Impact of Microencapsulation on Ocimum gratissimum L. Essential Oil: Antimicrobial, Antioxidant Activities, and Chemical Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Purified Cashew Gum
2.2.2. Essential Oil Extraction
2.2.3. Emulsion Preparation and Microencapsulation by Spray-Drying
2.2.4. Moisture Content, Water Activity (aw), Encapsulation Efficiency (EE), Particle Size, and Scanning Electron Microscopy (SEM)
2.2.5. Chemical Characterization by GC–MS Analysis
2.2.6. Antioxidant Activity
2.2.7. Antibacterial Activity
2.2.8. Minimum Inhibitory Concentration Determination
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Microencapsulated Essential Oil
3.1.1. Moisture Content, Water Activity, and Encapsulation Efficiency
3.1.2. Particle Size and Scanning Electron Microscopy
3.2. Chemical Characterization of Ocimum gratissimum Essential Oil
3.3. Chemical Characterization of Encapsulated Ocimum gratissimum Essential Oil
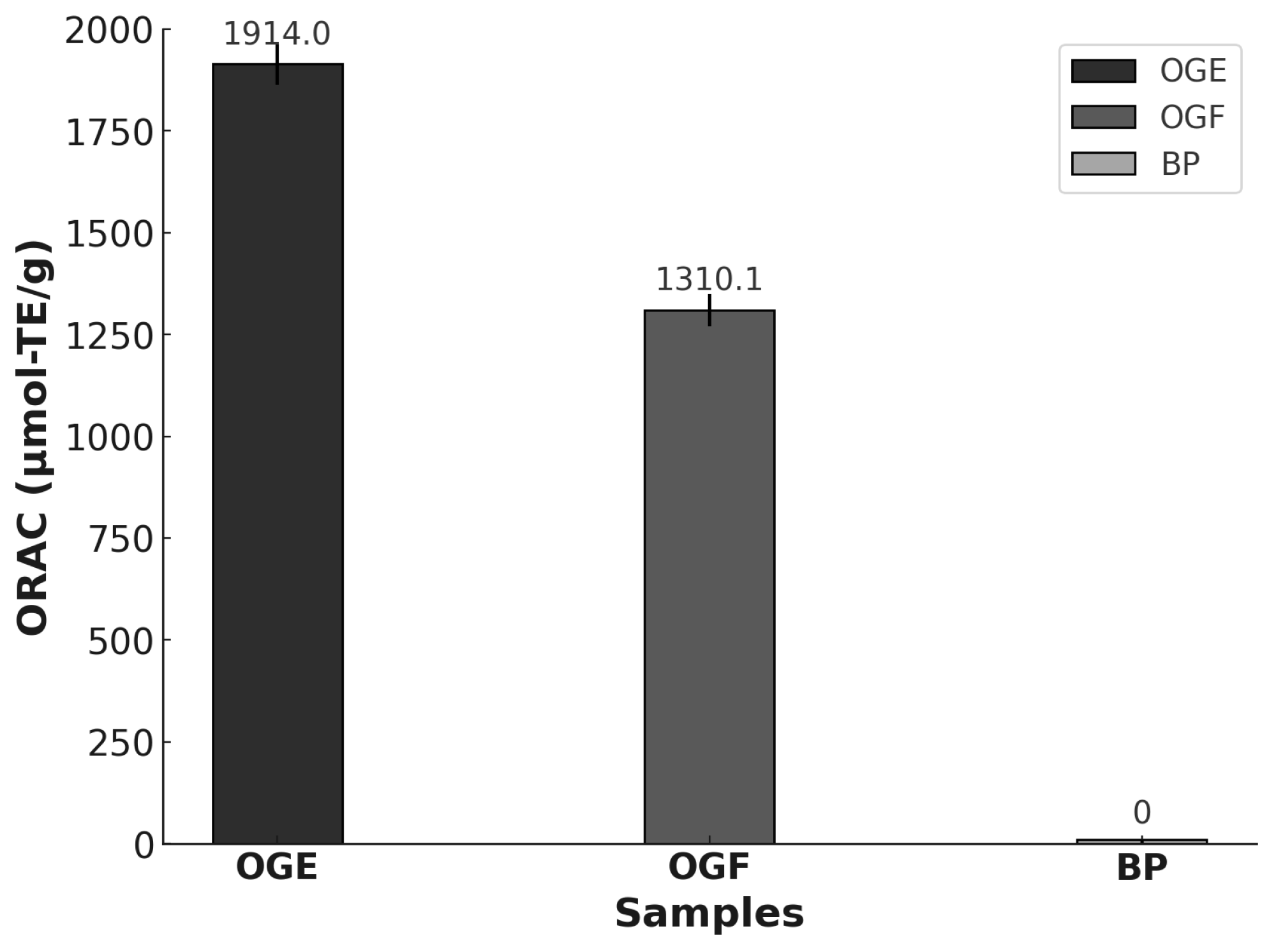
3.4. Antioxidant Activity
3.5. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araujo, T.D.S.; da Costa, J.M.A.R.; de Oliveira Silva Ribeiro, F.; de Jesus Oliveira, A.C.; do Nascimento Dias, J.; de Araujo, A.R.; Barros, A.B.; da Paixão Brito, M.; de Oliveira, T.M.; de Almeida, M.P.; et al. Nanoemulsion of Cashew Gum and Clove Essential Oil (Ocimum gratissimum Linn) Potentiating Antioxidant and Antimicrobial Activity. Int. J. Biol. Macromol. 2021, 193, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rosas, J.; Ferreira-Grosso, C.R.; Gómez-Aldapa, C.A.; Rangel-Vargas, E.; Rodríguez-Marín, M.L.; Guzmán-Ortiz, F.A.; Falfan-Cortes, R.N. Recent Advances in Microencapsulation of Natural Sources of Antimicrobial Compounds Used in Food—A Review. Food Res. Int. 2017, 102, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems—A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Eghbal, N.; Liao, W.; Dumas, E.; Azabou, S.; Dantigny, P.; Gharsallaoui, A. Microencapsulation of Natural Food Antimicrobials: Methods and Applications. Appl. Sci. 2022, 12, 3837. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An Overview of Natural Antimicrobials Role in Food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of Essential Oils in Food Safety: Antimicrobial and Antioxidant Applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical Composition and Antibacterial and Antioxidant Properties of Commercial Essential Oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Fernandes, V.F.; de Almeida, L.B.; Feijó, E.V.d.R.S.; Silva, D.d.C.; de Oliveira, R.A.; Mielke, M.S.; Costa, L.C.d.B. Light Intensity on Growth, Leaf Micromorphology and Essential Oil Production of Ocimum gratissimum. Rev. Bras. de Farmacogn. 2013, 23, 419–424. [Google Scholar] [CrossRef]
- Pereira, C.A.M.; Maia, J.F. Estudo Da Atividade Antioxidante Do Extrato e Do Óleo Essencial Obtidos Das Folhas de Alfavaca (Ocimum gratissimum L.). Cienc. e Tecnol. de Aliment. 2007, 27, 624–632. [Google Scholar] [CrossRef]
- Pandey, S. Antibacterial and Antifungal Activities of Ocimum gratissimum L. Int. J. Pharm. Pharm. Sci. 2017, 9, 26. [Google Scholar] [CrossRef]
