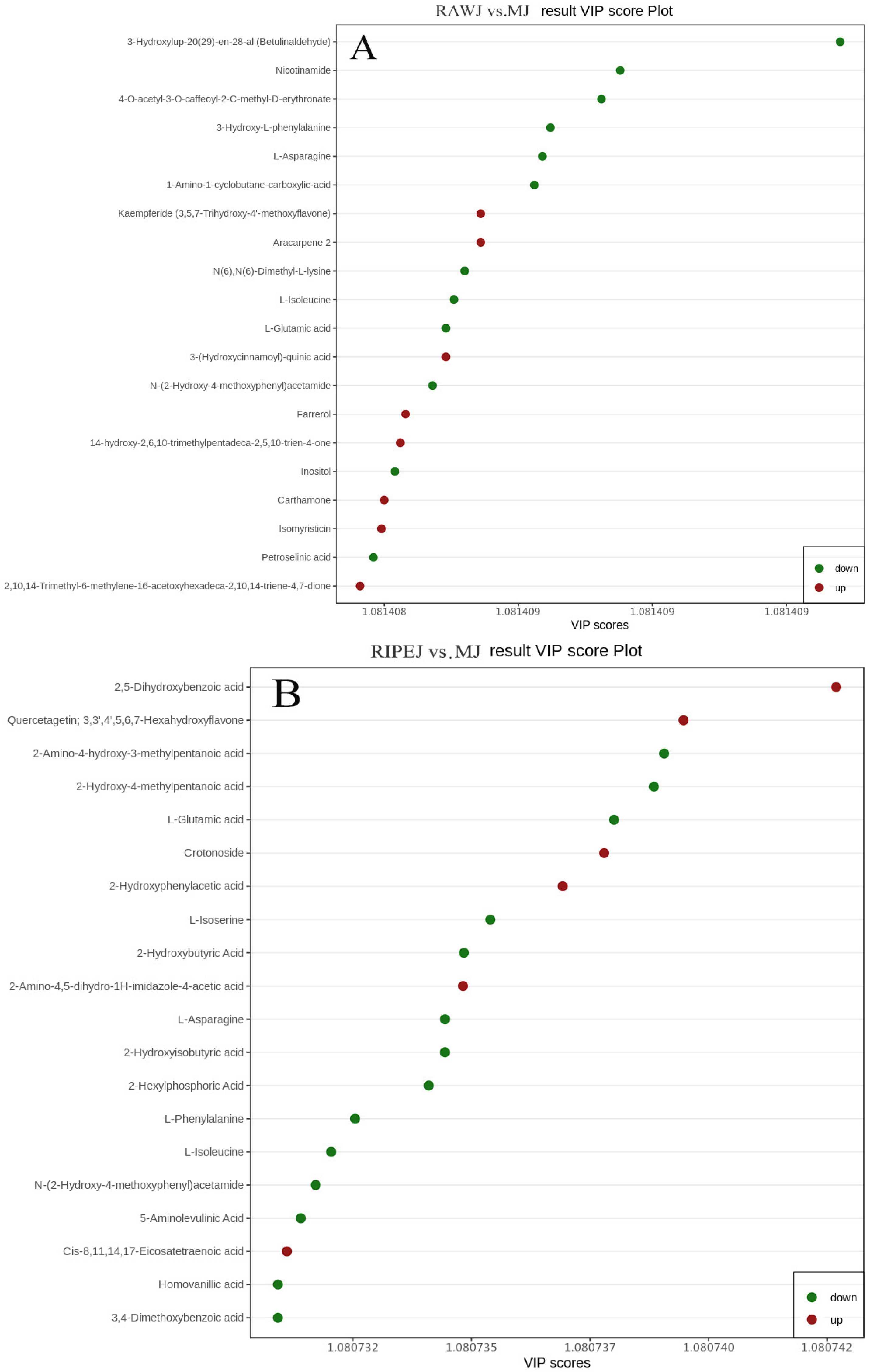
3.2.2. OPLS-DA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, F.J.; Wei, X.L.; Liu, H.Y.; Li, H.; Xia, Y.; Wu, D.T.; Zhang, P.Z.; Gandhi, G.R.; Li, H.-B.; Gan, R.Y. State-of-the-art review of dark tea: From chemistry to health benefits. Trends Food Sci. Technol. 2021, 109, 126–138. [Google Scholar] [CrossRef]
- Jia, W.; Fan, Z.; Du, A.; Li, Y.; Zhang, R.; Shi, Q.; Shi, L.; Chu, X. Recent advances in baijiu analysis by chromatography based technology–A review. Food Chem. 2020, 324, 126899. [Google Scholar] [CrossRef]
- Yue, C.; Zhao, Q.C. Research on Cordyceps militaris tea wine. China Brew. 2011, 30, 179–182. [Google Scholar]
- Zou, C.; Xu, Y. Fermentation process optimization and chemical composition analysis on black tea wine. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2021. [Google Scholar]
- He, Y.; Liu, Z.; Qian, M.; Yu, X.; Xu, Y.; Chen, S. Unraveling the chemosensory characteristics of strong-aroma type baijiu from different regions using comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry and descriptive sensory analysis. Food Chem. 2020, 331, 127335. [Google Scholar] [CrossRef]
- Song, S.; Pan, L.; Wu, X. Production of Dangshan Pear Wine by Liquid Fermentation Based on Orthogonal Design Optimal Experiments. J. Food Eng. Technol. 2015, 4, 17–23. [Google Scholar]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Ma, R.; Sui, L.; Zhang, J.; Hu, J.; Liu, P. Polyphasic characterization of yeasts and lactic acid bacteria metabolic contribution in semi-solid fermentation of Chinese baijiu (traditional fermented alcoholic drink): Towards the design of a tailored starter culture. Microorganisms 2019, 7, 147. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Hu, J.; Zhou, X.; Wang, M.; Zhang, J.; Liu, P. Microbiota Dynamics in Semisolid Fermentation of Light-Flavor Liquor with Corn as Main Material by Illumina MiSeq Sequencing. Res. Sq. 2020; preprint. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, Z.J.; Yang, F.Y.; Yu, Y.G. Development of a new type of Anhua black tea and its application: Black tea wine. J. Food Process. Preserv. 