Heavy Metal Nanoparticle Detection in Human and Formula Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. NP Chemical–Physical Identification
2.3. Statistical Analysis
3. Results
3.1. NP Content Determination in BM
3.2. NP Content Determination in FM
3.3. NP Content and Size: BM vs. FM
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnology 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Jiang, C.; Gao, S.; Liu, X.; Zhai, S. Fetotoxicity of Nanoparticles: Causes and Mechanisms. Nanomaterials 2021, 11, 791–816. [Google Scholar] [CrossRef] [PubMed]
- State of Global Air 2024: A Special Report on Global Exposure to Air Pollution and Its Health Impacts, with a Focus on Children’s Health. Health Effects Institute, Institute for Health Metrics and Evaluation. 2024; 1-35. Available online: https://www.stateofglobalair.org (accessed on 19 June 2024).
- Sun, L.; Zeng, X.; Li, J. Pollutants Release and Control during WEEE Recycling: A Critical Review. Procedia Environ. Sci. 2016, 31, 867–872. [Google Scholar] [CrossRef]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef]
- Ghazi, T.; Naidoo, P.; Naidoo, R.N.; Chuturgoon, A.A. Prenatal Air Pollution Exposure and Placental DNA Methylation Changes: Implications on Fetal Development and Future Disease Susceptibility. Cells 2021, 10, 3025. [Google Scholar] [CrossRef]
- Aysal, H.; Atasoy, N. Determination of Heavy Metal Ions (As, Pb, Cd) and Zinc Mineral (Zn) in Humans and Cows Milk in Bitlis Turkey. Rev. Chim. 2017, 68, 962–966. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, P.; Kaur, R.; Khetarpal, P. Increased Incidence of Spontaneous Abortions on Exposure to Cadmium and Lead: A Systematic Review and Meta-Analysis. Gynecol. Endocrinol. 2022, 38, 16–21. [Google Scholar] [CrossRef]
