Pasta Incorporating Olive Pomace: Impact on Nutritional Composition and Consumer Acceptance of a Prototype
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Pomace Samples
2.2. Pasta Samples
2.3. Chemicals and Reagents
2.4. Chemical Analysis
2.4.1. Proximate Analysis
2.4.2. Lipid Fraction Analysis
2.4.3. Antioxidant Screening
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis of Olive Pomace Samples
3.2. Chemical Analysis of Pasta Samples
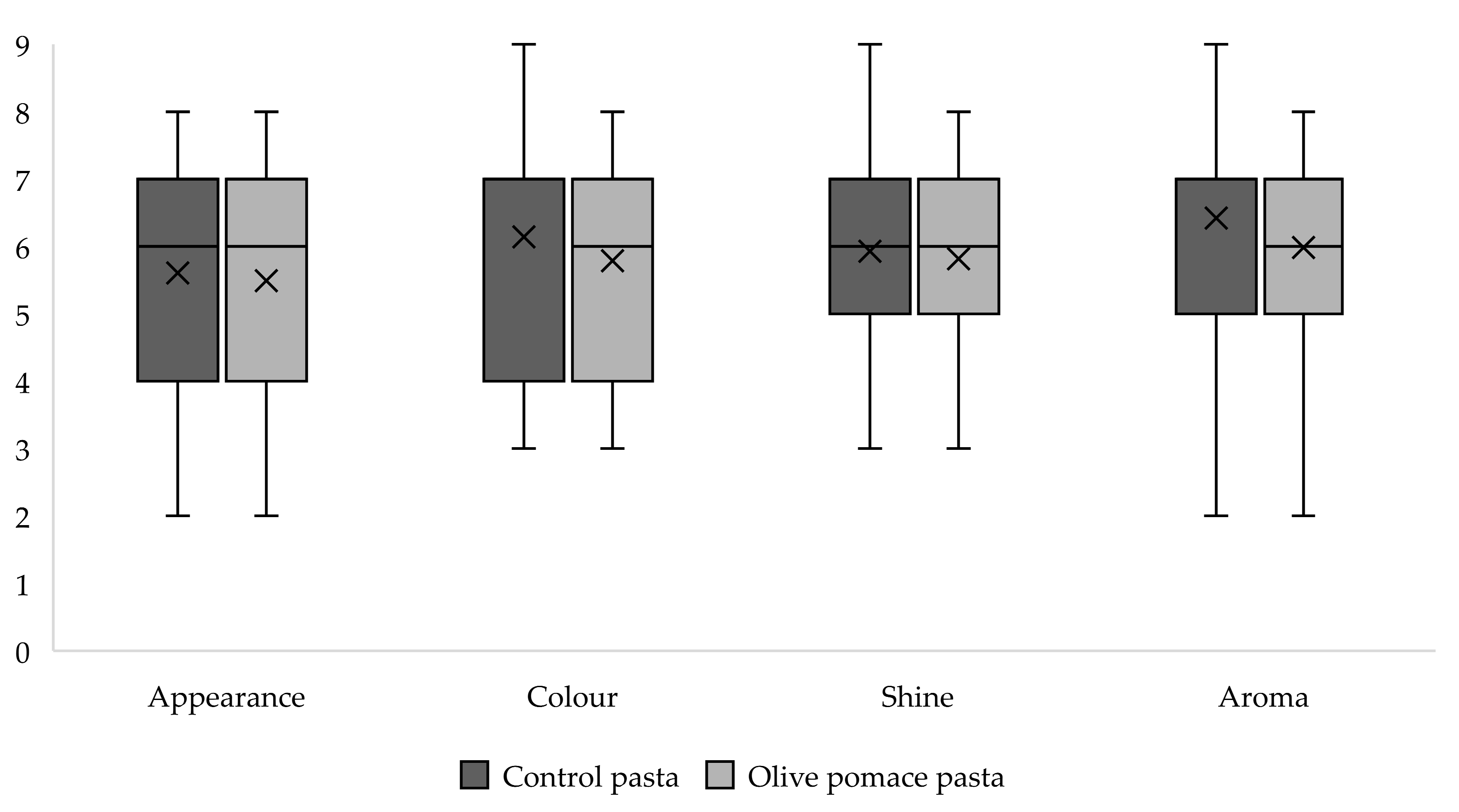
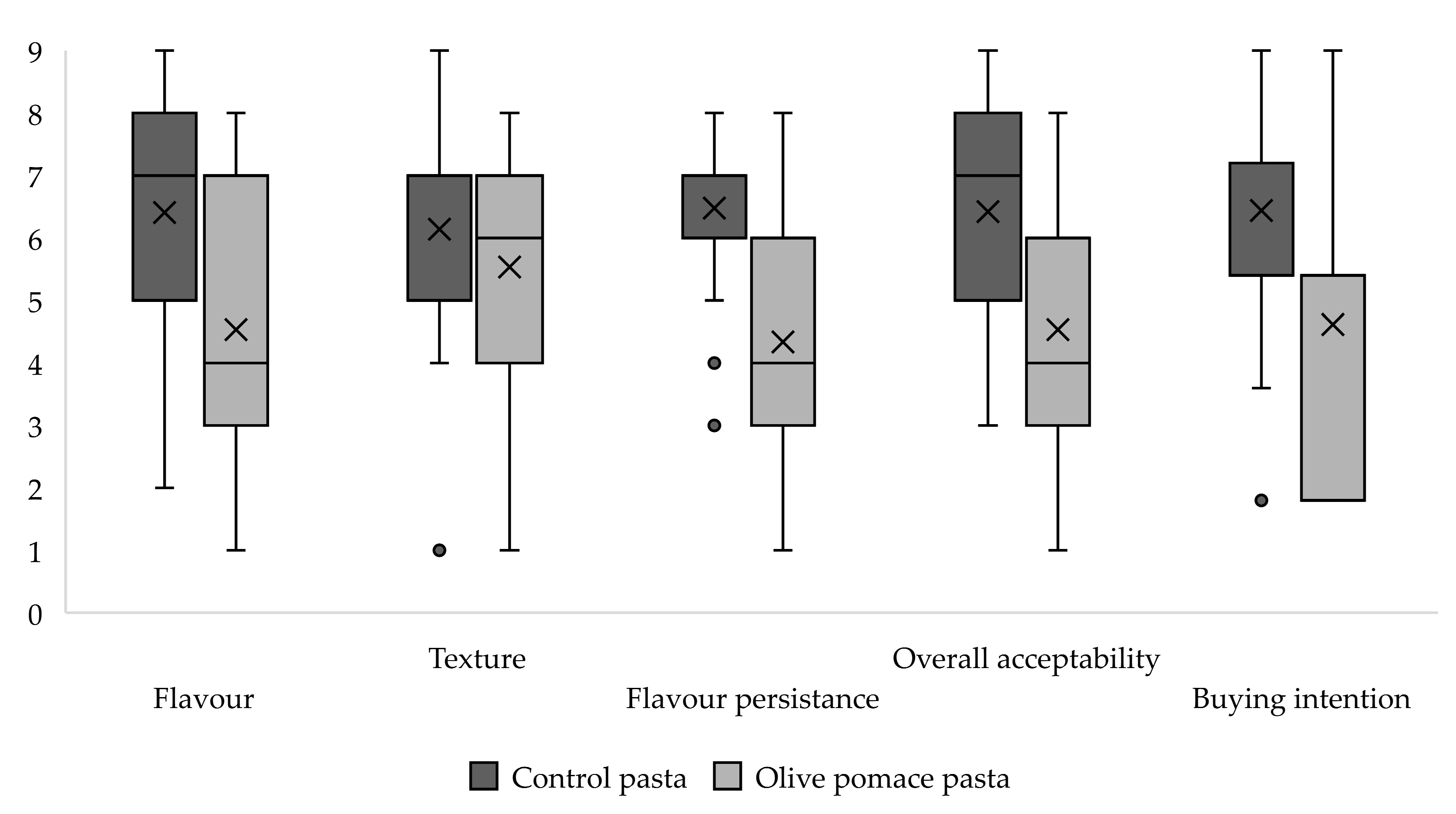
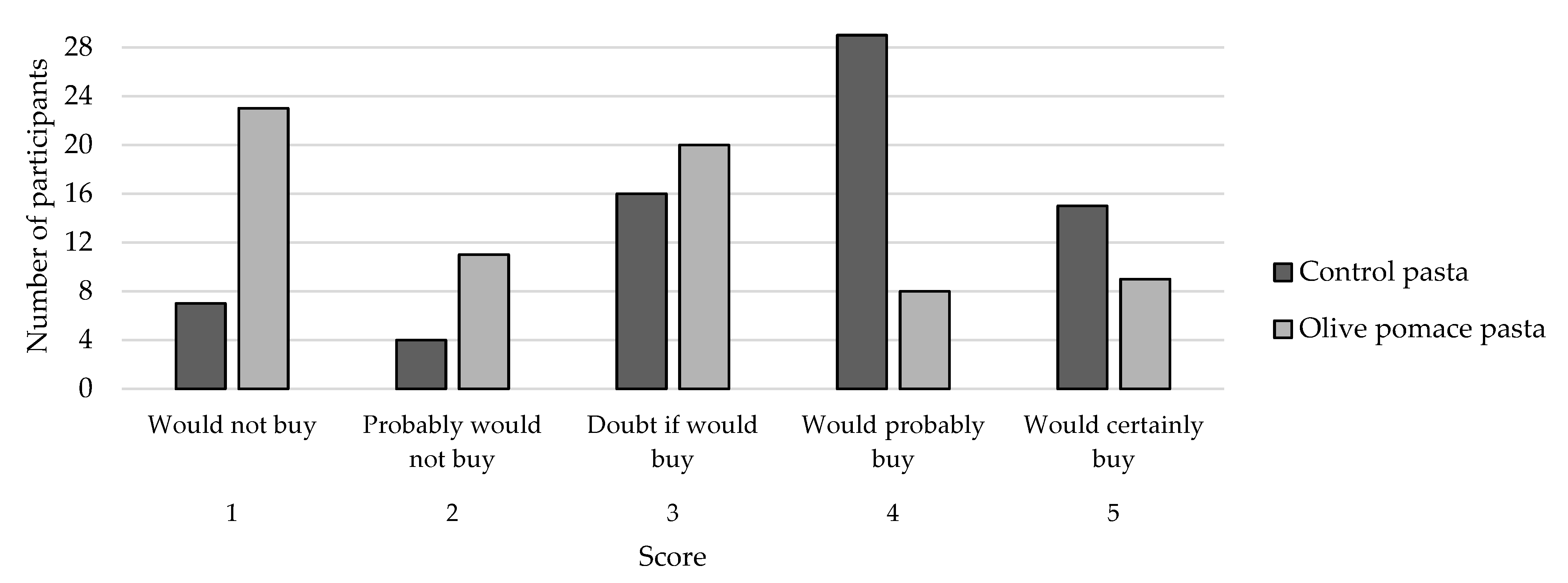
3.3. Sensory Analysis of Pasta Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017; Volume 296, pp. 1–180. [Google Scholar]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical composition and antimicrobial activity of a new olive pomace functional ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.M.; Machado, S.; Espírito Santo, L.; Costa, A.S.G.; Ranga, F.; Chiș, M.S.; Palmeira, J.D.; Oliveira, M.B.P.P.; Alves, R.C.; Ferreira, H. Changes in the Composition of Olive Pomace after Fermentation: A Preliminary Study. Fermentation 2024, 10, 287. [Google Scholar] [CrossRef]
- Esteve, C.; Marina, M.L.; García, M.C. Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chem. 2015, 167, 272–280. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Reszczyński, F.; Páscoa, R.N.M.J.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Influence of Olive Pomace Blending on Antioxidant Activity: Additive, Synergistic, and Antagonistic Effects. Molecules 2020, 26, 169. [Google Scholar] [CrossRef]
- Nunes, M.A.; Páscoa, R.N.M.J.; Alves, R.C.; Costa, A.S.G.; Bessada, S.; Oliveira, M.B.P.P. Fourier transform near infrared spectroscopy as a tool to discriminate olive wastes: The case of monocultivar pomaces. Waste Manag. 2020, 103, 378–387. [Google Scholar] [CrossRef]
- Rodrigues, R.; Lobo, J.C.; Ferreira, D.M.; Senderowicz, E.; Nunes, M.A.; Amaral, M.H.; Alves, R.C.; Oliveira, M.B.P.P. Chemical and Rheological Characterization of a Facial Mask Containing an Olive Pomace Fraction. Cosmetics 2023, 10, 64. [Google Scholar] [CrossRef]
- Sousa, M.M.; Ferreira, D.M.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Palmeira, J.D.; Nunes, M.A.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Effect of Different Time/Temperature Binomials on the Chemical Features, Antioxidant Activity, and Natural Microbial Load of Olive Pomace Paste. Molecules 2023, 28, 2876. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Barreto-Peixoto, J.; Andrade, N.; Machado, S.; Silva, C.; Lobo, J.C.; Nunes, M.A.; Álvarez-Rivera, G.; Ibáñez, E.; Cifuentes, A.; et al. Comprehensive analysis of the phytochemical composition and antitumoral activity of an olive pomace extract obtained by mechanical pressing. Food Biosci. 2024, 61, 104759. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Prestes, O.D.; Augusti, P.R.; Fonseca Moreira, J.C. Phenolic compounds and contaminants in olive oil and pomace—A narrative review of their biological and toxic effects. Food Biosci. 2023, 53, 102626. [Google Scholar] [CrossRef]