2021, 46, e15862. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Jia, W.; Wen, B.; Liao, S.; Zhao, Y.; Tang, Q.; Li, K.; Hua, Y.; Yang, Y.; et al. Dynamic changes in the major chemical and volatile components during the “Ziyan” tea wine processing. LWT Food Sci. Technol. 2023, 186, 115273. [Google Scholar] [CrossRef]
- Qi, W.; Wu, L.; Zhao, W.W.; Zhou, S.L. Response surface optimization of glutinous rice tea wine production process. China Brew. 2020, 39, 204–210. [Google Scholar]
- Li, Y.; Zhang, S.; Sun, Y. Measurement of catechin and gallic acid in tea wine with HPLC. Saudi J. Biol. Sci. 2020, 27, 214–221. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Liu, F.; Du, L.; Xiao, D. Development of Pu’er tea fermented wine. Liquor-Mak. Sci. Technol. 2015, 10, 103–106. [Google Scholar]
- GB/T 22111-2008.2008-06-17; Geographical Indication Products Pu’er Tea. Standards Press of China: Beijing, China, 2008.
- Zehra, K.; Yaren, A. Assessment of instrumental and sensory quality characteristics of the bread products enriched with Kombucha tea. Int. J. Gastron. Food Sci. 2022, 29, 100562. [Google Scholar]
- Yang, R.; Lin, W.; Liu, J.; Liu, H.; Fu, X.; Liu, H.; Han, Z.; Wang, L.; Wang, Y.; Ba, G. Formation mechanism and solution of Pu-erh tea cream based on non-targeted metabonomics. LWT 2023, 173, 114331. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Characterization, Antioxidant Potential, and harmacokinetics Properties of Henolic Compounds from Native Astralian Herbs and Fruits. Plants 2023, 12, 993. [Google Scholar] [CrossRef]
- Farag, M.A.; Kabbash, E.M.; Mediani, A.; Döll, S.; Esatbeyoglu, T.; Afifi, S.M. Comparative Metabolite Fingerprinting of Four Different Cinnamon Species Analyzed via UPLC–MS and GC–MS and Chemometric Tools. Molecules 2022, 27, 2935. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- GB/T 10781.3-2006; Rice Aromatic Chinese Spirits. Standards Press of China: Beijing, China, 2006.
- GB/T 23776-2018; Sensory Evaluation Method for Tea. Standards Press of China: Beijing, China, 2018.
- GB/T 22211-2008; Fragrance Rice Baijiu. Standards Press of China: Beijing, China, 2008.
