HPLC-MS/MS Phenolic Characterization of Olive Pomace Extracts Obtained Using an Innovative Mechanical Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Dry Matter Determination
2.3. Determination of Yield (YF) and Technological Retention Factor (TRF)
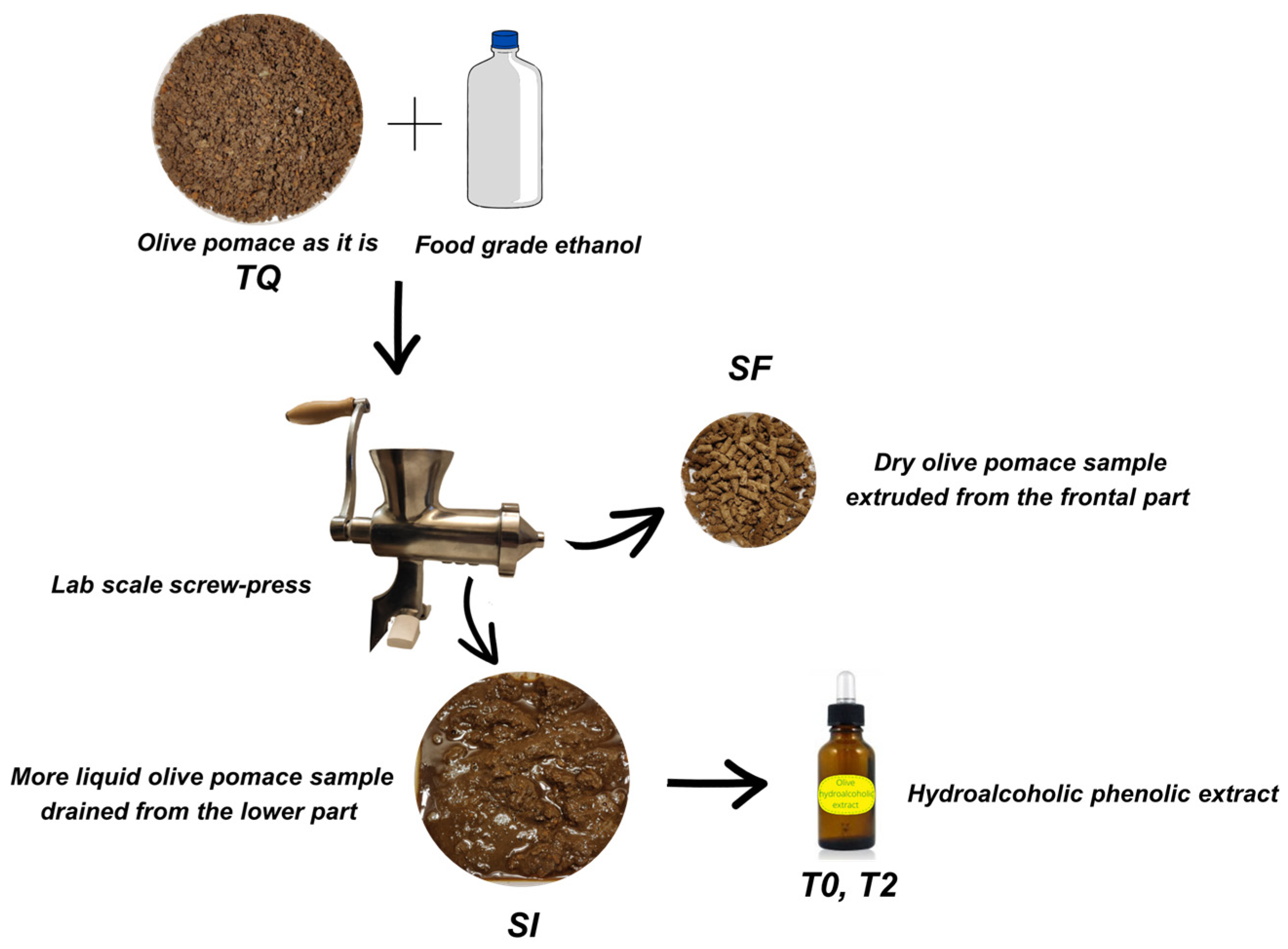
2.4. Phenolic Extraction Procedure
2.5. Determination of the Total Reducing Molecules by the Folin–Ciocâlteu Method
2.6. Phenolic Identification and Quantification by High-Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC-MS/MS)
2.7. Shelf-Life Study of the Hydroalcoholic Extract
2.8. Data Analysis
3. Results and Discussion
3.1. Moisture and Mass Yields
3.2. Characterization of Olive Pomace Fractions
3.2.1. Total Reducing Molecules of Olive Pomace Fractions
3.2.2. Identification and Quantification of the Phenolic by High-Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC-MS/MS)
3.3. Characterization and Shelf-Life of the Hydroalcoholic Extract
3.3.1. Total Reducing Molecules
3.3.2. Phenolic Quantification by High-Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC-MS/MS)
3.3.3. Sensory Analysis
3.4. Technological Retention Factor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ducom, G.; Gautier, M.; Pietraccini, M.; Tagutchou, J.P.; Lebouil, D.; Gourdon, R. Comparative analyses of three olive mill solid residues from different countries and processes for energy recovery by gasification. Renew. Energ. 2020, 145, 180–189. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Research and Innovation. A Sustainable Bioeconomy for Europe—Strengthening the Connection between Economy, Society and the Environment—Updated Bioeconomy Strategy, Publications Office. 2018. Available online: https://data.europa.eu/doi/10.2777/792130 (accessed on 5 October 2023).
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods-A review. J. Sci. Food Agric. 2021, 101, 1. [Google Scholar] [CrossRef]
- Türkekul, B.; Günden, C.; Abay, C.; Miran, B. Competitiveness of Mediterranean countries in the olive oil market. New Medit. 2010, 9, 41–46. [Google Scholar]
- European Commission. Market Situation in the Olive Oil and Table Olives Sectors. Committee for the Common Organisation of the Agricultural Markets—Arable Crops and Olive Oil; European Commission: Brussels, Belgium, 2023; Available online: https://agriculture.ec.europa.eu/system/files/2023-05/market-situation-olive-oil-table-olives_en.pdf (accessed on 2 December 2023).
