Impact of Mild Field Drought on the Aroma Profile and Metabolic Pathways of Fresh Tea (Camellia sinensis) Leaves Using HS-GC-IMS and HS-SPME-GC-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Collection of Tea Samples
2.2. Chemical Reagents
2.3. HS-GC-IMS Analysis
2.4. HS-SPME-GC-MS Analysis
2.4.1. Sample Preparation
2.4.2. HS-SPME Extraction of VOCs
2.4.3. GC-MS Analysis of VOCs
2.4.4. Identification and Quantification of VOCs
2.4.5. Odor Activity Value (OAV) Analysis of VOCs
2.5. Data Analysis
3. Results and Discussion
3.1. Qualitative and Quantitative Analysis of the VOCs by HS-GC-IMS
3.2. Identification of VOCs by HS-SPME-GC-MS
3.3. OAV Analysis of HS-SPME-GC–MS Data
3.4. Comparative Analysis of HS-GC-IMS and HS-SPME-GC-MS in Identifying VOCs
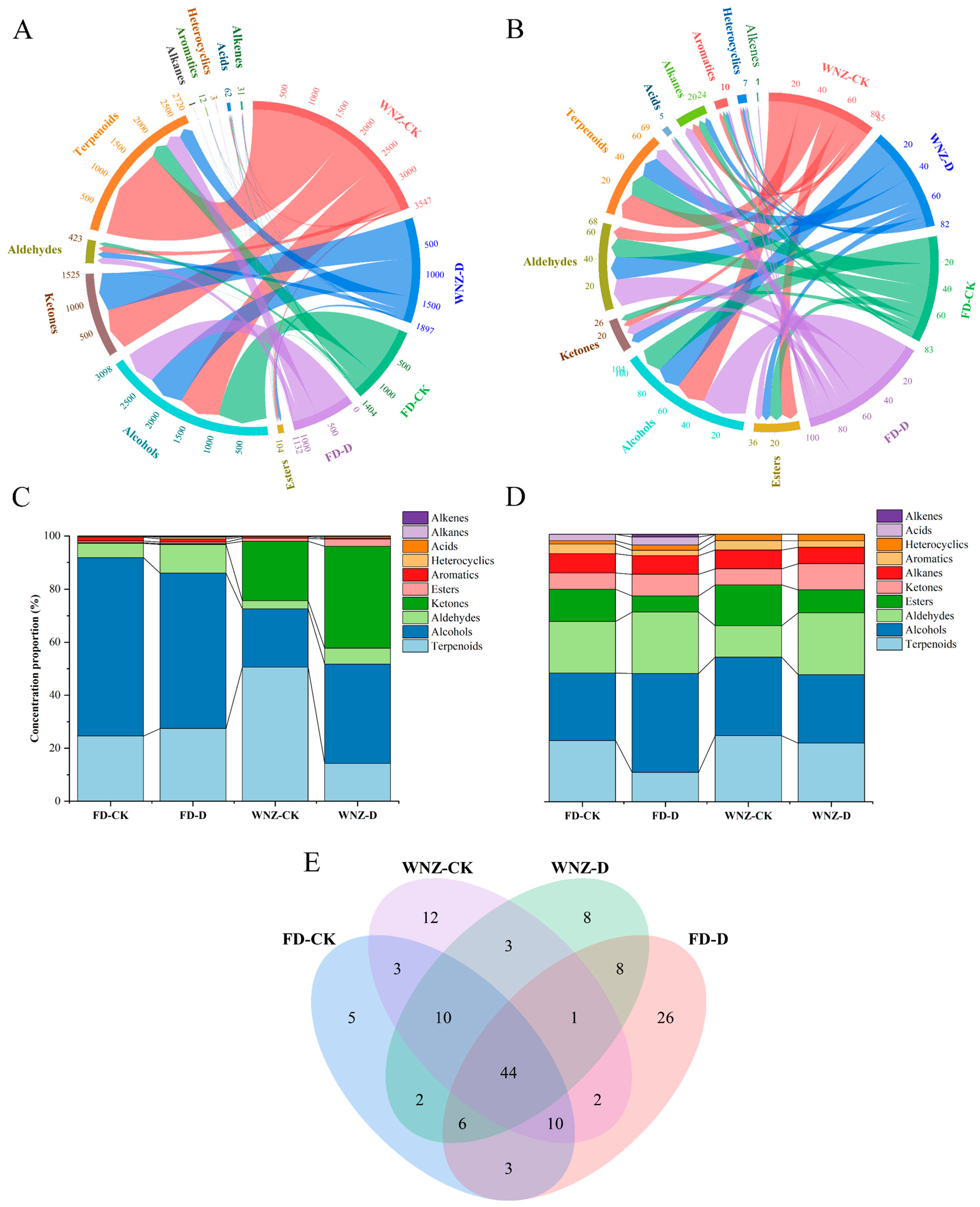
3.4.1. Comparative Analysis of VOC Quantities and Categories
3.4.2. PCA of All VOCs
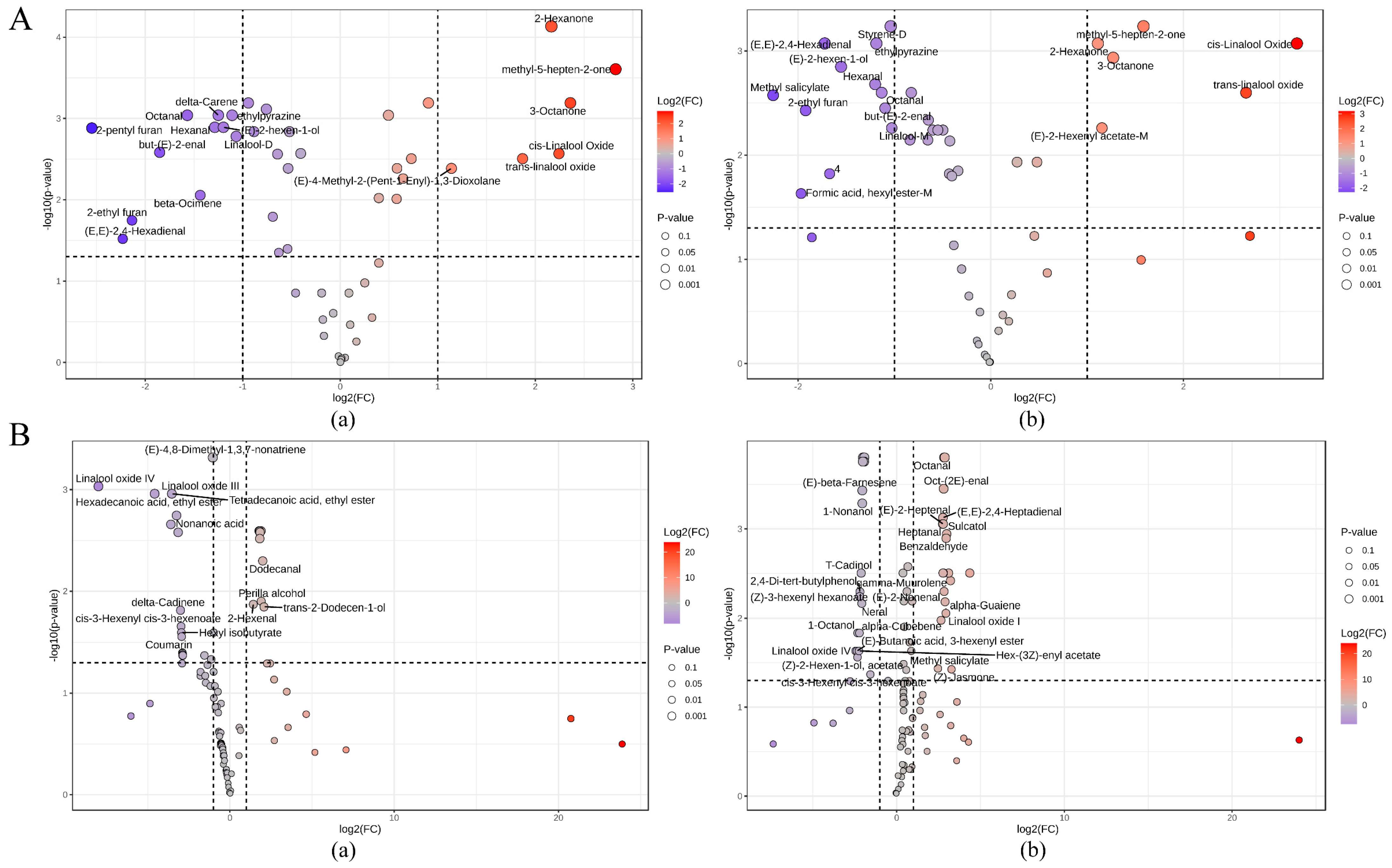
3.4.3. Identification of Aroma Markers Based on HS-GC-IMS and HS-SPME-GC–MS Data
3.4.4. VOC Change of Different Tea Cultivars Following Drought Stress
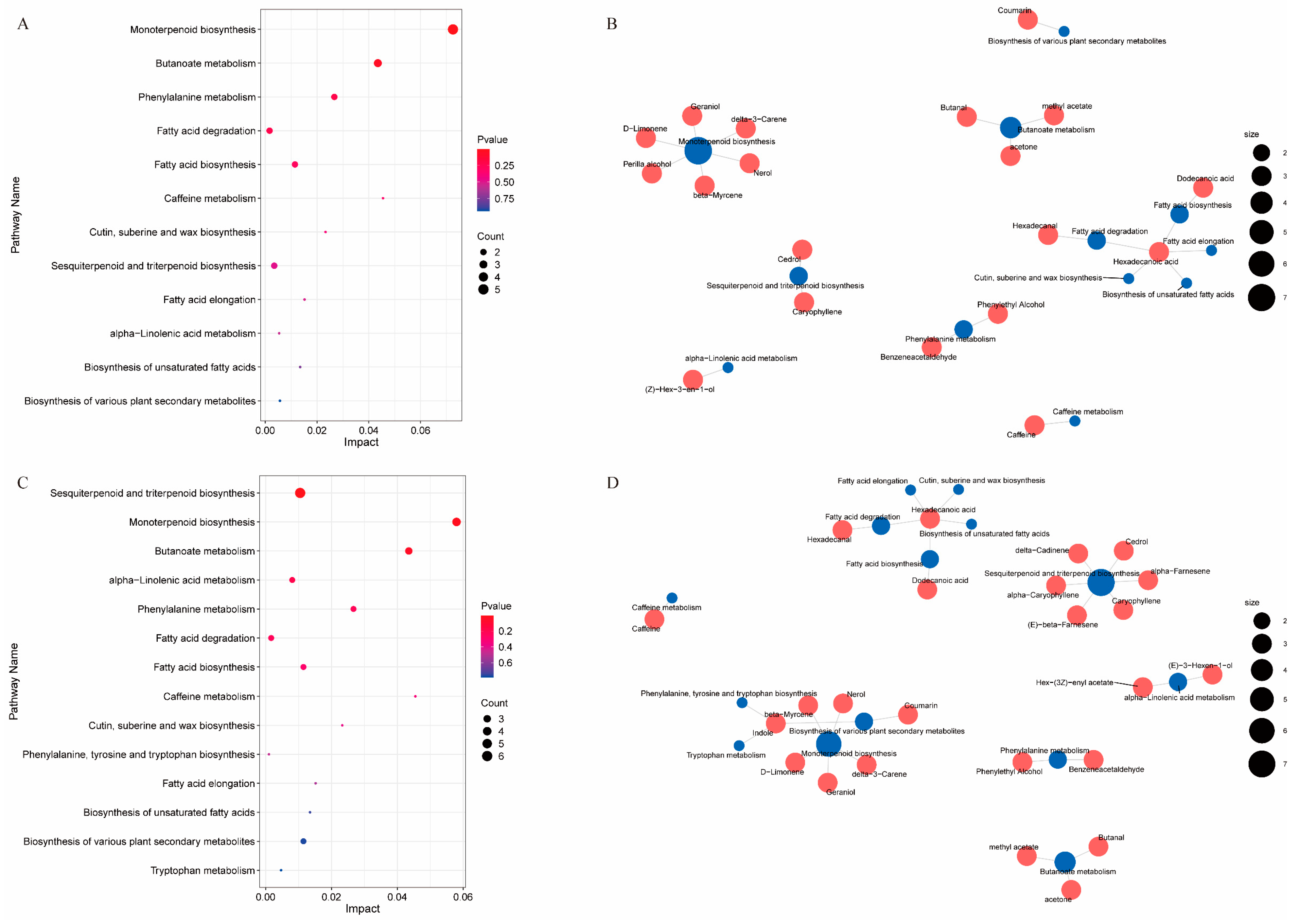
3.5. Analysis of Differential Metabolite KEGG Enrichment Pathways in Different Tea Cultivars Subjected to Drought Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Zhang, S.; Feng, Y.; Jiang, Y.; Yuan, H.; Shan, X.; Zhang, Q.; Niu, L.; Wang, S.; Zhou, Q.; et al. Seasonal variation in non-volatile flavor substances of fresh tea leaves (Camellia sinensis) by integrated lipidomics and metabolomics using UHPLC-Q-Exactive mass spectrometry. Food Chem. 2025, 462, 140986. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Zhao, D. Next-Generation Tea Beverages: Innovations in Formulation and Processing. J. Tea Sci. Res. 2024, 14, 112–122. [Google Scholar] [CrossRef]
- Xiang, L.; Zhu, C.; Qian, J.; Zhou, X.; Wang, M.; Song, Z.; Chen, C.; Yu, W.; Chen, L.; Zeng, L. Positive contributions of the stem to the formation of white tea quality-related metabolites during withering. Food Chem. 2024, 449, 139173. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Zhang, L.; Granvogl, M.; Ho, C.T.; Wan, X. Flavor of tea (Camellia sinensis): A review on odorants and analytical techniques. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3867–3909. [Google Scholar] [CrossRef]
- Fang, R.; Redfern, S.P.; Kirkup, D.; Porter, E.A.; Kite, G.C.; Terry, L.A.; Berry, M.J.; Simmonds, M.S.J. Variation of theanine, phenolic, and methylxanthine compounds in 21 cultivars of Camellia sinensis harvested in different seasons. Food Chem. 2017, 220, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhou, J.; He, C.; Qiu, L.; Zhang, D.; Yu, Z.; Wang, Y.; Ni, D.; Chen, Y. Non-targeted metabolomics characterization of flavor formation of Lichuan black tea processed from different cultivars in Enshi. Food Chem. X 2023, 19, 100809. [Google Scholar] [CrossRef]
