Antioxidant Peptides and Protein Hydrolysates from Tilapia: Cellular and In Vivo Evidences for Human Health Benefits
Abstract
:1. Introduction
2. Tilapia
3. Production of Antioxidant Hydrolysates and Peptides from Tilapia
4. Cellular Effects
5. In Vivo Effects
6. Molecular Characteristics and Structure–Activity Relationship
7. Potential Applications in Human Health
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, F.-C.; Xiao, J.; Wang, S.; Ee, K.-Y.; Chai, T.-T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Xu, B.; Dong, Q.; Yu, C.; Chen, H.; Zhao, Y.; Zhang, B.; Yu, P.; Chen, M. Advances in research on the activity evaluation, mechanism and structure-activity relationships of natural antioxidant peptides. Antioxidants 2024, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Habinshuti, I.; Nsengumuremyi, D.; Muhoza, B.; Ebenezer, F.; Yinka Aregbe, A.; Antoine Ndisanze, M. Recent and novel processing technologies coupled with enzymatic hydrolysis to enhance the production of antioxidant peptides from food proteins: A review. Food Chem. 2023, 423, 136313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Quan, Z.; Xiao, P.; Duan, J.-A. New insights into antioxidant peptides: An overview of efficient screening, evaluation models, molecular mechanisms, and applications. Antioxidants 2024, 13, 203. [Google Scholar] [CrossRef]
- Xiang, Z.; Xue, Q.; Gao, P.; Yu, H.; Wu, M.; Zhao, Z.; Li, Y.; Wang, S.; Zhang, J.; Dai, L. Antioxidant peptides from edible aquatic animals: Preparation method, mechanism of action, and structure-activity relationships. Food Chem. 2023, 404, 134701. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Wu, J. Marine proteins and peptides: Production, biological activities, and potential applications. Food Innov. Adv. 2023, 2, 69–84. [Google Scholar] [CrossRef]
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef]
- Jafar, I.; Asfar, M.; Mahendradatta, M.; Paradiman, A.Z.; Iqbal, M. Fish protein hydrolysate research trends over the last 5 years and future research predictions; a bibliometric analysis. Int. J. Pept. Res. Ther. 2024, 30, 34. [Google Scholar] [CrossRef]
- Yuan, Z.; Ye, X.; Hou, Z.; Chen, S. Sustainable utilization of proteins from fish processing by-products: Extraction, biological activities and applications. Trends Food Sci. Technol. 2024, 143, 104276. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Identification and characterization of novel antioxidant peptides from mackerel (Scomber japonicus) muscle protein hydrolysates. Food Chem. 2020, 323, 126809. [Google Scholar] [CrossRef]
- Wang, K.; Han, L.; Hong, H.; Pan, J.; Liu, H.; Luo, Y. Purification and identification of novel antioxidant peptides from silver carp muscle hydrolysate after simulated gastrointestinal digestion and transepithelial transport. Food Chem. 2021, 342, 128275. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Wang, Y.-M.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Antioxidant peptides from the protein hydrolysate of monkfish (Lophius litulon) muscle: Purification, identification, and cytoprotective function on HepG2 cells damage by H2O2. Mar. Drugs 2020, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Zhu, L.; Zhang, W.; Jin, W.; Bai, F.; Xu, P.; Wang, J.; Sun, Q.; Guo, Z.; Yuan, L. Novel peptides from sturgeon ovarian protein hydrolysates prevent oxidative stress-induced dysfunction in osteoblast cells: Purification, identification, and characterization. J. Agric. Food Chem. 2024, 72, 10076–10088. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.F.; Xi, Q.H.; Sheng, Y.; Wang, Y.M.; Wang, W.Y.; Chi, C.F.; Wang, B. Antioxidant peptides from monkfish swim bladders: Ameliorating NAFLD in vitro by suppressing lipid accumulation and oxidative stress via regulating AMPK/Nrf2 pathway. Mar. Drugs 2023, 21, 360. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.-M.; Li, L.-Y.; Chi, C.-F.; Wang, B. Twelve antioxidant peptides from protein hydrolysate of skipjack tuna (Katsuwonus pelamis) roe prepared by flavourzyme: Purification, sequence identification, and activity evaluation. Front. Nutr. 2022, 8, 813780. [Google Scholar] [CrossRef]
- Sun, L.; Chang, W.; Ma, Q.; Zhuang, Y. Purification of antioxidant peptides by high resolution mass spectrometry from simulated gastrointestinal digestion hydrolysates of Alaska Pollock (Theragra chalcogramma) skin collagen. Mar. Drugs 2016, 14, 186. [Google Scholar] [CrossRef]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides 2011, 32, 1496–1501. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Zhao, Y.-Q.; Zhao, G.-X.; Chi, C.-F.; Wang, B. Antioxidant peptides from collagen hydrolysate of redlip croaker (Pseudosciaena polyactis) scales: Preparation, characterization, and cytoprotective effects on H2O2-damaged HepG2 cells. Mar. Drugs 2020, 18, 156. [Google Scholar] [CrossRef]
- FAO. FishStat: Global Aquaculture Production—Quantity (1950–2022). In: FishStatJ. Available online: www.fao.org/fishery/en/statistics/software/fishstatj (accessed on 23 August 2024).
