Evaluation of Prebiotic Potential of Crude Polysaccharides Extracted from Wild Lentinus polychrous and Lentinus squarrosulus and Their Application for a Formulation of a Novel Lyophilized Synbiotic
Abstract
:1. Background
2. Materials and Methods
2.1. Collection of Wild Edible Mushrooms
2.2. Genomic DNA Extraction
2.3. Identification of Mushroom Species
2.4. Mushroom Preparation for Polysaccharide Extraction
2.5. Crude Polysaccharide Extraction
2.6. Determination of Total Carbohydrate Content
2.7. Determination of Reducing Sugar
2.8. Determination of Protein Content
2.9. Analysis of Monosaccharide Composition in Crude Polysaccharides
2.10. Determination of Hydrolysis Tolerance in Simulated Gastric Buffer
2.11. Antioxidant Activity by 1,1-Diphenyl-2-picrylhydrazyl Scavenging Ability
2.12. Determination of Probiotic Bacterial Growth Promoted by Crude Polysaccharides
2.13. Preparation of Lyophilized Synbiotic
2.14. Lyophilized Cells Observed Using Scanning Electron Microscopy
2.15. Survival Ability of Probiotic during Synbiotic Storage
3. Results
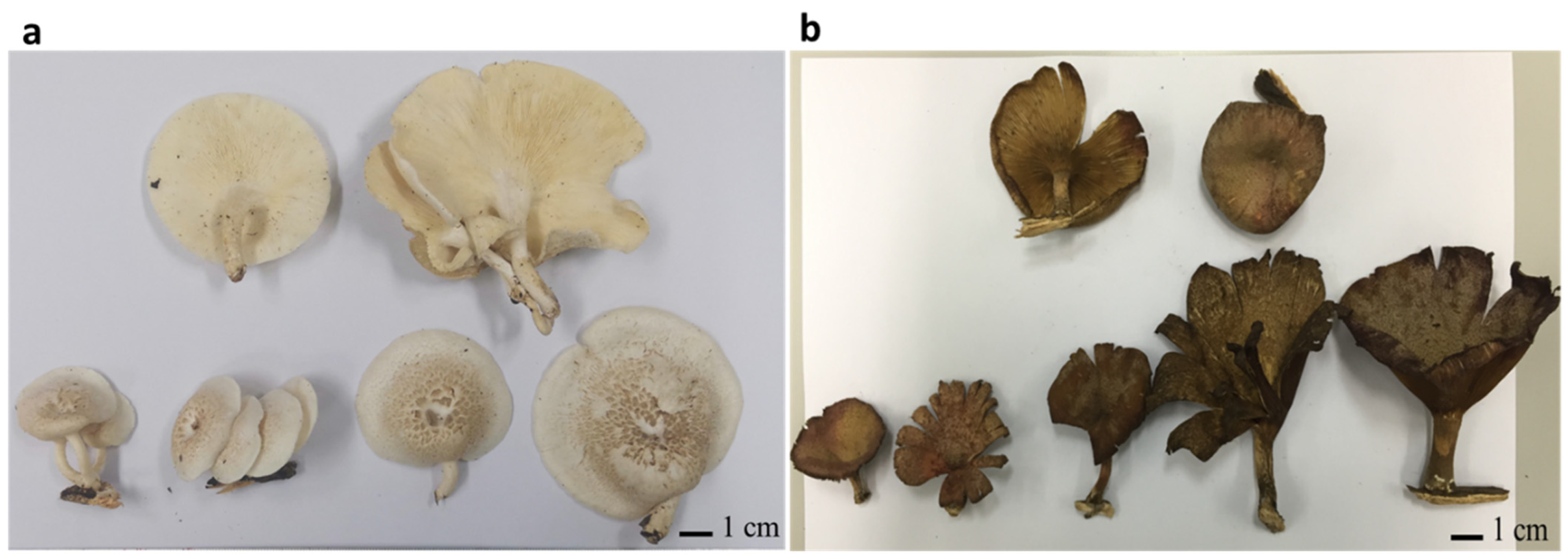
3.1. Wild Mushroom Collection and Fruiting Body Morphology Recording
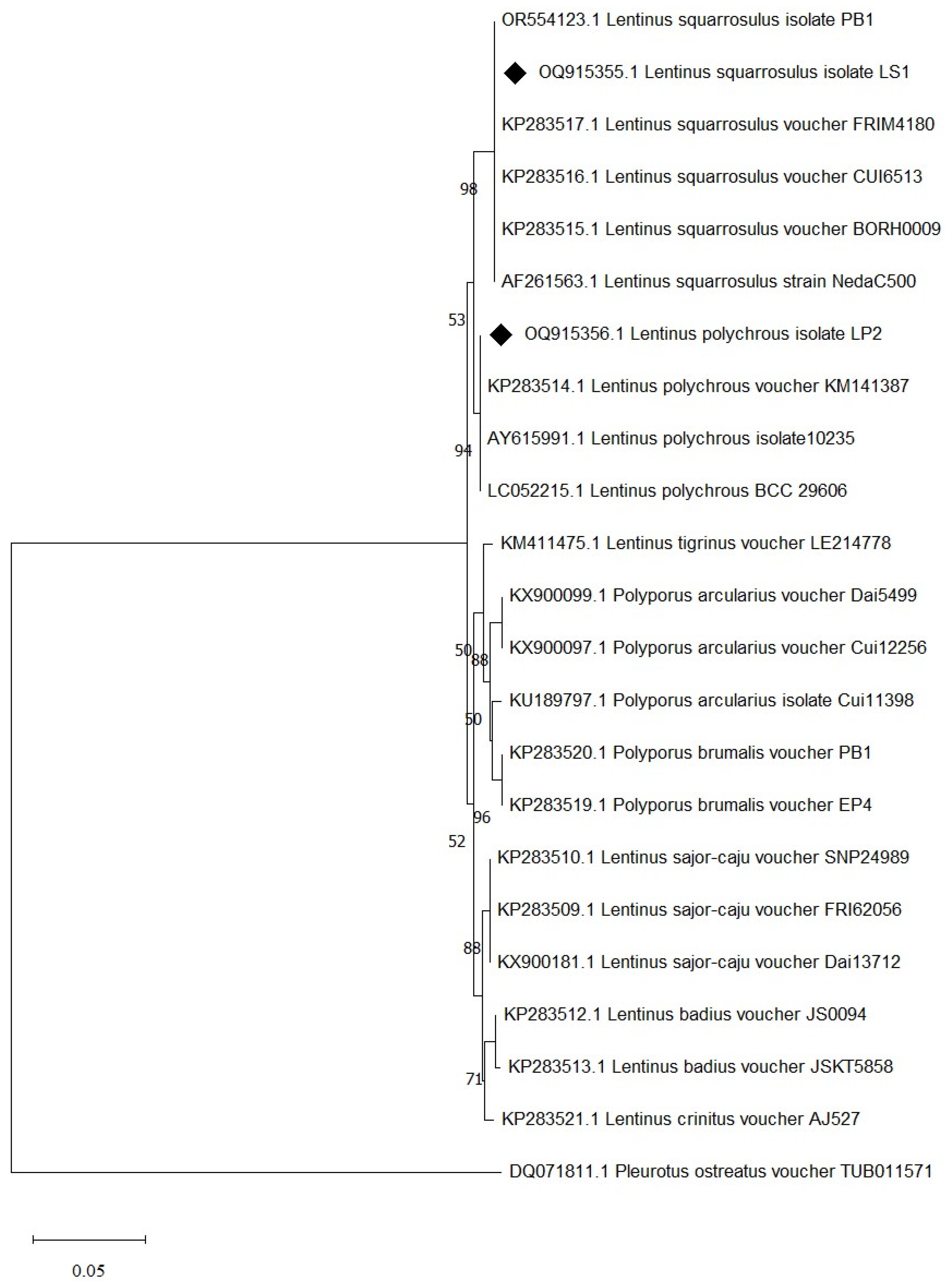
3.2. Molecular Identification of Mushroom Species
3.3. Deposition of Ribosomal RNA Gene Sequence in the NCBI Database
3.4. Yields of Crude Polysaccharide Extraction
3.5. Compound Component of Extracted Crude Polysaccharide
3.6. Monosaccharide Composition of CPS_UBU_LS1 and CPS_UBU_LP2
3.7. Resistance in Simulated Human Gastric Acidic pH Solution
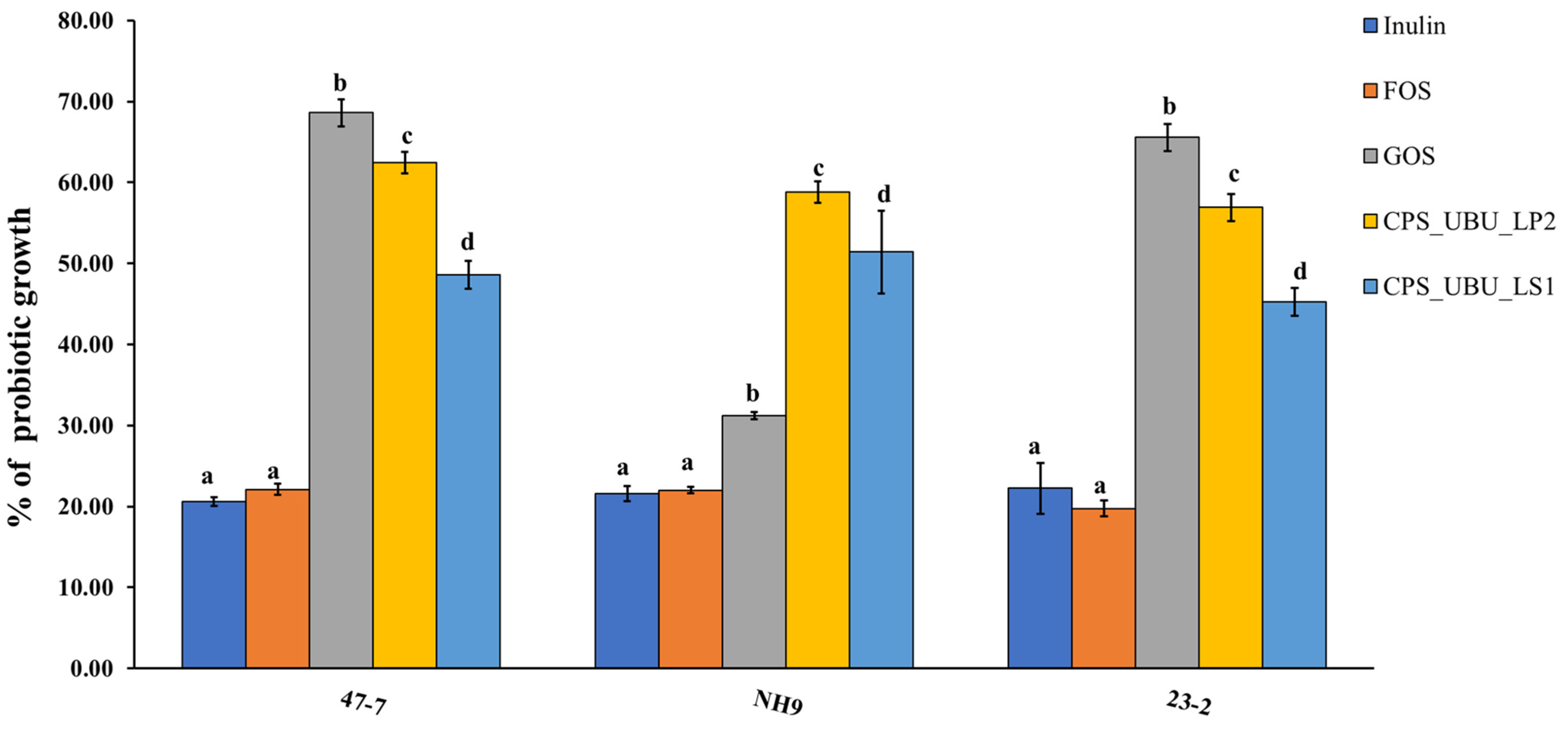
3.8. Potential of Crude Polysaccharides for Stimulating Probiotic Growth
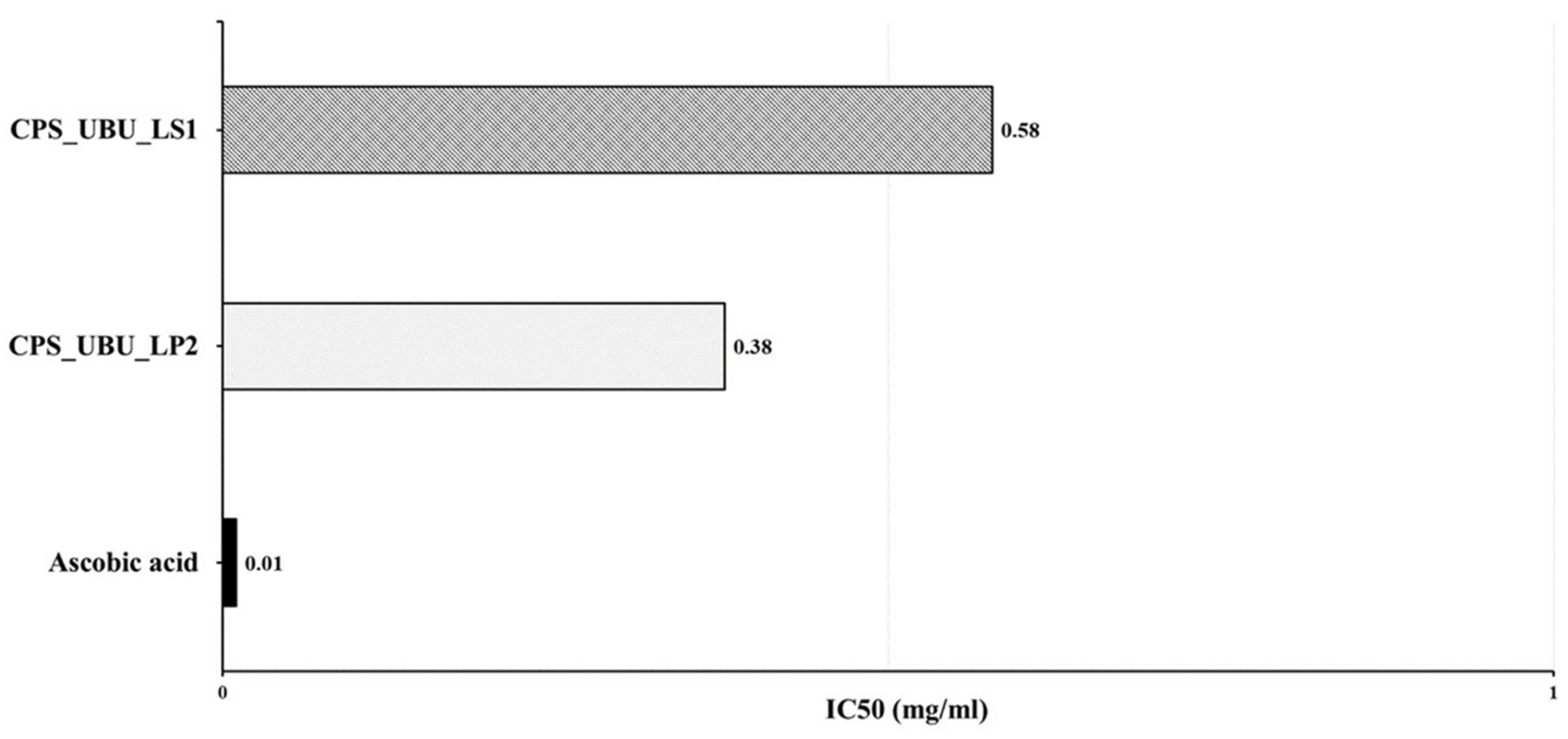
3.9. Antioxidant Activities of Crude Polysaccharides
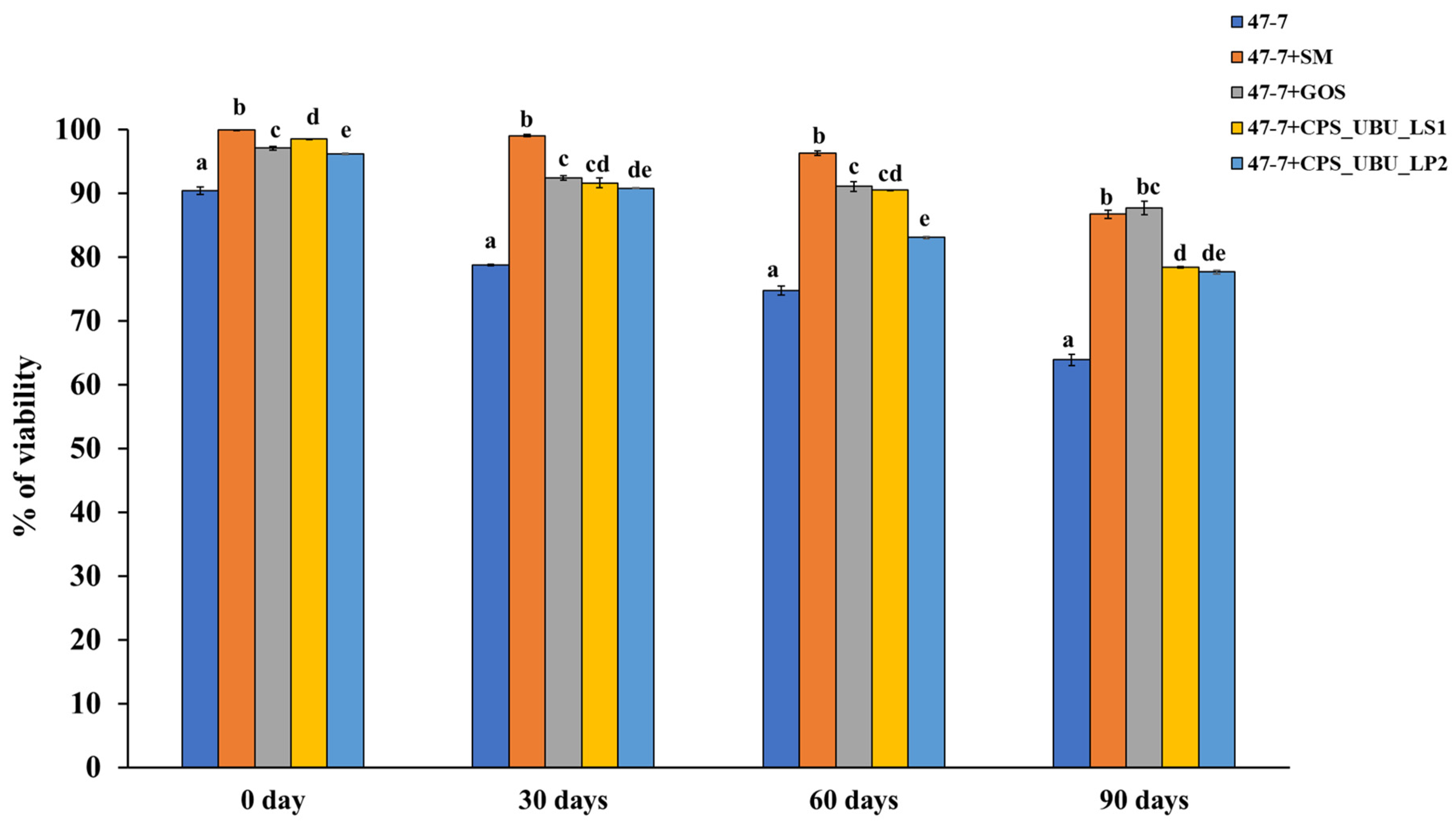