- Niu, M.; Li, R.; Li, X.; Yang, H.; Ding, J.; Zhou, X.; He, Y.; Xu, Y.; Qu, Q.; Liu, Z.; et al. Insights into the Metabolite Profiles of Two Camellia (Theaceae) Species in Yunnan Province through Metabolomic and Transcriptomic Analysis. Biomolecules 2024, 14, 1106. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Y.; Ding, Z. Global transcriptional analysis reveals the complex relationship between tea quality, leaf senescence and the responses to cold-drought combined stress in Camellia sinensis. Front. Plant Sci. 2016, 7, 227252. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, M.; Yu, X.; Wang, L.; Guo, C.; Ming, R.; Zhang, J. Transcriptome dynamics of Camellia sinensis in response to continuous salinity and drought stress. Tree Genet. Genomes 2017, 13, 1–17. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Chen, J.; Gao, X.; Shao, C.; Lv, Z.; Jiao, H.; Xu, H.; Shen, C. Survival strategies based on the hydraulic vulnerability segmentation hypothesis, for the tea plant [Camellia sinensis (L.) O. Kuntze] in long-term drought stress condition. Plant Physiol. Biochem. 2020, 156, 484–493. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Chen, J.; Shen, C. Flavonoid metabolites in tea plant (Camellia sinensis) stress response: Insights from bibliometric analysis. Plant Physiol. Biochem. 2023, 202, 107934. [Google Scholar] [CrossRef] [PubMed]
- Ran, W.; Li, Q.; Hu, X.; Zhang, D.; Yu, Z.; Chen, Y.; Wang, M.; Ni, D. Comprehensive analysis of environmental factors on the quality of tea (Camellia sinensis var. sinensis) fresh leaves. Sci. Hortic. 2023, 319, 112177. [Google Scholar] [CrossRef]
- Xie, J.; Wang, L.; Deng, Y.; Yuan, H.; Zhu, J.; Jiang, Y.; Yang, Y. Characterization of the key odorants in floral aroma green tea based on GC-E-Nose, GC-IMS, GC-MS and aroma recombination and investigation of the dynamic changes and aroma formation during processing. Food Chem. 2023, 427, 136641. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Sun, L.; Zhang, S.; Chen, Z.; Chen, R.; Li, Z.; Lai, X.; Zhang, Z.; Cao, J.; Li, Q.; et al. The formation mechanism of aroma quality of green and yellow teas based on GC-MS/MS metabolomics. Food Res. Int. 2023, 172, 113137. [Google Scholar] [CrossRef]