- Motas, M.; Jiménez, S.; Oliva, J.; Cámara, M.Á.; Pérez-Cárceles, M.D. Heavy Metals and Trace Elements in Human Breast Milk from Industrial/Mining and Agricultural Zones of Southeastern Spain. Int. J. Environ. Res. Public Health 2021, 18, 9289–9304. [Google Scholar] [CrossRef]
- Guideline: Counselling of Women to Improve Breastfeeding Practices; World Health Organization: Geneva, Switzerland, 2018. [PubMed]
- Dipasquale, V.; Serra, G.; Corsello, G.; Romano, C. Standard and Specialized Infant Formulas in Europe: Making, Marketing, and Health Outcomes. Nutr. Clin. Pract. 2020, 35, 273–281. [Google Scholar] [CrossRef]
- Green Corkins, K.; Shurley, T. What’s in the Bottle? A Review of Infant Formulas. Nutr. Clin. Pract. 2016, 31, 723–729. [Google Scholar] [CrossRef]
- Akhtar, S.; Shahzad, M.A.; Yoo, S.H.; Ismail, A.; Hameed, A.; Ismail, T.; Riaz, M. Determination of Aflatoxin M1 and Heavy Metals in Infant Formula Milk Brands Available in Pakistani Markets. Korean J. Food Sci. Anim. Resour. 2017, 37, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Macdonald, C.E.; Johnson, W.S. Colostrum and Milk-Derived Peptide Growth Factors for the Treatment of Gastrointestinal Disorders. Am. J. Clin. Nutr. 2000, 72, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Sager, M.; McCulloch, C.R.; Schoder, D. Heavy Metal Content and Element Analysis of Infant Formula and Milk Powder Samples Purchased on the Tanzanian Market: International Branded versus Black Market Products. Food Chem. 2018, 255, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z. Nanoparticles Induced Embryo–Fetal Toxicity. Toxicol. Ind. Health 2020, 36, 181–213. [Google Scholar] [CrossRef]
- Jie, C.; Zang, X.; Wu, Z.; Liu, J.; Wang, D. Translocation of Transition Metal Oxide Nanoparticles to Breast Milk and Offspring: The Necessity of Bridging Mother-Offspring-Integration Toxicological Assessments. Environ. Int. 2019, 133, 105153. [Google Scholar] [CrossRef]
