Abstract
Edible mushrooms, including wild mushrooms, are currently being investigated as natural sources to evaluate their prebiotic potential. This study aimed to evaluate the prebiotic potential of crude polysaccharides (CPSs) extracted from wild Lentinus squarrosulus UBU_LS1 and Lentinus polychrous UBU_LP2 and their application as cryoprotectants in the freeze-drying process to formulate a novel synbiotic product. Based on fruiting body morphology and molecular identification, two wild edible mushrooms named UBU_LS1 and UBU_LP2 were identified as Lentinus squarrosulus and Lentinus polychrous, respectively. L. squarrosulus UBU_LS1 and L. polychrous UBU_LP2 contained high amounts of CPS after hot water extraction. Monosaccharide component analysis showed that CPS_UBU_LS1 and CPS_UBU_LP2 were typical heteropolysaccharides. CPS_UBU_LS1 and CPS_UBU_LP2 showed hydrolysis tolerance to the simulated human gastric acidic pH solution, indicating that these CPSs are capable of reaching the lower gastrointestinal tract. Antioxidant activity determined using the 1,1-diphenyl-2-picrylhydrazyl assay revealed that the CPS_UBU_LS1 and CPS_UBU_LP2 displayed greater antioxidant activity comparable with that of ascorbic acid. It was found that CPS_UBU_LS1 and CPS_UBU_LP2 have a high potential for stimulating growth in all probiotic strains. Moreover, both CPS compounds could possibly be used as cryoprotectants in freeze drying, since the viability of the selected probiotic L. fermentum 47-7 exhibited cell survival of greater than 70% after 90 days of storage at 4 °C. These results highlight that wild edible mushrooms L. squarrosulus UBU_LS1 and L. polychrous UBU_LP2 are potential natural sources of prebiotics and can be applied as cryoprotectants in the freeze-drying process. The crude polysaccharide derived from this study could also be considered as a potent antioxidative compound. Therefore, our study provides evidence to support the application of CPSs from wild edible mushrooms in synbiotic product development and in various functional foods. Finally, further evaluation of these prebiotics, including the determination of the potential rehabilitation of beneficial gut microbes in diseased individuals, is currently being conducted by our research group.
1. Background
According to the definition provided by The International Scientific Association for Probiotics and Prebiotics (ISAPP), a prebiotic is defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [1]. Three criteria are used for selecting compounds to be matched with prebiotics: first, resistance to the acidic pH in the human stomach and hydrolysis by enzymatic systems in the lower intestine; second, utilization by intestinal microbiota; and third, selection to stimulate the growth of beneficial microbes, especially probiotic-associated species/strains [2]. Fructo-oligosaccharides (FOSs) and galacto-oligosaccharides (GOSs), well-known prebiotics, have been evaluated for their prebiotic potential in some diseases, including type-2 diabetes [3]. Fermentation of prebiotics by the intestinal microbiota results in the production of various short-chain fatty acids (SCFAs), which are beneficial not only in the local intestine but also in distal organs, such as the brain [4]. The positive immunomodulation of prebiotics is another benefit to the host’s health. For example, the purified fraction of Inonotus obliquus polysaccharide induced anticancer activities in mice. It accomplished this by inducing proinflammatory cytokines, promoting the proliferation of splenocytes and lymphocytes, increasing Bax expression, and inhibiting Bcl-2 expression [5]. These immunological cells and molecules facilitate apoptosis in tumor cells. As detailed in a previous report, the hot-water-extracted fractions of L. polychrous and Ganoderma lucidum polysaccharide exhibited anticancer, antiviral, and anti-inflammatory effects [6]. For these reasons, the isolation and characterization of prebiotics from other natural food sources, including edible mushrooms, is an important research topic.
Historically, edible mushrooms have been consumed by humans as natural foods, food supplements, and medicines. Edible mushrooms consumed as medicines contain not only good nutritional components but also excellent natural bioactive compounds with beneficial effects in humans [7]. These benefits include anti-inflammatory [8], antioxidant [9], antimicrobial [10,11], and immunomodulatory [12] effects, as well as effectiveness in the treatment of particular diseases, including diabetes [13], Alzheimer’s disease [14], coronary heart disease [15], and cancer [16]. Different bioactive compounds, including terpenoids, phenolic compounds, proteins, lipids, and polysaccharides, have been isolated from edible wild mushrooms and cultured mycelia [17]. Among these compounds, polysaccharides have been found to possess a vast array of activities, and there has been recent interest in employing mushroom polysaccharides for the modulation of human intestinal microbiota in particular intestinal-associated disorders or diseases; however, the evidence of these benefits has mostly been verified in some edible mushrooms, including Agrocybe cylindracea [18], Volvariella volvacea [19], Agaricus bisporus [20], Trametes versicolor [21], and Lentinus squarrosulus [22].
In addition to prebiotic properties, synbiotics, mixtures comprising live microorganisms and one or more substrates selectively utilized by host microorganisms that confer health benefits to the host, have been an attractive development [23]. The probiotics Lactobacillus and Bifidobacterium spp. that are incorporated in a synbiotic formula utilize prebiotics for their proliferation and may produce metabolic by-products, such as antimicrobial compounds and other organics. Therefore, the prebiotic properties of specific probiotic strains should be evaluated.
Several methods are used to prepare synbiotic products. Freeze drying or lyophilization is one of the most common methods used to prepare synbiotics [24]. However, this method uses deep, low temperature under high pressure, leading to the death of some probiotic populations. To overcome this limitation, it is necessary to use effective cryoprotectants during freeze drying. However, the use of crude polysaccharides extracted from edible mushrooms as cryoprotectants has not yet been reported.
In the present study, we evaluated the prebiotic properties of crude polysaccharides extracted from the wild edible mushrooms L. squarrosulus UBU_LS1 and L. polychrous UBU_LP2. Three probiotic strains, Limosilactobacillus fermentum 47-7, Lacticaseibacillus rhamnosus NH9, and Lacticaseibacillus rhamnosus 23-2, were used to evaluate growth promotion by crudely extracted prebiotics. Among these strains, L. fermentum 47-7 was selected as the model probiotic for synbiotic incorporation. Our results suggest the possibility of using crude polysaccharides from Lentinus species not only as prebiotics, but also as cryoprotectants. The prebiotics and formulated synbiotics derived in this study are being evaluated for their potential to modulate the human gut microbiota, and hopefully for further use as a novel medical technology for human gut microbiota rehabilitation.
2. Materials and Methods
2.1. Collection of Wild Edible Mushrooms
Wild mushrooms were collected from the local area of Ubon Ratchathani Province, Thailand. Mushrooms were kept in a collective paper bag and carried to the microbiology laboratory of the College of Medicine and Public Health, Ubon Ratchathani University, Warinchamrap, Ubon Ratchathani, Thailand. Subsequently, the fruiting body morphology of each mushroom was observed and recorded.
2.2. Genomic DNA Extraction
Fresh fruiting bodies of the mushrooms were selected for genomic DNA extraction. Approximately 100 mg of mushroom tissue was cut from a portion of the apical stalk of the fruiting bodies. The genomic DNA of mushrooms was extracted using the Prep Fungi/Yeast Genomic DNA Extraction Mini Kit according to the manufacturer’s instructions (Favorgen Biotech Corp., Ping Tung, Taiwan). The extracted DNA was stored at −20 °C until use.
2.3. Identification of Mushroom Species
The correct species of the collected mushrooms were identified by conventional polymerase chain reaction (PCR), DNA sequencing, and analysis, as previously described [25]. Genomic DNA extracted from each mushroom was used as the template. Table 1 shows the oligonucleotide primer pairs, LROR and LR6, and the PCR conditions used to amplify the target rRNA region of fungal genomic DNA, as previously reported [25]. All primers were purchased from Integrated DNA Technologies (Singapore). The AllTaq™ PCR Core Kit (Qiagen, Germantown, MD, USA) was used for PCR. The amplicons were verified through agarose gel electrophoresis and further purified using the HiYield Gel/PCR DNA Fragment Extraction Kit (RBC Bioscience, New Taipei City, Taiwan). DNA sequencing was performed at ATGC (Thailand Science Park, Pathumthani, Thailand) using the chain-termination DNA method. The amplicons were sequenced using the same primers used for the PCR. Nucleotide sequencing results were compared to those of fungal rDNA sequences obtained from the rRNA databases of the National Center for Biotechnology Information (NCBI) with highly similar sequence (megablast) parameter settings. A phylogenetic tree was constructed using MEGA version 11 [26]. The neighbor-joining method with 0.75 as the maximum sequence difference was used in this analysis.

Table 1.
Oligonucleotide primers and PCR conditions used in this study.
2.4. Mushroom Preparation for Polysaccharide Extraction
The entire fruiting body of each mushroom was soaked in distilled water to remove contaminated soil and other debris. Cleaned mushrooms were cut into small pieces using scissors and were further incubated at 50 °C with forced air circulation for two days. Dried mushrooms were crushed into a fine powder using a blender (BL-T60 model, Thai Toshiba Electric Industries, Nonthaburi, Thailand). Mushroom powder (10 g) was added to 40 mL of absolute ethanol (VWR, BDH Chemical, Singapore) and incubated at ambient temperature with shaking at 150 rpm for 18 h. After incubation, the mixture was centrifuged at 9000× g for 20 min at 4 °C. The debris was collected and incubated in an oven at 50 °C for 24 h until completely dry.
2.5. Crude Polysaccharide Extraction
Dried ethanol-washed mushroom powder (10 g) was mixed with 90 mL of distilled water. The mixture was incubated at 90 °C in a water bath for 4 h. To collect the clear supernatant, the mixture was centrifuged three times at 9000× g for 20 min. The upper phase of the solution was collected and mixed with absolute ethanol (VWR, BDH Chemical) at a 1:4 ratio (upper phase supernatant:absolute ethanol), and the mixture was further incubated at 4 °C for 16–18 h. The mixture was centrifuged at 9000× g for 15 min. The pellet of extracted polysaccharide was washed with absolute ethanol and dried at 50 °C for 4 h. Thereafter, the polysaccharide was crushed into a fine powder using a mortar and pestle. The extraction yields were calculated using the following equation:
The extracted polysaccharide powder was stored in a bottle in a silica bag at 4 °C until use.
2.6. Determination of Total Carbohydrate Content
The total carbohydrate content of the crude polysaccharide was determined as described previously [27]. Briefly, 50 μL of an appropriate dilution of crude polysaccharide was mixed with 150 μL of concentrated sulfuric acid (Fisher Scientific, Seoul, Republic of Korea) and was immediately mixed with 30 μL of 5% (w/v) phenol (Fisher Scientific). The mixture was maintained at 90 °C for 5 min. The reaction mixture was then cooled to ambient temperature. The absorbance of the reaction solution was measured at 490 nm using a microplate reader (Bio Chrom/EZ Read 2000, Biochrom Ltd., Waterbeach Cambridge, UK). A D-(+)-glucose solution (PanReac Applichem, Darmstadt, Germany) at different concentrations was used to calculate the standard concentration point.