- Chandra, M.; Sathiavelu, S. Waste management in the olive oil industry in the Mediterranean region by composting. Clean. Techn. Environ. Policy. 2009, 11, 293–298. [Google Scholar] [CrossRef]
- Espadas-Aldana, G.; Vialle, C.; Belaud, J.P.; Vaca-Garcia, C.; Sablayrolles, C. Analysis and trends for Life Cycle Assessment of olive oil production. Sustain. Prod. Consum. 2019, 19, 216–230. [Google Scholar] [CrossRef]
- Argun, M.; Arslan, F.N.; Ates, H.; Yel, E.; Çakmakcı, Ö.; Dağ, B. A pioneering study on the recovery of valuable functional compounds from olive pomace by using supercritical carbon dioxide extraction: Comparison of perlite addition and drying. Sep. Purif. Technol. 2023, 306 Pt A, 122593. [Google Scholar] [CrossRef]
- Sicari, V.; Custureri, I.M.G.; Tundis, R.; Loizzo, M.R. Comparison of Physicochemical Characteristics and Bioactivity of Olive Oil Mill Wastewaters from Traditional and Water-Saving ARA-Controlled Three-Phase Decanter. Sustainability 2023, 15, 3890. [Google Scholar] [CrossRef]
- Stempfle, S.; Roselli, L.; Carlucci, D.; Leone, A.; De Gennaro, B.C.; Giannoccaro, G. Toward the circular economy into the olive oil supply chain: A case study analysis of a vertically integrated firm. Front. Sustain. Food Syst. 2022, 6, 1005604. [Google Scholar] [CrossRef]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef] [PubMed]
- Khdai, A.; Abu-Rumman, G. Sustainable Environmental Management and Valorization Options for Olive Mill Byproducts in the Middle East and North Africa (MENA) Region. Processes 2020, 8, 671. [Google Scholar] [CrossRef]
- Stramarkou, M.; Missirli, T.V.; Kyriakopoulou, K.; Papadaki, S.; Angelis-Dimakis, A.; Krokida, M. The Recovery of Bioactive Compounds from Olive Pomace Using Green Extraction Processes. Resources 2023, 12, 77. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pawlowski, S.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P.; Velizarov, S. Valorization of olive pomace by a green integrated approach applying sustainable extraction and membrane-assisted concentration. Sci. Total Environ. 2019, 652, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Zhao, H.; Avena-Bustillos, R.; Wang, S. Extraction, Purification, and in vitro antioxidant activity evaluation of phenolic compounds in California Olive Pomace. Foods 2022, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef] [PubMed]