- Wei, F.; Luo, L.; Zeng, L. Characterization of key sweet taste compounds in Camellia nanchuanica black tea. LWT 2023, 182, 114858. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Dai, Z.-S.; Gao, Y.; Wang, F.; Chen, J.-X.; Feng, Z.-H.; Yin, J.-F.; Zeng, L.; Xu, Y.-Q. Untargeted metabolomics coupled with chemometrics for flavor analysis of Dahongpao oolong tea beverages under different storage conditions. LWT 2023, 185, 115128. [Google Scholar] [CrossRef]
- Yue, C.; Cao, H.; Zhang, S.; Shen, G.; Wu, Z.; Yuan, L.; Luo, L.; Zeng, L. Multilayer omics landscape analyses reveal the regulatory responses of tea plants to drought stress. Int. J. Biol. Macromol. 2023, 253, 126582. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, M.; Gao, T.; Jing, T.; Zhang, N.; Wang, J.; Zhang, X.; Huang, J.; Schwab, W.; Song, C. Amplification of early drought responses caused by volatile cues emitted from neighboring tea plants. Hortic. Res. 2021, 8, 243. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Zhou, Y.; Zhou, S.; Zhang, S.; Tong, H.; Zhao, A. Transcriptome and metabolome profiling unveiled mechanisms of tea (Camellia sinensis) quality improvement by moderate drought on pre-harvest shoots. Phytochemistry 2020, 180, 112515. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, K.; Wang, J.; Ding, Z.-T.; Wang, H.; Bi, C.-H.; Zhang, Y.-W.; Sun, H.-W. Proteomic analysis of Camellia sinensis (L.) reveals a synergistic network in the response to drought stress and recovery. J. Plant Physiol. 2017, 219, 91–99. [Google Scholar] [CrossRef]
- Huafu, W. Characteristic aroma components of Qimen Black Tea. J. Tea Sci. 1993, 13, 61–68. [Google Scholar]
- Ahmed, S.; Stepp, J.R.; Orians, C.; Griffin, T.; Matyas, C.; Robbat, A.; Cash, S.; Xue, D.; Long, C.; Unachukwu, U.; et al. Effects of Extreme Climate Events on Tea (Camellia sinensis) Functional Quality Validate Indigenous Farmer Knowledge and Sensory Preferences in Tropical China. PLoS ONE 2014, 9, e109126. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Liu, C.; Liu, K. Aromatic constituents in fresh leaves of Lingtou Dancong tea induced by drought stress. Front. Agric. China 2007, 1, 81–84. [Google Scholar] [CrossRef]
- Guo, X.; Schwab, W.; Ho, C.-T.; Song, C.; Wan, X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC–MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Wu, X.; Zhang, Y.; He, Z.; Zhang, Y.; Zhang, X.; Li, Q.; Huang, J.; Liu, Z. Pu-erh tea unique aroma: Volatile components, evaluation methods and metabolic mechanism of key odor-active compounds. Trends Food Sci. Technol. 2022, 124, 25–37. [Google Scholar] [CrossRef]
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Z.; Zhang, L.; Dai, H.; Wu, W.; Zheng, Z.; Lin, F.; Xu, J.; Huang, Y.; Sun, W. Characterization of volatile compounds and identification of key aroma compounds in different aroma types of Rougui Wuyi rock tea. Food Chem. 2024, 455, 139931. [Google Scholar] [CrossRef]