2.7. Determination of Reducing Sugar
The reducing sugar present in the extracted crude polysaccharide was assayed using a 3,5-dinitrosalicyclic acid (DNS) (PanReac Applichem) assay, as previously described [28]. The absorbance of the reaction mixture was measured at 540 nm using a microplate reader. A D-glucose solution was used to calculate the standard concentration.
2.8. Determination of Protein Content
The protein content of the extracted crude polysaccharides was determined as previously described [9]. The absorbance of the reaction mixture was measured at 595 nm using a microplate reader. Bovine serum albumin (Sigma-Aldrich, Singapore) was used to prepare the standard protein solution.
2.9. Analysis of Monosaccharide Composition in Crude Polysaccharides
The crude polysaccharide powder was dissolved in sterile distilled water, and the pH was adjusted to 6.5. The solution was filtered using a 0.45 μM pore size filter (Whatman PuradiscTM, Buckinghamshire, UK). The monosaccharide composition of the crude polysaccharide solution was analyzed using HPLC at the Sugars and Derivatives Analytical Laboratory (SDAL), Kasetsart University, Bangkok, Thailand. Standard compounds glucose, mannose, galactose, and fucose were used for peak identification and concentration estimation. The reaction was performed in triplicate.
2.10. Determination of Hydrolysis Tolerance in Simulated Gastric Buffer
The digestibility of the artificial gastric juice was evaluated as previously described [29]. Briefly, pH1 and pH5 of the hydrochloric acid buffer were used to simulate human gastric juice for the polysaccharide hydrolysis assay. The buffer was loaded into a polysaccharide solution (10 mg/mL) at an equal ratio and incubated at 37 °C for 2 h. Inulin was used as a comparative prebiotic. The reducing sugar released from the crude polysaccharide and the total sugar content were determined using the DNS assay and the phenol–sulfuric acid method, as previously described. The hydrolysis assay was performed after 0 and 2 h of incubation. The percentage hydrolysis was calculated using the following equation:
2.11. Antioxidant Activity by 1,1-Diphenyl-2-picrylhydrazyl Scavenging Ability
The scavenging ability was determined using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals as described by Inyod et al. [30]. The DPPH solution was prepared to a final concentration of 0.2 mM DPPH (Sigma-Aldrich) in absolute ethyl alcohol (VWN). The crude polysaccharide samples were dissolved in distilled water at a concentration range of 0–5 mg/mL. The sample and DPPH solution were mixed in a 2:1 ratio and incubated for 30 min in the dark before the absorbance at 517 nm was measured using a spectrophotometer. The antioxidant activity was determined by measuring the 50% inhibitory concentration (IC50, mg/mL) of DPPH radicals. The percentage of inhibition was calculated using the following equation where ascorbic acid was used as the reference antioxidant:
where Ablank is the absorbance of the control reaction (containing all reagents except the test compound) and Asample is the absorbance of the sample.
% Inhibition = (Ablank − Asample/Ablank) × 100
Inhibitory activity values were calculated for various concentrations of the polysaccharide samples in triplicate.
2.12. Determination of Probiotic Bacterial Growth Promoted by Crude Polysaccharides
Three probiotic strains, Limosilactobacillus fermentum 47-7, Lacticaseibacillus rhamnosus NH9, and Lacticaseibacillus rhamnosus 23-2, were used to evaluate growth promotion by crudely extracted prebiotics. A single colony of each strain was inoculated into 10 mL of MRS broth and was incubated at 37 °C for 18 h. Bacterial cell pellets were harvested by centrifugation at 9000× g for 10 min at 4 °C. The bacterial cell pellets were washed twice with cold sterile distilled water. The bacterial cells were adjusted by comparing the bacterial solution with McFarland No. 1.0 (3.0 × 108 CFU/mL). To collect bacterial cells, 200 μL of the adjusted bacterial cell solution was transferred into a microtube and centrifuged at 9000× g for 5 min. Bacterial cell pellets were collected and suspended with 200 μL MRS broth without glucose (MRSGlu−) supplemented with 20 mg/mL of crude polysaccharide, hereafter abbreviated as MRSGlu−/Pre. MRS with glucose (MRSGlu+) was used as a comparative medium. Fructo-oligosaccharide (FOS), galacto-oligosaccharide (GOS), and inulin (Inu) were purchased from Sigma and used as comparative prebiotics. The plates were incubated at 37 °C for 48 h. Bacterial growth was determined by measuring the turbidity in the wells at OD620 nm using a microplate reader 48 h after incubation. The experiment was performed in triplicate. The percentage bacterial growth for each MRSGlu/Pre ratio was calculated using the following equation:
2.13. Preparation of Lyophilized Synbiotic
To prepare the lyophilized synbiotic, the L. fermentum strain 47-7 was used as a model probiotic and incorporated into the synbiotic component. L. fermentum 47-7 was grown in 10 mL MRS broth and was incubated at 37 °C for 18 h. Bacterial cell pellets were collected by centrifugation and washed twice with cooled sterile distilled water. Bacterial cell numbers were adjusted to obtain an approximate bacterial cell number of 1.2 × 109 CFU/mL by comparing the bacterial solution with McFarland No. 4.0. Serial dilutions, followed by the plate count method, were used to confirm the viable bacterial cells. Bacterial cell pellets were harvested from 1 mL of the adjusted bacterial solution by centrifugation at 9000 rpm for 10 min. The bacterial cell pellet was suspended in 20 mL of a cryoprotectant medium, as shown in Table 2. The solution was kept on ice for 30 min and was further incubated at −20 °C for 18 h. The frozen solution was used to prepare a lyophilized powder using a freeze dryer (Alpha 1–4 LSCbasic, Martin Christ Company, Osterode am Harz, Germany) at the Faculty of Pharmacology, Ubon Ratchathani University, Ubon Ratchathani Province, Thailand.

Table 2.
Ingredients of cryoprotective media used for preparation of lyophilized synbiotic in freeze drying.
2.14. Lyophilized Cells Observed Using Scanning Electron Microscopy
After lyophilization, each powder was aliquoted and placed in a tube covered with carbon tape. The lyophilized sample was coated with gold at a process current of 10 mA for 1 min (Sputter Coater, SPI module, West Chester, PA, USA). The morphology of the gold-coated samples was observed using scanning electron microscopy at 10.0 kv (JEOL JSM-6010 LV, Peabody, MA, USA) at the College of Medicine and Public Health, Ubon Ratchathani University, Ubon Ratchathani Province, Thailand.
2.15. Survival Ability of Probiotic during Synbiotic Storage
The survival ability of the probiotic L. fermentum 47-7 was evaluated at 0, 30, 60, and 90 days of storage. Lyophilized powders from each sample were aliquoted from the storage tubes and weighed. The lyophilized powders were dissolved in 1 mL of sterile distilled water and used to prepare 10-fold dilutions. Next, 100 μL of an appropriate dilution was spread onto MRS agar plates. After 48 h of incubation at 37 °C, colonies were counted and calculated as colony-forming units (CFU per mL). The experiment was performed in triplicate.
3. Results
3.1. Wild Mushroom Collection and Fruiting Body Morphology Recording
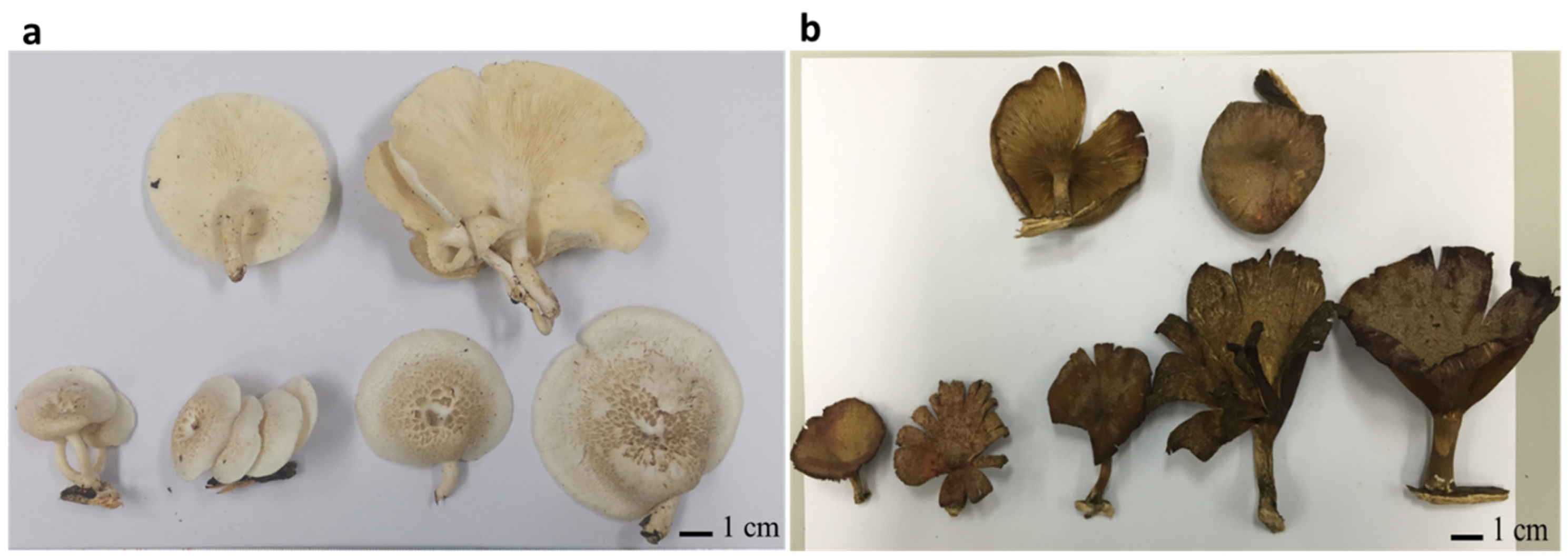
In the present study, two mushroom samples, UBU_LS1 and UBU_LP2, were collected separately from deadwood logs. UBU_LS1 was collected from a forest in Phibun Mangsahan district, Ubon Ratchathani Province, Thailand, at the following geographic coordinates: 15°13′47.4″ N 105°13′22.3″ E. UBU_LP2 was collected from Nong Ejem swamp in Ubon Ratchathani University, Warinchamrab district, Ubon Ratchathani Province, Thailand, at the following geographic coordinates: 15°07′43.0″ N 104°54′46.2″ E. Observation of fruiting body morphology revealed that UBU_LS1 had white and umbilicated sporocarps (Figure 1a), while UBU_LP2 had funnel-like brown-to-fuscous lamellae (Figure 1b).
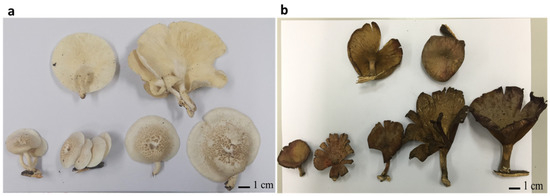
Figure 1.