- FAO. GLOBEFISH Highlights Second Issue 2021, Annual 2021 Statistics—A Quarterly Update on World Seafood Markets. Globefish Highlights. No. 2–2021; FAO: Rome, Italy, 2021. [Google Scholar]
- FAO. FishStat: Global Aquaculture Production—Value (1984–2022). In: FishStatJ. Available online: www.fao.org/fishery/en/statistics/software/fishstatj (accessed on 24 August 2024).
- Zhang, R.; Chen, J.; Jiang, X.; Yin, L.; Zhang, X. Antioxidant and hypoglycaemic effects of tilapia skin collagen peptide in mice. Int. J. Food Sci. Technol. 2016, 51, 2157–2163. [Google Scholar] [CrossRef]
- Li, D.D.; Li, W.J.; Kong, S.Z.; Li, S.D.; Guo, J.Q.; Guo, M.H.; Cai, T.T.; Li, N.; Chen, R.Z.; Luo, R.Q.; et al. Protective effects of collagen polypeptide from tilapia skin against injuries to the liver and kidneys of mice induced by D-galactose. Biomed. Pharmacother. 2019, 117, 109204. [Google Scholar] [CrossRef]
- Song, B.; Liu, D.; Liu, T.C.; Li, K.; Wang, S.; Liu, J.; Regenstein, J.M.; Wu, Y.; Zhou, P. The combined effect of commercial tilapia collagen peptides and antioxidants against UV-induced skin photoaging in mice. Food Funct. 2023, 14, 5936–5948. [Google Scholar] [CrossRef]
- Uddin, M.N.; Kabir, K.H.; Roy, D.; Hasan, M.T.; Sarker, M.A.; Dunn, E.S. Understanding the constraints and its related factors in tilapia (Oreochromis sp.) fish culture at farm level: A case from Bangladesh. Aquaculture 2021, 530, 735927. [Google Scholar] [CrossRef]
- Hemker, A.K.; Nguyen, L.T.; Karwe, M.; Salvi, D. Effects of pressure-assisted enzymatic hydrolysis on functional and bioactive properties of tilapia (Oreochromis niloticus) by-product protein hydrolysates. LWT 2020, 122, 109003. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Mi, C.X.; Chen, J.; Jiao, R.W.; Li, X.; Wang, Q.K.; He, Y.H.; Ren, D.D.; Wu, L.; Zhou, H. Contribution of amino acid composition and secondary structure to the antioxidant properties of tilapia skin peptides. J. Food Meas. Charact. 2024, 18, 1483–1498. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, D.; Nepomuceno, E.F.V.; Galvão, J.A.; Fabrício, L.F.F.; Gaziola, S.A.; Azevedo, R.A.; Vieira, T.M.F.S.; Oetterer, M. Enzymatic conversion of red tilapia (Oreochromis niloticus) by-products in functional and bioactive products. J. Aquat. Food Prod. Technol. 2023, 32, 269–291. [Google Scholar] [CrossRef]
- Zhang, X.; Noisa, P.; Yongsawatdigul, J. Identification and characterization of tilapia antioxidant peptides that protect AAPH-induced HepG2 cell oxidative stress. J. Funct. Foods 2021, 86, 104662. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.v.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Miao, W.; Wang, W. Trends of aquaculture production and trade: Carp, tilapia, and shrimp. Asian Fish. Sci. 2020, 33, 1–10. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Current state and future potential. In Tilapia Culture; El-Sayed, A.-F.M., Ed.; CABI Publishing: Oxfordshire, UK, 2006; pp. 1–24. [Google Scholar]
- Pillay, T.V.R.; Kutty, M.N. Aquaculture: Principles and Practices, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2005; p. 640. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Version (02/2024). 2024. Available online: www.fishbase.org (accessed on 1 July 2024).