3.10. Potential Crude Polysaccharides as Cryoprotectants in the Freeze-Drying Process and for Storage
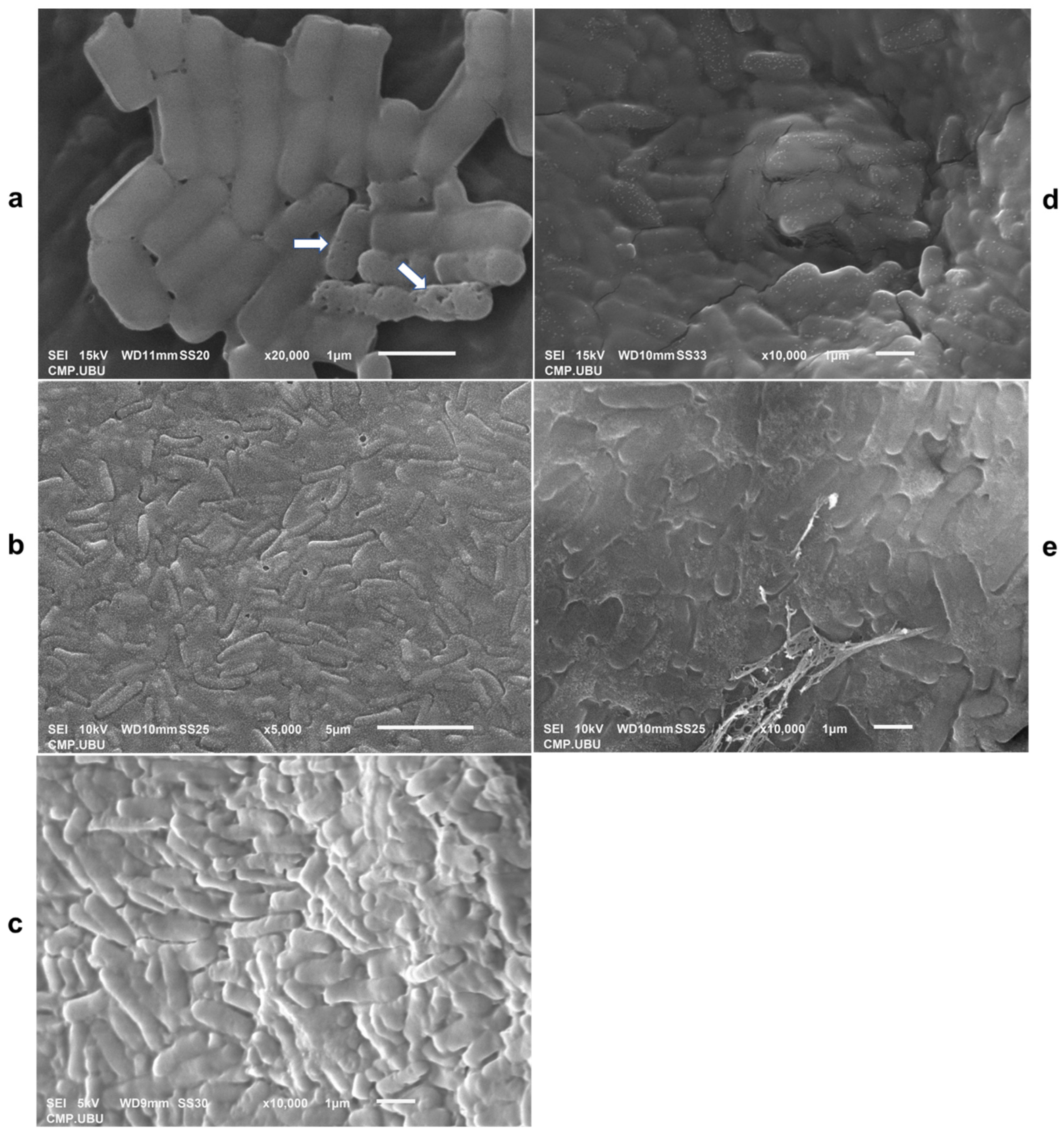
3.11. Lyophilized Cell Morphology Observed Using SEM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in healthy young population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef]
- He, Q.; Si, C.; Sun, Z.; Chen, Y.; Zhang, X. The Intervention of Prebiotics on Depression via the Gut—Brain Axis. Molecules 2022, 27, 3671. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Y.; Cui, Z.; Liu, J. Purification, characterization and biological activity of a novel polysaccharide from Inonotus obliquus. Int. J. Biol. Macromol. 2015, 79, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Fangkrathok, N.; Sripanidkulchai, K.; Junlatat, J.; Sripanidkulchai, B. Bioactivities of Lentinus polychrous and Ganoderma lucidum (Agaricomycetes) Polysaccharide Extracts. Int. J. Med. Mushrooms 2021, 23, 51–61. [Google Scholar] [CrossRef]
- Das, S.; Prakash, B. Chapter 11—Edible mushrooms: Nutritional composition and medicinal benefits for improvement in quality life. In Research and Technological Advances in Food Science; Prakash, B., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 269–300. [Google Scholar]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef]
- Thetsrimuang, C.; Khammuang, S.; Chiablaem, K.; Srisomsap, C.; Sarnthima, R. Antioxidant properties and cytotoxicity of crude polysaccharides from Lentinus polychrous Lév. Food Chem. 2011, 128, 634–639. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review. Viruses 2021, 13, 350. [Google Scholar] [CrossRef]
- Zhu, H.; Sheng, K.; Yan, E.; Qiao, J.; Lv, F. Extraction, purification and antibacterial activities of a polysaccharide from spent mushroom substrate. Int. J. Biol. Macromol. 2012, 50, 840–843. [Google Scholar] [CrossRef]
