Quality Change of Citri Reticulatae Pericarpium (Pericarps of Citrus reticulata ‘Chachi’) During Storage and Its Sex-Based In Vitro Digestive Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
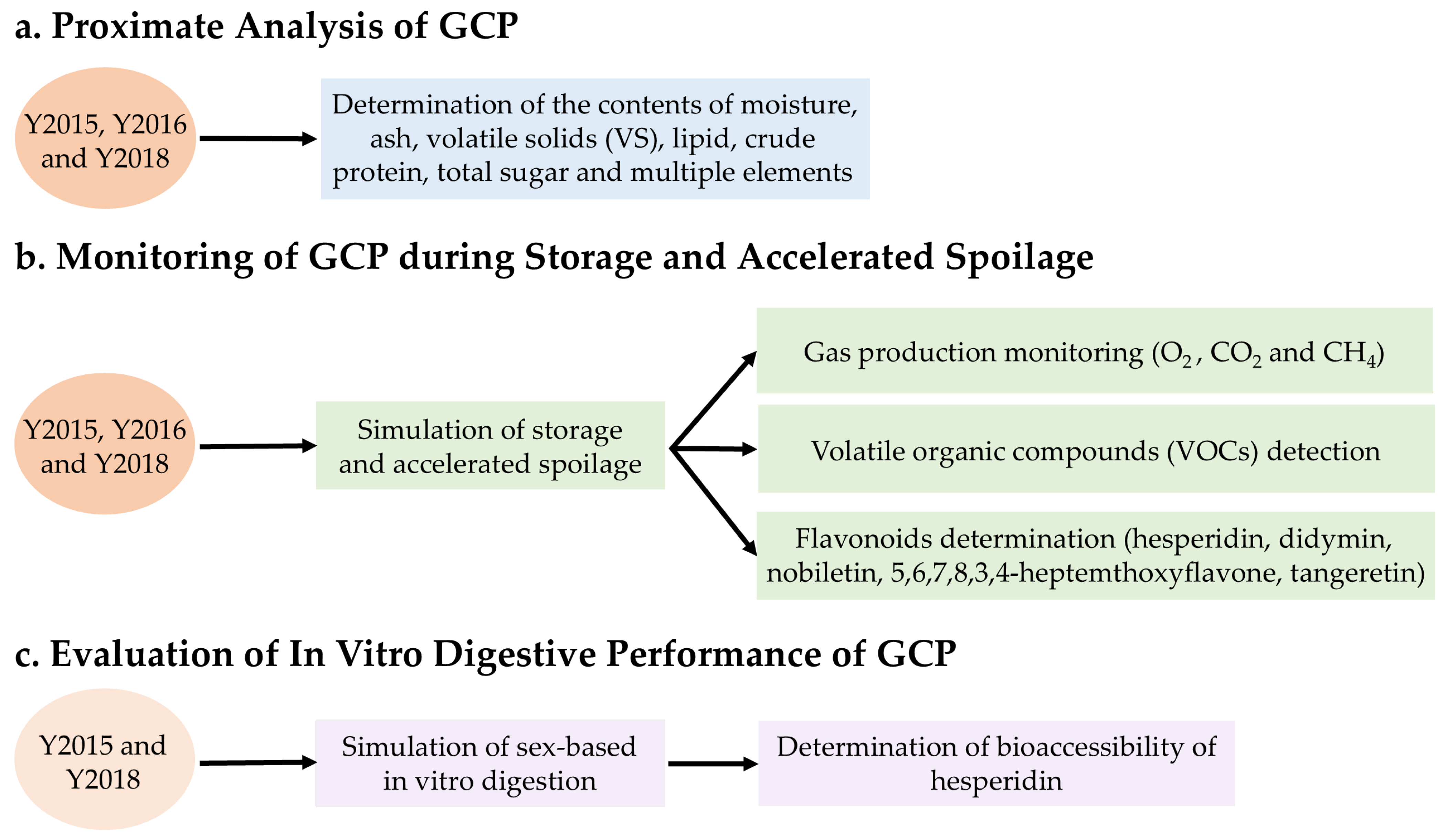
2.2. Proximate Analysis of GCP