- Wu, Y.; Li, T.; Huang, W.; Liu, Q.; Deng, G.; Zhang, J.; Wei, Y.; Wang, Y.; Ning, J. Investigation of the aroma profile and blending strategy of Lu’an Guapian teas during grain rain period by sensory evaluation combined with SBSE-GC–MS, GC–O and OAV. Food Chem. 2025, 463, 141167. [Google Scholar] [CrossRef]
- Ouyang, J.; Jiang, R.; Chen, H.; Liu, Q.; Yi, X.; Wen, S.; Huang, F.; Zhang, X.; Li, J.; Wen, H.; et al. Characterization of key odorants in ‘Baimaocha’ black teas from different regions. Food Chem. X 2024, 22, 101303. [Google Scholar] [CrossRef]
- Xiong, Z.; Feng, W.; Xia, D.; Zhang, J.; Wei, Y.; Li, T.; Huang, J.; Wang, Y.; Ning, J. Distinguishing raw pu-erh tea production regions through a combination of HS-SPME-GC-MS and machine learning algorithms. LWT 2023, 185, 115140. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Yan, F.; Tang, Y.; Yu, B.; Chen, B.; Lu, L.; Yuan, L.; Wu, Z.; Chen, H. Monitoring Changes in the Volatile Compounds of Tea Made from Summer Tea Leaves by GC-IMS and HS-SPME-GC-MS. Foods 2023, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, L.; Wen, S.; Chen, R.; Sun, S.; Lai, X.; Li, Q.; Zhang, Z.; Lai, Z.; Li, Z.; et al. Analysis of aroma quality changes of large-leaf black tea in different storage years based on HS-SPME and GC–MS. Food Chem. X 2023, 20, 100991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Liu, S.; Li, T.; Wei, Y.; Gu, Z.; Su, Z.; Ning, J.; Wang, Y.; Hou, Z. Characterization of the key volatile compounds in longjing tea (Camellia sinensis) with different aroma types at different steeping temperatures by GC-MS and GC-IMS. LWT 2024, 200, 116183. [Google Scholar] [CrossRef]
- Qiao, D.; Zhu, J.; Mi, X.; Xie, H.; Shu, M.; Chen, M.; Li, R.; Liu, S.; Wei, C. Effects of withering time of fresh leaves on the formation of flavor quality of Taiping Houkui tea. LWT 2023, 182, 114833. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of aroma-active volatiles in oolong tea infusions using GC–olfactometry, GC–FPD, and GC–MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Fu, Y.-Q.; Chen, J.-X.; Wang, F.; Feng, Z.-H.; Yin, J.-F.; Zeng, L.; Xu, Y.-Q.J.F.C. Effects of baking treatment on the sensory quality and physicochemical properties of green tea with different processing methods. Food Chem. 2022, 380, 132217. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, G.; Huang, L.; Ning, J. Sensory-directed flavor analysis reveals the improvement in aroma quality of summer green tea by osmanthus scenting. Food Chem. X 2024, 23, 101571. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter: Utrecht, The Netherlands, 2011. [Google Scholar]