Morphology of fresh fruiting bodies of Lentinus squarrosulus UBU_LS1 (a) and Lentinus polychrous UBU_LP2 (b). Scale bar represents 1 centimeter (cm).
3.2. Molecular Identification of Mushroom Species
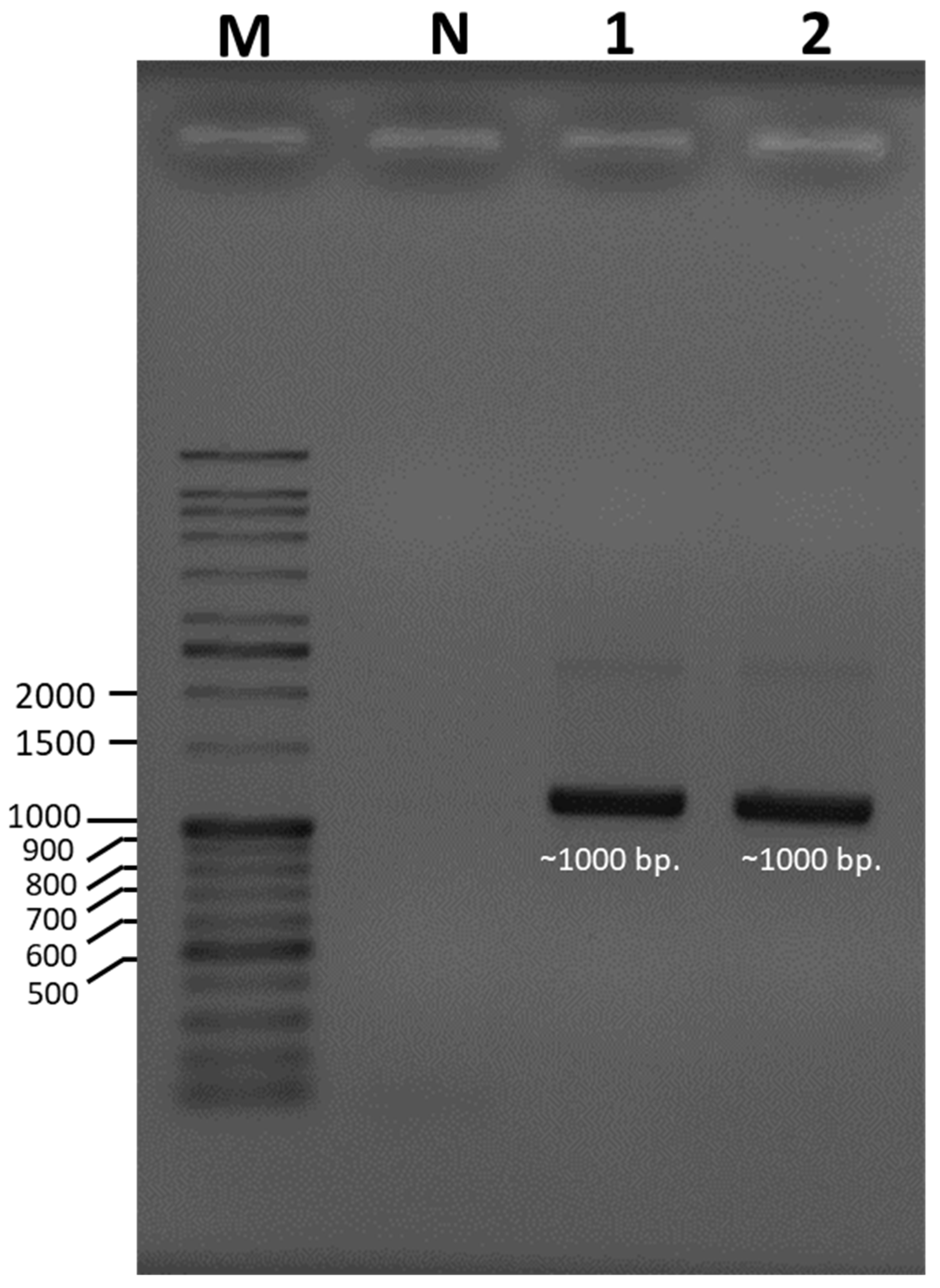
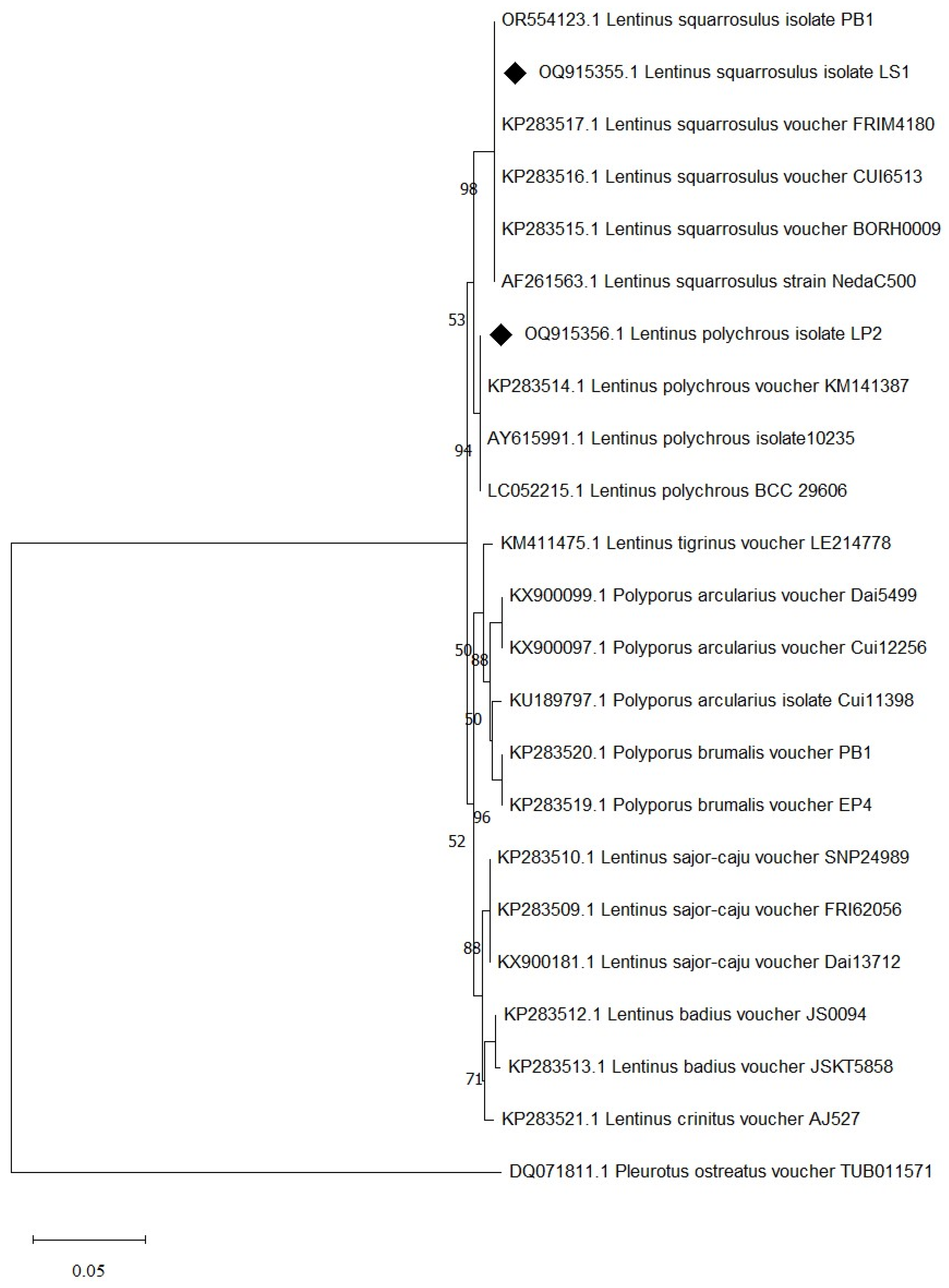
Species identification was carried out using a molecular-based technique in which a large subunit of the 28S ribosomal RNA gene (LSU-28S rRNA) was amplified, sequenced, and analyzed. As shown in Figure 2, an amplified product of approximately 1000 base pairs (bp) was obtained from both UBU_LS1 and UBU_LP2, and after nucleotide sequencing, it was found that these products contained 1050 and 1016 bp, respectively. Sequence comparison of the LSU-28S rRNA gene of UBU_LS1 with those held in the NCBI database demonstrated that the LSU-28S rRNA gene of UBU_LS1 had an identity similarity (100%, 1050/1050 identities) with the LSU-28S rRNA gene of L. squarrosulus FRIM4180 (GenBank accession No. KP283517.1). For UBU_LP2 LSU-28S rRNA gene analysis, we observed an identity similarity (100%, 1016/1016 identities) with Lentinus polychrous KM141387 (GenBank accession No. KP283514.1). Phylogenetic tree analysis of the LSU-28S rRNA gene revealed that UBU_LP2 and UBU_LS1 were closely related to L. polychrous and L. squarrosulus, respectively (Figure 3). Therefore, based on morphological appearance and rRNA gene sequencing and analysis, the two wild mushroom isolates, UBU_LS1 and UBU_LP2, were identified as Lentinus squarrosulus and Lentinus polychrous, respectively.
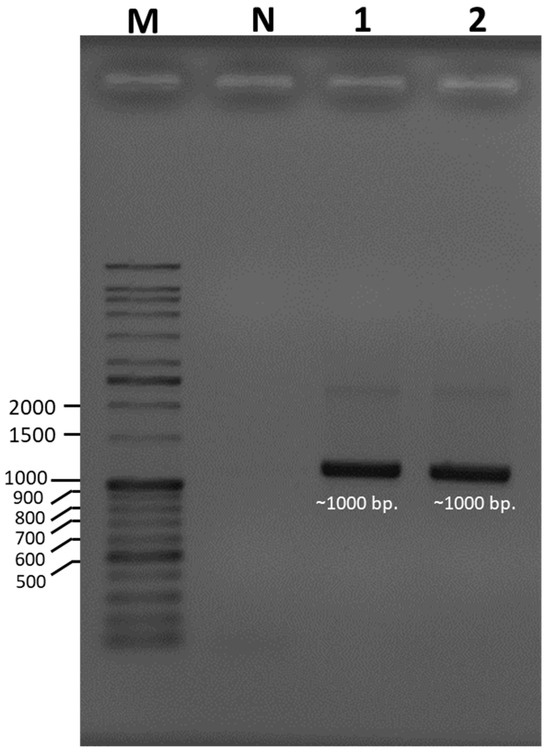
Figure 2.