- Hush, H.H. Quality and Quality Changes in Fresh Fish; FAO: Rome, Italy, 1995. [Google Scholar]
- FAO. GLOBEFISH Highlights Fourth Issue 2022, with January–June 2022 Statistics—International Markets for Fisheries and Aquaculture Products. Globefish Highlights. No. 4–2022; FAO: Rome, Italy, 2023. [Google Scholar]
- Fitzsimmons, K. Development of new products and markets for the global tilapia trade. In Proceedings of the New Dimensions in Farmed Tilapia, Proceedings of ISTA 6, Manila, Philippines, 12–16 September 2004; pp. 624–633. [Google Scholar]
- Karapanagiotidis, I.T. Chapter 16. Nutrient profiles of tilapia. In Tilapia in Intensive Co-Culture; Perschbacher, P.W., Stickney, R.R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 261–305. [Google Scholar]
- Quah, Y.; Tong, S.-R.; Bojarska, J.; Giller, K.; Tan, S.-A.; Ziora, Z.M.; Esatbeyoglu, T.; Chai, T.-T. Bioactive peptide discovery from edible insects for potential applications in human health and agriculture. Molecules 2023, 28, 1233. [Google Scholar] [CrossRef]
- Liping, S.; Qiuming, L.; Jian, F.; Xiao, L.; Yongliang, Z. Purification and characterization of peptides inhibiting MMP-1 activity with C terminate of Gly-Leu from simulated gastrointestinal digestion hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. J. Agric. Food Chem. 2018, 66, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, X.; Zhuang, Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Riyadi, P.H.; Atho’illah, M.F.; Tanod, W.A.; Rahmawati, I.S. Tilapia viscera hydrolysate extract alleviates oxidative stress and renal damage in deoxycorticosterone acetate-salt-induced hypertension rats. Vet. World 2020, 13, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Riyadi, P.H.; Suprayitno, E.; Aulanni’am; Sulistiati, T.D. Optimization of protein hydrolysate from visceral waste of Nile tilapia (Oreochromis niloticus) by response surface methodology. Aquac. Aquar. Conserv. Legis. 2019, 12, 2347–2358. [Google Scholar]
- Chen, M.F.; Gong, F.; Zhang, Y.Y.; Li, C.; Zhou, C.; Hong, P.; Sun, S.; Qian, Z.J. Preventive effect of YGDEY from tilapia fish skin gelatin hydrolysates against alcohol-induced damage in HepG2 cells through ROS-mediated signaling pathways. Nutrients 2019, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Noisa, P.; Yongsawatdigul, J. Chemical and cellular antioxidant activities of in vitro digesta of tilapia protein and its hydrolysates. Foods 2020, 9, 833. [Google Scholar] [CrossRef]
- Kangsanant, S.; Thongraung, C.; Jansakul, C.; Murkovic, M.; Seechamnanturakit, V. Purification and characterisation of antioxidant and nitric oxide inhibitory peptides from tilapia (Oreochromis niloticus) protein hydrolysate. Int. J. Food Sci. Technol. 2015, 50, 660–665. [Google Scholar] [CrossRef]
- Lin, S.B.; Chen, C.C.; Chen, L.C.; Chen, H.H. The bioactive composite film prepared from bacterial cellulose and modified by hydrolyzed gelatin peptide. J. Biomater. Appl. 2015, 29, 1428–1438. [Google Scholar] [CrossRef]
- Sierra, L.; Fan, H.; Zapata, J.; Wu, J. Antioxidant peptides derived from hydrolysates of red tilapia (Oreochromis sp.) scale. LWT 2021, 146, 111631. [Google Scholar] [CrossRef]
- Yao, H.; Wang, S.; Fu, B.; Xu, X.; Cheng, S.; Du, M. The potential benefits of Oreochromis mossambicus derived hydrophobic peptides in protecting the skin against UVA-induced damage. Food Biosci. 2024, 59, 104120. [Google Scholar] [CrossRef]