- Deepthi, S.; Venkatesan, J.; Kim, S.K.; Bumgardner, J.D.; Jayakumar, R. An Overview of Chitin or Chitosan/Nano Ceramic Composite Scaffolds for Bone Tissue Engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353. [Google Scholar] [CrossRef]
- Illuminato, I.; Friends of the Earth. Nanoparticles in Baby Formula: Tiny New Ingredients Are a Big Concern; Friends of the Earth: London, UK, 2016; pp. 1–31. [Google Scholar]
- Sonwani, S.; Madaan, S.; Arora, J.; Suryanarayan, S.; Rangra, D.; Mongia, N.; Vats, T.; Saxena, P. Inhalation Exposure to Atmospheric Nanoparticles and Its Associated Impacts on Human Health. Front. Sustain. Cities 2021, 3, 690444. [Google Scholar] [CrossRef]
- W.H.O. Household Air Pollution; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Brender, J.D.; Suarez, L.; Langlois, P.H.; Steck, M.; Zhan, F.B.; Moody, K. Are Maternal Occupation and Residential Proximity to Industrial Sources of Pollution Related? J. Occup. Environ. Med. 2008, 50, 834–839. [Google Scholar] [CrossRef]
- Enax, J.; Meyer, F.; Wiesche, E.; Epple, M. On the Application of Calcium Phosphate Micro- and Nanoparticles as Food Additive. Nanomaterials 2022, 12, 4075–4086. [Google Scholar] [CrossRef]
- Li, B.; Chua, S.L.; Yu, D.; Chan, S.H.; Li, A.D. Identification and Size Distribution of Silver Nanoparticles (AgNPs. Milk Migr. Study Breast Milk Storage Bags. Molecules 2022, 27, 2539–2560. [Google Scholar] [CrossRef]
- Montanari, S.; Gatti, A.M. Nanopathology: The Health Impact of Nanoparticles; Jenny Stanford Publishing: New York, NY, USA, 2011; 312p. [Google Scholar] [CrossRef]
- Schoepf, J.J.; Bi, Y.; Kidd, J.; Herckes, P.; Hristovski, K.; Westerhoff, P. Detection and Dissolution of Needle-like Hydroxyapatite Nanomaterials in Infant Formula. NanoImpact 2017, 5, 22–28. [Google Scholar] [CrossRef]