- Prakash, B.; Shukla, R.; Singh, P.; Mishra, P.K.; Dubey, N.K.; Kharwar, R.N. Efficacy of Chemically Characterized Ocimum gratissimum L. Essential Oil as an Antioxidant and a Safe Plant Based Antimicrobial against Fungal and Aflatoxin B1 Contamination of Spices. Food Res. Int. 2011, 44, 385–390. [Google Scholar] [CrossRef]
- Tsurunaga, Y.; Kanou, M.; Ikeura, H.; Makino, M.; Oowatari, Y.; Tsuchiya, I. Effect of Different Tea Manufacturing Methods on the Antioxidant Activity, Functional Components, and Aroma Compounds of Ocimum gratissimum. Lwt 2022, 169, 114058. [Google Scholar] [CrossRef]
- Sahouo, G.B.; Félix, T.Z.; Boti, J.-B.; Chopard, C.; Mahy, J.-P.; N’Guessan, Y.T. Anti-inflammatory and analgesic activities: Chemical constituents of essential oils of Ocimum gratissimum, Eucalyptus citriodora and Cymbopogon giganteus inhibited lipoxygenase L-1 and cyclooxygenase of PGHS. Bull. Chem. Soc. Ethiop 2003, 17, 191–197. [Google Scholar]
- Philippe, S.; Guy, A.; Paulin, A.; Issaka, Y. Chemical Composition and Antifungal Activity of Essential Oil of Fresh Leaves of Ocimum gratissimum from Benin against Six Mycotoxigenic Fungi Isolated from Traditional Cheese Wagashi. Int. Res. J. Biol. Sci. 2012, 1, 22–27. [Google Scholar]
- Chimnoi, N.; Reuk-ngam, N.; Chuysinuan, P.; Khlaychan, P.; Khunnawutmanotham, N.; Chokchaichamnankit, D.; Thamniyom, W.; Klayraung, S.; Mahidol, C.; Techasakul, S. Characterization of Essential Oil from Ocimum gratissimum Leaves: Antibacterial and Mode of Action against Selected Gastroenteritis Pathogens. Microb. Pathog. 2018, 118, 290–300. [Google Scholar] [CrossRef]
- Ugbogu, O.C.; Emmanuel, O.; Agi, G.O.; Ibe, C.; Ekweogu, C.N.; Ude, V.C.; Uche, M.E.; Nnanna, R.O.; Ugbogu, E.A. A Review on the Traditional Uses, Phytochemistry, and Pharmacological Activities of Clove Basil (Ocimum gratissimum L.). Heliyon 2021, 7, e08404. [Google Scholar] [CrossRef]
- Nakamura, C.V.; Ueda-Nakamura, T.; Bando, E.; Negrão Melo, A.F.; Garcia Cortez, D.A.; Dias Filho Filho, B.P. Antibacterial Activity of Ocimum gratissimum L. Essential Oil. Mem. Do Inst. Oswaldo Cruz 1999, 94, 675–678. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, Bioactivities, Mode of Action and Industrial Applications of Essential Oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Altay, Ö.; Köprüalan, Ö.; İlter, I.; Koç, M.; Ertekin, F.K.; Jafari, S.M. Spray Drying Encapsulation of Essential Oils; Process Efficiency, Formulation Strategies, and Applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 1139–1157. [Google Scholar] [CrossRef] [PubMed]
- Da Veiga, R.D.S.; Silva-Buzanello, R.A.; Corso, M.P.; Canan, C. Essential Oils Microencapsulated Obtained by Spray Drying: A Review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Botrel, D.A.; Fernandes, R.V.d.B.; Borges, S.V. Microencapsulation of Essential Oils Using Spray Drying Technology. In Microencapsulation and Microspheres for Food Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 235–251. ISBN 9780128003503. [Google Scholar]
- De Melo, A.; Da Rosa, C.; Sganzerla, W.G.; Nunes, M.; Noronha, C.; De Oliveira, B.M.; Villetti, M.A.; Bertoldi, F.; Barreto, P.L. Syntesis and Characterization of Zein Nanoparticles Loaded with Essential Oil of Ocimum gratissimum and Pimenta racemosa. Mater. Res. Express 2019, 6, 095084. [Google Scholar] [CrossRef]