Ethidium bromide-stained agarose gel showing the amplified product of a large subunit of the 28S ribosomal RNA gene (LSU-28S rRNA) of Lentinus squarrosulus UBU_LS1 and Lentinus polychrous UBU_LP2. Lane M contained the VC DNA ladder. Lanes 1 and 2 contained the amplified products of the LSU-28S rRNA genes of UBU_LS1 and UBU_LP2, respectively. Lane N contained the PCR product without any DNA template. The nucleotide size (base pairs, bp) of the DNA ladder is shown beside the gel.
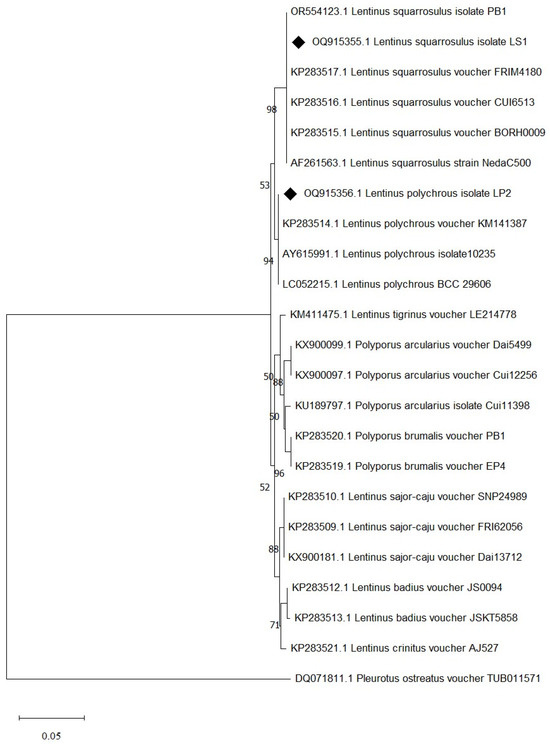
Figure 3.
Evolutionary relationships of taxa inferred from the neighbor-joining method using LSU-28S rRNA gene data. Bootstrap support value is greater than or equal to 50% is shown in each node. Evolutionary analyses were conducted using MEGA11. Pleurotus ostreatus was used as the outgroup. The LSU-28S rRNA of L. squarrosulus UBU_LS1 and L. polychrous UBU_LP2 are indicated by the symbol ♦ before their accession number.
3.3. Deposition of Ribosomal RNA Gene Sequence in the NCBI Database
The 28S large subunit ribosomal RNA gene sequences of UBU_LS1 and UBU_LP2 were deposited in the National Center for Biotechnology Information (NCBI) database under accession numbers OQ915355 and OQ915356, respectively.
3.4. Yields of Crude Polysaccharide Extraction
After extraction, the crude polysaccharides derived from L. squarrosulus UBU_LS1 (hereafter referred to as CPS_UBU_LS1) and L. polychrous UBU_LP2 (hereafter referred to as CPS_UBU_LP2) were dark brown in color (Figure 4). It was found that the extraction yield of CPS_UBU_LS1 and CPS_UBU_LP2 was 6.77 ± 0.02% and 7.14 ± 0.01% (Table 3), respectively.
Figure 4.
Appearance of crude polysaccharide CPS_UBU_LS1 (a) and CPS_UBU_LP2 (b).

Table 3.
Yield of crude polysaccharides and the amount of total carbohydrates, reducing sugars, polysaccharides, and protein present in crude polysaccharides CPS_UBU_LS1 and CPS_UBU_LP2 (n = 3).
3.5. Compound Component of Extracted Crude Polysaccharide
Table 3 lists the components of CPS_UBU_LS1 and CPS_UBU_LP2. The major components of both CPS_UBU_LS1 and CPS_UBU_LP2 were carbohydrates, and there were low amounts of protein and reducing sugars. CPS_UBU_LS1 and CPS_UBU_LP2 had high polysaccharide contents, which were approximately calculated as 93.53% and 92.61% of total carbohydrates.
3.6. Monosaccharide Composition of CPS_UBU_LS1 and CPS_UBU_LP2
The monosaccharide composition of mushroom extracts quantified by HPLC is shown in Table 4. It was found that polysaccharides of both CPS_UBU_LS1 and CPS_UBU_LP2 were composed of monosaccharides in varying concentrations. Fucose was found to be the most abundant monosaccharide in both CPS_UBU_LS1 and CPS_UBU_LP2 crude extracts, followed by mannose, glucose, and galactose at varying concentrations. These results indicated that the polysaccharides extracted from both UBU_LS1 and UBU_LP2 using hot water were heterologous polysaccharides.

Table 4.
Monosaccharide composition in crude polysaccharides CPS_UBU_LS1 and CPS_UBU_LP2.
3.7. Resistance in Simulated Human Gastric Acidic pH Solution
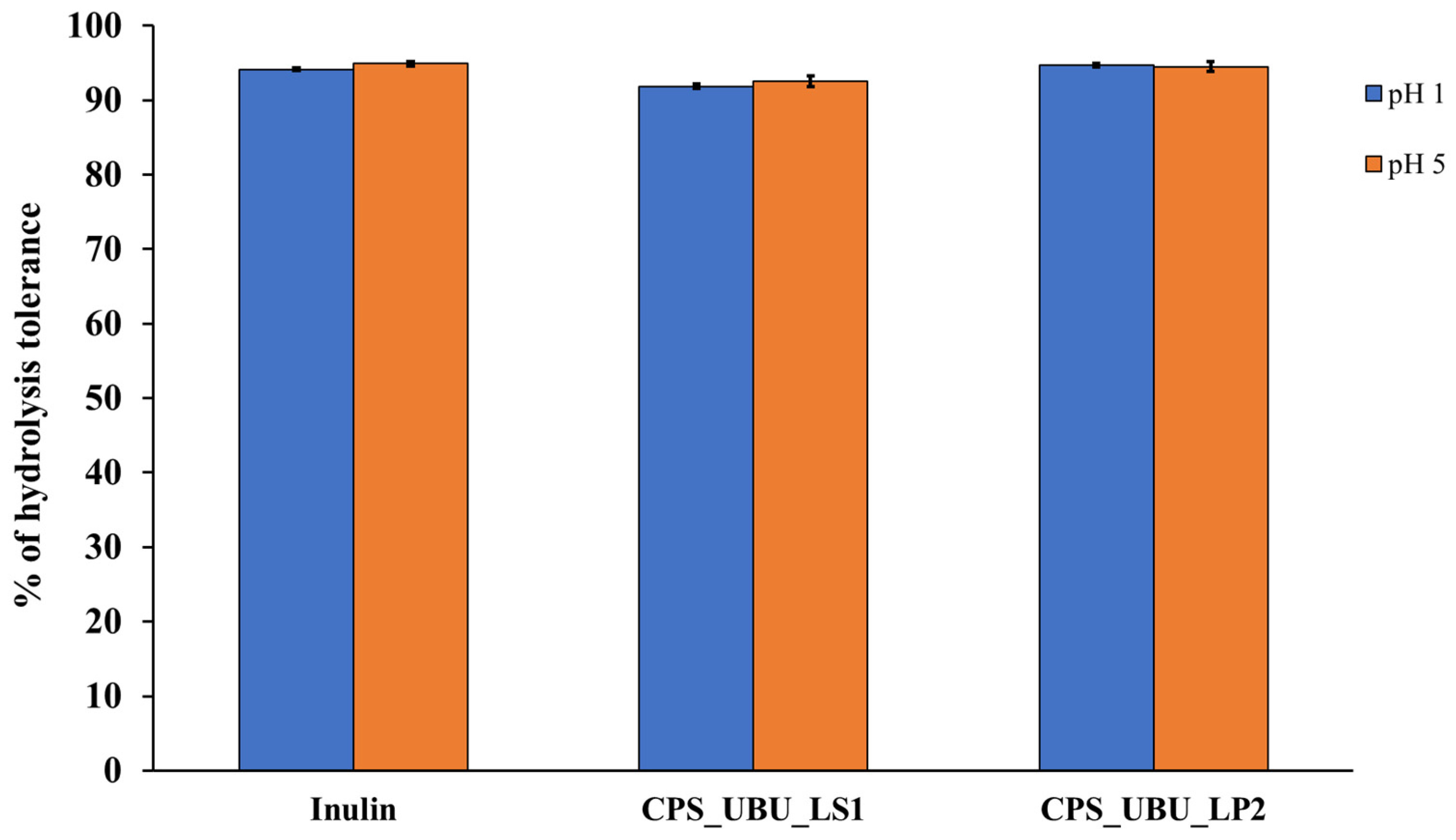
Figure 5 shows that all crude polysaccharides, including the prebiotic inulin, were highly resistant to hydrolysis at both pH1 and pH5 after 2 h of incubation. The results demonstrated that the crude polysaccharides of both CPS_UBU_LS1 and CPS_UBU_LP2 had a high hydrolysis tolerance of more than 90%. The crude polysaccharide of CPS_UBU_LP2 showed the highest hydrolysis tolerance at 95% at both pH1 and pH5, which was higher than that of CPS_UBU_LS1 and inulin.
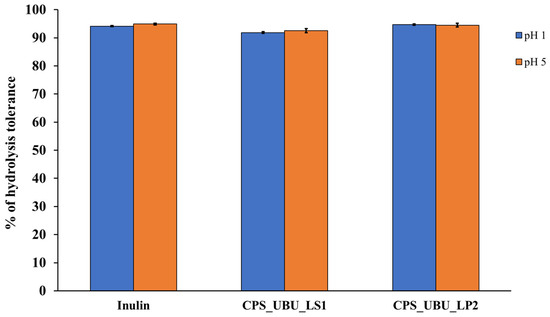
Figure 5.
The percentage of hydrolysis tolerance of crude polysaccharides CPS_UBU_LS1 and CPS_UBU_LP2. Inulin was used as reference prebiotic. (Raw data is shown in Supplement data for Figure 5 (Tables S2–S4)).