- European Food Safety Authority. The 2018 European Union Report on Pesticide Residues—Olive Oil. Available online: https://www.efsa.europa.eu/en/annual-pesticides-report-2018-olive-oil (accessed on 5 September 2024).
- Authority, E.F.S.; Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Medina Pastor, P. The 2021 European Union report on pesticide residues in food. EFSA J. 2023, 21, e07939. [Google Scholar] [CrossRef]
- Padalino, L.; D’Antuono, I.; Durante, M.; Conte, A.; Cardinali, A.; Linsalata, V.; Mita, G.; Logrieco, A.F.; Del Nobile, M.A. Use of Olive Oil Industrial By-Product for Pasta Enrichment. Antioxidants 2018, 7, 59. [Google Scholar] [CrossRef]
- Cecchi, L.; Schuster, N.; Flynn, D.; Bechtel, R.; Bellumori, M.; Innocenti, M.; Mulinacci, N.; Guinard, J.-X. Sensory Profiling and Consumer Acceptance of Pasta, Bread, and Granola Bar Fortified with Dried Olive Pomace (Pâté): A Byproduct from Virgin Olive Oil Production. J. Food Sci. 2019, 84, 2995–3008. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Bleve, G.; Selvaggini, R.; Veneziani, G.; Servili, M.; Mita, G. Bioactive Compounds and Stability of a Typical Italian Bakery Products “Taralli” Enriched with Fermented Olive Paste. Molecules 2019, 24, 3258. [Google Scholar] [CrossRef] [PubMed]
- Simonato, B.; Trevisan, S.; Tolve, R.; Favati, F.; Pasini, G. Pasta fortification with olive pomace: Effects on the technological characteristics and nutritional properties. LWT 2019, 114, 108368. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. 2020, 33, 100490. [Google Scholar] [CrossRef]
- Balli, D.; Cecchi, L.; Innocenti, M.; Bellumori, M.; Mulinacci, N. Food by-products valorisation: Grape pomace and olive pomace (pâté) as sources of phenolic compounds and fiber for enrichment of tagliatelle pasta. Food Chem. 2021, 355, 129642. [Google Scholar] [CrossRef]
- Lin, S.; Chi, W.; Hu, J.; Pan, Q.; Zheng, B.; Zeng, S. Sensory and nutritional properties of chinese olive pomace based high fibre biscuit. Emir. J. Food Agric. 2017, 29, 495–501. [Google Scholar] [CrossRef]
- Selim, K.A.; Badawy, W.; Smetanska, I. Utilization of Olive Pomace as a Source of Bioactive Compounds in Quality Improving of Toast Bread. Egypt. J. Food Sci. 2020, 48, 27–40. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Chiarello, E.; Di Nunzio, M.; Bordoni, A.; Gianotti, A. Colonic in Vitro Model Assessment of the Prebiotic Potential of Bread Fortified with Polyphenols Rich Olive Fiber. Nutrients 2021, 13, 787. [Google Scholar] [CrossRef] [PubMed]
- Simsek, M.; Süfer, Ö. Olive pomace from olive oil processing as partial flour substitute in breadsticks: Bioactive, textural, sensorial and nutritional properties. J. Food Process. Preserv. 2022, 46, e15705. [Google Scholar] [CrossRef]