- Onyebuchi, C.; Kavaz, D. Chitosan and N, N, N-Trimethyl Chitosan Nanoparticle Encapsulation of Ocimum gratissimum Essential Oil: Optimised Synthesis, in Vitro Release and Bioactivity. Int. J. Nanomed. 2019, 14, 7707–7727. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Fernandes, R.; Borges, S.; Botrel, D. Gum Arabic/Starch/Maltodextrin/Inulin as Wall Materials on the Microencapsulation of Rosemary Essential Oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef]
- Porto, B.C.; Cristianini, M. Evaluation of Cashew Tree Gum (Anacardium occidentale L.) Emulsifying Properties. LWT—Food Sci. Technol. 2014, 59, 1325–1331. [Google Scholar] [CrossRef]
- Amaral, R.G.; de Andrade, L.R.M.; Andrade, L.N.; Loureiro, K.C.; Souto, E.B.; Severino, P. Cashew Gum: A Review of Brazilian Patents and Pharmaceutical Applications with a Special Focus on Nanoparticles. Micromachines 2022, 13, 1137. [Google Scholar] [CrossRef]
- Ribeiro, A.; De Souza, F.; Bezerra, J.; Oliveira, C.; Nadvorny, D.; Soares, M.; Nunes, L.; Sobrinho, J. Gum’s Based Delivery Systems: Review on Cashew Gum and Its Derivates. Carbohydr. Polym. 2016, 147, 188–200. [Google Scholar] [CrossRef]
- Beirão-da-costa, S.; Duarte, C.; Bourbon, A.I.; Pinheiro, A.C.; Januário, M.I.N.; Vicente, A.A.; Beirão-da-costa, M.L.; Delgadillo, I. Inulin Potential for Encapsulation and Controlled Delivery of Oregano Essential Oil. Food Hydrocoll. 2013, 33, 199–206. [Google Scholar] [CrossRef]
- Fernandes, R.; Botrel, D.; Silva, E.; Borges, S.; Yoshida, M.I.; Monteiro de Paula, R. Cashew Gum and Inulin: New Alternative for Ginger Essential Oil Microencapsulation. Carbohydr. Polym. 2016, 153, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.A.; Grosso, C.R. Cashew Gum Microencapsulation Protects the Aroma of Coffee Extracts. J. Microencapsul. 2007, 25, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.C.T.; Leme, E.E.; Delarmelina, C.; Soares, A.A.; Figueira, G.M.; Sartoratto, A. Activity of Essential Oils from Brazilian Medicinal Plants on Escherichia Coli. J. Ethnopharmacol. 2007, 111, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; AOAC, Ed.; AOAC International: Rockville, MD, USA, 2007. [Google Scholar]
- Bhandari, B.R.; D’Arcy, B.R.; Padukka, I. Encapsulation of Lemon Oil by Paste Method Using β-Cyclodextrin: Encapsulation Efficiency and Profile of Oil Volatiles. J. Agric. Food Chem. 1999, 47, 5194–5197. [Google Scholar] [CrossRef]
- Fadini, A.L.; Alvim, I.; Paganotti, K.; Da Silva, L.; Oliveira Miguel, A.M.; Rodrigues, R. Optimization of the Production of Double-Shell Microparticles Containing Fish Oil. Food Sci. Technol. Int. 2018, 25, 359–369. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 9781932633214. [Google Scholar]
- Salvador, M.J.; Ferreira, E.O.; Mertens-Talcott, S.U.; De Castro, W.V.; Butterweck, V.; Derendorf, H.; Dias, D.A. Isolation and HPLC Quantitative Analysis of Antioxidant Flavonoids from Alternanthera Tenella Colla. Z. Fur. Naturforschung—Sect. C J. Biosci. 2006, 61, 19–25. [Google Scholar] [CrossRef]
- CLSI M02-A12: Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Twelfth Edition. Clin. Lab. Stand. Inst. 2015, 35, 73.