- Vitali Čepo, D.; Radić, K.; Jurmanović, S.; Jug, M.; Grdić Rajković, M.; Pedisić, S.; Moslavac, T.; Albahari, P. Valorization of Olive Pomace-Based Nutraceuticals as Antioxidants in Chemical, Food, and Biological Models. Molecules 2018, 23, 2070. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Guyot, S.; Marnet, N.; Lopes-da-Silva, J.A.; Renard, C.M.G.C.; Coimbra, M.A. Characterisation of phenolic extracts from olive pulp and olive pomace by electrospray mass spectrometry. J. Sci. Food Agric. 2005, 85, 21–23. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Chemical screening of olive biophenol extracts by hyphenated liquid chromatography. Anal. Chim. Acta 2007, 603, 176–189. [Google Scholar] [CrossRef]
- Malapert, A.; Reboul, E.; Loonis, M.; Dangles, O.; Tomao, V. Direct and Rapid Profiling of Biophenols in Olive Pomace by UHPLC-DAD-MS. Food Anal. Methods 2018, 11, 1001–1010. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive Mill and Winery Wastes as Viable Sources of Bioactive Compounds: A Study on Polyphenols Recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Phenolic extract obtained from steam-treated olive oil waste: Characterization and antioxidant activity. LWT Food Sci. Technol. 2013, 54, 114–124. [Google Scholar] [CrossRef]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, Isoverbascoside, and Their Derivatives Recovered from Olive Mill Wastewater as Possible Food Antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef]
- Romero, C.; García, P.; Brenes, M.; Aranzazu, A.; Garrido, A. Phenolic compounds in natural black Spanish olive varieties. Eur. Food Res. Technol. 2022, 215, 489–496. [Google Scholar] [CrossRef]
- D’Antuono, I.; Kontogianni, V.G.; Kotsiou, K.; Linsalata, V.; Logrieco, A.F.; Tasioula-Margari, M.; Cardinali, A. Polyphenolic characterization of olive mill wastewaters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res. Int. 2014, 65, 301–310. [Google Scholar] [CrossRef]
- Lozano-Sánchez, J.; Castro-Puyana, M.; Mendiola, J.; Segura-Carretero, A.; Cifuentes, A.; Ibáez, E. Recovering bioactive compounds from olive oil filter cake by advanced extraction techniques. Int. J. Mol. Sci. 2014, 15, 16270–16283. [Google Scholar] [CrossRef] [PubMed]
- Cea Pavez, I.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an Extract Rich in Phenolic Compounds from Olive Pomace by Pressurized Liquid Extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Castejón, M.L.; Rubio-Senent, F.; Fernández-Prior, Á.; Rodríguez-Gutiérrez, G.; Fernández-Bolaños, J. Isolation and structural determination of cis- and trans-p-coumaroyl secologanoside (comselogoside) from olive oil waste (alperujo). Photoisomerization with ultraviolet irradiation and antioxidant activities. Food Chem. 2024, 432, 137233. [Google Scholar] [CrossRef]