- Gatti, A.M.; Ristic, M.; Stanzani, S.; Lavezzi, A.M. Novel Chemical-Physical Autopsy Investigation in Sudden Infant Death and Sudden Intrauterine Unexplained Death Syndromes. Nanomedicine 2022, 17, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Pajewska-Szmyt, M.; Sinkiewicz-Darol, E.; Gadzała-Kopciuch, R. The Impact of Environmental Pollution on the Quality of Mother’s Milk. Env. Sci. Pollut. Res. Int. 2019, 26, 7405–7427. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency; World Health Organization. Minor and Trace Elements in Breast Milk: Report of a Joint WHO/IAEA Collaborative Study; World Health Organization: Geneva, Switzerland, 1989. [Google Scholar]
- Gatti, A.M.; Ballestri, M.; Bagni, A. Granulomatosis associated to porcelain wear debris. Am. J. Dent. 2002, 15, 369–372. [Google Scholar] [PubMed]
- Gatti, A.M.; Bosco, P.; Rivasi, F.; Bianca, S.; Ettore, G.; Gaetti, L.; Montanari, S.; Bartoloni, G.; Gazzolo, D. Heavy Metals Nanoparticles in Fetal Kidney and Liver Tissues. Front. Biosci. Elite Ed. 2011, 3, 221–226. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Y.; Sun, S.; Tian, T.; Zhu, M.; Ahmad, Z.; Yang, J.; Jin, J.; Zhang, H.; Chen, J.; et al. Accumulation Characteristics of Metals in Human Breast Milk and Association with Dietary Intake in Northeastern China. Sci. Total Environ. 2024, 912, 168515. [Google Scholar] [CrossRef]
- Gatti, A.M.; Montanari, S.N. The Nano-Bio-Interaction of Nanoparticles Inside the Human Body. In Nanoparticles’ Promises and Risks; Springer International Publishing: Cham, Switzerland, 2014; Volume Part II-5, pp. 70–85. [Google Scholar]
- Szukalska, M.; Merritt, T.A.; Lorenc, W.; Sroczyńska, K.; Miechowicz, I.; Komorowicz, I.; Mazela, J.; Barałkiewicz, D.; Florek, E. Toxic Metals in Human Milk in Relation to Tobacco Smoke Exposure. Environ. Res. 2021, 197, 111090. [Google Scholar] [CrossRef]