3.8. Potential of Crude Polysaccharides for Stimulating Probiotic Growth
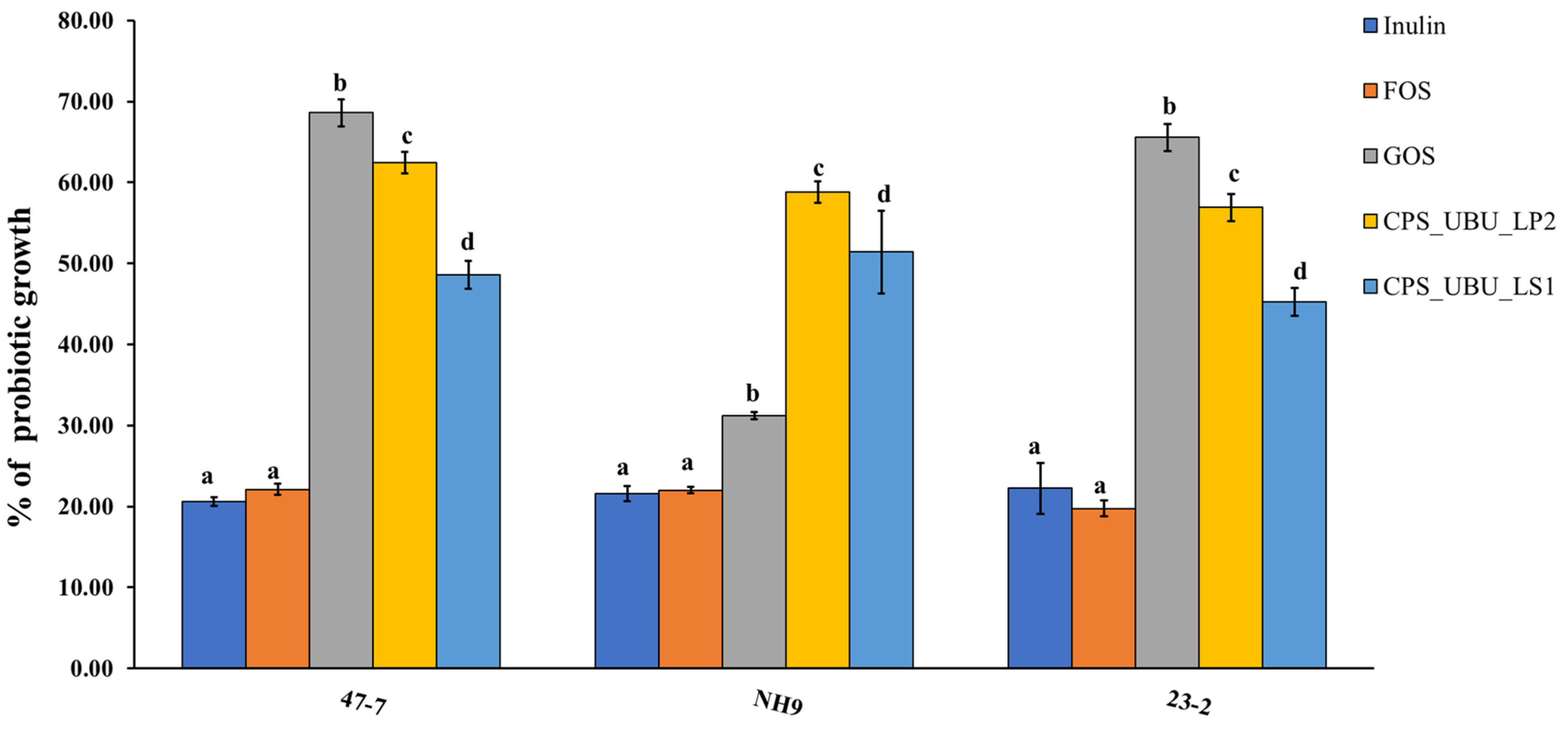
Figure 6 shows the potential of all crude polysaccharides, including comparative prebiotics (inulin, fructo-oligosaccharide (FOS), and galacto-oligosaccharide (GOS)), for stimulating the probiotic strains, 47-7, 23-2, and NH9. The percentage of growth of each strain was compared to that derived from MRS medium supplemented with glucose, which was taken as 100%. It was found that the crude polysaccharide CPS_UBU_LP2 had a high potential for stimulating growth in all probiotic strains, especially in the L. fermentum 47-7 strain, for which stimulation with CPS_UBU_LP2 was not significantly different from stimulation with GOS. Moreover, the crude polysaccharides CPS_UBU_LP2 and CPS_UBU_LS1 had the potential to stimulate all probiotic strains to significantly higher levels than that observed with stimulation by inulin and FOS.
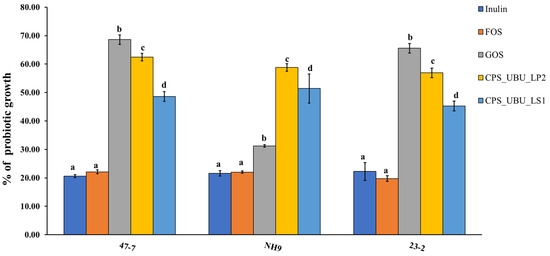
Figure 6.
Growth stimulation of probiotic bacteria by crude polysaccharides CPS_UBU_LS1 and CPS_UBU_LP2. Inulin, FOS, and GOS were used as reference prebiotics. Different letters indicate a significant difference (p < 0.05).
3.9. Antioxidant Activities of Crude Polysaccharides
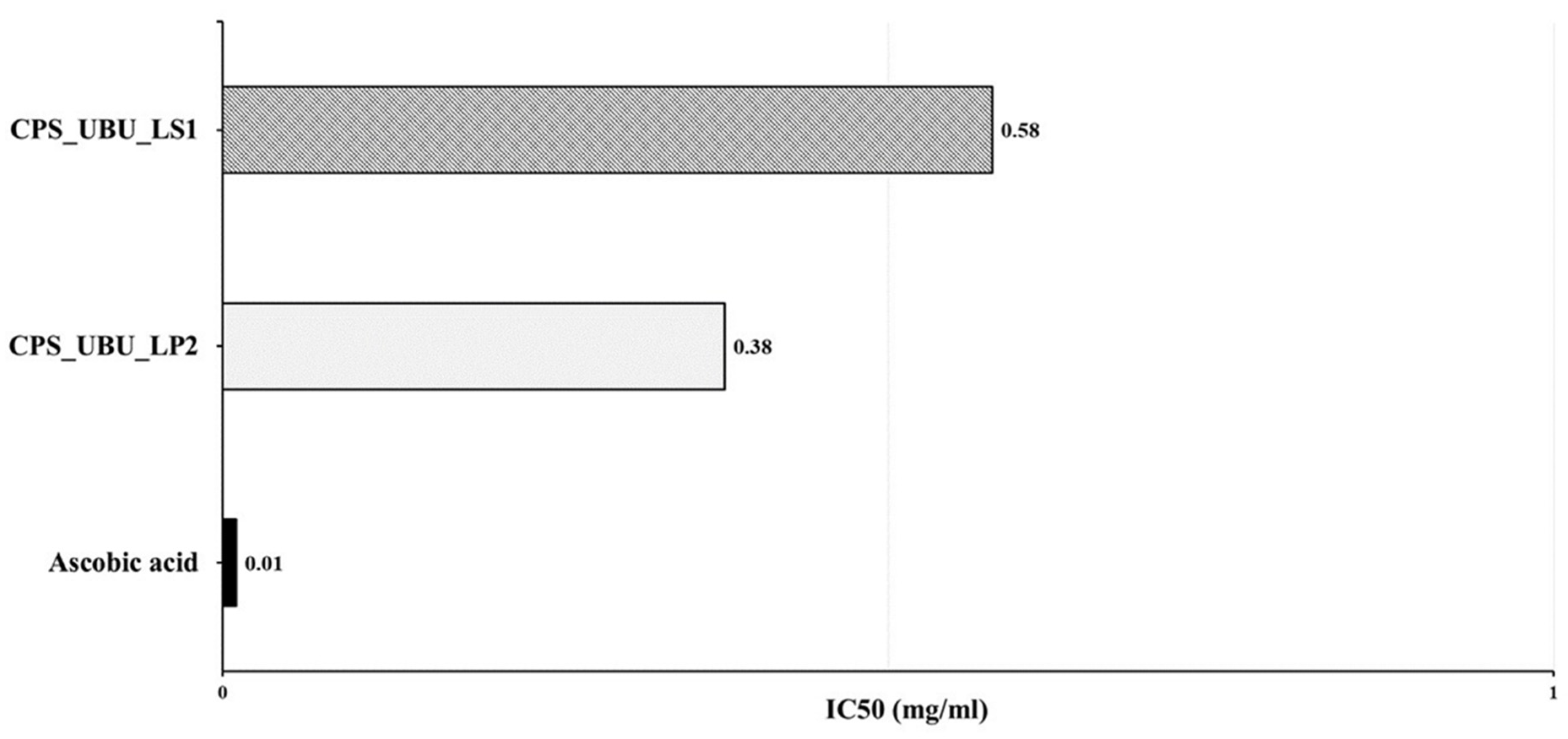
The antioxidant activities of the crude polysaccharides CPS_UBU_LS1 and CPS_UBU_LP2 were evaluated using DPPH radical scavenging assays. The antioxidant effectiveness was expressed as the IC50. As shown in Figure 7, the result indicated that CPS_UBU_LP2 and CPS_UBU_LS1 were potent DPPH scavengers with IC50 values of 0.38 and 0.58 mg/mL, respectively. The control (ascorbic acid) showed the highest antioxidant activity, with an IC50 value of 0.01 mg/mL.
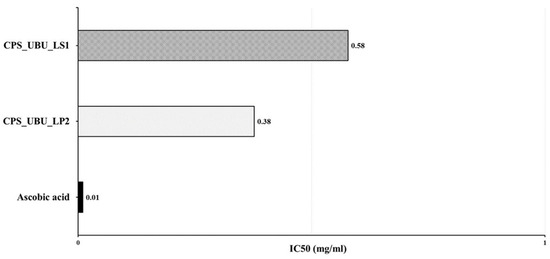
Figure 7.
IC50 values of crude polysaccharides CPS_UBU_LS1 and CPS_UBU_LP2 measured in DPPH assay. Ascorbic acid was used as reference antioxidant.