- Yang, J.; Liang, G.; Li, Z.; Liang, X.; Chen, Y. Analysis of aroma characteristics of fermented lingyun baihao tea based on odor activity value. J. Food Sci. 2023, 44, 336–343. [Google Scholar] [CrossRef]
- Munivenkatappa, N.; Sarikonda, S.; Rajagopal, R.; Balakrishnan, R.; Krishnappa Nagarathana, C. Variations in quality constituents of green tea leaves in response to drought stress under south Indian condition. Sci. Hortic. 2018, 233, 359–369. [Google Scholar] [CrossRef]
- Kong, W.; Zhu, Q.; Zhang, Q.; Zhu, Y.; Yang, J.; Chai, K.; Lei, W.; Jiang, M.; Zhang, S.; Lin, J. 5mC DNA methylation modification-mediated regulation in tissue functional differentiation and important flavor substance synthesis of tea plant (Camellia sinensis L.). Hortic. Res. 2023, 10, uhad126. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Wu, S.; Gu, D.; Zeng, L.; Yang, Z. Involvement of DNA methylation in regulating the accumulation of the aroma compound indole in tea (Camellia sinensis) leaves during postharvest processing. Food Res. Int. 2021, 142, 110183. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tan, H.; Huang, H.; Yu, J.; Zeng, L.; Liao, Y.; Wu, P.; Yang, Z. Light synergistically promotes the tea green leafhopper infestation-induced accumulation of linalool oxides and their glucosides in tea (Camellia sinensis). Food Chem. 2022, 394, 133460. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Yu, Z. Microwave pretreatment of camellia (Camellia oleifera Abel.) seeds: Effect on oil flavor. Food Chem. 2021, 364, 130388. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Jin, J.; Li, H.; Chen, F.; Fei, Y.; Wang, Y. Characterization of the flavor profile and dynamic changes in Chinese traditional fish sauce (Yu-lu) based on electronic nose, SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2024, 192, 114772. [Google Scholar] [CrossRef]
- Worley, B.; Halouska, S.; Powers, R. Utilities for quantifying separation in PCA/PLS-DA scores plots. Anal. Biochem. 2013, 433, 102–104. [Google Scholar] [CrossRef]
- Mahieu, B.; Qannari, E.M.; Jaillais, B. Extension and significance testing of Variable Importance in Projection (VIP) indices in Partial Least Squares regression and Principal Components Analysis. Chemom. Intell. Lab. Syst. 2023, 242, 104986. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Q.; Guo, F.; Wang, M.; Zhao, H.; Wang, Y.; Ni, D.; Wang, P. (Z)-3-Hexen-1-ol accumulation enhances hyperosmotic stress tolerance in Camellia sinensis. Plant Mol. Biol. 2020, 103, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, H.; Wang, X.; Qiu, Y.; Tian, L.; Qi, X.; Qu, L.Q. Cytochrome P450 family member CYP96B5 hydroxylates alkanes to primary alcohols and is involved in rice leaf cuticular wax synthesis. New Phytol. 2020, 225, 2094–2107. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.-H. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef]