- Cizmarova, B.; Hubkova, B.; Bolerazska, B.; Marekova, M.; Birkova, A. Caffeic acid: A brief overview of its presence, metabolism, and bioactivity. Bioact. Compd. Health Dis. 2020, 3, 74. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2022, 19, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Papoti, V.T.; Exarchou, V.; Tsimidou, M.Z.; Gerothanassis, I.P. Contribution of flavonoids to the overall radical scavenging activity of olive (Olea europaea L.) leaf polar extracts. J. Agric. Food Chem. 2010, 58, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.; Rahaman, A.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef]
- Fangxue, X.; Yujuan, L.; Mengmeng, Z.; Xiaozhi, X.; Xuelan, Z.; Chuncha, H. Structure Properties, Acquisition Protocols, and Biological Activities of Oleuropein Aglycone. Front. Chem. 2018, 6, 239. [Google Scholar] [CrossRef]
- Salamanca, A.; Almodóvar, P.; Jarama, I.; González-Hedström, D.; Prodanov, M.; Inarejos-García, A.M. Anti-influenza virus activity of the elenolic acid rich olive leaf (Olea europaea L.) extract Isenolic®. Antivir. Chem. Chemother. 2021, 29, 20402066211063391. [Google Scholar] [CrossRef]



| Sample | Dry Matter (%) | Wet Matter (w.m,g) | Wet Matter Yield | Dry Matter (d.m.,g) | Dry Matter Yield |
|---|---|---|---|---|---|
| TQ | 53.07 | 150.00 | 1.00 | 79.60 | 1.00 |
| SI | 36.53 | 108.32 | 0.72 | 39.57 | 0.50 |
| SF | 59.03 | 24.61 | 0.16 | 14.53 | 0.18 |
| Sample | Reducing Substance Content (mg Gallic Acid kg Olive Pomace−1 d.m.) | SD |
|---|---|---|
| TQ | 6750.6 b | 1126.5 |
| SI | 14741.5 a | 1356.5 |
| SF | 4164.2 c | 96.15 |
| Compound Name | Tag | Chem.Family | RT (min) | Precurson Ion [M-1] | Product Ion | Fragmentor Voltage | CE | Other Transitions | SI | SF | T0 | T2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quinic acid | ph1 | org-ac | 0.74 | 191 | 127 | 150 | 20 | 173 | x | x | x | x |
| Dihydroxyphenylglycol | ph2 | phen | 1.06 | 169 | 151 | 60 | 10 | x | x | x | x | |
| Verbascoside-rha isomer 1 | ph3 | hydr-ac | 1.18 | 477 | 161 | 100 | 10 | x | x | |||
| Hydroxylated DCMEA derivative | ph4 | irid | 1.86 | 199 | 111 | 60 | 10 | 155 | x | x | x | x |
| Vanillic acid hexoside | ph5 | org-ac | 2.05 | 329 | 167 | 150 | 10 | 123, 108 | x | x | x | x |
| Hydroxytyrosol glucoside isomer 1 | ph6 | phen | 2.11 | 315 | 153 | 150 | 10 | 123 | x | x | x | x |