- Ju, X.; Cheng, S.; Li, H.; Xu, X.; Wang, Z.; Du, M. Tyrosinase inhibitory effects of the peptides from fish scale with the metal copper ions chelating ability. Food Chem. 2022, 390, 133146. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Qian, Z.J.; Ryu, B.; Park, J.W.; Kim, S.K. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods 2010, 2, 107–117. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kim, J.A.; Ryu, B.; Kim, S.K. An antihypertensive peptide from tilapia gelatin diminishes free radical formation in murine microglial cells. J. Agric. Food Chem. 2011, 59, 12193–12197. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wei, H.; Yu, H.; Xing, Q.; Zou, Y.; Zhou, Y.; Peng, J. Fish skin gelatin hydrolysate production by ginger powder induces glutathione synthesis to prevent hydrogen peroxide induced intestinal oxidative stress via the Pept1-p62-Nrf2 cascade. J. Agric. Food Chem. 2018, 66, 11601–11611. [Google Scholar] [CrossRef]
- Raghavan, S.; Kristinsson, H.G.; Leeuwenburgh, C. Radical scavenging and reducing ability of tilapia (Oreochromis niloticus) protein hydrolysates. J. Agric. Food Chem. 2008, 56, 10359–10367. [Google Scholar] [CrossRef]
- Xiao, Z.; Liang, P.; Chen, J.; Chen, M.F.; Gong, F.; Li, C.; Zhou, C.; Hong, P.; Yang, P.; Qian, Z.J. A peptide YGDEY from tilapia gelatin hydrolysates inhibits UVB-mediated skin photoaging by regulating MMP-1 and MMP-9 expression in HaCaT cells. Photochem. Photobiol. 2019, 95, 1424–1432. [Google Scholar] [CrossRef]
- Lenzen, S.; Lushchak, V.I.; Scholz, F. The pro-radical hydrogen peroxide as a stable hydroxyl radical distributor: Lessons from pancreatic beta cells. Arch. Toxicol. 2022, 96, 1915–1920. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, H.; Wang, L.; Qian, H.; Qi, Y.; Miao, X.; Cheng, L.; Qi, X. Protective effect of ferulic acid against 2,2”²-azobis(2-amidinopropane) dihydrochloride-induced oxidative stress in PC12 cells. Cell. Mol. Biol. 2016, 62, 109–116. [Google Scholar]
- Preda, S.; Anastasescu, C.; Balint, I.; Umek, P.; Sluban, M.; Negrila, C.C.; Angelescu, D.G.; Bratan, V.; Rusu, A.; Zaharescu, M. Charge separation and ROS generation on tubular sodium titanates exposed to simulated solar light. Appl. Surf. Sci. 2019, 470, 1053–1063. [Google Scholar] [CrossRef]
- Ahmad, A.; Olah, G.; Szczesny, B.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, A mitochondrially targeted hydrogen sulfide donor, exerts protective effects in renal epithelial cells subjected to oxidative stress in vitro and in acute renal injury in vivo. Shock 2016, 45, 88–97. [Google Scholar] [CrossRef]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Li, J.; Guan, H. The antioxidant effects of complexes of tilapia fish skin collagen and different marine oligosaccharides. J. Ocean. Univ. China 2010, 9, 399–407. [Google Scholar] [CrossRef]
- Zapata, J.E.; Gómez-Sampedro, L.J. Antioxidant and antiproliferative activity of enzymatic hydrolysates from red tilapia (Oreochromis spp.) viscera. Biotechnol. Rep. 2024, 42, e00832. [Google Scholar] [CrossRef]
- Kong, J.; Hu, X.M.; Cai, W.W.; Wang, Y.M.; Chi, C.F.; Wang, B. Bioactive peptides from skipjack tuna cardiac arterial bulbs (II): Protective function on UVB-irradiated HaCaT cells through antioxidant and anti-apoptotic mechanisms. Mar. Drugs 2023, 21, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, J.; Qin, X.; Peng, Z.; Lin, H. Novel antioxidant peptides from Crassostrea hongkongensis improve photo-oxidation in UV-induced HaCaT cells. Mar. Drugs 2022, 20, 100. [Google Scholar] [CrossRef]
- He, Y.L.; Liu, Y.; Lin, L.; Mo, Y.; Li, H.; Zhou, C.; Hong, P.; Qian, Z.J. Antioxidant peptide ETT from Isochrysis zhanjiangensis attenuate skin aging by maintaining homeostasis and promoting collagen generation. Algal Res. 2024, 82, 103615. [Google Scholar] [CrossRef]
- Vincenzi, M.; Mercurio, F.A.; Leone, M. Virtual screening of peptide libraries: The search for peptide-based therapeutics using computational tools. Int. J. Mol. Sci. 2024, 25, 1798. [Google Scholar] [CrossRef]