- Azadfar, E.; Elhami Rad, A.H.; Sharifi, A.; Armin, M. Effect of Olive Pomace Fiber on the Baking Properties of Wheat Flour and Flat Bread (Barbari Bread) Quality. J. Food Process. Preserv. 2023, 2023, 1405758. [Google Scholar] [CrossRef]
- Cardinali, F.; Belleggia, L.; Reale, A.; Cirlini, M.; Boscaino, F.; Di Renzo, T.; Del Vecchio, L.; Cavalca, N.; Milanović, V.; Garofalo, C.; et al. Exploitation of Black Olive (Olea europaea L. cv. Piantone di Mogliano) Pomace for the Production of High-Value Bread. Foods 2024, 13, 460. [Google Scholar] [CrossRef]
- Dahdah, P.; Cabizza, R.; Farbo, M.G.; Fadda, C.; Del Caro, A.; Montanari, L.; Hassoun, G.; Piga, A. Effect of partial substitution of wheat flour with freeze-dried olive pomace on the technological, nutritional, and sensory properties of bread. Front. Sustain. Food Syst. 2024, 8, 1400339. [Google Scholar] [CrossRef]
- Dahdah, P.; Cabizza, R.; Farbo, M.G.; Fadda, C.; Mara, A.; Hassoun, G.; Piga, A. Improving the Rheological Properties of Dough Obtained by Partial Substitution of Wheat Flour with Freeze-Dried Olive Pomace. Foods 2024, 13, 478. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; Del Nobile, M.A.; Conte, A. Fish burger enriched by olive oil industrial by-product. Food Sci. Nutr. 2017, 5, 837–844. [Google Scholar] [CrossRef]
- Panza, O.; Lacivita, V.; Palermo, C.; Conte, A.; Del Nobile, M.A. Food By-Products to Extend Shelf Life: The Case of Cod Sticks Breaded with Dried Olive Paste. Foods 2020, 9, 1902. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Stübler, A.-S.; Heinz, V.; Aganovic, K. Development of food products. Curr. Opin. Green Sustain. Chem. 2020, 25, 100356. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J. Application of sensory descriptive analysis and consumer studies to investigate traditional and authentic foods: A review. Foods 2019, 8, 54. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, S.; Surasani, V.K.R. Quinoa protein isolate supplemented pasta: Nutritional, physical, textural and morphological characterization. LWT 2021, 135, 110045. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 21st ed.; Association of Official Analytical Chemists: Arlington VA, USA, 2019. [Google Scholar]
- Tontisirin, K. Chapter 2: Methods of food analysis. In Food Energy: Methods of Analysis and Conversion Factors: Report of a Technical Workshop; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- European Commission. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers; European Commission: Luxembourg, 2011; Volume 54, pp. 18–61.
- Alves, R.; Casal, S.; Oliveira, M. Determination of vitamin E in coffee beans by HPLC using a micro-extraction method. Food Sci. Technol. Int. 2009, 15, 57–63. [Google Scholar] [CrossRef]
- Melo, D.; Álvarez-Ortí, M.; Nunes, M.A.; Costa, A.S.G.; Machado, S.; Alves, R.C.; Pardo, J.E.; Oliveira, M.B.P.P. Whole or defatted sesame seeds (Sesamum indicum L.)? The effect of cold pressing on oil and cake quality. Foods 2021, 10, 2108. [Google Scholar] [CrossRef]
