Potential Skin Health Benefits of Abalone By-Products Suggested by Their Effects on MAPKS and PI3K/AKT/NF-kB Signaling Pathways in HDF and HaCaT Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. AVU Preparation
2.1.1. AVU Extraction
2.1.2. Analysis of the Chemical Composition of AVU
2.1.3. Analysis of Microorganisms
2.1.4. Profile of Amino Acid Composition
2.2. In Vitro Skin Health Effects
2.2.1. Cell Culture
2.2.2. Determinations of Procollagen Type 1 C-Peptide and Hyaluronan Levels Using an Enzyme-Linked Immunosorbent Assay
2.2.3. Reverse Transcription Polymerase Chain Reaction Analysis
2.2.4. Western Blotting
2.3. Statistical Analysis
3. Results
3.1. Chemical Composition and Microbiological Testing of AVU
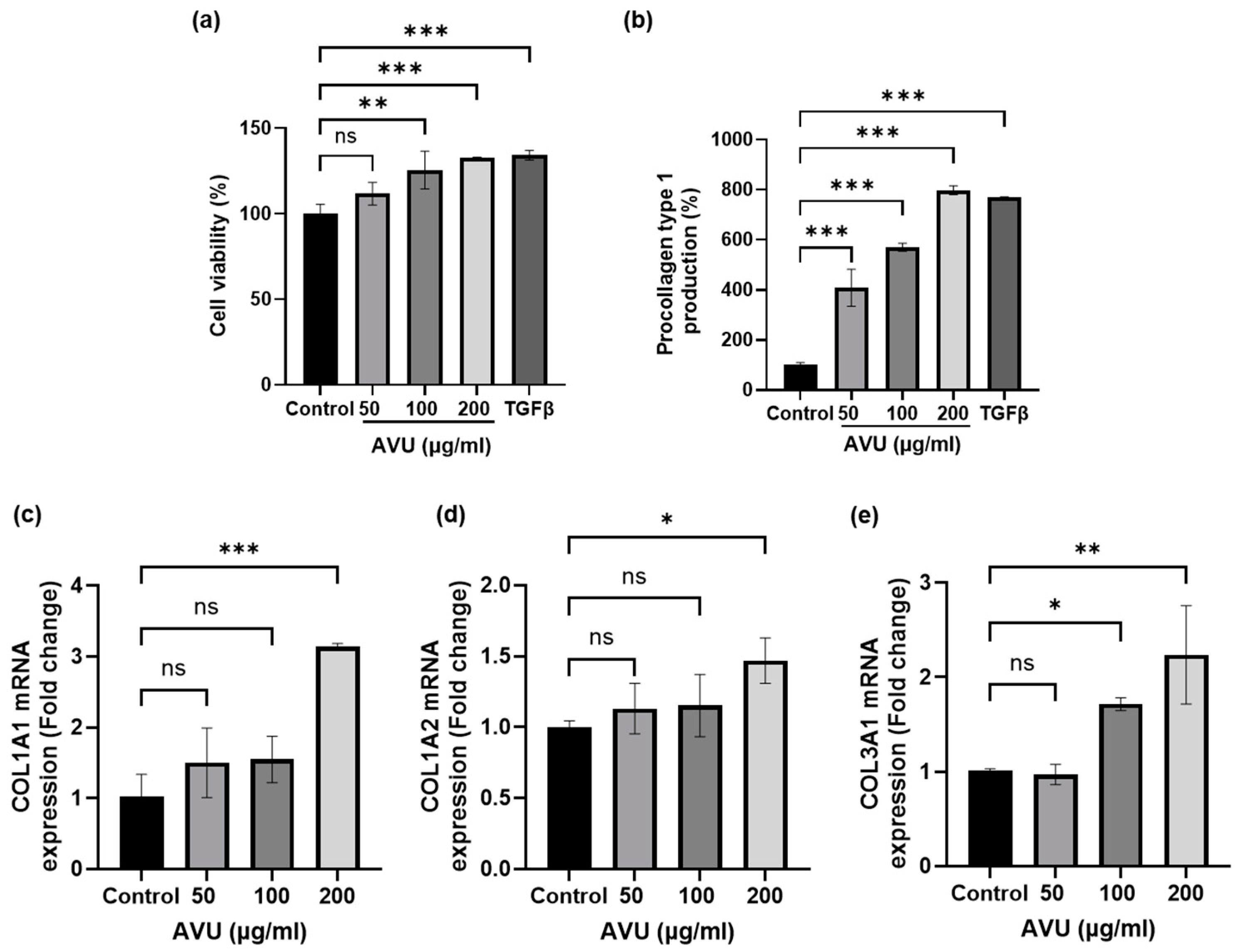
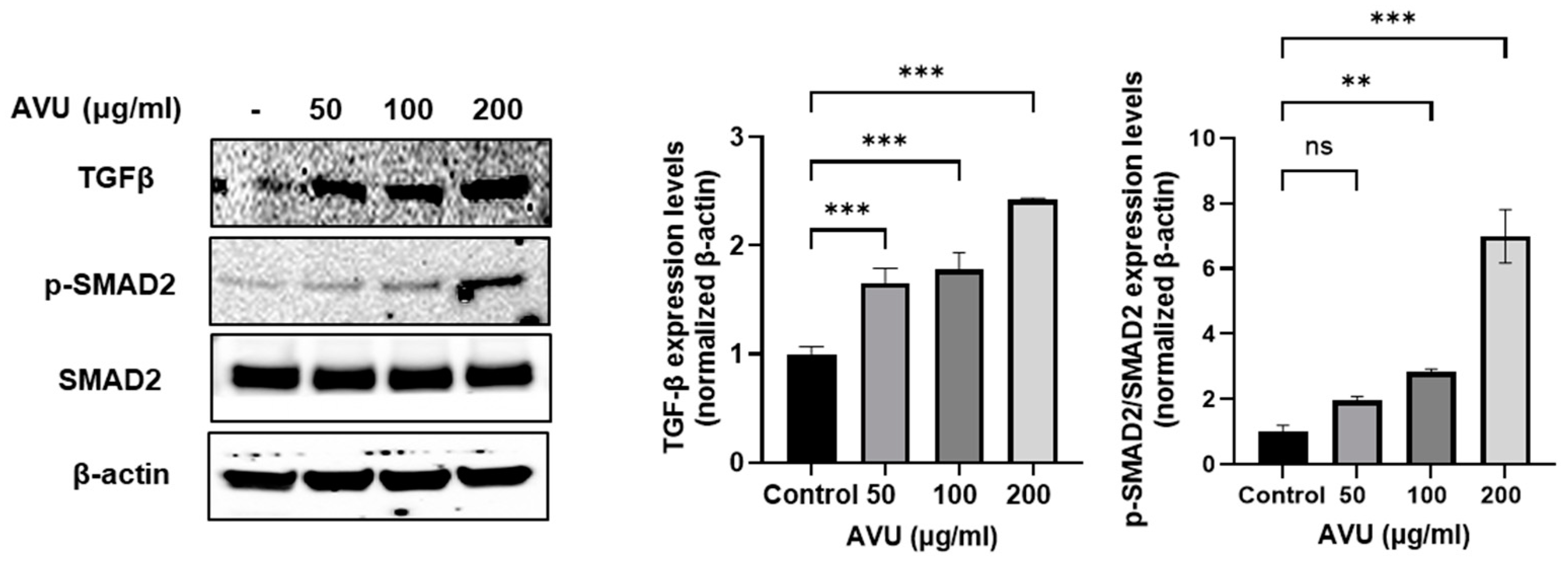
3.2. Collagen Synthesis Effects of AVU in HDF Cells
3.3. Moisturizing Effect of AVU in HaCaT Cells
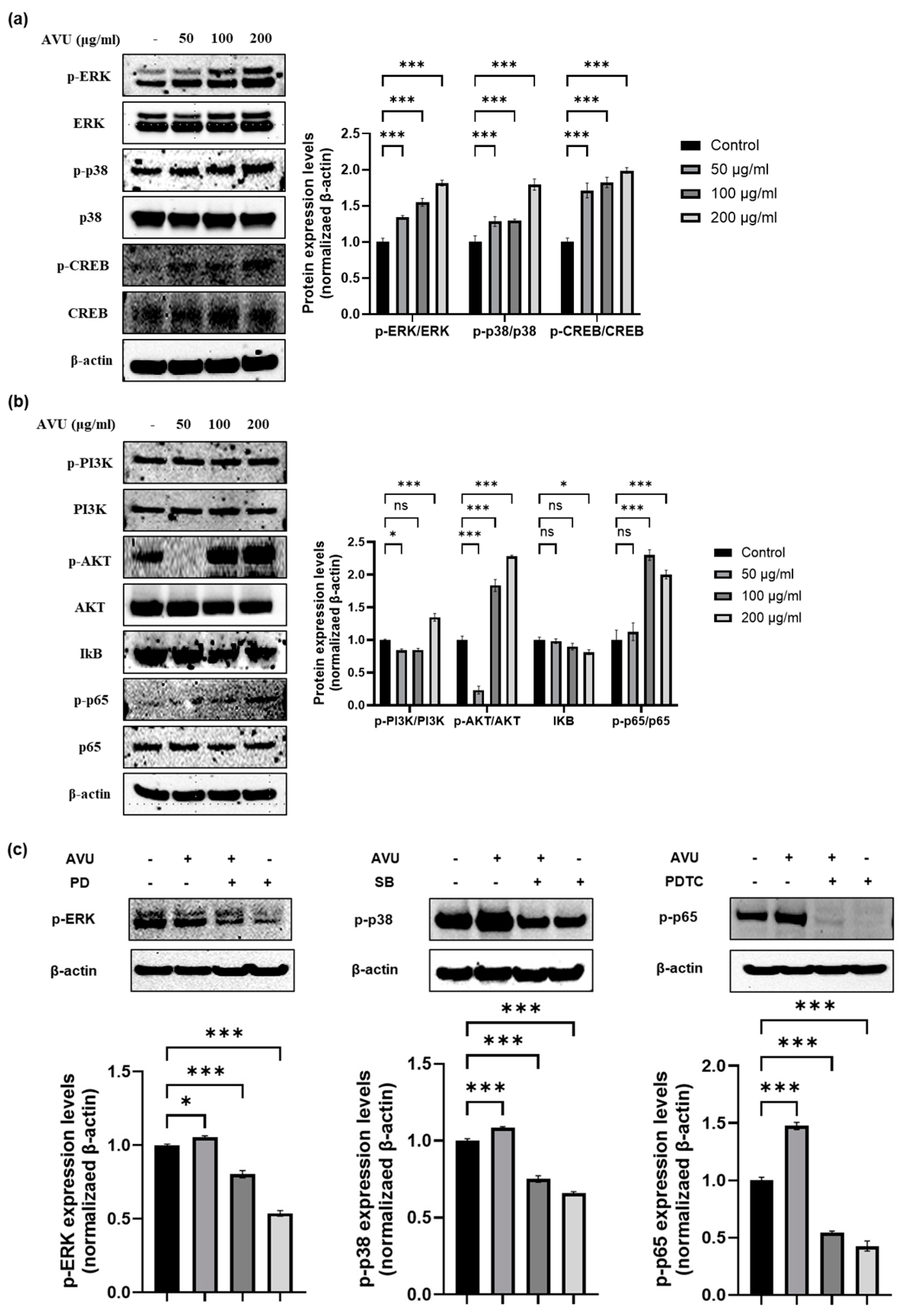
3.4. Moisturizing Effect of AVU through the MAPK and PI3K/Akt/NF-kB Signaling Pathway in HaCaT Cells
3.5. Role of Hyaluronic Acid Production in AVU-Induced EKR, p38 and NF-kB Activation in HaCaT Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivera-Pérez, C.; Ponce González, X.P.; Hernández-Savedra, N.Y.J.S.R. Antimicrobial and anticarcinogenic activity of bioactive peptides derived from abalone viscera (Haliotis fulgens and Haliotis corrugata). Sci. Rep. 2023, 13, 15185. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, N.; Sasaki, A.; Maizawa, T.; Hamada-Sato, N.J.N. Abalone viscera fermented with Aspergillus oryzae 001 prevents pressure elevation by inhibiting angiotensin converting enzyme. Nutrients 2023, 15, 947. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.