- Liao, S.F.; Liang, C.H.; Ho, M.Y.; Hsu, T.L.; Tsai, T.I.; Hsieh, Y.S.; Tsai, C.M.; Li, S.T.; Cheng, Y.Y.; Tsao, S.M.; et al. Immunization of fucose-containing polysaccharides from Reishi mushroom induces antibodies to tumor-associated Globo H-series epitopes. Proc. Natl. Acad. Sci. USA 2013, 110, 13809–13814. [Google Scholar] [CrossRef]
- Shamim, M.Z.; Mishra, A.K.; Kausar, T.; Mahanta, S.; Sarma, B.; Kumar, V.; Mishra, P.K.; Panda, J.; Baek, K.H.; Mohanta, Y.K. Exploring Edible Mushrooms for Diabetes: Unveiling Their Role in Prevention and Treatment. Molecules 2023, 28, 2837. [Google Scholar] [CrossRef]
- Li, I.C.; Chang, H.H.; Lin, C.H.; Chen, W.P.; Lu, T.H.; Lee, L.Y.; Chen, Y.W.; Chen, Y.P.; Chen, C.C.; Lin, D.P. Prevention of Early Alzheimer’s Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lan, J.; Dai, X.; Ye, J.; Zhou, S. A phase I/II study of Ling Zhi mushroom Ganoderma lucidum (W. Curt.: Fr.) Lloyd (Aphyllophoromycetideae) extract in patients with type II diabetes mellitus. Int. J. Med. Mushrooms 2004, 6. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Marras, E.; Ferrario, N.; Vivona, V.; Prini, P.; Vignati, F.; Perletti, G. Anti-Cancer Potential of Edible/Medicinal Mushrooms in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 10120. [Google Scholar] [CrossRef] [PubMed]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Choonpicharn, S.; Tanreuan, K.; Lumyong, S. Characterization of Polysaccharides from Wild Edible Mushrooms from Thailand and Their Antioxidant, Antidiabetic, and Antihypertensive Activities. Int. J. Med. Mushrooms 2020, 22, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Huang, R.; Huang, A.; Wang, J.; Liu, W.; Wu, S.; Chen, M.; Chen, M.; Xie, Y.; Jiao, C.; et al. Polysaccharide from Agrocybe cylindracea prevents diet-induced obesity through inhibiting inflammation mediated by gut microbiota and associated metabolites. Int. J. Biol. Macromol. 2022, 209, 1430–1438. [Google Scholar] [CrossRef]