3.10. Potential Crude Polysaccharides as Cryoprotectants in the Freeze-Drying Process and for Storage
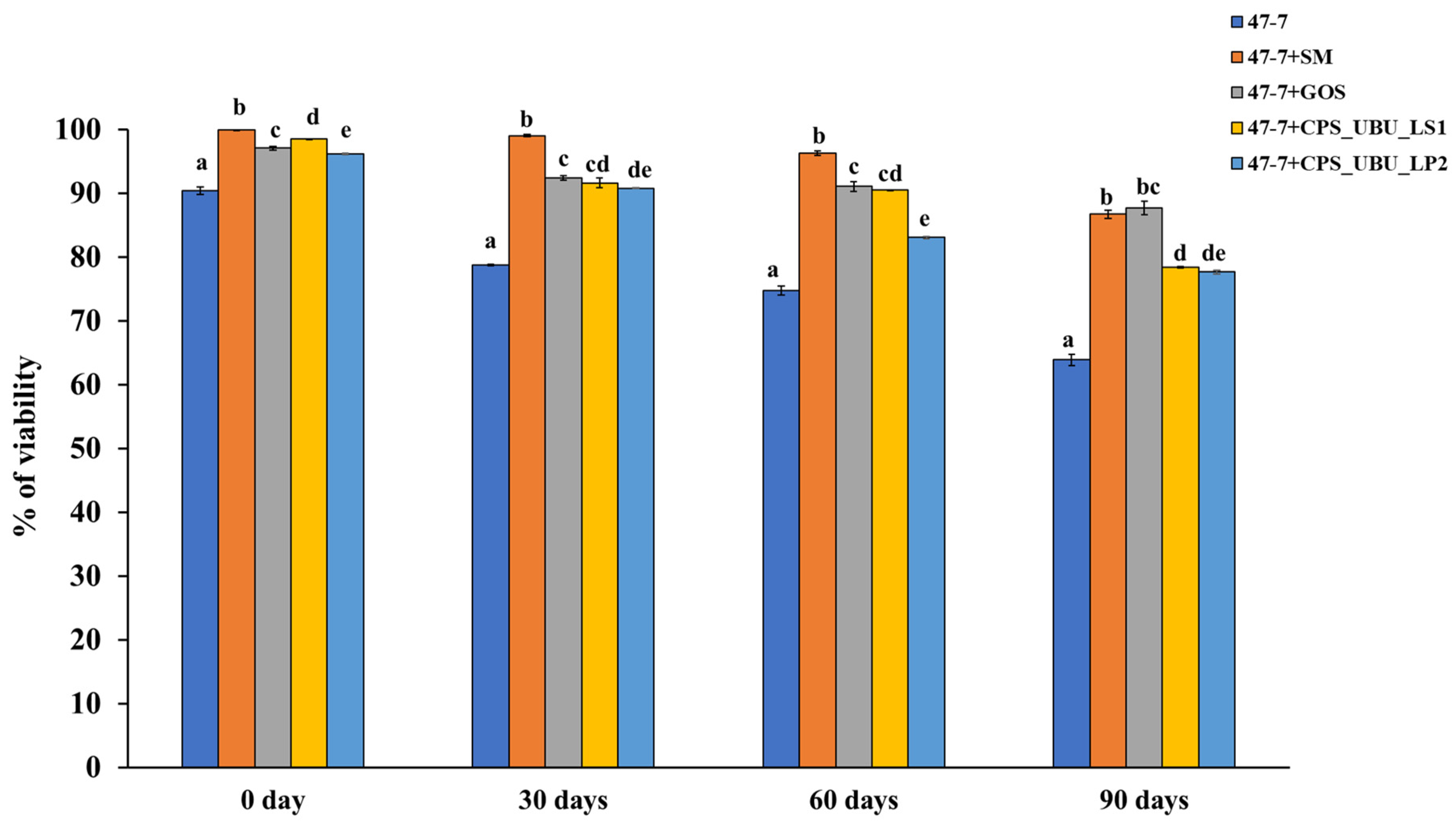
Crude polysaccharides CPS_UBU_LS1 and CPS_UBU_LP2 were used to formulate lyophilized synbiotic products, and L. fermentum 47-7 was selected as a model probiotic. After freeze drying, bacterial viability at 0, 30, 60, and 90 days was determined using the 10-fold dilution and plate count technique. The cell viability was expressed as colony-forming units (CFU) per ml. At 0 days (immediately after freeze drying), it was shown that all lyophilized cells had a high survival percentage of more than 90%, even for the lyophilized cells without a cryoprotectant (Figure 8). The greatest survival (99.90%) on day 0 was derived for freeze-dried 47-7 formulated with skim milk (SM) as the cryoprotectant (47-7+SM). After 90 days of storage at 4 °C, all freeze-dried cells exhibited a slow reduction in viability up to 90 days of storage. The freeze-dried L. fermentum 47-7 with a cryoprotectant derived from CPS_UBU_LP2 and CPS_UBU_LS1 exhibited viability of 77.64% (1.55 × 1010 CFU/mL) and 78.40% (1.57 × 1010 CFU/mL), respectively. Moreover, we found that the viability of freeze-dried 47-7 formulated with crude polysaccharides CPS_UBU_LP2 and CPS_UBU_LS1 was slightly enhanced upon the addition of SM to the formula (data are shown in Supplementary data: Table S1, Figure S1).
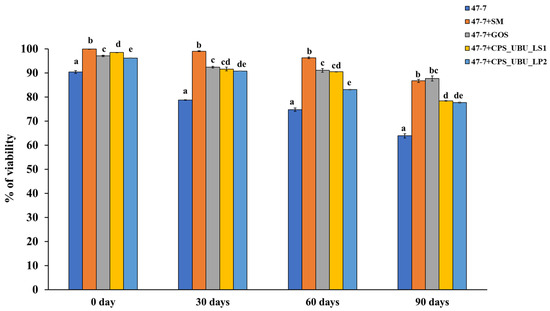
Figure 8.
Viability of probiotic L. fermentum 47-7 formulated with and without prebiotic after freeze drying and storage for 90 days. The small letters indicate significant differences (p < 0.05) from the control (without prebiotic).
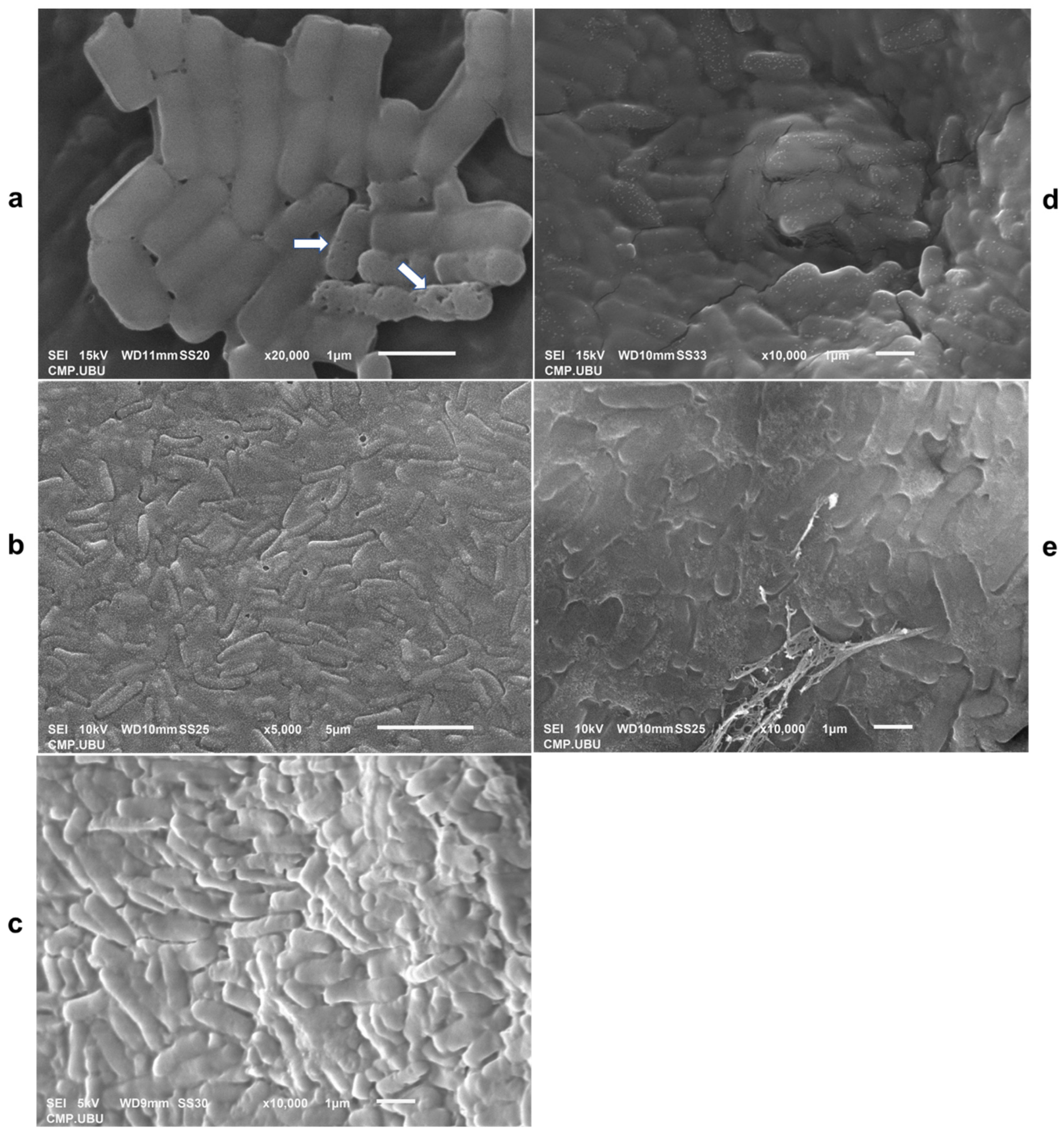
3.11. Lyophilized Cell Morphology Observed Using SEM
Scanning electron micrographs of L. fermentum 47-7 in different cryoprotective media are shown in Figure 9. The cryoprotective media of skim milk (Figure 9b) and crude polysaccharides, CPS_UBU-LS1 (Figure 9d) and CPS_UBU-LP2 (Figure 9e), enveloped the probiotic L. fermentum 47-7 inside the lyophilized powder. In addition, the reference prebiotic GOS had a smooth surface that coated the bacterial cells (Figure 9c). The lyophilized L. fermentum 47-7 cells without any cryoprotectant showed some damage presenting as pores in bacterial cells (Figure 9a). These SEM images confirmed that all wall materials facilitated the viability of probiotic cells during the lyophilization process at high temperatures and pressures.
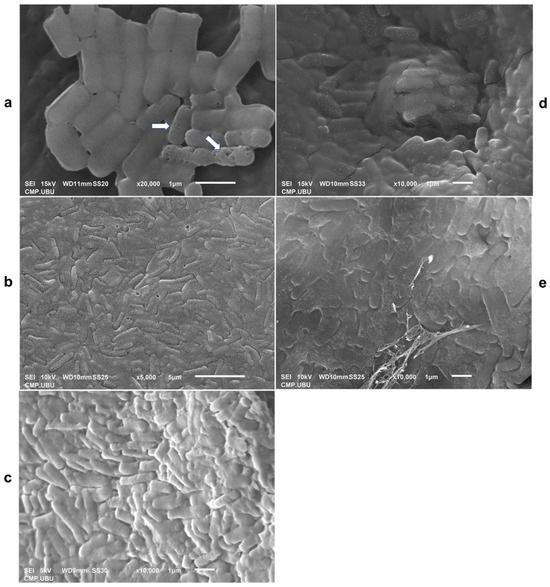
Figure 9.
Scanning electron micrographs of lyophilized cells of L. fermentum 47-7 without cryoprotective media (a) and with skim milk (SM) (b), galacto-oligosaccharide (GOS) (c), CPS_UBU_LS1 (d), and CPS_UBU_LP2 (e). The damaged bacterial cells are indicated by arrows.
4. Discussion
In this study, wild edible mushrooms were collected from a local area in Ubon Ratchathani Province, Thailand, and their properties and applications as cryoprotectants in the freeze-drying process for synbiotic product development were determined. However, there is serious evidence that eating wild mushrooms without correctly distinguishing between poisonous and edible mushrooms may cause illness or death [31]. Thus, not only the morphological identification of fresh fruiting bodies, but also a reliable method for the precise identification of mushrooms must be developed to ensure that mushroom samples are edible and safe. The molecular method of PCR followed by nucleotide sequence analysis, as previously described by Raja et al. (2017), was used in our study [25]. Our results demonstrated that the LROR/LR6 primers used to analyze the nucleotide regions of the 28S rRNA large subunit accurately identified the wild mushroom sample isolates UBU_LS1 and UBU_LP2 as L. squarrosulus and L. polychrous, respectively.