- Pilicheva, B.; Uzunova, Y.; Katsorov, P. Comparative Study on Microencapsulation of Lavender (Lavandula angustifolia Mill.) and Peppermint (Mentha piperita L.) Essential Oils via Spray-Drying Technique. Molecules 2021, 26, 7467. [Google Scholar] [CrossRef]
- Fernandes, R.V.d.B.; Silva, E.K.; Borges, S.V.; de Oliveira, C.R.; Yoshida, M.I.; da Silva, Y.F.; do Carmo, E.L.; Azevedo, V.M.; Botrel, D.A. Proposing Novel Encapsulating Matrices for Spray-Dried Ginger Essential Oil from the Whey Protein Isolate-Inulin/Maltodextrin Blends. Food Bioprocess Technol. 2017, 10, 115–130. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Alencar, D.; De Souza, E.; Almeida, E.; Da Silva, A.; Oliveira, H.; Cavalcanti, M. Microencapsulation of Cymbopogon Citratus D.C. Stapf Essential Oil with Spray Drying: Development, Characterization, and Antioxidant and Antibacterial Activities. Foods 2022, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.D.; Hashib, S.A.; Ibrahim, U.K.; Rahman, N.A. Development of Carrier Material for Food Applications in Spray Drying Technology: An Overview. Mater. Today Proc. 2021, 47, 1371–1375. [Google Scholar] [CrossRef]
- Ré, M.I. Microencapsulation by Spray Drying. Dry. Technol. 1998, 16, 1195–1236. [Google Scholar] [CrossRef]
- Reineccius, G.A. The Spray Drying of Food Flavors. Dry. Technol. 2004, 22, 1289–1324. [Google Scholar] [CrossRef]