- ISO. ISO 12966: Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters: Part 2: Preparation of Methyl Esters of Fatty Acids; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Ferreira, D.M.; Nunes, M.A.; Santo, L.E.; Machado, S.; Costa, A.S.G.; Álvarez-Ortí, M.; Pardo, J.E.; Oliveira, M.B.P.P.; Alves, R.C. Characterization of Chia Seeds, Cold-Pressed Oil, and Defatted Cake: An Ancient Grain for Modern Food Production. Molecules 2023, 28, 723. [Google Scholar] [CrossRef] [PubMed]
- Pasten, A.; Uribe, E.; Stucken, K.; Rodríguez, A.; Vega-Gálvez, A. Influence of Drying on the Recoverable High-Value Products from Olive (cv. Arbequina) Waste Cake. Waste Biomass Valorization 2019, 10, 1627–1638. [Google Scholar] [CrossRef]
- Teterycz, D.; Sobota, A.; Kozłowicz, K.; Zarzycki, P. Substitution of semolina durum with common wheat flour in egg and eggless pasta. Acta Sci. Pol. Technol. Aliment. 2019, 18, 439–451. [Google Scholar] [CrossRef]
- Bonilla, J.C.; Erturk, M.Y.; Kokini, J.L. Understanding the role of gluten subunits (LMW, HMW glutenins and gliadin) in the networking behavior of a weak soft wheat dough and a strong semolina wheat flour dough and the relationship with linear and non-linear rheology. Food Hydrocoll. 2020, 108, 106002. [Google Scholar] [CrossRef]
- Cecchini, C.; Bresciani, A.; Menesatti, P.; Pagani, M.A.; Marti, A. Assessing the Rheological Properties of Durum Wheat Semolina: A Review. Foods 2021, 10, 2947. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.M.; de Oliveira, N.M.; Chéu, M.H.; Meireles, D.; Lopes, L.; Oliveira, M.B.; Machado, J. Updated Organic Composition and Potential Therapeutic Properties of Different Varieties of Olive Leaves from Olea europaea. Plants 2023, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Lee, J.C.-Y. Vitamin E: Where Are We Now in Vascular Diseases? Life 2022, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Melo, D.; Álvarez-Ortí, M.; Nunes, M.A.; Espírito Santo, L.; Machado, S.; Pardo, J.E.; Oliveira, M.B.P.P. Nutritional and Chemical Characterization of Poppy Seeds, Cold-Pressed Oil, and Cake: Poppy Cake as a High-Fibre and High-Protein Ingredient for Novel Food Production. Foods 2022, 11, 3027. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Oliveira, N.M.D.; Lopes, L.; Machado, J.; Oliveira, M.B. Potential Therapeutic Properties of the Leaf of Cydonia oblonga Mill. Based on Mineral and Organic Profiles. Plants 2022, 11, 2638. [Google Scholar] [CrossRef]
- Shishavan, N.G.; Masoudi, S.; Mohamadkhani, A.; Sepanlou, S.G.; Sharafkhah, M.; Poustchi, H.; Mohamadnejad, M.; Hekmatdoost, A.; Pourshams, A. Dietary intake of fatty acids and risk of pancreatic cancer: Golestan cohort study. Nutr. J. 2021, 20, 69. [Google Scholar] [CrossRef]
- Lee, D.M.; Sevits, K.J.; Battson, M.L.; Wei, Y.; Cox-York, K.A.; Gentile, C.L. Monounsaturated fatty acids protect against palmitate-induced lipoapoptosis in human umbilical vein endothelial cells. PLoS ONE 2019, 14, e0226940. [Google Scholar] [CrossRef]