- Hu, W.; Di, Q.; Liang, T.; Zhou, N.; Chen, H.; Zeng, Z.; Luo, Y.; Shaker, M. Effects of in vitro simulated digestion and fecal fermentation of polysaccharides from straw mushroom (Volvariella volvacea) on its physicochemical properties and human gut microbiota. Int. J. Biol. Macromol. 2023, 239, 124188. [Google Scholar] [CrossRef]
- Giannenas, I.; Tsalie, E.; Chronis, E.; Mavridis, S.; Tontis, D.; Kyriazakis, I. Consumption of Agaricus bisporus mushroom affects the performance, intestinal microbiota composition and morphology, and antioxidant status of turkey poults. Anim. Feed. Sci. Technol. 2011, 165, 218–229. [Google Scholar] [CrossRef]
- Yu, Z.T.; Liu, B.; Mukherjee, P.; Newburg, D.S. Trametes versicolor extract modifies human fecal microbiota composition in vitro. Plant Foods Hum. Nutr. 2013, 68, 107–112. [Google Scholar] [CrossRef]
- Ayimbila, F.; Siriwong, S.; Nakphaichit, M.; Keawsompong, S. In vitro gastrointestinal digestion of Lentinus squarrosulus powder and impact on human fecal microbiota. Sci. Rep. 2022, 12, 2655. [Google Scholar] [CrossRef]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Fonseca, F.; Cenard, S.; Passot, S. Freeze-Drying of Lactic Acid Bacteria. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2015; pp. 477–488. [Google Scholar]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Su, C.-H.; Lai, M.-N.; Ng, L.-T. Effects of different extraction temperatures on the physicochemical properties of bioactive polysaccharides from Grifola frondosa. Food Chem. 2017, 220, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Inyod, T.; Ayimbila, F.; Payapanon, A.; Keawsompong, S. Antioxidant activities and prebiotic properties of the tropical mushroom Macrocybe crassa. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100298. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Zhang, Y.; Zhou, J.; Yin, Y.; He, Q.; Jiang, S.; Ma, P.; Zhang, Y.; Wen, K.; et al. Mushroom Poisoning Outbreaks—China, 2020. China CDC Wkly. 2021, 3, 41–45. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.-C.; Chang, J.-S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Juda, M.; Malm, A. The preliminary study of prebiotic potential of Polish wild mushroom polysaccharides: The stimulation effect on Lactobacillus strains growth. Eur. J. Nutr. 2018, 57, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.Y.; Yang, S.C.; Kim, J.; Lim, T.; Cho, H.; Hwang, K.T.J.L. Effects of non-traditional extraction methods on extracting bioactive compounds from chaga mushroom (Inonotus obliquus) compared with hot water extraction. LWT 2019, 110, 80–84. [Google Scholar] [CrossRef]
- Chen, X.; Ji, H.; Xu, X.; Liu, A. Optimization of polysaccharide extraction process from grifola frondosa and its antioxidant and anti-tumor research. J. Food Meas. Charact. 2019, 13, 144–153. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, W.; Zhang, Y. Fungal polysaccharides. Adv. Pharmacol. 2020, 87, 277–299. [Google Scholar] [PubMed]
- Ayimbila, F.; Keawsompong, S. Functional composition and antioxidant property of crude polysaccharides from the fruiting bodies of Lentinus squarrosulus. 3 Biotech 2021, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Smiderle, F.R.; Ruthes, A.C.; van Arkel, J.; Chanput, W.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J.L.D. Polysaccharides from Agaricus bisporus and Agaricus brasiliensis show similarities in their structures and their immunomodulatory effects on human monocytic THP-1 cells. BMC Complement. Altern. Med. 2011, 11, 58. [Google Scholar] [CrossRef]