- Guan, L.; Xia, D.; Hu, N.; Zhang, H.; Wu, H.; Jiang, Q.; Li, X.; Sun, Y.; Wang, Y.; Wang, Z. OsFAR1 is involved in primary fatty alcohol biosynthesis and promotes drought tolerance in rice. Planta 2023, 258, 24. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Sunitha, V.; Raghavendra, A.S. Methyl salicylate is the most effective natural salicylic acid ester to close stomata while raising reactive oxygen species and nitric oxide in Arabidopsis guard cells. Plant Physiol. Biochem. 2020, 157, 276–283. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, C.; Hu, N.; Zhu, Y.; He, Z.; Sun, Y.; Wang, Z.; Wang, Y. ECERIFERUM1-6A is required for the synthesis of cuticular wax alkanes and promotes drought tolerance in wheat. Plant Physiol. 2022, 190, 1640–1657. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dou, Y.; Geng, H.; Fu, J.; Dan, Z.; Liang, T.; Cheng, M.; Zhao, W.; Zeng, Y.; Hu, Z.; et al. OsGRP3 Enhances Drought Resistance by Altering Phenylpropanoid Biosynthesis Pathway in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2022, 23, 7045. [Google Scholar] [CrossRef]
- Fini, A.; Guidi, L.; Ferrini, F.; Brunetti, C.; Di Ferdinando, M.; Biricolti, S.; Pollastri, S.; Calamai, L.; Tattini, M. Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: An excess light stress affair? J. Plant Physiol. 2012, 169, 929–939. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Fan, X.; Li, R.; Yu, C.; Peng, Z.; Gao, Y.; Liu, Z.; Duan, L. A novel plant growth regulator B2 mediates drought resistance by regulating reactive oxygen species, phytohormone signaling, phenylpropanoid biosynthesis, and starch metabolism pathways in Carex breviculmis. Plant Physiol. Biochem. 2024, 213, 108860. [Google Scholar] [CrossRef]
- Geng, D.; Shen, X.; Xie, Y.; Yang, Y.; Bian, R.; Gao, Y.; Li, P.; Sun, L.; Feng, H.; Ma, F.; et al. Regulation of phenylpropanoid biosynthesis by MdMYB88 and MdMYB124 contributes to pathogen and drought resistance in apple. Hortic. Res. 2020, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Jin, J.; Wang, J.; Gao, T.; Luo, Y.; Jing, T.; Hu, Y.; Pan, Y.; Lu, M.; Schwab, W.; et al. Eugenol functions as a signal mediating cold and drought tolerance via UGT71A59-mediated glucosylation in tea plants. Plant J. 2022, 109, 1489–1506. [Google Scholar] [CrossRef]
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/search2.html (accessed on 7 September 2024).