- INE. Produção Record de Azeite na Campanha de 2021 Contrasta com Cenário de Seca e de Aumento do Preço dos Meios de Produção na Agricultura-JANEIRO de 2022. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_destaques&DESTAQUESdest_boui=526211517&DESTAQUESmodo=2 (accessed on 27 March 2024).
- Rahmani, R.; Aydi, S.S.; El Arbi, K.; Ahmed, F.B.; Hzemi, A.; Debouba, M.; Aydi, S. Volatile and non-volatile profiles of olive pomace and its potential uses. J. Oasis Agric. Sustain. Dev. 2024, 6, 44–51. [Google Scholar] [CrossRef]
- Celeiro. Available online: https://www.celeiro.pt/produtos/alimentacao/massas (accessed on 16 July 2024).
- Ervanário Portuense. Available online: https://www.ervanarioportuense.pt/c/alimentacao/arroz-farinhas-leguminosas-massas-sementes/massa/ (accessed on 16 July 2024).
- Oliveira, B.C.C.; Machado, M.; Machado, S.; Costa, A.S.G.; Bessada, S.; Alves, R.C.; Oliveira, M.B.P.P. Algae Incorporation and Nutritional Improvement: The Case of a Whole-Wheat Pasta. Foods 2023, 12, 3039. [Google Scholar] [CrossRef]

| Formulation | OP70 % | OP70 (g) | Wheat Semolina (g) | Wheat Flour (g) | Water (mL) |
|---|---|---|---|---|---|
| 1 | - | - | 200 | - | 110 |
| 2 | - | - | - | 200 | 110 |
| 3 | 10% | 20 | 180 | - | 110 |
| 4 | 10% | 20 | - | 180 | 110 |
| 5 | 7.5% | 15 | 185 | - | 110 |
| 6 | 7.5% | 15 | - | 185 | 110 |
| Sample | FOP | OP40 | OP70 | p-Value |
|---|---|---|---|---|
| Fat (g/100 g) | 9.23 ± 0.26 b | 16.69 ± 0.14 a | 16.58 ± 0.19 a | *** |
| Ash (g/100 g) | 4.03 ± 0.07 c | 6.46 ± 0.07 a | 5.65 ± 0.10 b | *** |
| Dietary fibre (g/100 g) | 54.68 ± 0.54 a | 47.31 ± 0.27 b | 47.23 ± 0.05 b | *** |
| Insoluble fibre (g/100 g) | 51.43 ± 0.31 a | 42.66 ± 0.33 b | 42.42 ± 0.53 b | *** |
| Soluble fibre (g/100 g) | 3.25 ± 0.23 | 4.65 ± 0.06 | 4.80 ± 0.48 | |
| Protein (g/100 g) | 4.25 ± 0.09 b | 6.61 ± 0.09 a | 6.59 ± 0.39 a | *** |
| Carbohydrates (g/100 g) | 27.81 ± 0.22 a | 22.93 ± 0.16 c | 25.09 ± 0.26 b | *** |
| α-Tocopherol (mg/kg) | 42.46 ± 2.94 c | 58.87 ± 1.04 b | 70.83 ± 1.07 a | *** |
| β-Tocopherol (mg/kg) | 1.36 ± 0.03 c | 2.19 ± 0.02 b | 2.48 ± 0.08 a | *** |
| γ-Tocopherol (mg/kg) | 2.48 ± 0.09 b | 3.90 ± 0.02 a | 4.06 ± 0.05 a | *** |
| γ-Tocotrienol (mg/kg) | 3.71 ± 0.21 c | 6.12 ± 0.08 b | 6.63 ± 0.08 a | *** |
| δ-Tocopherol (mg/kg) | 1.29 ± 0.01 b | 2.25 ± 0.04 a | 2.34 ± 0.04 a | *** |
| Total vitamin E (mg/kg) | 51.30 ± 3.26 c | 73.33 ± 1.18 b | 86.34 ± 1.21 a | *** |
| C16:0 Palmitic acid (relative %) | 10.40 ± 0.06 | 10.59 ± 0.11 | 10.47 ± 0.15 | |
| C16:1c Palmitoleic acid (relative %) | 0.48 ± 0.04 | 0.54 ± 0.05 | 0.46 ± 0.06 | |
| C18:0 Stearic acid (relative %) | 3.38 ± 0.19 | 3.36 ± 0.24 | 3.65 ± 0.08 | |
| C18:1n9c Oleic acid (relative %) | 76.19 ± 0.24 | 76.28 ± 0.89 | 75.04 ± 0.09 | |
| C18:2n6c Linoleic acid (relative %) | 8.70 ± 0.49 | 8.38 ± 0.69 | 9.44 ± 0.12 | |
| C20:0 Arachidic acid (relative %) | 0.31 ± 0.02 | 0.27 ± 0.04 | 0.32 ± 0.03 | |
| C18:3n3c α-Linolenic acid (relative %) | 0.45 ± 0.07 | 0.49 ± 0.07 | 0.53 ± 0.02 | |