- Thetsrimuang, C.; Khammuang, S.; Sarnthima, R. Antioxidant Activity of Crude Polysaccharides from Edible Fresh and Dry Mushroom Fruiting Bodies of Lentinus sp. Strain RJ-2. Int. J. Pharmacol. 2011, 7, 58–65. [Google Scholar] [CrossRef]
- Sawangwan, T.; Wansanit, W.; Pattani, L.; Noysang, C. Study of prebiotic properties from edible mushroom extraction. Agric. Nat. Resour. 2018, 52, 519–524. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Yin, C.; Noratto, G.D.; Fan, X.; Chen, Z.; Yao, F.; Shi, D.; Gao, H. The Impact of Mushroom Polysaccharides on Gut Microbiota and Its Beneficial Effects to Host: A Review. Carbohydr. Polym. 2020, 250, 116942. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Angelova, G.; Brazkova, M.; Mihaylova, D.; Slavov, A.; Petkova, N.; Blazheva, D.; Deseva, I.; Gotova, I.; Dimitrov, Z.; Krastanov, A. Bioactivity of Biomass and Crude Exopolysaccharides Obtained by Controlled Submerged Cultivation of Medicinal Mushroom Trametes versicolor. J. Fungi 2022, 8, 738. [Google Scholar] [CrossRef] [PubMed]
- Shaari, M.Y.; Shuhaimi, M.; Ariff, A.; Bakar, F.; Abdul Khalil, K.; Othaman, M.; Abd Manap, Y. Effect of Ganoderma lucidum polysaccharides on the growth of Bifidobacterium spp. as assessed using real-time PCR. Int. Food Res. J. 2012, 19, 1199–1205. [Google Scholar]
- Xu, Y.; Wu, Y.-j.; Sun, P.-l.; Zhang, F.-m.; Linhardt, R.J.; Zhang, A.-q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef]
- Gul, L.B.; Gul, O.; Yilmaz, M.T.; Dertli, E.; Con, A.H. Optimization of cryoprotectant formulation to enhance the viability of Lactobacillus brevis ED25: Determination of storage stability and acidification kinetics in sourdough. J. Food Process. Preserv. 2020, 44, e14400. [Google Scholar] [CrossRef]