-S.; Choi, K.S.; Chun, J.; Kho, K.-H.; Son, S.A.; Shim, S.-Y. Anti-inflammatory Effects of Abalone (Haliotis discus hannai) Viscera via Inhibition of ROS Production in LPS-Stimulated RAW 264.7 cells. Microbiol. Biotechnol. Lett. 2022, 50, 22–30. [Google Scholar] [CrossRef]
- Chung, W.H.; Coorey, R.; Takechi, R.; Howieson, J.J.L. Compositional and nutritional evaluation of viscera from commercially harvested wild-caught Australian abalones (Haliotis spp.). LWT 2024, 191, 115590. [Google Scholar] [CrossRef]
- Gong, F.; Chen, M.-F.; Chen, J.; Li, C.; Zhou, C.; Hong, P.; Sun, S.; Qian, Z. Boiled abalone by-product peptide exhibits antitumor activity in HT1080 cells and HUVECs by suppressing the metastasis and angiogenesis in vitro. J. Agric. Food Chem. 2019, 67, 8855–8867. [Google Scholar] [CrossRef]
- Kim, E.-A.; Kang, N.; Kim, J.; Yang, H.-W.; Ahn, G.; Heo, S.-J. Anti-inflammatory effect of turbo cornutus viscera ethanolic extract against lipopolysaccharide-stimulated inflammatory response via the regulation of the JNK/NF-kB signaling pathway in murine macrophage RAW 264.7 cells and a zebrafish model: A preliminary study. Foods 2022, 11, 364. [Google Scholar] [CrossRef]
- Yamanushi, M.; Shimura, M.; Nagai, H.; Hamada-Sato, N.J. Antihypertensive effects of abalone viscera fermented with Lactiplantibacillus pentosus SN001 via angiotensin-converting enzyme inhibition. Food Chem. 2022, 13, 100239. [Google Scholar] [CrossRef]
- Perera, C.O.; Alzahrani, M.A. Ultrasound as a pre-treatment for extraction of bioactive compounds and food safety: A review. LWT 2021, 142, 111114. [Google Scholar] [CrossRef]