- Peters, K.; Unger, R.E.; Gatti, A.M.; Sabbioni, E.; Tsaryk, R.; Kirkpatrick, C.J. Metallic Nanoparticles Exhibit Paradoxical Effects on Oxidative Stress and Pro-Inflammatory Response in Endothelial Cells in Vitro. Int. J. Immunopathol. Pharmacol. 2007, 20, 685–695. [Google Scholar] [CrossRef]
- Lucarelli, M.; Monari, E.; Gatti, A.M.; Boraschi, D. Modulation of Defence Cell Function by Nanoparticles in Vitro. Key Eng. Mater. 2004, 254–256, 907–910. [Google Scholar]
- Georgieff, M.K.; Krebs, N.F.; Cusick, S.E. The Benefits and Risks of Iron Supplementation in Pregnancy and Childhood. Annu. Rev. Nutr. 2019, 39, 121–146. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Nagaev, E.I.; Matveyeva, T.A.; Binhi, V.N.; Burmistrov, D.E.; Serov, D.A.; Astashev, M.E.; Simakin, A.V.; Uvarov, O.V.; Khabatova, V.V.; et al. Investigation of Aggregation and Disaggregation of Self-Assembling Nano-Sized Clusters Consisting of Individual Iron Oxide Nanoparticles upon Interaction with HEWL Protein Molecules. Nanomaterials 2022, 12, 3960–3985. [Google Scholar] [CrossRef]
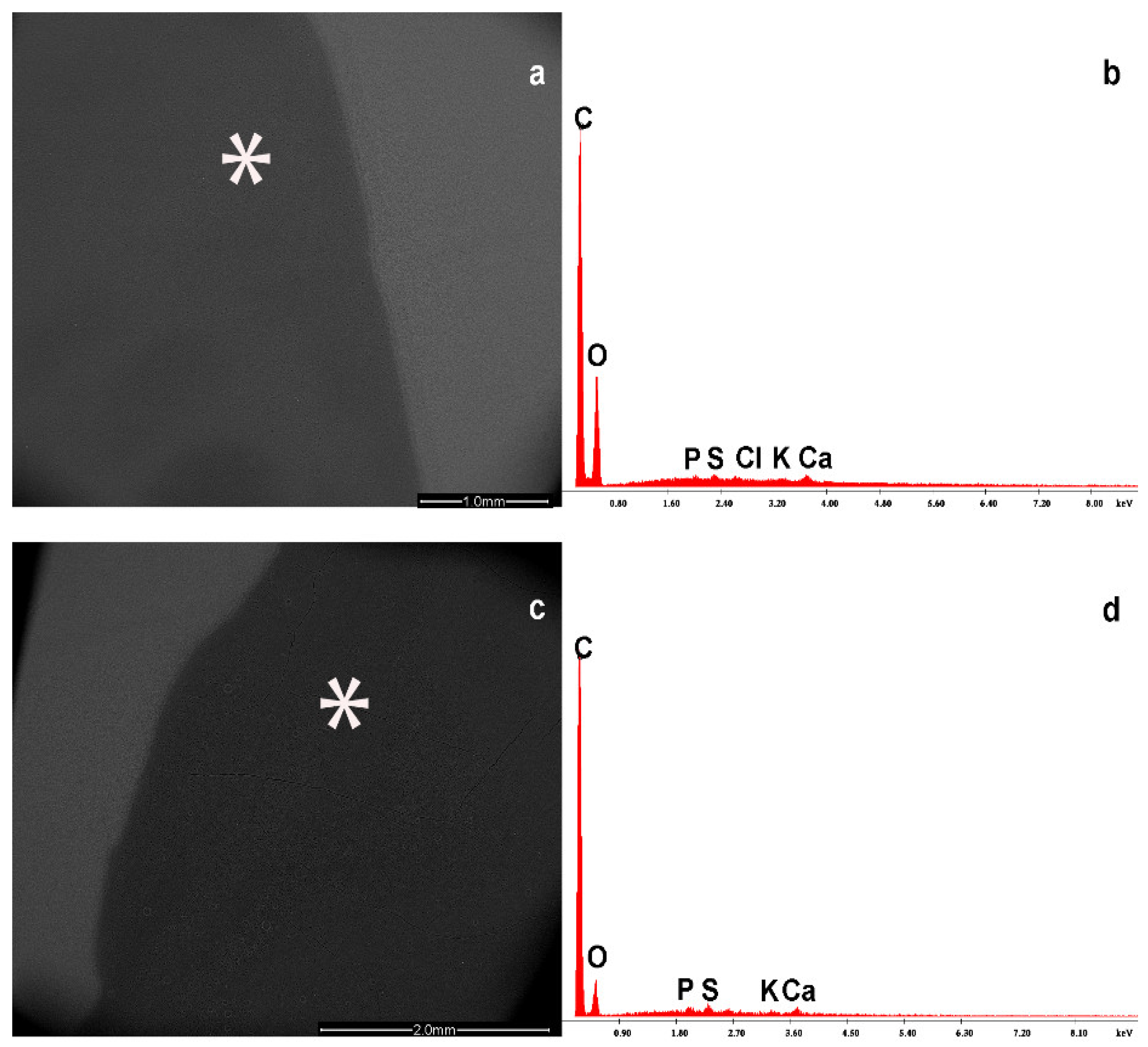
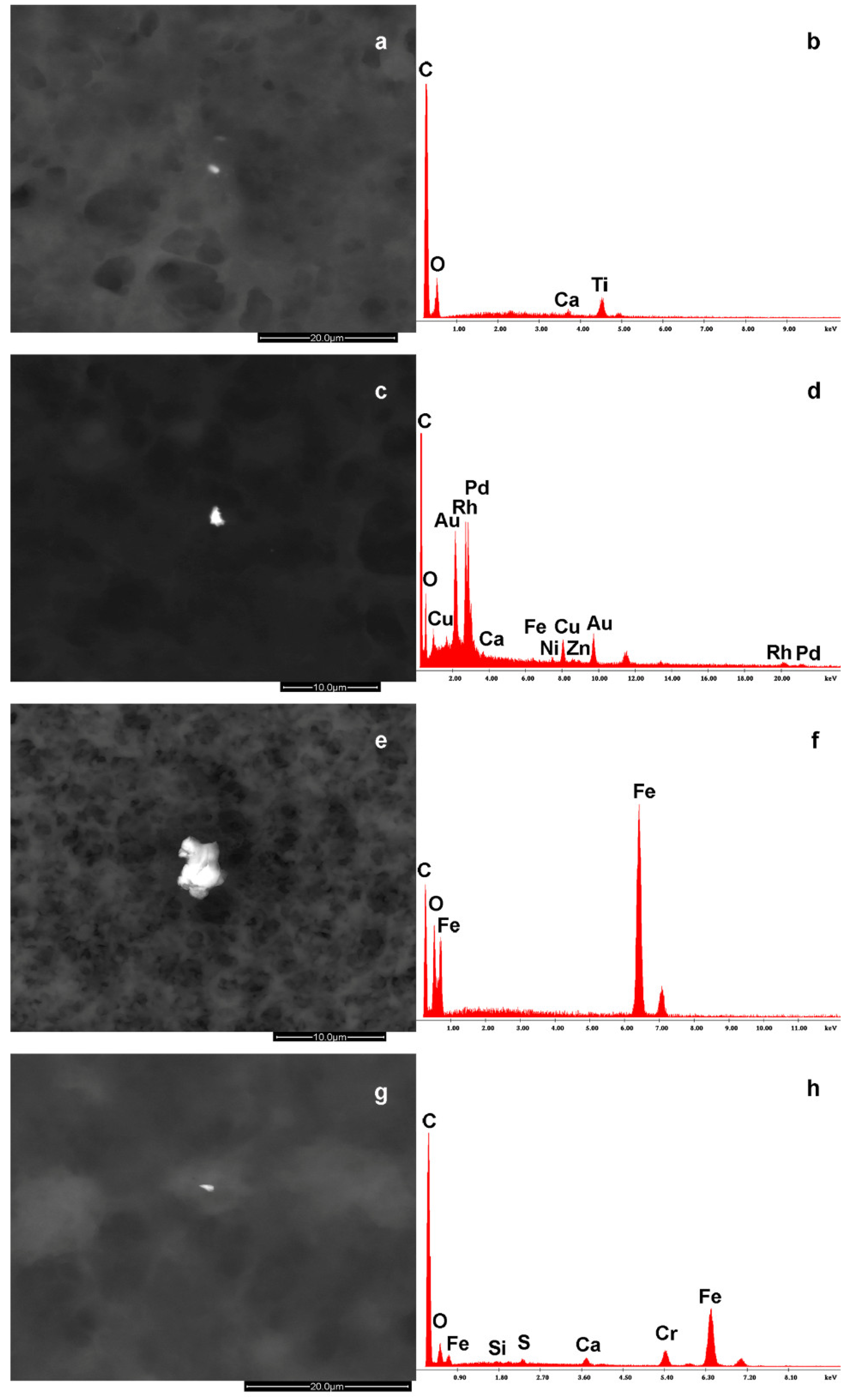
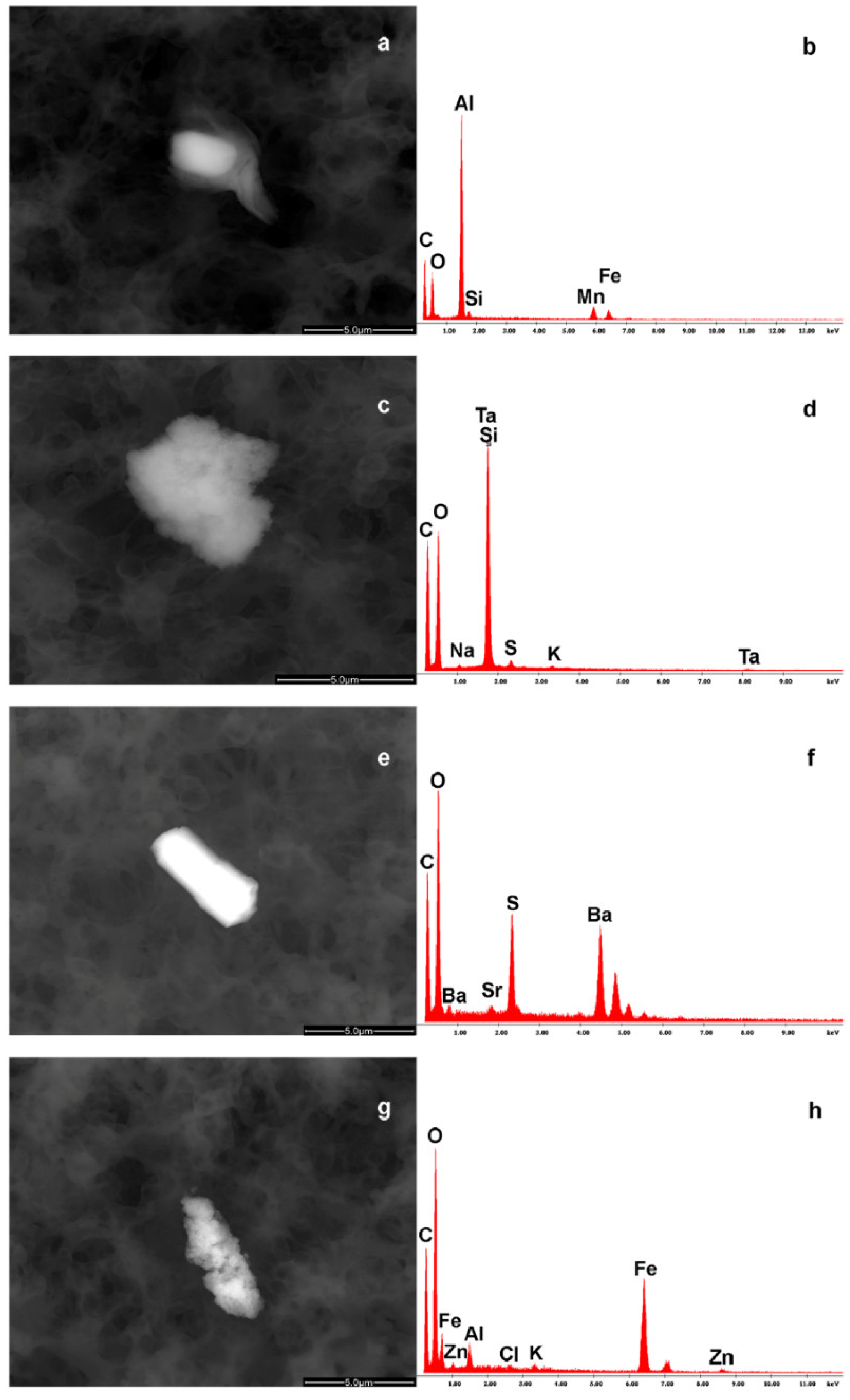
| Parameters | NP (n = 19) |
|---|---|
| Mother Characteristics | |
| Maternal age, (y) | 34 ± 3 |
| Weight, (Kg) | 63 ± 3 |
| Height, (cm) | 166 ± 5 |
| Exclusive breastfeeding, n/total | 19/19 |
| Newborn Characteristics | |
| GA, (wks) | 37 ± 3 |
| BW, (g) | 2895 ± 188 |
| Gender, (M/F) | 9/10 |
| Delivery mode | |
| Caesarean section, (n/total) | 0/19 |
| Vaginal, (n/total) | 19/19 |
| Apgar score > 7 | |
| at 1 min, n/total | 19/19 |
| at 5 min, n/total | 19/19 |
| Particle Composition | Breast Milk | Formula Milk |