- Nag, A.; Das, S. Effect of trehalose and lactose as cryoprotectant during freeze-drying, in vitro gastro-intestinal transit and survival of microencapsulated freeze-dried Lactobacillus casei 431 cells. Int. J. Dairy Technol. 2013, 66, 162–169. [Google Scholar] [CrossRef]
- Han, D.J.; Jun, S.J.; Lee, B.H.; Yoo, S.H. Cryoprotective effect of turanose on lyophilized Lactobacillus paracasei subsp. paracasei, L. casei 431. Food Sci. Biotechnol. 2022, 31, 343–347. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Ma, L.; Chen, H.; Zhu, W.; Wang, Z. Effect of different drying methods on physicochemical properties and antioxidant activities of polysaccharides extracted from mushroom Inonotus obliquus. Food Res. Int. 2013, 50, 633–640. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Lai, P.; Tang, B.; Wu, L. Influence of drying methods on the physicochemical properties and nutritional composition of instant Tremella fuciformis. Food Sci. Technol. 2020, 40. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
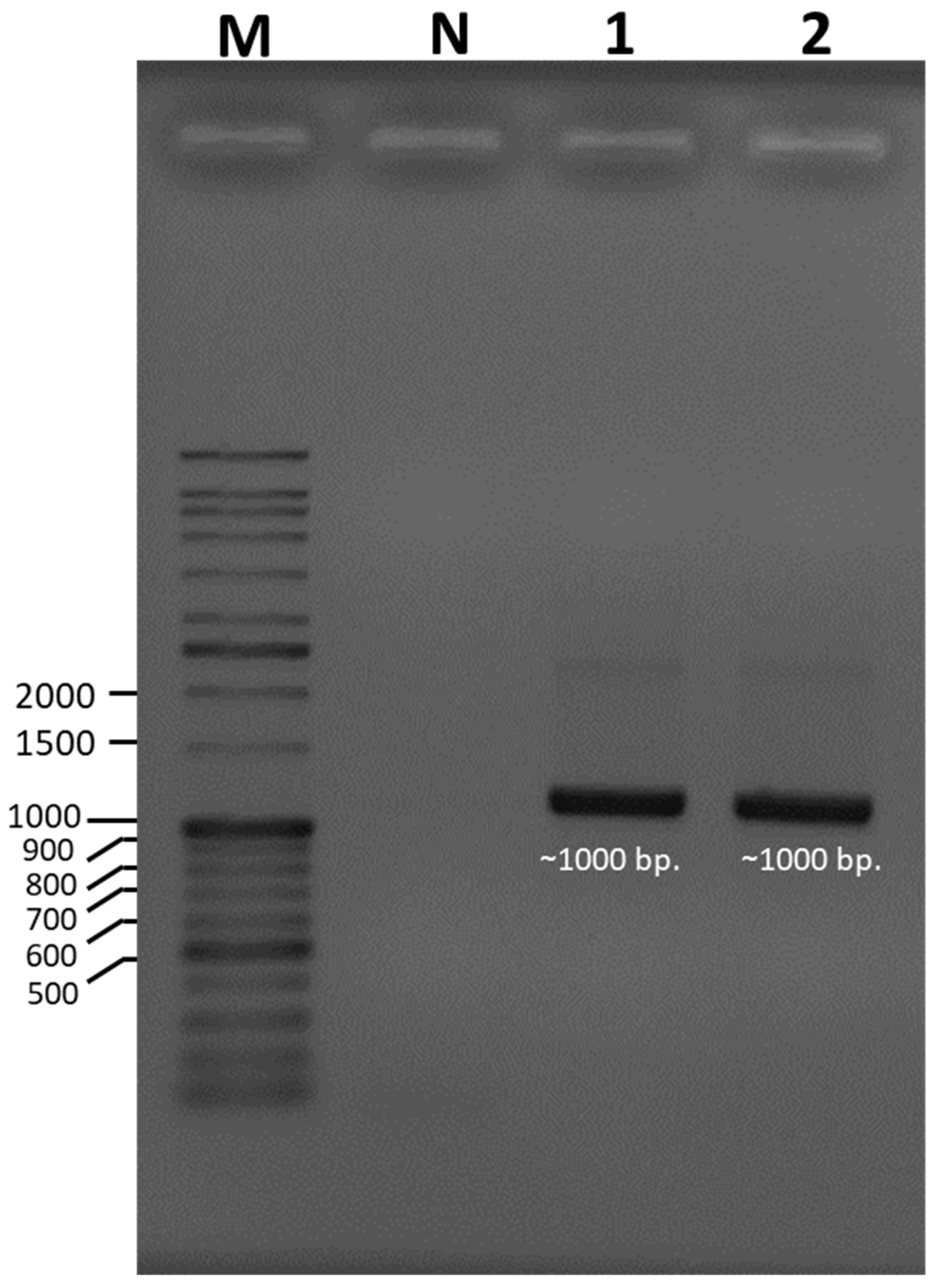

| Primer | Amplified DNA Size (bp.) | Sequence (5′→3′) | PCR Condition | Reference |
|---|---|---|---|---|
| Large subunit (LSU, 28S) of the rRNA | ||||
| LROR | ~1000 bp | ACCCGCTGAACTTAAGC | Step 1: 1 cycle of 95 °C, 5 min Step 2: 35 cycles of 94 °C, 30 s; 52 °C, 30 s; 72 °C, 1 min Step 3: 1 cycle of 72 °C, 8 min | [25] |
| LR6 | CGCCAGTTCTGCTTACC | |||
| Cryoprotective Media | L. fermentum 47-7 (CFU/mL) | Ingredients (mg) | |||
|---|---|---|---|---|---|
| Skim Milk | GOS | CPS_UBU_LS1 | CPS_UBU_LP2 | ||
| 47-7 | 2 × 1010 | - | - | - | - |
| 47-7+SM | 2 × 1010 | 500 | - | - | - |
| 47-7+GOS | 2 × 1010 | - | 500 | - | - |
| 47-7+CPS_UBU_LS1 | 2 × 1010 | - | - | 500 | - |
| 47-7+CPS_UBU_LP2 | 2 × 1010 | - | - | - | 500 |
| Crude Polysaccharide | Yield a (%) | Total Carbohydrate (mg/g) | Reducing Sugar (mg/g) | Polysaccharide (mg/g) | Total Protein (mg/g) |