- Majid, I.; Nayik, G.A.; Nanda, V. Ultrasonication and food technology: A review. Cogent Food Agric. 2015, 1, 1071022. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. IFSET 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Starek, A.; Kobus, Z.; Sagan, A.; Chudzik, B.; Pawlat, J.; Kwiatkoski, M.; Terebun, P.; Andrejko, D. Influence of ultrasound on selected microoranisms, chemical and structural chages in fresh tomato juice. Sci. Rep. 2021, 11, 3488. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lu, Y.; He, Q. Recent advances on bioactive compound, health benefits, and potential applications of jujube (Ziziphu Jujuba Mill.): A perspective of by-products valorization. Trends Food Sci. Technol. 2024, 145, 104368. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, W.J.; Park, S.Y.; Kim, H.; Chung, D.K. In vitro anti-wrinkle and skin-moisturizing effects of evening primrose (Oenothera biennis) sprout and identification of its active components. Processes 2021, 9, 145. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Zhong, W.; Liang, F.; Guo, Y.; Wang, Y.; Wang, Z.J. Moisturizing and antioxidant effects of Artemisia argyi essence liquid in HaCaT keratinocytes. Int. J. Mol. Sci. 2023, 24, 6809. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinol 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Lee, J.-E.; Kim, Y.-A.; Yu, S.; Park, S.Y.; Kim, K.H.; Kang, N.J. 6-Anhydro–L-galactose increases hyaluronic acid production via the EGFR and AMPKα signaling pathway in HaCaT keratinocytes. J. Dermatol. Sci. 2019, 96, 90–98. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, S.B.; Choi, J.M.; Kim, K.M.; Cho, B.K.; Cho, D.H.; Park, H.J. IL-18 downregulates collagen production in human dermal fibroblasts via the ERK pathway. J. Investig. Dermatol. 2010, 130, 706–715. [Google Scholar] [CrossRef]