- Xiong, X.; Liang, J.; Xu, Y.; Liu, J.; Liu, Y. The wound healing effects of the Tilapia collagen peptide mixture TY001 in streptozotocin diabetic mice. J. Sci. Food Agric. 2020, 100, 2848–2858. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Zhuang, Y. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis niloticus) gelatin peptides. J. Funct. Foods 2013, 5, 154–162. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L. Preparation of reactive oxygen scavenging peptides from tilapia (Oreochromis niloticus) skin gelatin: Optimization using response surface methodology. J. Food Sci. 2011, 76, C483–C489. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, H.; Chi, Y.; Deng, R.; He, Q. Structural characterization, erythrocyte protection, and antifatigue effect of antioxidant collagen peptides from tilapia (Oreochromis nilotica L.) skin. Food Funct. 2020, 11, 10149–10160. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, L.; Zhao, D.; Wang, X.; Xia, Y.; Li, B.; Liu, C.; Zuo, X. Tilapia skin peptides, a by-product of fish processing, ameliorate DSS-induced colitis by regulating inflammation and inhibiting apoptosis. Front. Nutr. 2022, 9, 988758. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.-y.; Liu, Y.; Shan, L.-t.; Xu, Y.-q.; Liang, J.; Lai, Y.-H.; Hsiao, C.-D. Evaluation of collagen mixture on promoting skin wound healing in zebrafish caused by acetic acid administration. Biochem. Biophys. Res. Commun. 2018, 505, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, L.; Hu, Z.; Kong, S.; Zhang, Z.; Li, G. Optimized preparation of gastric acid-response sulfhydryl functionalized chitosan/alginate/tilapia peptide hydrogel and its protective effects on alcohol-induced liver and brain injury. RSC Adv. 2021, 11, 34544–34557. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, L.; He, Y.; Wang, Z.; Xu, J.; Ma, H. Hydrolysis kinetics and antioxidant activity of collagen under simulated gastrointestinal digestion. J. Funct. Foods 2014, 11, 493–499. [Google Scholar] [CrossRef]
- Wang, L.; Ding, L.; Xue, C.; Ma, S.; Du, Z.; Zhang, T.; Liu, J. Corn gluten hydrolysate regulates the expressions of antioxidant defense and ROS metabolism relevant genes in H2O2-induced HepG2 cells. J. Funct. Foods 2018, 42, 362–370. [Google Scholar] [CrossRef]
- Wang, B.; Li, B. Effect of molecular weight on the transepithelial transport and peptidase degradation of casein-derived peptides by using Caco-2 cell model. Food Chem. 2017, 218, 1–8. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G.; Pratt, C.W. Fundamentals of Biochemistry: Life at the Molecular Level, 5th ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Hou, H.; Li, B.; Zhao, X.; Zhuang, Y.; Ren, G.; Yan, M.; Cai, Y.; Zhang, X.; Chen, L. The effect of pacific cod (Gadus macrocephalus) skin gelatin polypeptides on UV radiation-induced skin photoaging in ICR mice. Food Chem. 2009, 115, 945–950. [Google Scholar] [CrossRef]
- Ranathunga, S.; Rajapakse, N.; Kim, S.-K. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur. Food Res. Technol. 2006, 222, 310–315. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Bandara, N.; Wu, J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem. 2015, 188, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-Y.; Lee, J.-H.; Samarakoon, K.; Kim, J.-S.; Jeon, Y.-J. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food Chem. Toxicol. 2013, 52, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H.; Li, H. Overview of antioxidant peptides derived from marine resources: The sources, characteristic, purification, and evaluation methods. Appl. Biochem. Biotechnol. 2015, 176, 1815–1833. [Google Scholar] [CrossRef] [PubMed]
- Abuine, R.; Rathnayake, A.U.; Byun, H.G. Biological activity of peptides purified from fish skin hydrolysates. Fish. Aquat. Sci. 2019, 22, 10. [Google Scholar] [CrossRef]