- Melo, R.S.; Azevedo, Á.M.A.; Pereira, A.M.G.; Rocha, R.R.; Cavalcante, R.M.B.; Matos, M.N.C.; Lopes, P.H.R.; Gomes, G.A.; Rodrigues, T.H.S.; Dos Santos, H.S.; et al. Chemical Composition and Antimicrobial Effectiveness of Ocimum gratissimum l. Essential Oil against Multidrug-Resistant Isolates of Staphylococcus aureus and Escherichia coli. Molecules 2019, 24, 3864. [Google Scholar] [CrossRef]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and Antimicrobial Activity of Essential Oils from Aromatic Plants Used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Sharma, A.; Bhardwaj, G.; Sohal, H.S.; Gohain, A. Eugenol; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 9780323897792. [Google Scholar]
- Nhan, N.P.; Thanh, V.; Cang, M.; Lam, T.D.; Huong, N.; Nhan, L.T.; TTruc, T.; Tran, Q.T.; Bach, L.G. Microencapsulation of Lemongrass (Cymbopogon citratus) Essential Oil Via Spray Drying: Effects of Feed Emulsion Parameters. Processes 2020, 8, 40. [Google Scholar] [CrossRef]
- Radünz, M.; Cristina, H.; Mota, T.; Francine, C.; Nunes, P.; Antonio, F.; De Barros, P.; Dal, J.; José, P.; Filho, S.; et al. Antimicrobial Potential of Spray Drying Encapsulated Thyme (Thymus vulgaris) Essential Oil on the Conservation of Hamburger-like Meat Products. Int. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef]
- Van, C.K.; Thuong, P.; Nguyen, N.; Nguyen, T.T.; Giang, L. Microencapsulation of Citrus latifolia Peel Essential Oil by Spray-Drying Using Maltodextrin: Characterization, Antimicrobial Activities, and Release Profile. LWT 2024, 197, 115825. [Google Scholar] [CrossRef]