- Xue, N.; Liu, Y.; Jin, J.; Ji, M.; Chen, X.J.I. Chlorogenic acid prevents UVA-induced skin photoaging through regulating collagen metabolism and apoptosis in human dermal fibroblasts. Int. J. Mol. Sci. 2022, 23, 6941. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, H.J.; Woo, K.-J.; Cho, D.; Bang, S.I. Lipo-PGE1 suppresses collagen production in human dermal fibroblasts via the ERK/Ets-1 signaling pathway. PLoS ONE 2017, 12, e0179614. [Google Scholar] [CrossRef]
- Kezic, S.; Kammeyer, A.; Calkoen, F.; Fluhr, J.W.; Bos, J.D. Natural moisturizing factor components in the stratum corneum as biomarkers of filaggrin genotype: Evaluation of minimally invasive methods. Br. J. Dermatol. 2009, 161, 1098–1104. [Google Scholar] [CrossRef]
- Lee, J.-O.; Hwang, S.-H.; Shen, T.; Kim, J.H.; You, L.; Hu, W.; Cho, J.Y. Enhancement of skin barrier and hydration-related molecules by protopanaxatriol in human keratinocytes. J. Ginseng Res. 2021, 45, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kwon, M.S.; Oh, S.R.; Jeon, S.H.; Lee, P.J.; Park, S.K.; Kim, T.J.; Kim, Y.M. Zerumbone treatment upregulates hyaluronic acid synthesis via the MAPK, CREB, STAT3, and NF-κB signaling pathways in HaCaT cells. Biotechnol. Bioprocess Eng. 2022, 27, 51–60. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kim, E.-A.; Gunasekara, U.K.D.S.S.; Abeytunga, D.T.U.; Nanayakkara, C.; De Silva, E.D.; et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. ALGAE 2017, 32, 75–86. [Google Scholar] [CrossRef]
- Code, K.F.J.K. Korea Food and Drug Administration; Korean Ministry of Food and Drug Safety: Seoul, Republic of Korea, 2002; pp. 352–355.