- Karnila, R.; Dewita, E.; Yoswaty, D.; Yoswaty, D.; Putri, T.; Yunus, A. Antioxidant activity on protein hydrolysate peptide of mudskipper fish (Periophthalmodon schlosseri) using alcalase enzyme. Food Sci. Technol. 2023, 43, 1–8. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Wong, F.C.; Chai, T.T. Bioactive peptides and protein hydrolysates as lipoxygenase inhibitors. Biology 2023, 12, 917. [Google Scholar] [CrossRef]
- Wong, F.-C.; Chow, Y.-L.; Tan, S.-A.; Tian, L.; Bai, W.; Chai, T.-T. Inhibition of myeloperoxidase by food-derived peptides: A review of current research and future prospects. Food Biosci. 2024, 60, 104458. [Google Scholar] [CrossRef]
- Amadio, P.; Sandrini, L.; Zarà, M.; Barbieri, S.S.; Ieraci, A. NADPH-oxidases as potential pharmacological targets for thrombosis and depression comorbidity. Redox Biol. 2024, 70, 103060. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. The inhibitory activity of natural products to xanthine oxidase. Chem. Biodivers. 2023, 20, e202300005. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Zhang, N.; Li, Y.; Cai, Z.; Li, G.; Liu, Z.; Liu, Z.; Wang, Y.; Shao, X.; et al. Anti-aging activity and their mechanisms of natural food-derived peptides: Current advancements. Food Innov. Adv. 2023, 2, 272–290. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zheng, J.; Bu, T.; He, G.; Wu, J. Safety considerations on food protein-derived bioactive peptides. Trends Food Sci. Technol. 2020, 96, 199–207. [Google Scholar] [CrossRef]
- Ha, N.M.; Tran, S.H.; Shim, Y.-H.; Kang, K. Caenorhabditis elegans as a powerful tool in natural product bioactivity research. Appl. Biol. Chem. 2022, 65, 18. [Google Scholar] [CrossRef]
- Li, C.; Xu, W.; Zhang, X.; Cui, X.; Tsopmo, A.; Li, J. Antioxidant peptides derived from millet bran promote longevity and stress resistance in Caenorhabditis elegans. Plant Foods Hum. Nutr. 2023, 78, 790–795. [Google Scholar] [CrossRef]
- Chen, Q.; Nie, X.; Huang, W.; Wang, C.; Lai, R.; Lu, Q.; He, Q.; Yu, X. Unlocking the potential of chicken liver byproducts: Identification of antioxidant peptides through in silico approaches and anti-aging effects of a selected peptide in Caenorhabditis elegans. Int. J. Biol. Macromol. 2024, 272, 132833. [Google Scholar] [CrossRef]
- Giraldo, N.D.; Sánchez, J.D.; López, A.; González, N.; Balaguer, F.; Redondo, J.; Llopis, S.; Barrena, M.; Klotz, B. Functional assessment of a whey protein hydrolysate: In vivo responses in lipid regulation, oxidant defense, and neural protection in Caenorhabditis elegans. Int. Dairy J. 2024, 154, 105921. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Akaberi, S.; Gusbeth, C.; Silve, A.; Senthilnathan, D.S.; Navarro-López, E.; Molina-Grima, E.; Müller, G.; Frey, W. Effect of pulsed electric field treatment on enzymatic hydrolysis of proteins of Scenedesmus almeriensis. Algal Res. 2019, 43, 101656. [Google Scholar] [CrossRef]
- Bargeman, G.; Houwing, J.; Recio, I.; Koops, G.H.; Van Der Horst, C. Electro-membrane filtration for the selective isolation of bioactive peptides from an αs2-casein hydrolysate. Biotechnol. Bioeng. 2002, 80, 599–609. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. BIOPEP-UWM database—Present and future. Curr. Opin. Food Sci. 2024, 55, 101108. [Google Scholar] [CrossRef]
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; García-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B.; et al. AnOxPePred: Using deep learning for the prediction of antioxidative properties of peptides. Sci. Rep. 2020, 10, 21471. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Temussi, P.A. The good taste of peptides. J. Pept. Sci. 2012, 18, 73–82. [Google Scholar] [CrossRef]