| Verbascoside-rha isomer 2 | ph7 | hydr-ac | 2.18 | 477 | 161 | 100 | 10 | x | x | x | x | |
| Hydroxytyrosol glucoside isomer 2 | ph8 | phen | 2.25 | 315 | 153 | 150 | 10 | 123 | x | x | x | x |
| Hydroxytyrosol | ph9 | phen | 2.32 | 153 | 123 | 100 | 10 | x | x | x | x | |
| p-Coumaroyl aldarate | ph10 | hydr-ac | 2.39 | 355 | 209 | 100 | 10 | 191, 147, 129 | x | x | ||
| 1-Beta-glucosyl-acyclodihydroelenolic acid | ph11 | irid | 2.55 | 407 | 313 | 150 | 10 | 389, 375, 357, 161 | x | x | x | x |
| Caffeoyl-hexoside | ph12 | hydr-ac | 3.62 | 341 | 179 | 150 | 10 | 135 | x | x | x | x |
| Chlorogenic acid | ph13 | hydr-ac | 3.77 | 353 | 191 | 100 | 10 | x | x | x | x | |
| p-Cumaroyl-hexoside | ph14 | hydr-ac | 3.86 | 325 | 119 | 100 | 30 | 163 | x | x | x | x |
| Oleoside | ph15 | irid | 3.90 | 389 | 345 | 150 | 10 | 209 | x | x | x | x |
| 3,4-DHPEA-DEDA | ph16 | irid | 4.00 | 319 | 195 | 100 | 30 | x | x | |||
| Caffeic acid | ph17 | hydr-ac | 4.36 | 179 | 135 | 100 | 10 | x | x | x | x | |
| Oleoside deoxyriboside | ph18 | irid | 4.91 | 505 | 389 | 150 | 10 | 345, 121 | x | x | x | x |
| Oleuropein aglycone | ph19 | irid | 5.22 | 377 | 197 | 100 | 10 | 153 | x | x | x | x |
| Loganic acid glucoside | ph20 | irid | 5.36 | 537 | 375 | 60 | 10 | 179 | x | x | x | x |
| 4-Hydroxyphenylacetic acid | ph21 | org-ac | 5.74 | 151 | 108 | 60 | 20 | x | x | x | x | |
| p-Coumaric acid | ph22 | hydr-ac | 6.11 | 163 | 119 | 100 | 10 | x | x | x | x | |
| Hydroxyverbascoside | ph23 | hydr-ac | 6.14 | 639 | 621 | 150 | 20 | 529, 459 | x | x | x | x |
| 4-HPEA-DEDA | ph24 | irid | 7.30 | 303 | 285 | 100 | 10 | 179 | x | x | ||
| Rutin | ph25 | flav | 7.39 | 609 | 301 | 100 | 30 | 179 | x | x | x | x |
| Luteolin-O-rutinoside isomer 1 | ph26 | flav | 7.59 | 593 | 285 | 150 | 30 | x | x | x | x | |
| Luteolin-glucoside isomer 1 | ph27 | flav | 7.83 | 447 | 285 | 150 | 20 | x | x | x | x | |
| Verbascoside | ph28 | hydr-ac | 7.98 | 623 | 161 | 100 | 30 | 461 | x | x | x | x |
| Luteolin-O-rutinoside isomer 2 | ph29 | flav | 7.98 | 593 | 285 | 150 | 30 | 447 | x | x | x | x |
| Elenolic acid | ph30 | irid | 8.43 | 241 | 139 | 60 | 10 | 165, 127, 121, 101 | x | x | x | x |
| Luteolin-O-rutinoside isomer 3 | ph31 | flav | 8.46 | 593 | 285 | 150 | 30 | x | x | x | x | |
| Nüzhenide | ph32 | irid | 8.62 | 685 | 523 | 100 | 10 | 453, 421, 403, 299 | x | x | x | x |
| Luteolin-glucoside isomer 2 | ph33 | flav | 9.10 | 447 | 285 | 150 | 20 | x | x | x | x | |