- Kang, N.; Kim, E.-A.; Park, A.; Heo, S.-Y.; Heo, J.-H.; Heo, S.-J. Antiviral potential of fucoxanthin, an edible carotenoid purified from Sargassum siliquastrum, against Zika virus. Mar. Drugs 2024, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiratchayamaethasakul, C.; Lee, S.-H.J.I. Protocatechuic aldehyde attenuates UVA-induced photoaging in human dermal fibroblast cells by suppressing MAPKs/AP-1 and NF-κB signaling pathways. Int. J. Mol. Sci. 2020, 21, 4619. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.; Bae, I.-H.; Lim, S.H.; Jung, K.; Roh, J.; Kim, W. AP collagen peptides prevent cortisol-induced decrease of collagen type I in human dermal fibroblasts. Int. J. Mol. Sci. 2021, 22, 4788. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.-L.; Vonica-Țincu, A.L.; Loghin, F.J.M. Advantages of hyaluronic acid and its combination with other bioactive ingredients in cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, W.; Zhou, L.; Xiong, Y.; Liu, Q.; Shi, X.; Tian, J.J. The moisturizing effect of Capparis spinosa fruit extract targeting filaggrin synthesis and degradation. J. Cosmet. Dermatol. 2023, 22, 651–660. [Google Scholar] [CrossRef]
- Yang, H.-L.; Chen, S.-J.; Pandey, S.; Wu, I.-C.; Chung, Y.-T.; Vadivalagan, C.; Hseu, J.-H.; Hseu, Y.-C. The skin hydration and anti-inflammatory potential of zerumbone, a natural sesquiterpene of Zingiber zerumbet, enhanced Src/ERK-mediated HAS-2/AQP-3 and inhibited NFκB/AP-1 expression in UVB-irradiated human keratinocytes. J. Funct. Foods 2023, 111, 105890. [Google Scholar] [CrossRef]
- Song, K.-M.; Ha, S.J.; Lee, J.-E.; Kim, S.-H.; Kim, Y.H.; Kim, Y.; Hong, S.P.; Jung, S.K.; Lee, N.H. High yield ultrasonication extraction method for Undaria pinnatifida sporophyll and its anti-inflammatory properties associated with AP-1 pathway suppression. LWT 2015, 64, 1315–1322. [Google Scholar] [CrossRef]
- Ghelichi, S.; Sørensen, A.M.; Hajfathalian, M.; Jacobsen, C. Effect of post-extraction ultrasonication on compositional features and antioxidant activities of enzymatic/alkaline extracts of Palmaria palmata. Mar. Drugs 2024, 22, 179. [Google Scholar] [CrossRef] [PubMed]
- Tsurumachi, M.; Kamata, Y.; Tominaga, M.; Ishikawa, J.; Hideshima, T.; Shimizu, E.; Kaneko, T.; Suga, Y.; Takamori, K.J.J. Increased production of natural moisturizing factors and bleomycin hydrolase activity in elderly human skin. J. Dermatol. Sci. 2023, 110, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Arezki, N.R.; Williams, A.C.; Cobb, A.J.A.; Brown, M.B.J.I.J. Design, synthesis and characterization of linear unnatural amino acids for skin moisturization. Int. J. Cosmet. Sci. 2017, 39, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Wendisch, V.F. Amino Acid Biosynthesis–Pathways, Regulation and Metabolic Engineering; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2007; Volume 5. [Google Scholar]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M.J.M. Collagen based materials in cosmetic applications: A review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef]