- Saha, B.C.; Hayashi, K. Debittering of protein hydrolyzates. Biotechnol. Adv. 2001, 19, 355–370. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Penjamras, P.; Kristinsson, H.G. Chemical compositions and muddy flavour/odour of protein hydrolysate from Nile tilapia and broadhead catfish mince and protein isolate. Food Chem. 2014, 142, 210–216. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Kishimura, H. Antioxidant and sensory properties of protein hydrolysate derived from Nile tilapia (Oreochromis niloticus) by one- and two-step hydrolysis. J. Food Sci. Technol. 2015, 52, 3336–3349. [Google Scholar] [CrossRef]
- Leksrisompong, P.; Gerard, P.; Lopetcharat, K.; Drake, M. Bitter taste inhibiting agents for whey protein hydrolysate and whey protein hydrolysate beverages. J. Food Sci. 2012, 77, S282–S287. [Google Scholar] [CrossRef]
- Sarabandi, K.; Gharehbeglou, P.; Jafari, S.M. Spray-drying encapsulation of protein hydrolysates and bioactive peptides: Opportunities and challenges. Drying Technol. 2020, 38, 577–595. [Google Scholar] [CrossRef]
- Bahnasawy, M.H. Effect of dietary protein levels on growth performance and body composition of monosex Nile tilapia, Oreochromis niloticus L. reared in fertilized tanks. Pak. J. Nutr. 2009, 8, 674–678. [Google Scholar] [CrossRef]
- Wachirattanapongmetee, K.; Katekaew, S.; Sae-Eaw, A.; Thawornchinsombut, S. Production factors affecting antioxidant peptides from tilapia processing byproducts. Food Sci. Technol. 2019, 39, 181–187. [Google Scholar] [CrossRef]

| Aquaculture Type | Tilapia Species | Year | ||||
|---|---|---|---|---|---|---|
| 2000 | 2005 | 2010 | 2015 | 2020 | ||
| Inland Aquaculture | Nile tilapia (Oreochromis niloticus) | 1001.5 | 1721.3 | 2637.4 | 4000.9 | 4407.2 |
| Tilapias nei (Oreochromis spp.) | 123.9 | 199.3 | 449.6 | 929.9 | 1069.9 | |
| Marine and Coastal Aquaculture | Nile tilapia (Oreochromis niloticus) | 1.6 | 5.3 | 20.3 | 49.8 | 107.4 |
| Tilapia Part | Enzyme Used for Hydrolysis | Hydrolysis Condition (pH; Temperature; Duration) | DH (%) | Yield (%) | Reference |
|---|---|---|---|---|---|
| Muscle | Alcalase® | pH 8; 50 °C; 10 h | 35.0 | 12.4 | [29] |
| Muscle | Flavourzyme® | pH 7; 55 °C; 1 h | 13.3 | - | [48] |
| Dorsal meat | Alcalase® followed by pepsin and pancreatin | Alcalase®: pH 8; 50 °C; 2, 6, 10 and 16 h Pepsin: pH 3; 37 °C; 2 h Pancreatin: pH 7; 37 °C; 2 h | 40–55 | - | [47] |
| Skin | Alcalase® followed by Pronase E® | Alcalase®: pH 8; 50 °C; 30 min Pronase E®: pH 8; 50 °C; 2 h | - | - | [49] |
| Skin | Pepsin followed by pancreatin | Pepsin: pH 2.5; 37 °C; 1 h Pancreatin: pH 7.5; 37 °C; 2 h | - | - | [42] |
| Skin | Multifect neutral® followed by Properase E® | Multifect neutral®: pH 8.0; 35 °C; 4.5 h Properase E®: pH 9.0; 55 °C; 4.5 h | 22.1 | - | [43,46] |
| Skin | Neutral protease and papain | pH 7; 50 °C; 3 h | - | - | [23] |
| Scales | Alcalase® | pH 8; 58.5 °C; 55 min | 12 | 86 | [50] |
| Scales | Trypsin | pH 7; 55 °C; 4 h | - | - | [51,52] |
| Viscera | Alcalase® | pH 8; 55 °C; 1.5 h | 41.5 | - | [44,45] |
| Purification Strategy | Peptide Identification | Peptide Sequence | Reference |
|---|---|---|---|
| GFC, RP-HPLC | MS/MS |
| [42] |
| GFC, RP-HPLC | LC-MS/MS |
| [48] |
| GFC, RPC | LC–MS/MS |
| [29] |
| AEC, RP-HPLC | MS/MS |
| [53,54] |
| GFC, CEC, RP-HPLC | MS/MS |
| [43,46] |
| Part of Tilapia | Species | Protease Used for Hydrolysis | Sample Dose | Cell Model | Key Findings | Reference |
|---|---|---|---|---|---|---|
| Muscle | Oreochromis niloticus |
| 7.5–25% |
|
| [56] |
| Skin | Oreochromis niloticus | Alcalase® followed with Pronase E® | 1.56–12.5 mg/mL | AAPH-treated Detroit 551 cells |
| [49] |
| Skin | Oreochromis niloticus | Ginger protease | 0.5–10 mg/mL | H2O2-treated IPEC-J2 cells |
| [55] |
| Scales | Oreochromis sp. | Alcalase® | 2 mg/mL | Angiotensin II-treated A7r5 cells |