| Caffeoyl-6-secologanoside | ph34 | hydr-ac | 9.20 | 551 | 161 | 100 | 30 | 507, 389, 341, 281, 252, 221, 179 | x | x | x | x |
| Luteolin-glucoside isomer 3 | ph35 | flav | 9.81 | 447 | 285 | 150 | 20 | x | x | x | x | |
| 10-Hydroxy-DCMO aglycone | ph36 | irid | 9.91 | 335 | 199 | 100 | 10 | 155, 111 | x | x | x | |
| Oleuroside | ph37 | irid | 10.22 | 539 | 275 | 150 | 20 | 469, 377, 307 | x | x | x | x |
| Comselogoside | ph38 | hydr-ac | 10.40 | 535 | 145 | 150 | 20 | 491, 389, 345, 307, 265, 163 | x | x | x | x |
| Ligstroside derivative | ph39 | irid | 10.89 | 655 | 291 | 150 | 20 | 361, 259 | x | x | x | x |
| Ligstroside aglicone | ph40 | irid | 11.25 | 361 | 137 | 100 | 10 | x | x | |||
| Luteolin | ph41 | flav | 11.84 | 285 | 133 | 150 | 30 | 151 | x | x | x | x |
| Apigenin | ph42 | flav | 13.76 | 269 | 227 | 150 | 20 | 161 | x | x | x | x |
| Analyte | LOD (mg L−1) | LOQ (mg L−1) | Concentration Range (mg L−1) |
|---|---|---|---|
| Verbascoside | 0.01 | 0.04 | 0.10–22.30 |
| Rutin | 0.01 | 0.04 | 0.10–24.80 |
| Oleuropein A | 0.01 | 0.03 | 0.10–99.00 |
| 99.00–831.90 | |||
| Luteolin-7-glucoside | 0.003 | 0.01 | 0.10–24.60 |
| Luteolin | 0.001 | 0.002 | 0.10–24.60 |
| Apigenin | 0.01 | 0.02 | 0.10–4.80 |
| Quinic acid | 0.02 | 0.08 | 0.25–101.00 |
| Caffeic acid B | 0.02 | 0.07 | 0.10–5.00 |
| 0.10–25.20 | |||
| Hydroxytyrosol | 0.02 | 0.06 | 0.10–24.70 |
| Samples Average Concentrations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound Name | Tag | TQ (mg/kg d.m.) A | SD | SI (mg/kg d.m.) | SD | SF (mg/kg d.m.) | SD | T0 (mg/L) | SD | T2 (mg/L) | SD |
| Quinic acid | ph1 | 130.78 | 9.62 | 121.92 | 5.64 | 172.16 | 5.4 | 172.54 | 11.74 | 163.71 | 12.81 |
| Dihydroxyphenylglycol | ph2 | 0.14 | 0.02 | 0.26 | 0.03 | 0.31 | 0.06 | 0.71 | 0.07 | 0.61 | 0.09 |
| Verbascoside-rha isomer 1 B | ph3 | 0.65 | 0.15 | 0.38 | 0.05 | 0.07 | 0.04 | ND | - | ND | - |
| Hydroxylated DCMEA derivative C | ph4 | 81.55 | 15.39 | 84.78 | 5.87 | 34.41 | 2.16 | 20.99 | 2.71 | 19.6 | 2.94 |
| Vanillic acid hexoside | ph5 | 2.62 | 0.16 | 2.51 | 0.14 | 1.61 | 0.06 | 1.41 | 0.04 | 1.31 | 0.12 |
| Hydroxytyrosol glucoside 1 | ph6 | 2.78 | 0.40 | 6.84 | 0.35 | 4.5 | 0.1 | 3.09 | 0.07 | 2.73 | 0.23 |
| Verbascoside-rha isomer 2 B | ph7 | 0.24 | 0.13 | 1.04 | 0.11 | 0.12 | 0.04 | 0.54 | 0.09 | 0.51 | 0.10 |
| Hydroxytyrosol glucoside 2 | ph8 | 7.57 | 0.93 | 11 | 0.48 | 5.31 | 0.20 | 3.17 | 0.25 | 2.88 | 0.30 |