|---|---|---|
| Basic milk composition | ||
| C | + | + |
| O | + | + |
| Ca | + | + |
| K | + | + |
| Cl | + | + |
| P | + | + |
| Mg | + | − |
| Na | + | + |
| Particle composition | ||
| Ba | + | + |
| S | + | + |
| Ti | + | + |
| Zn | + | + |
| Ta | − | + |
| Rh | + | − |
| Pd | + | − |
| Au | + | − |
| Cu | + | − |
| Ni | + | − |
| Fe | + | + |
| Cr | + | − |
| Si | + | + |
| Al | + | + |
| Mn | + | − |
| Sr | − | + |
| Breast Milk | Particle Size |
|---|---|
| BaS, COSBaCa, COTiCa, COSCaKClP, COZnTi,CRhPdAuOCuCaNiFeZn | 1–2 μm |
| FeCO, COClSK | 2–5 μm |
| COCaPSCl, CFeOCrCaSSi, CSiOCaS | 3–6 μm |
| COCaClK, CFeOTiCaAlMg | 4 μm |
| COCaS, COSiAlKCaSMgNa | 2 μm |
| Formula Milk | |
| COCaPSK | 0.5 μm |
| COCaPS, AlCOMnFeSi, CCaOPSK | 3–5 μm |
| COCaSPK, CCaOSPKCl, COTiCaPSK | 3–5 μm |
| COCaKPS, SiOCSTaKNa, CFeO | 3–7 μm |
| COCaKSP, OCSBaSr | 6 μm |
| COCaPSK, OCFeAlZnKCl | 4–7 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, A.M.; D’Adamo, E.; Botondi, V.; Montanari, S.; Colletti, E.; Gagliardi, L.; Ciotti, S.; Abdelhameed, A.S.; Gazzolo, F.; Maconi, A.; et al. Heavy Metal Nanoparticle Detection in Human and Formula Milk. Foods 2024, 13, 3178. https://doi.org/10.3390/foods13193178
Gatti AM, D’Adamo E, Botondi V, Montanari S, Colletti E, Gagliardi L, Ciotti S, Abdelhameed AS, Gazzolo F, Maconi A, et al. Heavy Metal Nanoparticle Detection in Human and Formula Milk. Foods. 2024; 13(19):3178. https://doi.org/10.3390/foods13193178
Chicago/Turabian StyleGatti, Antonietta Morena, Ebe D’Adamo, Valentina Botondi, Stefano Montanari, Erika Colletti, Luigi Gagliardi, Sabina Ciotti, Ali Saber Abdelhameed, Francesca Gazzolo, Antonio Maconi, and et al. 2024. "Heavy Metal Nanoparticle Detection in Human and Formula Milk" Foods 13, no. 19: 3178. https://doi.org/10.3390/foods13193178