- Bentayeb, K.; Vera, P.; Rubio, C.; Nerín, C. The Additive Properties of Oxygen Radical Absorbance Capacity (ORAC) Assay: The Case of Essential Oils. Food Chem. 2014, 148, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Salvador, M.J.; De Lourenço, C.C.; Andreazza, N.L.; Pascoal, A.C.R.F.; Stefanello, M.É.A. Antioxidant Capacity and Phenolic Content of Four Myrtaceae Plants of the South of Brazil. Nat. Prod. Commun. 2011, 6, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Nehme, R.; Andrés, S.; Pereira, R.B.; Jemaa, M.B.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential Oils in Livestock: From Health to Food Quality Essential. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R. Chemical Composition, in Vitro Antimicrobial and Antioxidant Activities of the Essential Oils of Ocimum Gratissimum, O. Sanctum and Their Major Constituents. Indian J. Pharm. Sci. 2013, 75, 457–462. [Google Scholar] [CrossRef]
- Lacerda, E.C.Q.; Calado, V.M.D.A.; Monteiro, M.; Finotelli, P.V.; Torres, A.G.; Perrone, D. Starch, Inulin and Maltodextrin as Encapsulating Agents Affect the Quality and Stability of Jussara Pulp Microparticles. Carbohydr. Polym. 2016, 151, 500–510. [Google Scholar] [CrossRef]
- Da Costa, S.B.; Duarte, C.; Bourbon, A.I.; Pinheiro, A.C.; Serra, A.T.; Martins, M.M.; Januário, M.I.N.; Vicente, A.A.; Delgadillo, I.; Duarte, C.; et al. Effect of the Matrix System in the Delivery and in Vitro Bioactivity of Microencapsulated Oregano Essential Oil. J. Food Eng. 2012, 110, 190–199. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G. Essential Oil Nanoemulsions as Antimicrobial Agents in Food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Yoplac, I.; Vargas, L.; Robert, P.; Hidalgo, A. Heliyon Characterization and Antimicrobial Activity of Microencapsulated Citral with Dextrin by Spray Drying. Heliyon 2021, 7, e06737. [Google Scholar] [CrossRef]
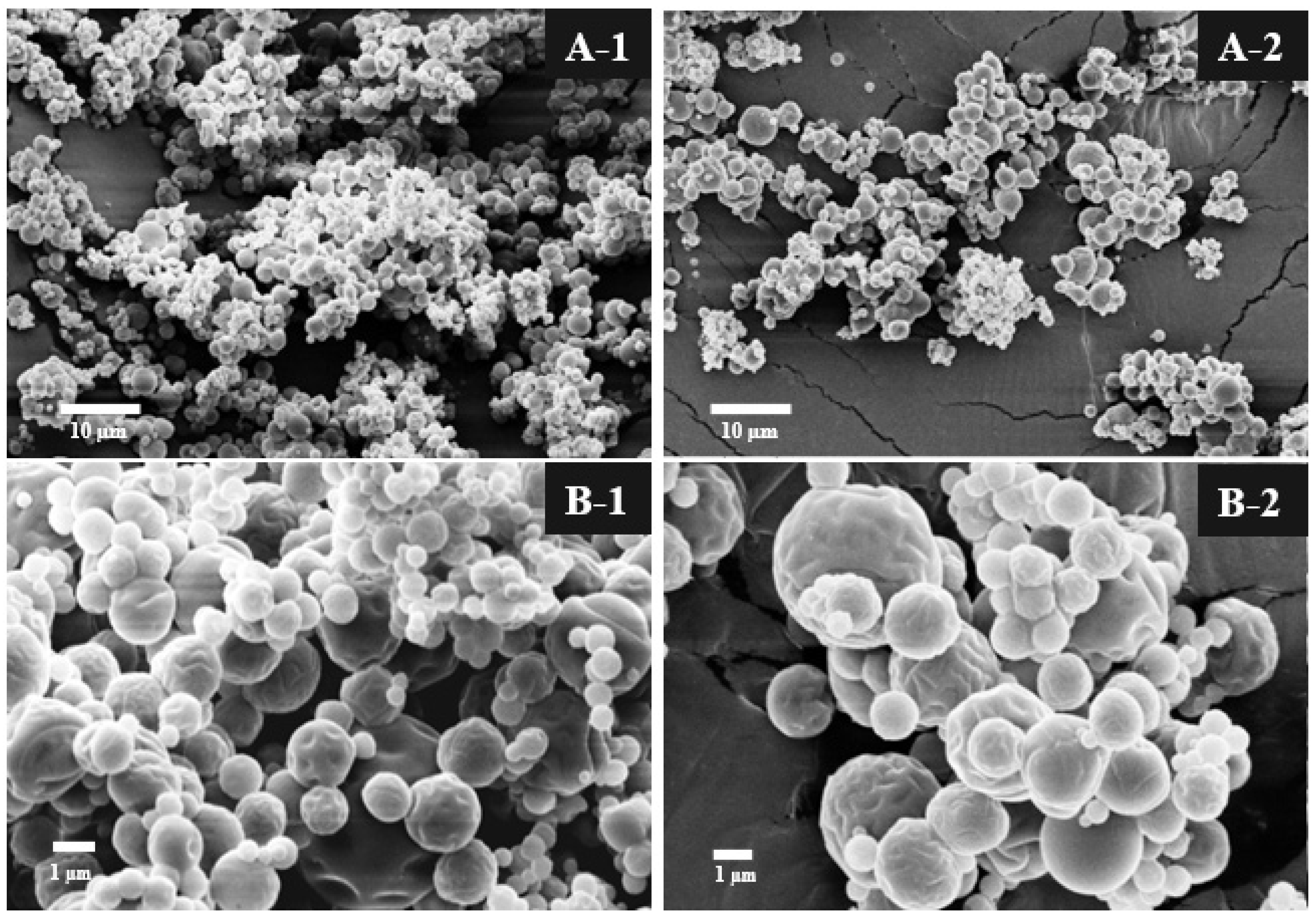
| Properties | OGE * |
|---|---|
| Moisture g/100 g (dry basis) | 3.91% ± 0.11 |
| EE (%) | 45.2% ± 0.4 |
| aw | 0.25 ± 0.02 |
| D4.3 (µm) | 7.32 ± 0.81 |
| Span | 1.25 ± 0.05 |
| D10 (µm) | 3.27 ± 0.04 |
| D50 (µm) | 6.29 ± 0.17 |
| D90 (µm) | 11.11 ± 0.48 |
| Compound Name | OGF | OGE | |||||
|---|---|---|---|---|---|---|---|
| RT | RI | %A | RT | RI | %A | ||
| 1 | Ethylbenzene | 3.88 | 858 | 0.3 | - | - | - |
| 2 | Para- xylene | 4.02 | 867 | 0.1 | - | - | - |
| 3 | α—thujene | 5.16 | 925 | 0.1 | - | - | - |
| 4 | Sabinene | 6.33 | 971 | 0.1 | - | - | - |
| 5 | Myrcene | 6.79 | 989 | 0.1 | - | - | - |
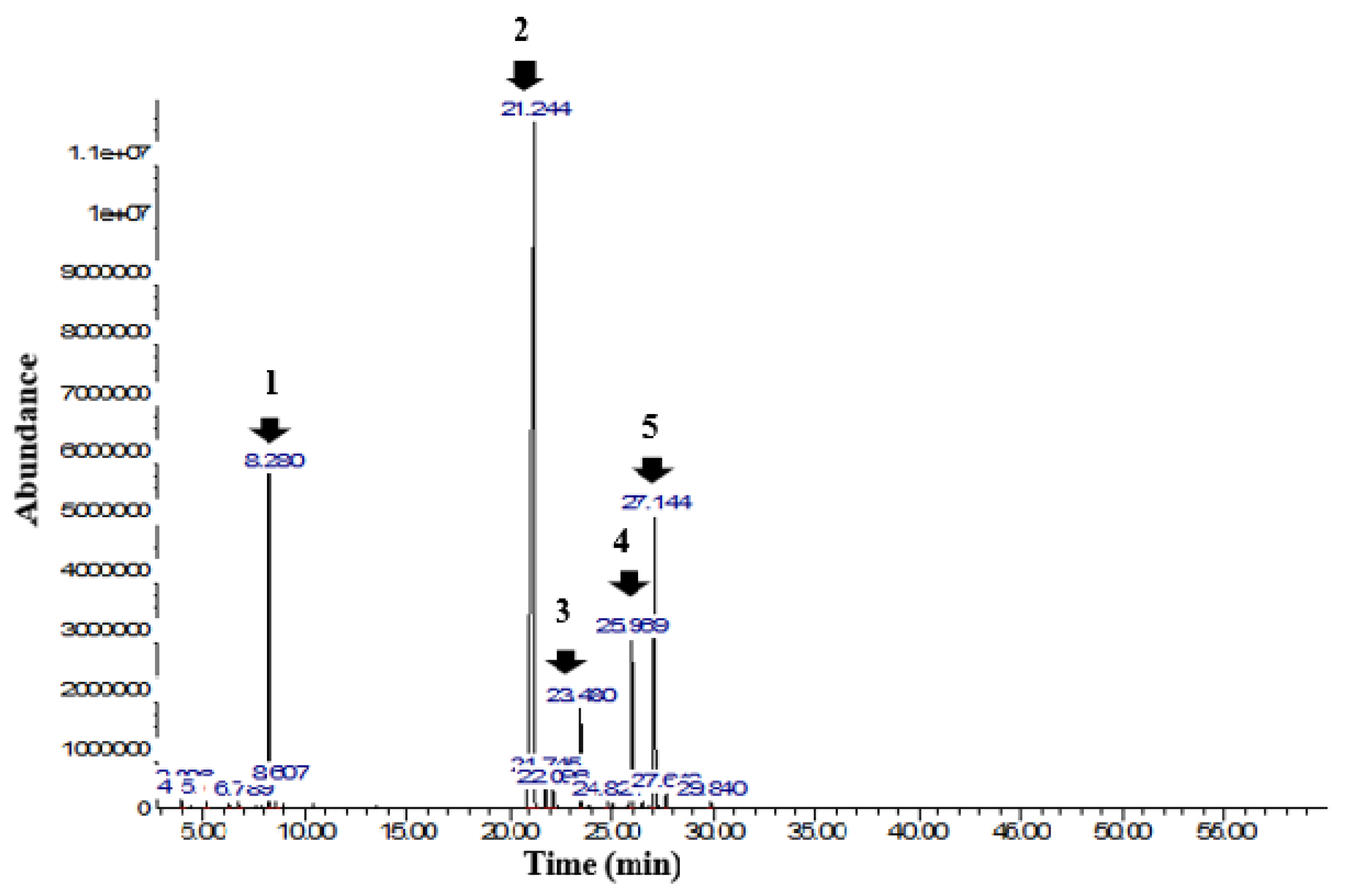
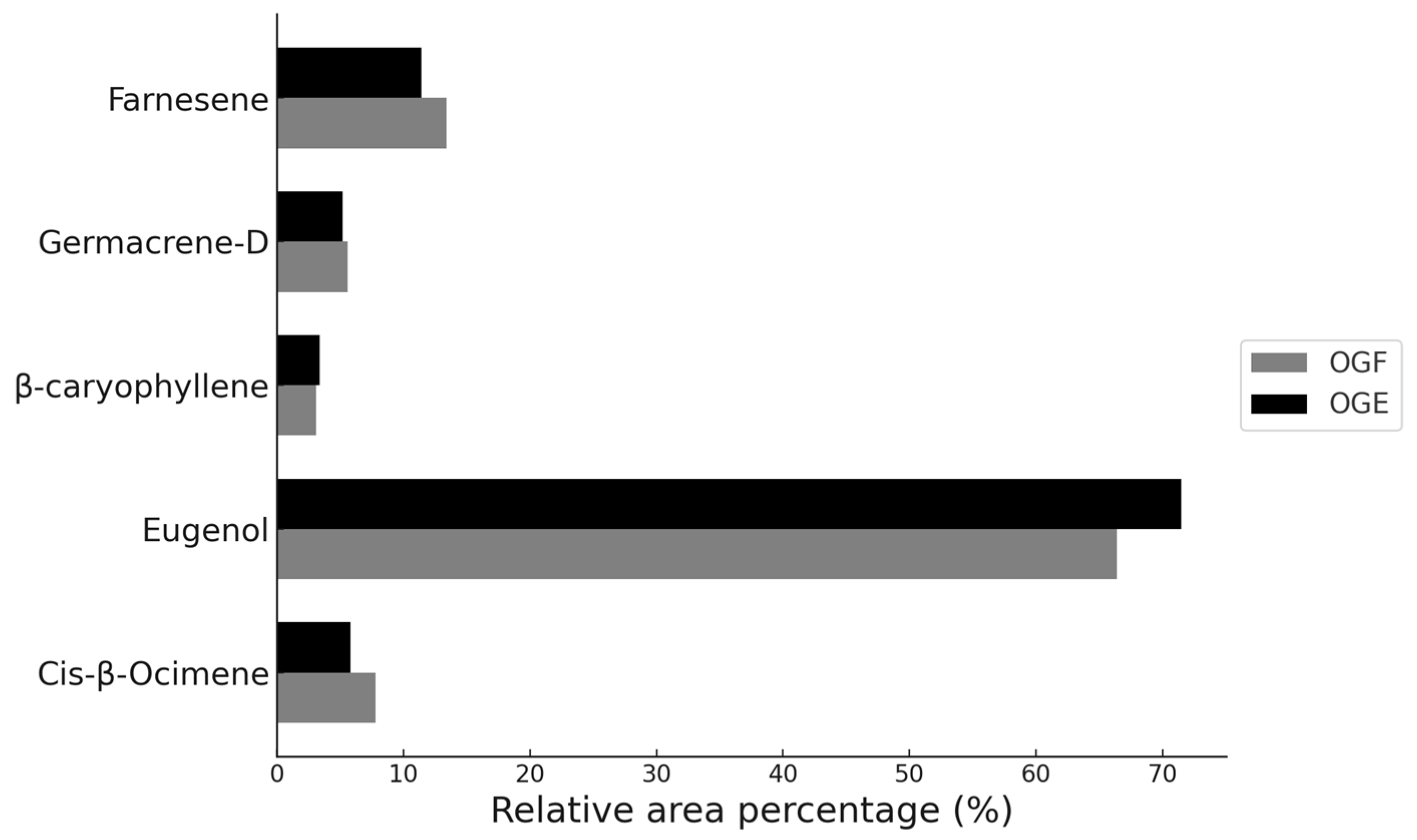
| 6 | Cis-β-Ocimene | 8.28 | 1035 | 7.8 | 8.24 | 1034 | 5.8 |
| 7 | Trans-Ocimene | 8.61 | 1045 | 0.5 | 8.56 | 1043 | 0.4 |
| 8 | Eugenol | 21.25 | 1362 | 66.4 | 21.31 | 1363 | 71.5 |
| 9 | Copaene | 21.74 | 1374 | 0.9 | 21.71 | 1373 | 1 |
| 10 | Bourbonene | 22.09 | 1382 | 0.6 | 22.06 | 1381 | 0.6 |
| 11 | β-caryophyllene | 23.48 | 1416 | 3.1 | 23.45 | 1415 | 3.4 |
| 12 | Humulene | 24.82 | 1450 | 0.2 | - | - | - |
| 13 | Germacrene-D | 25.97 | 1478 | 5.6 | 25.93 | 1477 | 5.2 |