- Wu, W.; Jiang, X.; Zhu, Q.; Yuan, Y.; Chen, R.; Wang, W.; Liu, A.; Wu, C.; Ma, C.; Li, J.; et al. Metabonomics analysis of the flavor characteristics of Wuyi Rock Tea (Rougui) with “rock flavor” and microbial contributions to the flavor. Food Chem. 2024, 450, 139376. [Google Scholar] [CrossRef]
- Lapczynski, A.; Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on dehydrolinalool. Food Chem. Toxicol. 2008, 46, S117–S120. [Google Scholar] [CrossRef]
- Wang, B.; Yu, M.; Tang, Y.; Wang, Y.; Xia, T.; Song, H. Characterization of odor-active compounds in Dahongpao Wuyi Rock Tea (Camellia sinensis) by sensory-directed flavor analysis. J. Food Compos. Anal. 2023, 123, 105612. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, P.; Xia, W.; Jiang, Q.; Liu, S.; Xu, Y. Characterization of key aroma compounds in low-salt fermented sour fish by gas chromatography-mass spectrometry, odor activity values, aroma recombination and omission experiments. Food Chem. 2022, 397, 133773. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Z.; Lan, X.; Wang, C.; Chen, W.; Zhan, S.; Sun, Y.; Su, W.; Lin, C.-C.; Liu, W.; et al. Unveiling the aromatic intricacies of Wuyi Rock Tea: A comparative study on sensory attributes and odor-active compounds of Rougui and Shuixian varieties. Food Chem. 2024, 435, 137470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, J.; Wang, J.; Zhao, Z. Identification of volatile and odor-active compounds in Maojian herbal tea (Dracocephalum rupestre Hance). J. Food Compos. Anal. 2024, 135, 106643. [Google Scholar] [CrossRef]
- Chen, W.; Hu, D.; Miao, A.; Qiu, G.; Qiao, X.; Xia, H.; Ma, C. Understanding the aroma diversity of Dancong tea (Camellia sinensis) from the floral and honey odors: Relationship between volatile compounds and sensory characteristics by chemometrics. Food Control. 2022, 140, 109103. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Hu, C.; Yu, B.; Wan, C.; Chen, B.; Lu, L.; Yuan, L.; Wu, Z.; Chen, H. The flavor substances changes in Fuliang green tea during storage monitoring by GC–MS and GC-IMS. Food Chem. X 2024, 21, 101047. [Google Scholar] [CrossRef]





| NO. | Volatile Compounds | Odor Characteristics | OT (μg/L) | OAVs | |||
|---|---|---|---|---|---|---|---|
| FD-CK | FD-D | WNZ-CK | WNZ-D | ||||
| 1 | Hex-(3Z)-enyl acetate | Green, fruity | 13 | 0.19 | - | 1.58 | 0.25 |
| 2 | Methyl salicylate | Minty, green | 40 | 0.12 | 0.17 | 0.27 | 1.18 |
| 3 | 1-Hexanol | Herbal, fruity | 5.6 | 0.62 | 0.63 | 0.64 | 0.64 |
| 4 | 1-Heptanol | Green, leafy, violet | 5.4 | 0.68 | 0.79 | 0.7 | 0.7 |
| 5 | 1-Octen-3-ol | Earthy, mushroom | 1.5 | 2.36 | 2.53 | 2.35 | 2.76 |
| 6 | (Z)-Hex-3-en-1-ol | Green, grassy | 3.9 | 1.66 | - | - | - |
| 7 | 1-Octanol | Waxy, green, orange | 125.8 | 0.03 | 0.06 | 0.1 | - |
| 8 | Linalool | Floral, citrus, rose | 0.22 | 3155.46 | 1577.42 | 2307.87 | 2190.92 |
| 9 | 1-Nonanol | Floral, rose, orange | 45.5 | - | - | 0.16 | - |
| 10 | Geraniol | Floral, rose | 1.1 | 119.45 | 115.99 | 130.9 | 99.64 |
| 11 | 6-methyl-5-Hepten-2-one | Citrus, lemon | 0.16 | - | 1.68 | - | 3.06 |
| 12 | 1-Octen-3-one | Earthy, herbal, mushroom | 0.003 | - | - | - | 113.76 |
| 13 | (Z)-Jasmone | Floral, woody, herbal | 0.26 | 9.31 | 0.16 | - | 1.81 |
| 14 | (E)-alpha-Ionone | Violet | 0.1 | - | 5.09 | 2.76 | 29.44 |
| 15 | beta-Damascone | Fruity, floral, berry | 0.002 | 9.32 | 19.18 | 60.44 | 157.58 |