| Hydroxytyrosol | ph9 | 4.25 | 0.37 | 4.39 | 0.43 | 3.04 | 0.20 | 2.68 | 0.29 | 2.43 | 0.20 |
| p-Coumaroyl aldarate | ph10 | 0.29 | 0.07 | 0.47 | 0.08 | 0.09 | 0.02 | ND | - | ND | - |
| 1-beta-Glucosyl-acyclodihydroelenolic acid | ph11 | 33.75 | 3.02 | 37.04 | 1.26 | 15.03 | 1.73 | 13.21 | 0.23 | 11.58 | 1.83 |
| Caffeoyl-hexoside | ph12 | 0.12 | 0.01 | 0.24 | 0.02 | 0.11 | 0.02 | 0.08 | 0.01 | 0.07 | 0.01 |
| Chlorogenic acid | ph13 | 0.29 | 0.04 | 0.3 | 0.04 | 0.06 | 0.01 | 0.04 | 0.02 | 0.02 | 0 |
| p-Cumaroyl-hexoside | ph14 | 2.33 | 0.18 | 2.76 | 0.08 | 1.26 | 0.04 | 1.12 | 0.06 | 1.02 | 0.08 |
| Oleoside | ph15 | 2.89 | 0.64 | 3.63 | 0.63 | 0.68 | 0.21 | 0.6 | 0.18 | 0.5 | 0.17 |
| 3,4-DHPEA-DEDA D | ph16 | TR | - | TR | - | 1.66 | 0.26 | ND | - | ND | - |
| Caffeic acid | ph17 | 0.22 | 0.05 | 0.46 | 0.03 | 0.13 | 0.02 | 0.09 | 0.01 | 0.07 | 0.01 |
| Oleoside deoxyriboside | ph18 | 3.00 | 0.77 | 3.76 | 0.56 | 0.51 | 0.14 | TR | - | TR | - |
| Oleuropein aglycone | ph19 | 765.72 | 223.78 | 1224.53 | 153.93 | 118.97 | 9.22 | 111.95 | 4.53 | 94.76 | 8.34 |
| Loganic acid glucoside | ph20 | 2.30 | 0.31 | 4.41 | 0.24 | 1.71 | 0.18 | 1.57 | 0.18 | 1.25 | 0.19 |
| 4-Hydroxyphenylacetic acid | ph21 | 0.26 | 0.03 | 0.34 | 0.04 | 0.92 | 0.05 | 0.05 | 0.01 | 0.05 | 0.01 |
| p-Coumaric acid | ph22 | 0.42 | 0.04 | 0.4 | 0.01 | 0.56 | 0.01 | 0.05 | 0.01 | 0.03 | 0.01 |
| Hydroxyverbascoside | ph23 | 3.86 | 0.29 | 4.1 | 0.53 | 1.02 | 0.24 | 1.69 | 0.15 | 1.53 | 0.23 |
| 4-HPEA-DEDA E | ph24 | 0.47 | 0.22 | 1.18 | 0.15 | 2.16 | 0.18 | ND | - | ND | - |
| Rutin | ph25 | 9.14 | 1.59 | 14.8 | 1.81 | 3.82 | 0.27 | 2.81 | 0.22 | 2.32 | 0.24 |
| Luteolin-O-rutinoside isomer 1 | ph26 | 2.13 | 0.21 | 4.03 | 0.32 | 0.99 | 0.07 | 0.92 | 0.08 | 0.74 | 0.06 |
| Luteolin-glucoside isomer 1 | ph27 | 12.02 | 1.05 | 19.99 | 1.57 | 9.51 | 0.69 | 4.47 | 0.34 | 3.15 | 1.63 |
| Verbascoside | ph28 | 12.23 | 4.79 | 4.64 | 0.67 | 1.16 | 0.11 | 0.27 | 0.06 | 0.2 | 0.04 |
| Luteolin-O-rutinoside isomer 2 | ph29 | 0.5 | 0.11 | 1.04 | 0.12 | 0.27 | 0.03 | 0.13 | 0.05 | 0.05 | 0.02 |
| Elenolic acid | ph30 | 866.6 | 170.96 | 1321 | 83.74 | 472.81 | 18.61 | 268.38 | 34.5 | 213.8 | 13.05 |
| Luteolin-O-rutinoside isomer 3 | ph31 | 0.13 | 0.06 | 0.26 | 0.03 | TR | 0 | TR | - | TR | - |
| Nüzhenide | ph32 | 4.97 | 0.73 | 11.66 | 1.18 | 3.75 | 0.44 | 3.91 | 0.43 | 3.35 | 0.34 |
| Luteolin-glucoside isomer 2 | ph33 | 12.92 | 1.24 | 13.04 | 0.6 | 6.1 | 0.51 | 3.51 | 0.27 | 3.17 | 0.28 |