| 14 | Farnesene | 27.15 | 1508 | 13.4 | 27.08 | 1506 | 11.4 |
| 15 | δ—Cadinene | 27.65 | 1521 | 0.5 | 27.6 | 1519 | 0.7 |
| 16 | Caryophyllene oxide | 29.84 | 1578 | 0.3 | - | - | - |
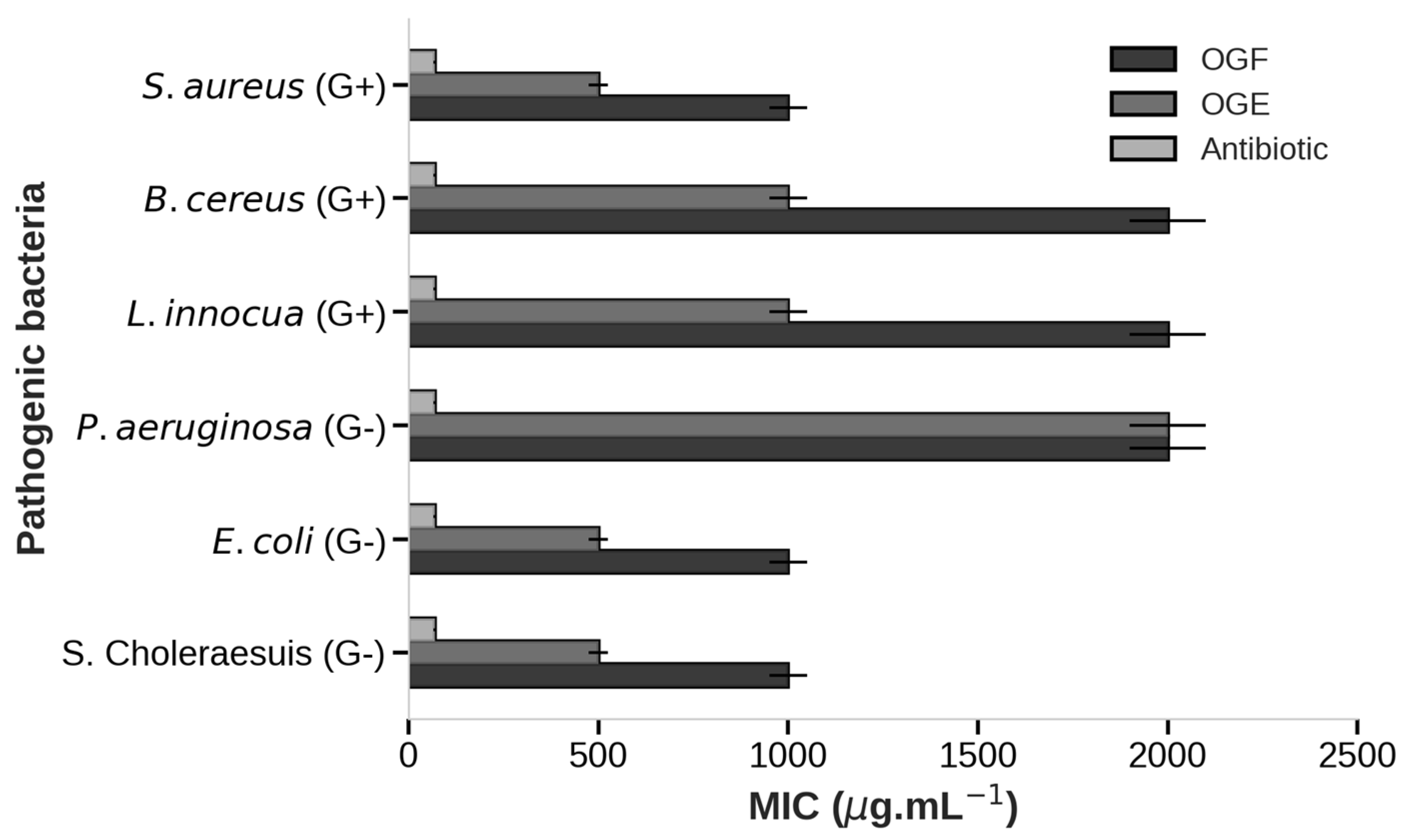
| Pathogenic Bacteria | OGF | OGE |
|---|---|---|
| Salmonella choleraesuis (G−) | >2000 | 1000 |
| Escherichia coli (G−) | 2000 | 1000 |
| Pseudomona aeruginosa (G−) | >2000 | 2000 |
| Listeria innocua (G+) | >2000 | 2000 |
| Bacillus cereus (G+) | >2000 | 2000 |
| Staphylococcus aureus (G+) | 2000 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granados, A.D.P.F.; Duarte, M.C.T.; Noguera, N.H.; Lima, D.C.; Rodrigues, R.A.F. Impact of Microencapsulation on Ocimum gratissimum L. Essential Oil: Antimicrobial, Antioxidant Activities, and Chemical Composition. Foods 2024, 13, 3122. https://doi.org/10.3390/foods13193122
Granados ADPF, Duarte MCT, Noguera NH, Lima DC, Rodrigues RAF. Impact of Microencapsulation on Ocimum gratissimum L. Essential Oil: Antimicrobial, Antioxidant Activities, and Chemical Composition. Foods. 2024; 13(19):3122. https://doi.org/10.3390/foods13193122
Chicago/Turabian StyleGranados, Angela Del Pilar Flores, Marta Cristina Teixeira Duarte, Nathan Hargreaves Noguera, Dyana Carla Lima, and Rodney Alexandre Ferreira Rodrigues. 2024. "Impact of Microencapsulation on Ocimum gratissimum L. Essential Oil: Antimicrobial, Antioxidant Activities, and Chemical Composition" Foods 13, no. 19: 3122. https://doi.org/10.3390/foods13193122