| 16 | (E)-beta-Ionone | Foral, fruity, woody | 0.007 | 217.88 | 8.6 | 746.08 | 658.21 |
| 17 | Hexanal | Green, grassy | 5 | 0.61 | 1.34 | 0.81 | 1.24 |
| 18 | 2-Hexenal | Green, almond, fruity | 30 | 0.16 | 0.66 | 0.14 | 0.67 |
| 19 | Heptanal | Green, fatty, herbal | 2.8 | 0.71 | 0.7 | - | 0.68 |
| 20 | (E)-2-Heptenal | Green, vegetable, fatty | 13 | - | 0.14 | - | 0.16 |
| 21 | (E,E)-2,4-Heptadienal | Fatty, green | 0.032 | - | 58.5 | - | 59.41 |
| 22 | Octanal | Aldehydic, citrus | 0.587 | 4.02 | 4.45 | - | 4.13 |
| 23 | Oct-(2E)-enal | Fatty, green, herbal | 0.2 | - | - | - | 9.83 |
| 24 | Benzeneacetaldehyde | Green, floral, hyacinth | 5.2 | - | 0.19 | - | 0.2 |
| 25 | Nonanal | Aldehydic, orange, rose | 1.1 | 2.79 | 3.18 | 2.78 | - |
| 26 | (E)-2-Nonenal | Fatty, cucumber, citrus | 0.19 | 10.01 | - | - | 10.78 |
| 27 | Decanal | Aldehydic | 3 | 0.74 | 0.96 | 0.71 | 0.91 |
| 28 | (E)-2-Decenal | Fatty, earthy, green | 0.3 | 7.9 | 7.82 | 7.48 | 10.78 |
| 29 | Nona-(2E,4E)-dienal | Fatty, melon, green | 0.1 | - | - | - | 19.16 |
| 30 | Neral | Citrus, lemon | 53 | 0.04 | 0.04 | 0.62 | - |
| 31 | Citral | Citrus, lemon, sweet | 40 | 1.06 | 1.43 | 1.42 | 1.43 |
| 32 | Undecanal | Aldehydic, floral, citrus | 12.5 | 0.16 | - | - | - |
| 33 | Dodecanal | Aldehydic, floral, citrus | 0.13 | - | 16.5 | - | - |
| 34 | Tridecanal | Aldehydic, citrus, grapefruit | 10 | - | 0.19 | - | - |
| 35 | beta-Myrcene | Spicy, peppery, woody | 1.2 | 3.85 | 2.83 | 3.85 | 3.1 |
| 36 | D-Limonene | Citrus, orange | 34 | 0.1 | 0.09 | 0.1 | - |
| 37 | (E)-beta-Ocimene | Herbal, citrus, woody | 34 | 0.13 | 0.09 | 0.11 | 0.16 |
| 38 | alpha-Farnesene | Woody, citrus, herbal | 87 | - | - | - | 0.24 |
| 39 | delta-Cadinene | Woody, spicy, burnt | 1.5 | 2.36 | - | 3.38 | 2.4 |
| 40 | Cedrol | Woody, cedarwood | 0.5 | 6.54 | 6.03 | 5.75 | - |
| 41 | Linalool oxide I | Woody, flowery, earthy | 100 | 0.98 | 1.4 | 1.3 | 6.41 |
| 42 | Linalool oxide II | Woody, flowery | 190 | 0.76 | 0.95 | 0.36 | 5.72 |
| 43 | Coumarin | Tonka | 25 | 0.14 | - | 0.15 | 0.17 |
| 44 | Naphthalene | Pungent, dry resinous | 6 | 0.59 | 0.6 | 0.58 | 0.59 |
| 45 | Indole | Flowery | 40 | - | - | 0.13 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Dong, F.; Li, Y.; Lu, F.; Wang, B.; Zhou, T.; Zhao, D.; Huang, M.; Wang, F. Impact of Mild Field Drought on the Aroma Profile and Metabolic Pathways of Fresh Tea (Camellia sinensis) Leaves Using HS-GC-IMS and HS-SPME-GC-MS. Foods 2024, 13, 3412. https://doi.org/10.3390/foods13213412
Liu X, Dong F, Li Y, Lu F, Wang B, Zhou T, Zhao D, Huang M, Wang F. Impact of Mild Field Drought on the Aroma Profile and Metabolic Pathways of Fresh Tea (Camellia sinensis) Leaves Using HS-GC-IMS and HS-SPME-GC-MS. Foods. 2024; 13(21):3412. https://doi.org/10.3390/foods13213412
Chicago/Turabian StyleLiu, Xiaohui, Fabao Dong, Yucai Li, Fu Lu, Botao Wang, Taicen Zhou, Degang Zhao, Mingzheng Huang, and Feifei Wang. 2024. "Impact of Mild Field Drought on the Aroma Profile and Metabolic Pathways of Fresh Tea (Camellia sinensis) Leaves Using HS-GC-IMS and HS-SPME-GC-MS" Foods 13, no. 21: 3412. https://doi.org/10.3390/foods13213412
APA StyleLiu, X., Dong, F., Li, Y., Lu, F., Wang, B., Zhou, T., Zhao, D., Huang, M., & Wang, F. (2024). Impact of Mild Field Drought on the Aroma Profile and Metabolic Pathways of Fresh Tea (Camellia sinensis) Leaves Using HS-GC-IMS and HS-SPME-GC-MS. Foods, 13(21), 3412. https://doi.org/10.3390/foods13213412