| C24:0 Lignoceric acid (relative %) | 0.07 ± 0.02 | 0.08 ± 0.01 | 0.09 ± 0.01 | |
| ∑SFAs Saturated fatty acids (relative %) | 14.17 ± 0.19 | 14.30 ± 0.14 | 14.52 ± 0.10 | |
| ∑MUFAs Monounsaturated fatty acids (relative %) | 76.67 ± 0.27 a, b | 76.82 ± 0.85 a | 75.50 ± 0.03 b | *** |
| ∑PUFAs Polyunsaturated fatty acids (relative %) | 9.16 ± 0.46 | 8.88 ± 0.75 | 9.97 ± 0.11 | |
| C16:0 Palmitic acid (g/100 g) | 0.96 ± 0.00 b | 1.77 ± 0.02 a | 1.74 ± 0.02 a | *** |
| C16:1c Palmitoleic acid (g/100 g) | 0.04 ± 0.00 b | 0.09 ± 0.01 a | 0.08 ± 0.01 a | *** |
| C18:0 Stearic acid (g/100 g) | 0.31 ± 0.01 b | 0.56 ± 0.03 a | 0.60 ± 0.01 a | *** |
| C18:1n9c Oleic acid (g/100 g) | 7.03 ± 0.02 c | 12.73 ± 0.12 a | 12.44 ± 0.01 b | *** |
| C18:2n6c Linoleic acid (g/100 g) | 0.80 ± 0.04 b | 1.40 ± 0.09 a | 1.57 ± 0.02 a | *** |
| C20:0 Arachidic acid (g/100 g) | 0.03 ± 0.00 b | 0.04 ± 0.00 a | 0.05 ± 0.00 a | * |
| C18:3n3c α-Linolenic acid (g/100 g) | 0.04 ± 0.01 b | 0.08 ± 0.01 a | 0.09 ± 0.00 a | ** |
| C24:0 Lignoceric acid (g/100 g) | 0.01 ± 0.00 b | 0.01 ± 0.00 a | 0.02 ± 0.00 a | ** |
| ∑SFAs Saturated fatty acids (g/100 g) | 1.31 ± 0.01 b | 2.39 ± 0.02 a | 2.41 ± 0.02 a | ** |
| ∑MUFAs Monounsaturated fatty acids (g/100 g) | 7.08 ± 0.02 c | 12.82 ± 0.12 a | 12.52 ± 0.01 b | ** |
| ∑PUFAs Polyunsaturated fatty acids (g/100 g) | 0.85 ± 0.04 b | 1.48 ± 0.10 a | 1.65 ± 0.02 a | *** |
| n6/n3 | 19.56 ± 3.03 | 17.10 ± 1.16 | 17.78 ± 0.52 | |
| PUFAs/SFAs | 0.65 ± 0.03 | 0.62 ± 0.04 | 0.69 ± 0.01 | |
| TPCs (g GAEs/100 g) | 3.92 ± 0.49 a | 2.73 ± 0.15 c | 3.38 ± 0.15 b | ** |
| TFC (g CEs/100 g) | 3.00 ± 0.39 b | 2.26 ± 0.13 c | 3.46 ± 0.11 a | ** |
| FRAP (g FSEs/100 g) | 5.37 ± 0.95 b | 4.84 ± 0.34 b | 7.49 ± 0.33 a | *** |
| DPPH•-SA (g TEs/100 g) | 1.50 ± 0.23 a | 1.18 ± 0.09 b | 1.27 ± 0.12 a,b | * |
| Sample | UCP | CCP | UOP70P | COP70P | p-Value |
|---|---|---|---|---|---|
| Energy (kJ/100 g) | 928 ± 5 b | 933 ± 3 b | 989 ± 3 a | 995 ± 4 a | *** |
| Energy (kcal/100 g) | 397 ± 1 a,b | 397 ± 0 a | 395 ± 0 b | 397 ± 0 a | * |
| Fat (g/100 g) | 0.79 ± 0.13 b | 0.70 ± 0.02 b | 2.10 ± 0.05 a | 2.10 ± 0.05 a | *** |
| Ash (g/100 g) | 0.58 ± 0.01 c | 0.44 ± 0.02 d | 0.96 ± 0.01 a | 0.75 ± 0.01 b | *** |
| Dietary fibre (g/100 g) | 2.52 ± 0.12 b | 2.48 ± 0.18 b | 5.93 ± 0.04 a | 5.45 ± 0.43 a | ** |
| Insoluble fibre (g/100 g) | 0.95 ± 0.03 b | 1.00 ± 0.02 b | 2.88 ± 0.10 a | 2.55 ± 0.36 a | * |
| Soluble fibre (g/100 g) | 1.58 ± 0.15 b | 1.48 ± 0.20 b | 3.05 ± 0.05 a | 2.90 ± 0.09 a | ** |
| Protein (g/100 g) | 9.64 ± 0.02 b | 10.48 ± 0.15 a | 9.11 ± 0.19 c | 10.14 ± 0.13 a | * |
| Carbohydrates (g/100 g) | 86.46 ± 0.14 a | 85.89 ± 0.18 a | 81.90 ± 0.22 b | 81.56 ± 0.18 b | *** |
| α-Tocopherol (mg/kg) | 0.58 ± 0.02 b | 0.69 ± 0.01 b | 7.38 ± 0.11 a | 7.32 ± 0.07 a | *** |
| α-Tocotrienol (mg/kg) | 0.28 ± 0.00 b | 0.30 ± 0.00 b | 1.49 ± 0.08 a | 1.49 ± 0.02 a | *** |