| Caffeoyl-6-secologanoside | ph34 | 1.51 | 0.28 | 3.48 | 0.6 | 0.41 | 0.02 | 0.62 | 0.04 | 0.52 | 0.05 |
| Luteolin-glucoside isomer 3 | ph35 | 8.22 | 0.8 | 8.4 | 0.34 | 4.42 | 0.39 | 2.36 | 0.19 | 2.07 | 0.14 |
| 10-Hydroxy-DCMO aglycone F | ph36 | 6.07 | 3.11 | 5.42 | 0.73 | 1.67 | 0.13 | 0.1 | 0.05 | TR | - |
| Oleuroside | ph37 | 6.47 | 0.6 | 5.35 | 0.76 | 2.24 | 0.32 | 1.32 | 0.2 | 0.95 | 0.09 |
| Comselogoside | ph38 | 5.55 | 0.89 | 9.06 | 1.69 | 2.4 | 0.37 | 1.7 | 0.1 | 1.31 | 0.18 |
| Ligstroside derivative | ph39 | 0.24 | 0.17 | 0.75 | 0.16 | 0.09 | 0.09 | TR | - | TR | - |
| Ligstroside aglicone | ph40 | 1.84 | 0.4 | 2.48 | 0.4 | 0.23 | 0.08 | ND | - | ND | - |
| Luteolin | ph41 | 8.73 | 1.28 | 10.22 | 0.41 | 7.33 | 0.21 | 1.07 | 0.08 | 1.07 | 0.06 |
| Apigenin | ph42 | 1.56 | 0.29 | 1.50 | 0.1 | 1.15 | 0.07 | 0.24 | 0.04 | 0.21 | 0.05 |
| TOT G | - | 2007.34 | 416.65 | 2953.83 | 244.36 | 884.76 | 29.22 | 627.37 | 49.21 | 537.55 | 34.00 |
| Sample | Defects or Other Negative Attributes | Vanilla | Caramel | Red Fruits | Olive Fruits |
|---|---|---|---|---|---|
| T0 | 0 | 4 | 1 | 1 | 2 |
| T2 | 0 | 2 | 2 | 3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoletto, I.; García Salas, P.; Valli, E.; Bendini, A.; Ferioli, F.; Pasini, F.; Sánchez Villasclaras, S.; García-Ruiz, R.; Gallina Toschi, T. HPLC-MS/MS Phenolic Characterization of Olive Pomace Extracts Obtained Using an Innovative Mechanical Approach. Foods 2024, 13, 285. https://doi.org/10.3390/foods13020285
Grigoletto I, García Salas P, Valli E, Bendini A, Ferioli F, Pasini F, Sánchez Villasclaras S, García-Ruiz R, Gallina Toschi T. HPLC-MS/MS Phenolic Characterization of Olive Pomace Extracts Obtained Using an Innovative Mechanical Approach. Foods. 2024; 13(2):285. https://doi.org/10.3390/foods13020285
Chicago/Turabian StyleGrigoletto, Ilaria, Patricia García Salas, Enrico Valli, Alessandra Bendini, Federico Ferioli, Federica Pasini, Sebastián Sánchez Villasclaras, Roberto García-Ruiz, and Tullia Gallina Toschi. 2024. "HPLC-MS/MS Phenolic Characterization of Olive Pomace Extracts Obtained Using an Innovative Mechanical Approach" Foods 13, no. 2: 285. https://doi.org/10.3390/foods13020285
APA StyleGrigoletto, I., García Salas, P., Valli, E., Bendini, A., Ferioli, F., Pasini, F., Sánchez Villasclaras, S., García-Ruiz, R., & Gallina Toschi, T. (2024). HPLC-MS/MS Phenolic Characterization of Olive Pomace Extracts Obtained Using an Innovative Mechanical Approach. Foods, 13(2), 285. https://doi.org/10.3390/foods13020285