- You, J.; Li, H.; Wang, Q.; Xu, F.; Lin, S.; Wang, X.; Huang, S.; Sheng, Y.; Zhu, B.; Zhang, Q.; et al. Establishment of male and female Eucommia fingerprints by UPLC combined with OPLS-DA model and its application. Chem. Biodivers. 2023, 20, e202201054. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Arroo, R.; Sari, S.; Huang, S. Flavonoids as Inducers of Apoptosis and Autophagy in Breast Cancer. In Discovery and Development of Anti-Breast Cancer Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Ding, L.L.; Lin, X.Y. Status quo and research and development of bitter phytonutrients. Food Res. Dev. 2003, 1, 57–59. [Google Scholar]
- Chen, C.H.; Wu, M.C.; Hou, C.Y.; Jiang, C.M.; Huang, C.M.; Wang, Y.T. Effect of phenolic acid on antioxidant activity of wine and inhibition of pectin methyl esterase. J. Inst. Brew. 2009, 115, 328–333. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, H.; Deng, Y. Simultaneous determination of catechins, caffeine and Gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 2002, 57, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.Q.; Tukhvatshin, M.; Zheng, D.; Zhang, Q.; Yang, J.; Ye, N. Determination of phenolic acid compounds in tea by high performance liquid chromatography. Chin. J. Tea 2019, 6, 60. [Google Scholar]
- Wang, S.; Kai, L.; Yang, J.; Ji, X.; Qian, H.; Xu, Y.; Du, H. Effects of short-chain fatty acids and their ethyl esters in strong-flavored liquor on the human body. Food Ferment. Ind. 2020, 46, 7. [Google Scholar]
- Chen, H.; Wu, Y.; Wang, J.; Hong, J.; Tian, W.; Zhao, D.; Sun, J.; Huang, M.; Li, H.; Zheng, F.; et al. Uncover the flavor code of roasted sesame for sesame flavor baijiu: Advance on the revelation of aroma compounds in sesame flavor baijiu by means of modern separation technology and molecular sensory evaluation. Foods 2022, 11, 11. [Google Scholar] [CrossRef]
- Procopio, S.; Sprung, P.; Becker, T. Effect of amino acid supply on the transcription of flavour-related genes and aroma compound production during lager yeast fermentation. LWT Food Sci. Technol. 2015, 63, 289–297. [Google Scholar] [CrossRef]
- Wen, C.K.; Chen, X.; Wang, Y.F.; Fang, S.L.; Xia, Y.F.; Li, Q.; Li, X.Q.; Chen, M.B. Research Progress on functional components of rice wine. China Brew. 2019, 38, 5–8. [Google Scholar]
- Yasuda, S.; Horinaka, M.; Iizumi, Y.; Goi, W.; Sukeno, M.; Sakai, T. Oridonin inhibits SASP by blocking p38 and NF-κB pathways in senescent cells. Biochem. Biophys. Res. Commun. 2022, 590, 55–62. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.; Zhang, X.; Rao, J.; Xin, P.; Yin, J.; Song, Q. Determination of terpenoids in yam wine by liquid–liquid extraction coupled with gas chromatography-mass spectrometry. Food Ferment. Ind. 2019, 45, 213–217. [Google Scholar]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metab. 2016, 4, 97–103. [Google Scholar] [CrossRef]
- Yuan, Z.; Qin, Y.; Yang, F.; Deng, Z. Ultrasound-assisted technology to develop a new type of dark tea wine. Food Sci. Technol. 2021, 11, 46. [Google Scholar]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi- and Megavariate Data Analysis: Part I: Basic Principles and Applications; Umetrics Inc.: Wilmington, MA, USA, 2006. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Niu, M.; Yang, H.; Zhou, X.; Ding, J.; Xu, Y.; Lv, C.; Li, J. Analysis of Metabolite Differences in Different Tea Liquors Based on Broadly Targeted Metabolomics. Foods 2024, 13, 2800. https://doi.org/10.3390/foods13172800
Li X, Niu M, Yang H, Zhou X, Ding J, Xu Y, Lv C, Li J. Analysis of Metabolite Differences in Different Tea Liquors Based on Broadly Targeted Metabolomics. Foods. 2024; 13(17):2800. https://doi.org/10.3390/foods13172800
Chicago/Turabian StyleLi, Xiongyu, Miao Niu, Hongyan Yang, Xianxiu Zhou, Jianliang Ding, Yawen Xu, Caiyou Lv, and Jiahua Li. 2024. "Analysis of Metabolite Differences in Different Tea Liquors Based on Broadly Targeted Metabolomics" Foods 13, no. 17: 2800. https://doi.org/10.3390/foods13172800