| β-Tocopherol (mg/kg) | 0.68 ± 0.02 c | 1.01 ± 0.01 b | 2.10 ± 0.04 a | 2.09 ± 0.02 a | *** |
| γ-Tocopherol (mg/kg) | 0.47 ± 0.01 c | 0.60 ± 0.00 b | 0.91 ± 0.01 a | 0.91 ± 0.01 a | *** |
| β-Tocotrienol (mg/kg) | 4.92 ± 0.17 c | 7.61 ± 0.04 b | 14.14 ± 0.33 a | 14.14 ± 0.19 a | *** |
| γ-Tocotrienol (mg/kg) | ND | ND | 1.01 ± 0.09 a | 1.06 ± 0.09 a | *** |
| Total vitamin E (mg/kg) | 6.93 ± 0.21 c | 10.21 ± 0.04 b | 27.03 ± 0.58 a | 27.00 ± 0.35 a | *** |
| C16:0 Palmitic acid (relative %) | 19.93 ± 0.40 a | 19.39 ± 0.09 a | 13.63 ± 0.22 c | 14.85 ± 0.35 b | ** |
| C16:1c Palmitoleic acid (relative %) | ND | ND | 0.40 ± 0.01 | ND | |
| C18:0 Stearic acid (relative %) | 1.54 ± 0.10 b | 1.16 ± 0.07 b | 2.78 ± 0.03 a | 2.70 ± 0.26 a | *** |
| C18:1n9c Oleic acid (relative %) | 14.68 ± 0.49 c | 12.47 ± 0.46 d | 53.39 ± 0.40 a | 50.52 ± 0.23 b | *** |
| C18:2n6c Linoleic acid (relative %) | 60.32 ± 0.46 b | 63.12 ± 0.70 a | 27.61 ± 0.22 d | 30.20 ± 0.51 c | *** |
| C20:0 Arachidic acid (relative %) | ND | ND | 0.21 ± 0.02 | ND | |
| C18:3n3c Linolenic acid (relative %) | 3.12 ± 0.20 a | 3.20 ± 0.14 a | 1.55 ± 0.01 b | 1.72 ± 0.03 b | *** |
| C20:1n9c cis-11-eicosenoic acid (relative %) | 0.41 ± 0.03 b | 0.67 ± 0.06 a | 0.25 ± 0.02 c | ND | |
| C22:0 Behenic acid (relative %) | ND | ND | 0.17 ± 0.01 | ND | |
| ∑SFAs Saturated fatty acids (relative %) | 21.47 ± 0.49 a | 20.55 ± 0.08 b | 16.79 ± 0.20 c | 17.56 ± 0.42 c | * |
| ∑MUFAs Monounsaturated fatty acids (relative %) | 15.09 ± 0.45 c | 13.13 ± 0.50 d | 54.04 ± 0.40 a | 50.52 ± 0.23 b | ** |
| ∑PUFAs Polyunsaturated fatty acids (relative %) | 63.44 ± 0.46 b | 66.32 ± 0.56 a | 29.16 ± 0.22 d | 31.92 ± 0.52 c | *** |
| C16:0 Palmitic acid (mg/100 g) | 156.89 ± 2.54 c | 136.30 ± 0.49 d | 286.27 ± 3.76 b | 311.94 ± 6.09 a | ** |
| C16:1c Palmitoleic acid (mg/100 g) | ND | ND | 8.46 ± 0.23 | ND | |
| C18:0 Stearic acid (mg/100 g) | 12.13 ± 0.62 b | 8.17 ± 0.42 b | 58.32 ± 0.44 a | 56.80 ± 4.52 a | *** |
| C18:1n9c Oleic acid (mg/100 g) | 115.55 ± 3.13 c | 87.65 ± 2.65 d | 1121.33 ± 6.78 a | 1061.05 ± 3.92 b | *** |
| C18:2n6c Linoleic acid (mg/100 g) | 474.83 ± 2.98 c | 443.82 ± 4.00 d | 579.93 ± 3.80 b | 634.20 ± 8.73 a | ** |
| C20:0 Arachidic acid (mg/100 g) | ND | ND | 4.50 ± 0.31 | ND | |
| C18:3n3c α-Linolenic acid (mg/100 g) | 24.53 ± 1.26 c | 22.48 ± 0.80 c | 32.55 ± 0.12 b | 36.07 ± 0.58 a | ** |
| C20:1n9c cis-11-eicosenoic acid (mg/100 g) | 3.24 ± 0.21 b | 4.69 ± 0.34 a | 5.22 ± 0.37 a | ND | ** |
| C22:0 Behenic acid (mg/100 g) | ND | ND | 3.62 ± 0.22 | ND | |
| ∑SFAs Saturated fatty acids (mg/100 g) | 169.02 ± 3.13 c | 144.47 ± 0.47 d | 352.71 ± 3.51 b | 368.74 ± 7.22 a | * |
| ∑MUFAs Monounsaturated fatty acids (mg/100 g) | 118.79 ± 2.92 c | 92.33 ± 2.87 d | 1135.01 ± 6.86 a | 1061.05 ± 3.92 b | ** |
| ∑PUFAs Polyunsaturated fatty acids (mg/100 g) | 499.36 ± 2.93 c | 466.30 ± 3.20 d | 612.49 ± 3.80 b | 670.26 ± 8.96 a | *** |
| n6/n3 | 19.41 ± 1.03 | 19.77 ± 0.90 | 17.81 ± 0.14 | 17.59 ± 0.30 | |