| [50] |
| Scales | Oreochromis niloticus | Trypsin | 50–500 µg/mL | UVA radiation-treated HFF-1 cells |
| [51] |
| Dorsal meat | Oreochromis niloticus | Alcalase® followed with pepsin and pancreatin | 0.1–5.0 mg/mL | AAPH-treated HepG2 cells |
| [47] |
| Part of Tilapia Hydrolyzed | Protease Used for Hydrolysis | Peptide Identified | Sample Dose | Cell Model | Key Findings | Reference |
|---|---|---|---|---|---|---|
| Scale | Alcalase® |
| 20–200 µg/mL [53] 1–20 µg/mL [54] |
|
| [53,54] |
| Muscle | Flavourzyme® |
| 100 µg/mL | H2O2-treated RAW 264.7 cells |
| [48] |
| 100 µg/mL | Lipopolysaccharide-treated RAW 264.7 cells |
| |||
| Skin | Pepsin followed with pancreatin |
| 50–200 µg/mL | UVB radiation-treated MEF cells |
| [42,43] |
| Skin | Multifect neutral® followed with Properase E® |
| 10–100 µM | UVB radiation-treated HaCaT cells |
| [43,57] |
| 10–100 µM | Ethanol-treated HepG2 cells |
| [43,46] | |||
| Muscle | Alcalase® |
| 10–100 µM | AAPH-treated HepG2 cells |
| [29] |
| Sample | Effective Dosage (per kg Body Weight) | In Vivo Model | Key Findings | Reference |
|---|---|---|---|---|
| Collagen polypeptides (<3 kDa) prepared from tilapia skin using neutral protease and papain | 500–2000 mg | Mouse model of aging induced by D-galactose |
| [23] |
| Tilapia gelatin hydrolysate prepared with Properase E® | 100–200 mg | UV-induced skin photoaging mice |
| [70,71] |
| Commercial tilapia collagen peptide powder, in combination with antioxidant supplements | Collagen peptide, 1.2 g; vitamin C, 100 mg; vitamin E, 2.66 mg; astaxanthin, 2.5 mg | UV-induced skin photoaging mice |
| [24] |
| Tilapia skin collagen hydrolysate prepared with Alcalase® | 0.85–1.70 g | Alloxan-induced diabetic mice |
| [22] |
| Tilapia collagen peptide mixture TY001 | 20–60 g | STZ-induced diabetic mice |
| [69] |
| Tilapia viscera hydrolysate extract prepared with Alcalase® | 150–300 mg | DOCA-salt-induced hypertensive rats |
| [44] |
| Tilapia skin collagen hydrolysate prepared with Alcalase® | 250–2500 mg | Mice subjected to exhaustive swimming assay |
| [72] |
| Peptide | Molecular Mass (Da) | Hydrophobic Amino Acid Residue (%) b | Reference |
|---|---|---|---|
| EKP | 372.42 a | 33.3 | [29] |
| EKL | 388.46 a | 33.3 | |
| ALSC | 392.48 a | 50.0 | |
| ELSC | 450.51 a | 25.0 | |
| HKPA | 451.53 a | 50.0 | |
| SLCH | 458.54 a | 25.0 | |
| ASLCH | 529.62 a | 40.0 | |
| LPGYF | 595.70 a | 60.0 | |
| LEVPGY | 676.77 a | 66.7 | |
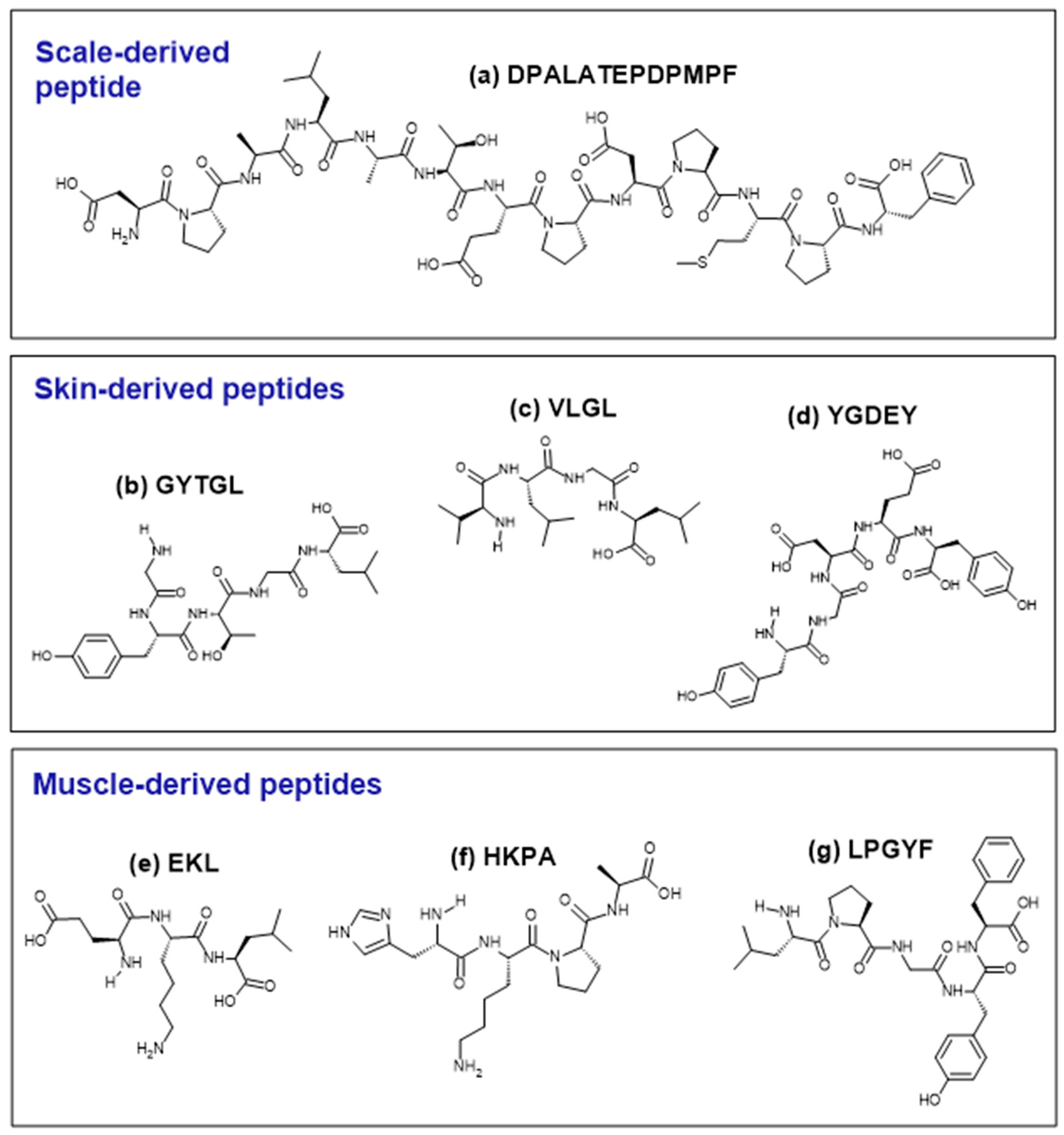
| VLGL | 401.28 | 100.0 | [42,43] |
| GYTGL | 510.26 | 60.0 | |
| LGATGL | 531.31 | 83.3 | |
| YGDEY | 645.21 | 20.0 | [43,46,57] |
| DPALATEPDPMPF | 1382.57 | 69.2 | [53,54] |
| AFAVIDQDKSGFIEEDELKLFLQNFSAGARAGDSDGDGKIGVDEFAALVK | 6309.46 | 56.0 | [48] |
| KAFAVIDQDKSGFIEEDELKLFLQNFSAGARAGDSDGDGKIGVDEFAALVK | 6334.49 | 54.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, W.-J.; Wong, F.-C.; Abd Manan, F.; Chow, Y.-L.; Ooi, A.-L.; Ong, M.-K.; Zhang, X.; Chai, T.-T. Antioxidant Peptides and Protein Hydrolysates from Tilapia: Cellular and In Vivo Evidences for Human Health Benefits. Foods 2024, 13, 2945. https://doi.org/10.3390/foods13182945
Ng W-J, Wong F-C, Abd Manan F, Chow Y-L, Ooi A-L, Ong M-K, Zhang X, Chai T-T. Antioxidant Peptides and Protein Hydrolysates from Tilapia: Cellular and In Vivo Evidences for Human Health Benefits. Foods. 2024; 13(18):2945. https://doi.org/10.3390/foods13182945
Chicago/Turabian StyleNg, Wen-Jie, Fai-Chu Wong, Fazilah Abd Manan, Yit-Lai Chow, Ai-Lin Ooi, Mei-Kying Ong, Xuewu Zhang, and Tsun-Thai Chai. 2024. "Antioxidant Peptides and Protein Hydrolysates from Tilapia: Cellular and In Vivo Evidences for Human Health Benefits" Foods 13, no. 18: 2945. https://doi.org/10.3390/foods13182945