Currently, a wide range of polysaccharide extraction methods are available for different mushroom species. These methods include conventional hot water extraction (HWA), subcritical water extraction (SWE), enzyme-assisted extraction (EAE), ultrasonic-assisted extraction (UAE), microwave-assisted extraction (MAE), ultrasonic–microwave synergistic extraction (UMSE), pulsed electric field-assisted extraction (PEFAE), and aqueous two-phase extraction (ATPE) [32]. Among these methods, HWA is widely used because of its simplicity and because it does not require specialized equipment. Herein, polysaccharides derived from the dried fruiting bodies of L. squarrosulus UBU_LS1 and L. polychrous UBU_LP2 were extracted using HWA, followed by absolute ethanol precipitation. The extraction yield of crude polysaccharides from L. polychrous UBU_LP2 was slightly higher than that from L. squarrosulus UBU_LS1. This was attributed to species-dependent factors. Our results are in agreement with a previous report by Nowak et al., who demonstrated that using the same protocol for different species resulted in variable yields [33]. Thus, a species-dependent factor affects the polysaccharide extraction yield. The extraction yield of both L. squarrosulus UBU_LS1 and L. polychrous UBU_LP2 exceeded the previous report that used the same mushroom species and different species such as Grifola frondose (3.35 ± 0.16%) [29] and Inonotus obliquus (3.81 ± 0.34%) [34]. However, it has been demonstrated that the extraction yield derived using the HWE method may be increased if the protocol is optimized. As previously reported for polysaccharide extraction from wild G. frondose, a higher extraction yield was obtained when the extraction temperature and time and the ratio of liquid to raw material were optimized [35]. Moreover, repeated extractions (up to six) enhanced the polysaccharide yield without altering polysaccharide properties [32].
In addition to the quantity of extracted polysaccharides, the composition of crude polysaccharides also influences their properties [36]. Both CPS_UBU_LS1 and CPS_UBU_LP2 contained a high carbohydrate content, which was mostly polysaccharides, and very low amounts of reducing sugars and proteins. Thus, the crude extracts derived from both mushrooms could be considered as crude polysaccharides. High-performance liquid chromatography (HPLC) analysis of the monosaccharide components revealed that CPS_UBU_LS1 and CPS_UBU_LP2, composed of glucose, galactose, fucose, and mannose with varying concentrations of these fucoses, were greater, indicating a typical heteropolysaccharide. It has been reported that crude polysaccharides from L. squarrosulus extracted by HWA are composed of galactose, glucose, and mannose as the major monosaccharides [37]. Using a similar HWA extraction method, the crude polysaccharide content of Agaricus bisporus and Agaricus brasiliensis was mainly constituted of glucose, mannose, galactose, and methyl-galactose, with low amounts of fucose and ribose. Among these monosaccharides, fucose, in a formulation with fucogalactan, is presumed to play an important role in immunomodulatory activities [38]. Moreover, Thetsrimuang et al. (2011) found that mannose was a major monosaccharide in crude polysaccharides extracted from Lentinus sp. [39]. Mannose is an essential monosaccharide for biological activities, such as antioxidation, in various polysaccharides, including those of Lentinus species [9,40]. Xylose has been reported as a minor monosaccharide that may be an essential component of biological properties in L. squarrosulus crude polysaccharide extracts [38]. Thus, CPS_UBU_LS1 and CPS_UBU_LP2 were reasonable for further elucidation of their properties, including the influence of polysaccharide composition and structure on their biological activities.
Prebiotics are attractive candidates comprising crude and pure polysaccharides extracted from edible mushrooms. Some edible cultivatable and wild mushrooms have been examined for their prebiotic potential and other properties, such as antioxidant activity, cytotoxicity against cancer cells [9], and pathogenic growth inhibition [40]. Two types of well-characterized prebiotic fructans (inulin and fructo-oligosaccharide (FOS)) and galacto-oligosaccharides (GOSs) have been used for health promotion and for treating some gastrointestinal disorders [41]. Another major goal of using prebiotics in humans is to modulate beneficial microorganisms, mainly bacteria, in the large intestine [42]. Thus, to reach the large intestine, prebiotics must resist the acidic conditions of the stomach and the enzymatic system in the intestine. Our study demonstrated that CPS_UBU_LS1 and CPS_UBU_LP2 had resisted hydrolysis by simulated human gastric acidic solution at both pH 1 and pH 5, as polysaccharide activity was retained at more than 90%. The retained polysaccharide activities of these two crude prebiotics were comparable to those of inulin, which was used as the reference prebiotic. These results indicated that CPS_UBU_LS1 and CPS_UBU_LP2 are suitable for application as prebiotics in humans.
In addition to hydrolytic resistance under acidic conditions, stimulation of beneficial bacteria, particularly probiotic strains, is a major property of candidate prebiotics [2]. CPS_UBU _LS1 and CPS_UBU_LP2 stimulated three probiotic strains, L. fermentum 47-7, L. rhamnosus NH9, and L. rhamnosus 23-2. The percentage of growth stimulation of these probiotic strains with CPS_UBU_LS1 and CPS_UBU_LP2 was significantly higher than that of inulin and FOS and comparable to that of GOS. Our results are in concordance with previous findings by Nowak et al., who found that crude polysaccharides extracted from wild edible mushrooms could act as prebiotics that stimulate the growth of probiotic bacterial strains [33]. Other mushrooms, such as Pleurotus ostreatus, Pleurotus eryngii, and Trametes versicolor, reportedly contain polysaccharides that stimulate probiotic lactobacilli and Bifidobacterium growth [43,44]. Moreover, the crude polysaccharide and its purified fraction from the medicinal mushroom Ganoderma lucidum have potential as prebiotics to stimulate Bifidobacterium species in in vitro and human fecal fermentation assays [45]. Thus, based on this evidence, crude mushrooms can be used as a source of prebiotics, and further studies are required to evaluate their potential in vivo, especially in healthy humans and/or those with particular diseases.
In the DPPH radical scavenging assay, a low IC50 value indicates a potent DPPH scavenger. The crude polysaccharides CPS_UBU_LS1 and CPS_UBU_LP2 could be considered as potent antioxidative compounds as they showed low IC50 values of 0.5 and 0.38, respectively. These values were higher than those of ascorbic acid, a reference antioxidant compound. The potent DPPH scavenging activity of the polysaccharides extracted from L. squarrosulus was enhanced by increasing the concentration of polysaccharides in the reaction. This indicates the dose-dependent properties of polysaccharides as DPPH scavengers [38]. Moreover, it has been reported that the antioxidant activity of polysaccharides could be enhanced by the chemical modification of polysaccharide structure, for example by sulfation, resulting in a high solubility of crude polysaccharides and leading to greater antioxidant activity [46]. As detailed in a previous report, the polysaccharide properties, including solubility, net charge, and side chain, play an important role in their antioxidant activity [47]. Thus, further analysis of CPS_UBU_LS1 and CPS_UBU_LP2 properties, as mentioned above, is required to understand their function.
Many commercial functional food products, particularly synbiotics, have attracted considerable consumer attention. The final synbiotic product is mostly non-encapsulated, encapsulated, or pilled. Thus, the production chain and storage are crucial processes. Freeze drying or lyophilization is a widely used method for preserving viable bacteria, including probiotics, in a dried form [24]. This process requires two main components: the target probiotic strain and wall material or one or more effective cryoprotectants. Some cryoprotectants, such as monosaccharides (glucose, galactose, and mannose), disaccharides (trehalose, sucrose, turanose, and lactose), standard prebiotics (FOS, GOS, and lactulose), and other substances including skim milk, have been used to enhance the survival of specific probiotics during freeze drying [48,49,50]. According to the definition of synbiotics, the compounds acting as cryoprotectants used in synbiotic formulations must be “food-grade” [51]. Freeze drying does not affect the antioxidant activity of crude polysaccharides extracted from the mushroom I. obliquus [52]. Moreover, it was reported that freeze drying did not alter the quality, including the polysaccharide properties, of products derived from the mushroom Tremella fuciformis [53]. Thus, polysaccharides provide an attractive option for use as cryoprotectants. Our result showed that CPS_UBU_LS1 and CPS_UBU_LP2 preserved the survival ability of the selected probiotic L. fermentum 47-7 for up to 90 days of storage at 4 °C at the viability of 1.55 × 1010 CFU/mL and 1.57 × 1010 CFU/mL, respectively. The number of viable cells was comparable to that obtained from lyophilized cells treated with SM and GOS. Additionally, the number of viable cells in our study showed adequate probiotic benefits [54]. It has been reported that monosaccharides, such as glucose and mannose, found in crude polysaccharide played an importance role in the viability during freeze drying and storage. The lyophilized cells were enveloped inside the cryoprotectant, which may be the protective mechanism by which the crude polysaccharide preserves probiotics, as damaged cells were not observed under SEM in the samples, except for lyophilized 47-7 cells alone.
5. Conclusions
In this study, two wild edible mushrooms collected from the local area of Ubon Ratchathani Province, Thailand, were successfully identified at the species level. We demonstrated that wild edible mushrooms of L. squarrosulus and L. polychrous can be a natural source of prebiotics and applied as cryoprotectants in the freeze-drying process for the development of a synbiotic product. Moreover, these crude polysaccharides exhibited antioxidative potential. Although our result demonstrated the potential prebiotic of crude polysaccharides extracted from wild edible mushrooms, the prebiotic properties need to be evaluated ex vivo and in vivo in the next research steps. Further evaluation of these prebiotics, including the elucidation of the polysaccharide structure–function relationship and the determination of the potential rehabilitation of beneficial gut microbes in diseased individuals, is currently being conducted by our research group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13020287/s1, Figure S1: Viability of probiotic L. fermentum 47-7 formulated with different ingredients of cryoprotective media after freeze drying and storage for 90 days; Table S1: Viability of probiotic L. fermentum 47-7 formulated with different ingredients of cryoprotective media after freeze drying and storage for 90 days. Table S2: Contents of reducing sugar releasing in gastric buffer pH 1 and 5 (Raw data). Table S3: The percent of polysaccharide hydrolysis in gastric buffer pH 1 and 5 (Raw data). Table S4: The percent of polysaccharide hydrolysis tolerance in gastric buffer pH 1 and 5 (Raw data).