- Reilly, D.M.; Lozano, J.J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthet. Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Li, J.; Tong, T.; Ko, D.-O.; Chung, D.-O.; Jeong, W.-C.; Kim, J.-E.; Kang, S.-G. Antioxidant and anti-skin-aging effects of abalone viscera extracts in human dermal fibroblasts. Korean J. Food Preserv. 2012, 19, 463–469. [Google Scholar] [CrossRef]
- Dhandapani, S.; Wang, R.; Cheol Hwang, K.; Kim, H.; Kim, Y.-J. Enhanced skin anti-inflammatory and moisturizing action of gold nanoparticles produced utilizing Diospyros kaki fruit extracts. Arab. J. Chem. 2023, 16, 104551. [Google Scholar] [CrossRef]
- Li, L.; Xue, Y.; Zhang, H.; Liu, Y.; Yi, F.; Dong, Y.J. A new polysaccharide isolated from Dendrobium officinale, stimulates aquaporin-3 expression in human keratinocytes. Food Sci. Technol. 2020, 41, 90–95. [Google Scholar] [CrossRef]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.-T.; Jang, Y.; Myung, S.-W.; Lee, J.M.; Kim, H.-S.; Ko, S.-C.; Lee, S.-H. In vitro screening of elastase, collagenase, hyaluronidase, and tyrosinase inhibitory and antioxidant activities of 22 halophyte plant extracts for novel cosmeceuticals. Fish. Aquat. Sci. 2020, 23, 6. [Google Scholar] [CrossRef]


| Proximate Composition (%) | |
| Yield | 6.53 ± 0.25 |
| Protein contents | 39.01 ± 1.76 |
| Polysaccharide contents | 8.63 ± 0.22 |
| Total phenolic contents | 1.48 ± 0.08 |
| Microorganisms (/mL) | |
| Total bacteria count | 0 |
| Coliform bacteria | 0 |
| Total fungal count | 0 |
| Staphylococcus aureus | 0 |
| Amino acid composition (%) | |
| Aspartic acid | 2.80 |
| Glutamic acid | 5.82 |
| Serine | 2.00 |
| Glutamine | 1.99 |
| Histidine | 0.44 |
| Glycine | 6.07 |
| Threonine | 1.63 |
| Arginine | 7.33 |
| Alanine | 65.30 |
| Tyrosine | 0.68 |
| Valine | 1.60 |
| Isoleucine | 0.99 |
| Lysine | 2.38 |
| Proline | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.-A.; Kang, N.; Heo, J.-H.; Park, A.; Heo, S.-Y.; Ko, C.-I.; Ahn, Y.-S.; Ahn, G.; Heo, S.-J. Potential Skin Health Benefits of Abalone By-Products Suggested by Their Effects on MAPKS and PI3K/AKT/NF-kB Signaling Pathways in HDF and HaCaT Cells. Foods 2024, 13, 2902. https://doi.org/10.3390/foods13182902
Kim E-A, Kang N, Heo J-H, Park A, Heo S-Y, Ko C-I, Ahn Y-S, Ahn G, Heo S-J. Potential Skin Health Benefits of Abalone By-Products Suggested by Their Effects on MAPKS and PI3K/AKT/NF-kB Signaling Pathways in HDF and HaCaT Cells. Foods. 2024; 13(18):2902. https://doi.org/10.3390/foods13182902
Chicago/Turabian StyleKim, Eun-A, Nalae Kang, Jun-Ho Heo, Areumi Park, Seong-Yeong Heo, Chang-Ik Ko, Yong-Seok Ahn, Ginnae Ahn, and Soo-Jin Heo. 2024. "Potential Skin Health Benefits of Abalone By-Products Suggested by Their Effects on MAPKS and PI3K/AKT/NF-kB Signaling Pathways in HDF and HaCaT Cells" Foods 13, no. 18: 2902. https://doi.org/10.3390/foods13182902