|---|---|---|---|---|---|
| CPS_UBU_LS1 | 6.77 ± 0.02 | 313.99 ± 0.83 | 20.28 ± 1.92 | 293.68 ± 4.59 | 28.23 ± 0.38 |
| CPS_UBU_LP2 | 7.14 ± 0.01 | 364.65 ± 1.68 | 26.94 ± 0.96 | 337.71 ± 7.53 | 31.46 ± 0.36 |
| Crude Polysaccharide | Glucose (mM) | Galactose (mM) | Fucose (mM) | Mannose (mM) |
|---|---|---|---|---|
| CPS_UBU_LS1 | 0.88 | 0.22 | 2.13 | 1.10 |
| CPS_UBU_LP2 | 1.13 | 0.11 | 2.04 | 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panya, M.; Kaewraemruaen, C.; Saenwang, P.; Pimboon, P. Evaluation of Prebiotic Potential of Crude Polysaccharides Extracted from Wild Lentinus polychrous and Lentinus squarrosulus and Their Application for a Formulation of a Novel Lyophilized Synbiotic. Foods 2024, 13, 287. https://doi.org/10.3390/foods13020287
Panya M, Kaewraemruaen C, Saenwang P, Pimboon P. Evaluation of Prebiotic Potential of Crude Polysaccharides Extracted from Wild Lentinus polychrous and Lentinus squarrosulus and Their Application for a Formulation of a Novel Lyophilized Synbiotic. Foods. 2024; 13(2):287. https://doi.org/10.3390/foods13020287
Chicago/Turabian StylePanya, Marutpong, Chamraj Kaewraemruaen, Phairo Saenwang, and Patcharin Pimboon. 2024. "Evaluation of Prebiotic Potential of Crude Polysaccharides Extracted from Wild Lentinus polychrous and Lentinus squarrosulus and Their Application for a Formulation of a Novel Lyophilized Synbiotic" Foods 13, no. 2: 287. https://doi.org/10.3390/foods13020287
APA StylePanya, M., Kaewraemruaen, C., Saenwang, P., & Pimboon, P. (2024). Evaluation of Prebiotic Potential of Crude Polysaccharides Extracted from Wild Lentinus polychrous and Lentinus squarrosulus and Their Application for a Formulation of a Novel Lyophilized Synbiotic. Foods, 13(2), 287. https://doi.org/10.3390/foods13020287