Author Contributions
M.P. prepared research proposal for grant receival, designed and performed the experiments, analyzed data, and performed manuscript preparation and submission. C.K. performed the experiments and data analysis. P.S. prepared SEM for observing lyophilized cells. P.P. provided equipment and culture medium and extracted crude polysaccharides. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Science, Research and Innovation Fund (Fundamental Fund, FF65) (grant to MP). Funding for English editing and publication fee was supported by the College of Medicine and Public Health and Ubon Ratchathani University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
We thank Sureepawn Kroongtong for preparing experimental equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in healthy young population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef]
- He, Q.; Si, C.; Sun, Z.; Chen, Y.; Zhang, X. The Intervention of Prebiotics on Depression via the Gut—Brain Axis. Molecules 2022, 27, 3671. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Y.; Cui, Z.; Liu, J. Purification, characterization and biological activity of a novel polysaccharide from Inonotus obliquus. Int. J. Biol. Macromol. 2015, 79, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Fangkrathok, N.; Sripanidkulchai, K.; Junlatat, J.; Sripanidkulchai, B. Bioactivities of Lentinus polychrous and Ganoderma lucidum (Agaricomycetes) Polysaccharide Extracts. Int. J. Med. Mushrooms 2021, 23, 51–61. [Google Scholar] [CrossRef]
- Das, S.; Prakash, B. Chapter 11—Edible mushrooms: Nutritional composition and medicinal benefits for improvement in quality life. In Research and Technological Advances in Food Science; Prakash, B., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 269–300. [Google Scholar]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef]
- Thetsrimuang, C.; Khammuang, S.; Chiablaem, K.; Srisomsap, C.; Sarnthima, R. Antioxidant properties and cytotoxicity of crude polysaccharides from Lentinus polychrous Lév. Food Chem. 2011, 128, 634–639. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review. Viruses 2021, 13, 350. [Google Scholar] [CrossRef]
- Zhu, H.; Sheng, K.; Yan, E.; Qiao, J.; Lv, F. Extraction, purification and antibacterial activities of a polysaccharide from spent mushroom substrate. Int. J. Biol. Macromol. 2012, 50, 840–843. [Google Scholar] [CrossRef]
- Liao, S.F.; Liang, C.H.; Ho, M.Y.; Hsu, T.L.; Tsai, T.I.; Hsieh, Y.S.; Tsai, C.M.; Li, S.T.; Cheng, Y.Y.; Tsao, S.M.; et al. Immunization of fucose-containing polysaccharides from Reishi mushroom induces antibodies to tumor-associated Globo H-series epitopes. Proc. Natl. Acad. Sci. USA 2013, 110, 13809–13814. [Google Scholar] [CrossRef]
- Shamim, M.Z.; Mishra, A.K.; Kausar, T.; Mahanta, S.; Sarma, B.; Kumar, V.; Mishra, P.K.; Panda, J.; Baek, K.H.; Mohanta, Y.K. Exploring Edible Mushrooms for Diabetes: Unveiling Their Role in Prevention and Treatment. Molecules 2023, 28, 2837. [Google Scholar] [CrossRef]
- Li, I.C.; Chang, H.H.; Lin, C.H.; Chen, W.P.; Lu, T.H.; Lee, L.Y.; Chen, Y.W.; Chen, Y.P.; Chen, C.C.; Lin, D.P. Prevention of Early Alzheimer’s Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lan, J.; Dai, X.; Ye, J.; Zhou, S. A phase I/II study of Ling Zhi mushroom Ganoderma lucidum (W. Curt.: Fr.) Lloyd (Aphyllophoromycetideae) extract in patients with type II diabetes mellitus. Int. J. Med. Mushrooms 2004, 6. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Marras, E.; Ferrario, N.; Vivona, V.; Prini, P.; Vignati, F.; Perletti, G. Anti-Cancer Potential of Edible/Medicinal Mushrooms in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 10120. [Google Scholar] [CrossRef] [PubMed]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Choonpicharn, S.; Tanreuan, K.; Lumyong, S. Characterization of Polysaccharides from Wild Edible Mushrooms from Thailand and Their Antioxidant, Antidiabetic, and Antihypertensive Activities. Int. J. Med. Mushrooms 2020, 22, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Huang, R.; Huang, A.; Wang, J.; Liu, W.; Wu, S.; Chen, M.; Chen, M.; Xie, Y.; Jiao, C.; et al. Polysaccharide from Agrocybe cylindracea prevents diet-induced obesity through inhibiting inflammation mediated by gut microbiota and associated metabolites. Int. J. Biol. Macromol. 2022, 209, 1430–1438. [Google Scholar] [CrossRef]
- Hu, W.; Di, Q.; Liang, T.; Zhou, N.; Chen, H.; Zeng, Z.; Luo, Y.; Shaker, M. Effects of in vitro simulated digestion and fecal fermentation of polysaccharides from straw mushroom (Volvariella volvacea) on its physicochemical properties and human gut microbiota. Int. J. Biol. Macromol. 2023, 239, 124188. [Google Scholar] [CrossRef]
- Giannenas, I.; Tsalie, E.; Chronis, E.; Mavridis, S.; Tontis, D.; Kyriazakis, I. Consumption of Agaricus bisporus mushroom affects the performance, intestinal microbiota composition and morphology, and antioxidant status of turkey poults. Anim. Feed. Sci. Technol. 2011, 165, 218–229. [Google Scholar] [CrossRef]
- Yu, Z.T.; Liu, B.; Mukherjee, P.; Newburg, D.S. Trametes versicolor extract modifies human fecal microbiota composition in vitro. Plant Foods Hum. Nutr. 2013, 68, 107–112. [Google Scholar] [CrossRef]
- Ayimbila, F.; Siriwong, S.; Nakphaichit, M.; Keawsompong, S. In vitro gastrointestinal digestion of Lentinus squarrosulus powder and impact on human fecal microbiota. Sci. Rep. 2022, 12, 2655. [Google Scholar] [CrossRef]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Fonseca, F.; Cenard, S.; Passot, S. Freeze-Drying of Lactic Acid Bacteria. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2015; pp. 477–488. [Google Scholar]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Su, C.-H.; Lai, M.-N.; Ng, L.-T. Effects of different extraction temperatures on the physicochemical properties of bioactive polysaccharides from Grifola frondosa. Food Chem. 2017, 220, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Inyod, T.; Ayimbila, F.; Payapanon, A.; Keawsompong, S. Antioxidant activities and prebiotic properties of the tropical mushroom Macrocybe crassa. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100298. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Zhang, Y.; Zhou, J.; Yin, Y.; He, Q.; Jiang, S.; Ma, P.; Zhang, Y.; Wen, K.; et al. Mushroom Poisoning Outbreaks—China, 2020. China CDC Wkly. 2021, 3, 41–45. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.-C.; Chang, J.-S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Juda, M.; Malm, A. The preliminary study of prebiotic potential of Polish wild mushroom polysaccharides: The stimulation effect on Lactobacillus strains growth. Eur. J. Nutr. 2018, 57, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.Y.; Yang, S.C.; Kim, J.; Lim, T.; Cho, H.; Hwang, K.T.J.L. Effects of non-traditional extraction methods on extracting bioactive compounds from chaga mushroom (Inonotus obliquus) compared with hot water extraction. LWT 2019, 110, 80–84. [Google Scholar] [CrossRef]
- Chen, X.; Ji, H.; Xu, X.; Liu, A. Optimization of polysaccharide extraction process from grifola frondosa and its antioxidant and anti-tumor research. J. Food Meas. Charact. 2019, 13, 144–153. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, W.; Zhang, Y. Fungal polysaccharides. Adv. Pharmacol. 2020, 87, 277–299. [Google Scholar] [PubMed]
- Ayimbila, F.; Keawsompong, S. Functional composition and antioxidant property of crude polysaccharides from the fruiting bodies of Lentinus squarrosulus. 3 Biotech 2021, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Smiderle, F.R.; Ruthes, A.C.; van Arkel, J.; Chanput, W.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J.L.D. Polysaccharides from Agaricus bisporus and Agaricus brasiliensis show similarities in their structures and their immunomodulatory effects on human monocytic THP-1 cells. BMC Complement. Altern. Med. 2011, 11, 58. [Google Scholar] [CrossRef]
- Thetsrimuang, C.; Khammuang, S.; Sarnthima, R. Antioxidant Activity of Crude Polysaccharides from Edible Fresh and Dry Mushroom Fruiting Bodies of Lentinus sp. Strain RJ-2. Int. J. Pharmacol. 2011, 7, 58–65. [Google Scholar] [CrossRef]
- Sawangwan, T.; Wansanit, W.; Pattani, L.; Noysang, C. Study of prebiotic properties from edible mushroom extraction. Agric. Nat. Resour. 2018, 52, 519–524. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Yin, C.; Noratto, G.D.; Fan, X.; Chen, Z.; Yao, F.; Shi, D.; Gao, H. The Impact of Mushroom Polysaccharides on Gut Microbiota and Its Beneficial Effects to Host: A Review. Carbohydr. Polym. 2020, 250, 116942. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Angelova, G.; Brazkova, M.; Mihaylova, D.; Slavov, A.; Petkova, N.; Blazheva, D.; Deseva, I.; Gotova, I.; Dimitrov, Z.; Krastanov, A. Bioactivity of Biomass and Crude Exopolysaccharides Obtained by Controlled Submerged Cultivation of Medicinal Mushroom Trametes versicolor. J. Fungi 2022, 8, 738. [Google Scholar] [CrossRef] [PubMed]
- Shaari, M.Y.; Shuhaimi, M.; Ariff, A.; Bakar, F.; Abdul Khalil, K.; Othaman, M.; Abd Manap, Y. Effect of Ganoderma lucidum polysaccharides on the growth of Bifidobacterium spp. as assessed using real-time PCR. Int. Food Res. J. 2012, 19, 1199–1205. [Google Scholar]
- Xu, Y.; Wu, Y.-j.; Sun, P.-l.; Zhang, F.-m.; Linhardt, R.J.; Zhang, A.-q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef]
- Gul, L.B.; Gul, O.; Yilmaz, M.T.; Dertli, E.; Con, A.H. Optimization of cryoprotectant formulation to enhance the viability of Lactobacillus brevis ED25: Determination of storage stability and acidification kinetics in sourdough. J. Food Process. Preserv. 2020, 44, e14400. [Google Scholar] [CrossRef]
- Nag, A.; Das, S. Effect of trehalose and lactose as cryoprotectant during freeze-drying, in vitro gastro-intestinal transit and survival of microencapsulated freeze-dried Lactobacillus casei 431 cells. Int. J. Dairy Technol. 2013, 66, 162–169. [Google Scholar] [CrossRef]
- Han, D.J.; Jun, S.J.; Lee, B.H.; Yoo, S.H. Cryoprotective effect of turanose on lyophilized Lactobacillus paracasei subsp. paracasei, L. casei 431. Food Sci. Biotechnol. 2022, 31, 343–347. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Ma, L.; Chen, H.; Zhu, W.; Wang, Z. Effect of different drying methods on physicochemical properties and antioxidant activities of polysaccharides extracted from mushroom Inonotus obliquus. Food Res. Int. 2013, 50, 633–640. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Lai, P.; Tang, B.; Wu, L. Influence of drying methods on the physicochemical properties and nutritional composition of instant Tremella fuciformis. Food Sci. Technol. 2020, 40. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).