| PUFAs/SFAs | 0.34 ± 0.01 b | 0.31 ± 0.00 b | 0.58 ± 0.00 a | 0.55 ± 0.02 a | *** |
| TPCs (g GAEs/100 g) | 0.090 ± 0.008 c | 0.030 ± 0.002 d | 0.239 ± 0.013 a | 0.198 ± 0.010 b | *** |
| TFC (g CEs/100 g) | 0.001 ± 0.001 c | 0.007 ± 0.000 c | 0.234 ± 0.018 a | 0.212 ± 0.017 b | * |
| FRAP (g FSEs/100 g) | 0.281 ± 0.013 c | 0.254 ± 0.010 c | 3.470 ± 0.187 a | 3.011 ± 0.133 b | *** |
| DPPH•-SA (g TEs/100 g) | ND | ND | 0.102 ± 0.016 | 0.092 ± 0.021 |
| Sample | CCP | COP70P | p-Value |
|---|---|---|---|
| Energy (kJ/100 g) | 354 ± 1 b | 573 ± 2 a | * |
| Energy (kcal/100 g) | 150 ± 0 b | 228 ± 0 a | * |
| Moisture (g/100 g) | 62.1 ± 0.8 a | 42.5 ± 0.8 b | * |
| Fat (g/100 g) | 0.27 ± 0.01 b | 1.21 ± 0.03 a | * |
| Ash (g/100 g) | 0.17 ± 0.01 b | 0.43 ± 0.01 a | * |
| Dietary fibre (g/100 g) | 0.94 ± 0.07 b | 3.13 ± 0.25 a | * |
| Insoluble fibre (g/100 g) | 0.38 ± 0.01 b | 1.47 ± 0.20 a | * |
| Soluble fibre (g/100 g) | 0.56 ± 0.08 b | 1.67 ± 0.05 a | * |
| Protein (g/100 g) | 3.97 ± 0.06 b | 5.84 ± 0.07 a | * |
| Carbohydrates (g/100 g) | 32.56 ± 0.07 b | 46.93 ± 0.10 a | * |
| α-Tocopherol (mg/kg) | 0.26 ± 0.00 b | 4.21 ± 0.04 a | * |
| α-Tocotrienol (mg/kg) | 0.11 ± 0.00 b | 0.85 ± 0.01 a | * |
| β-Tocopherol (mg/kg) | 0.38 ± 0.00 b | 1.20 ± 0.01 a | * |
| γ-Tocopherol (mg/kg) | 0.23 ± 0.00 b | 0.52 ± 0.01 a | * |
| β-Tocotrienol (mg/kg) | 2.89 ± 0.01 b | 8.13 ± 0.11 a | * |
| γ-Tocotrienol (mg/kg) | ND | 0.61 ± 0.05 | |
| Total vitamin E (mg/kg) | 3.87 ± 0.01 b | 15.54 ± 0.20 a | * |
| TPCs (g GAEs/100 g) | 0.011 ± 0.001 b | 0.116 ± 0.005 a | * |
| TFC (g CEs/100 g) | 0.002 ± 0.002 b | 0.122 ± 0.008 a | * |
| FRAP (g FSEs/100 g) | 0.096 ± 0.004 b | 1.732 ± 0.076 a | * |
| DPPH•-SA (g TEs/100 g) | ND | 0.054 ± 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, D.M.; Oliveira, B.C.C.; Barbosa, C.; Costa, A.S.G.; Nunes, M.A.; Oliveira, M.B.P.P.; Alves, R.C. Pasta Incorporating Olive Pomace: Impact on Nutritional Composition and Consumer Acceptance of a Prototype. Foods 2024, 13, 2933. https://doi.org/10.3390/foods13182933
Ferreira DM, Oliveira BCC, Barbosa C, Costa ASG, Nunes MA, Oliveira MBPP, Alves RC. Pasta Incorporating Olive Pomace: Impact on Nutritional Composition and Consumer Acceptance of a Prototype. Foods. 2024; 13(18):2933. https://doi.org/10.3390/foods13182933
Chicago/Turabian StyleFerreira, Diana Melo, Bárbara C. C. Oliveira, Carla Barbosa, Anabela S. G. Costa, Maria Antónia Nunes, Maria Beatriz P. P. Oliveira, and Rita C. Alves. 2024. "Pasta Incorporating Olive Pomace: Impact on Nutritional Composition and Consumer Acceptance of a Prototype" Foods 13, no. 18: 2933. https://doi.org/10.3390/foods13182933
APA StyleFerreira, D. M., Oliveira, B. C. C., Barbosa, C., Costa, A. S. G., Nunes, M. A., Oliveira, M. B. P. P., & Alves, R. C. (2024). Pasta Incorporating Olive Pomace: Impact on Nutritional Composition and Consumer Acceptance of a Prototype. Foods, 13(18), 2933. https://doi.org/10.3390/foods13182933