2.3. Monitoring of GCP During Storage and Accelerated Spoilage
2.3.1. Gas Production During Shelf Life
2.3.2. Emission of VOCs During Storage and Accelerated Spoilage
2.3.3. Changes in Flavonoid Levels During Storage and Accelerated Spoilage
2.4. Evaluation of In Vitro Digestive Performance of GCP
2.4.1. Sex-Based In Vitro Gastrointestinal Digestion of GCP Decoction
2.4.2. Determination of Bioaccessibility of Hesperidin in Digestive Samples
2.5. Experimental Design and Data Analysis
3. Results and Discussion
3.1. Proximate Analysis of GCP
3.2. Monitoring of GCP During Storage and Accelerated Spoilage
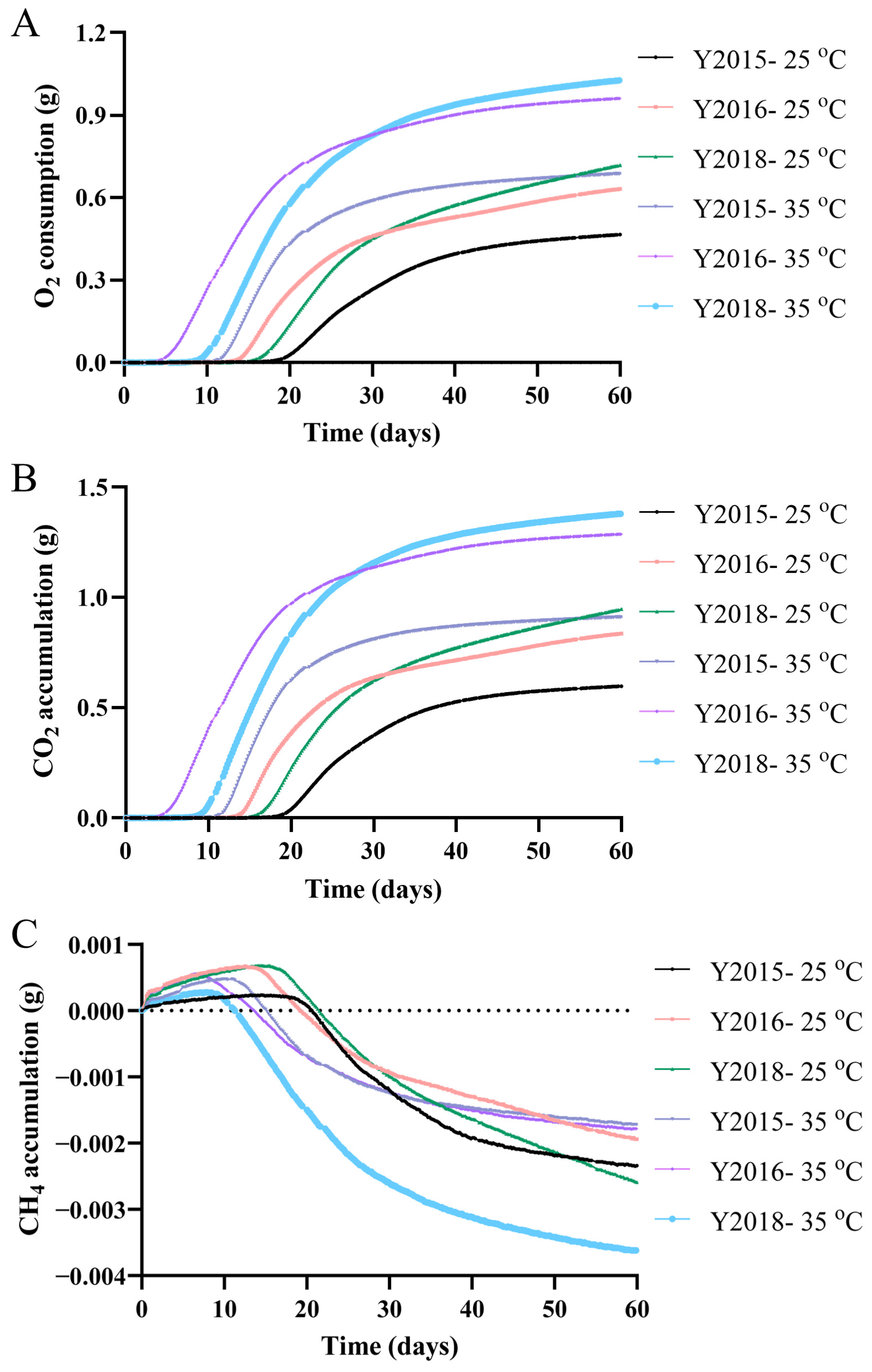
3.2.1. Gas Production During Shelf Life
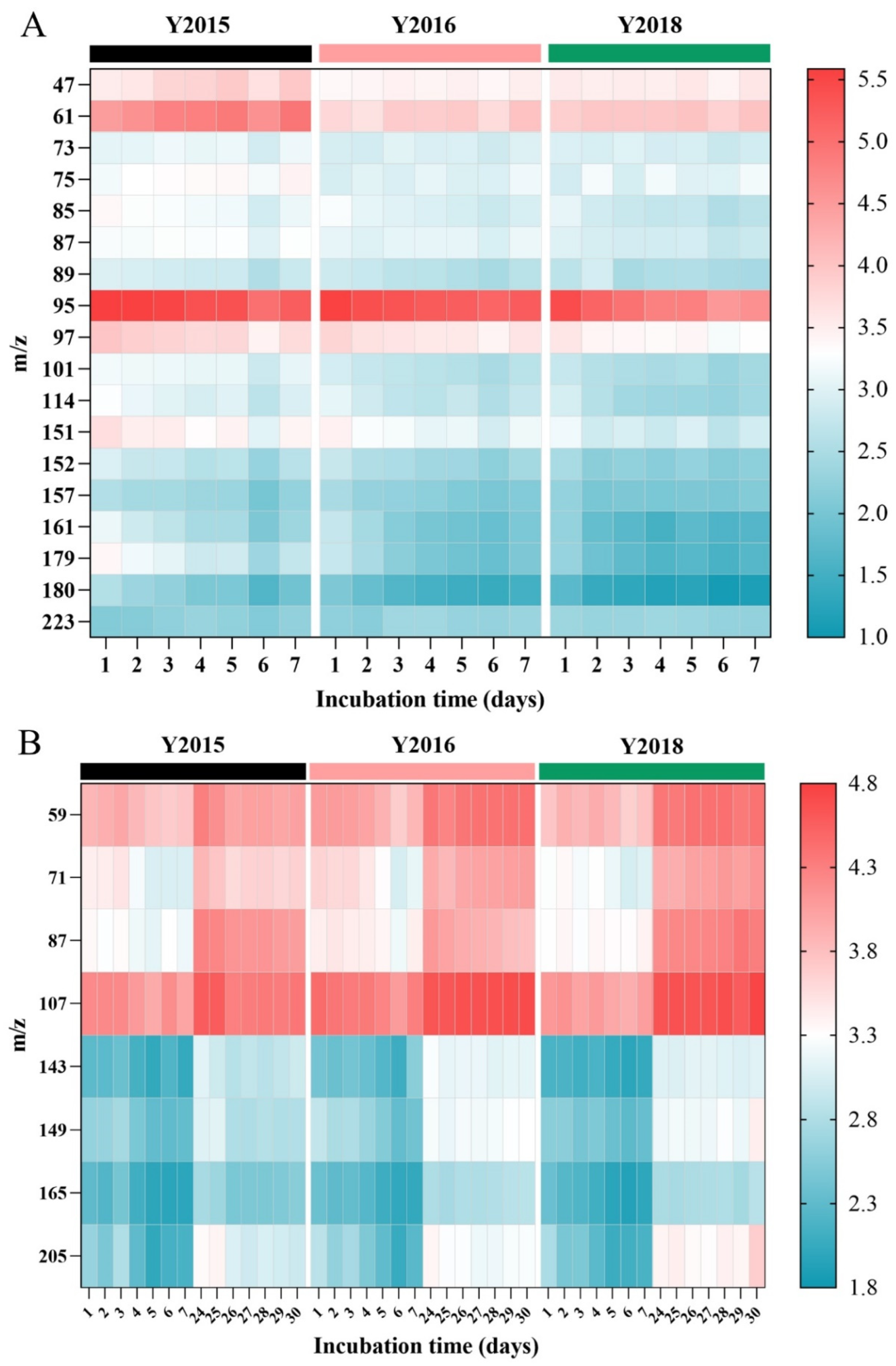
3.2.2. Emission of VOCs During Storage and Accelerated Spoilage
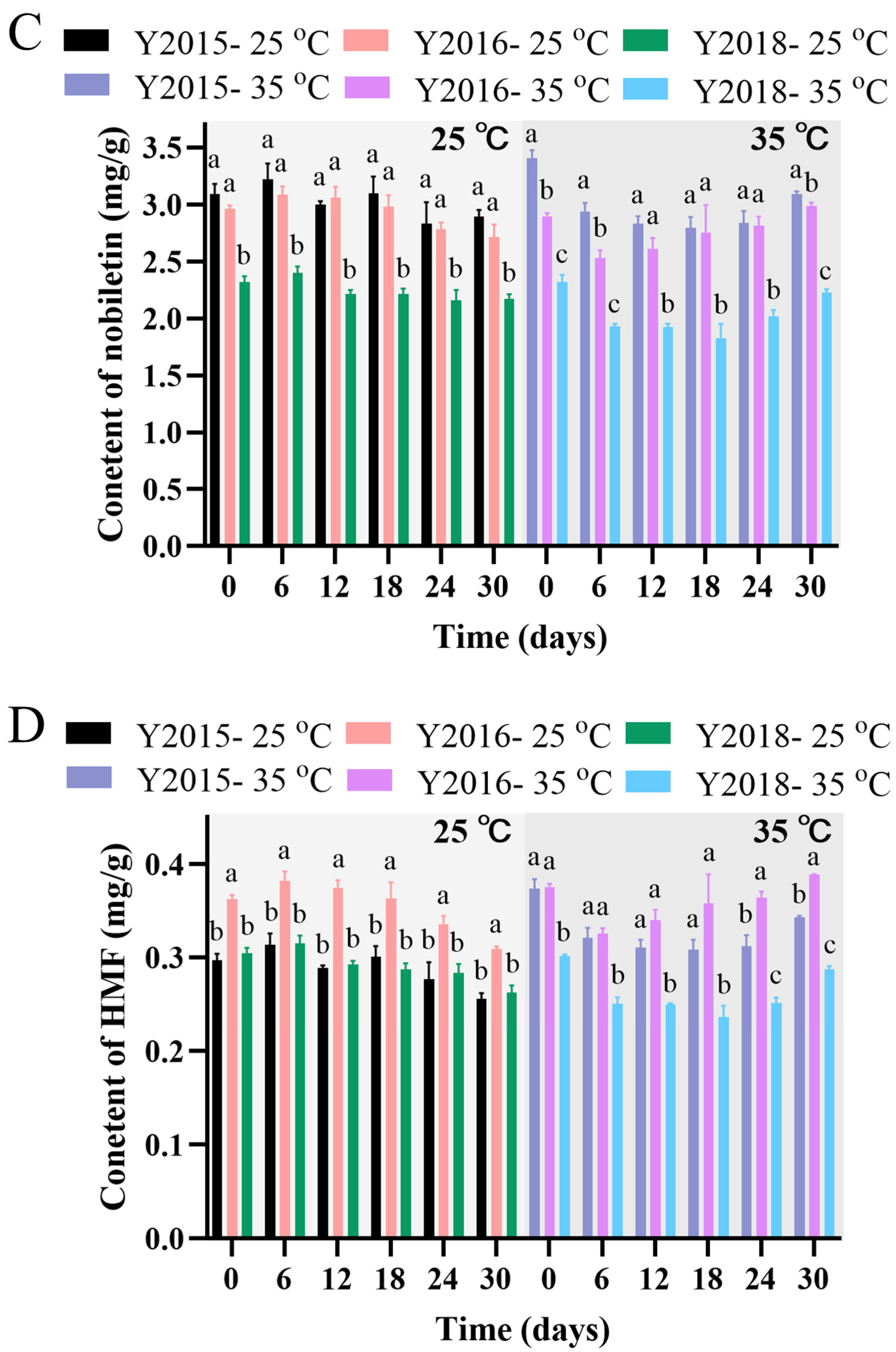
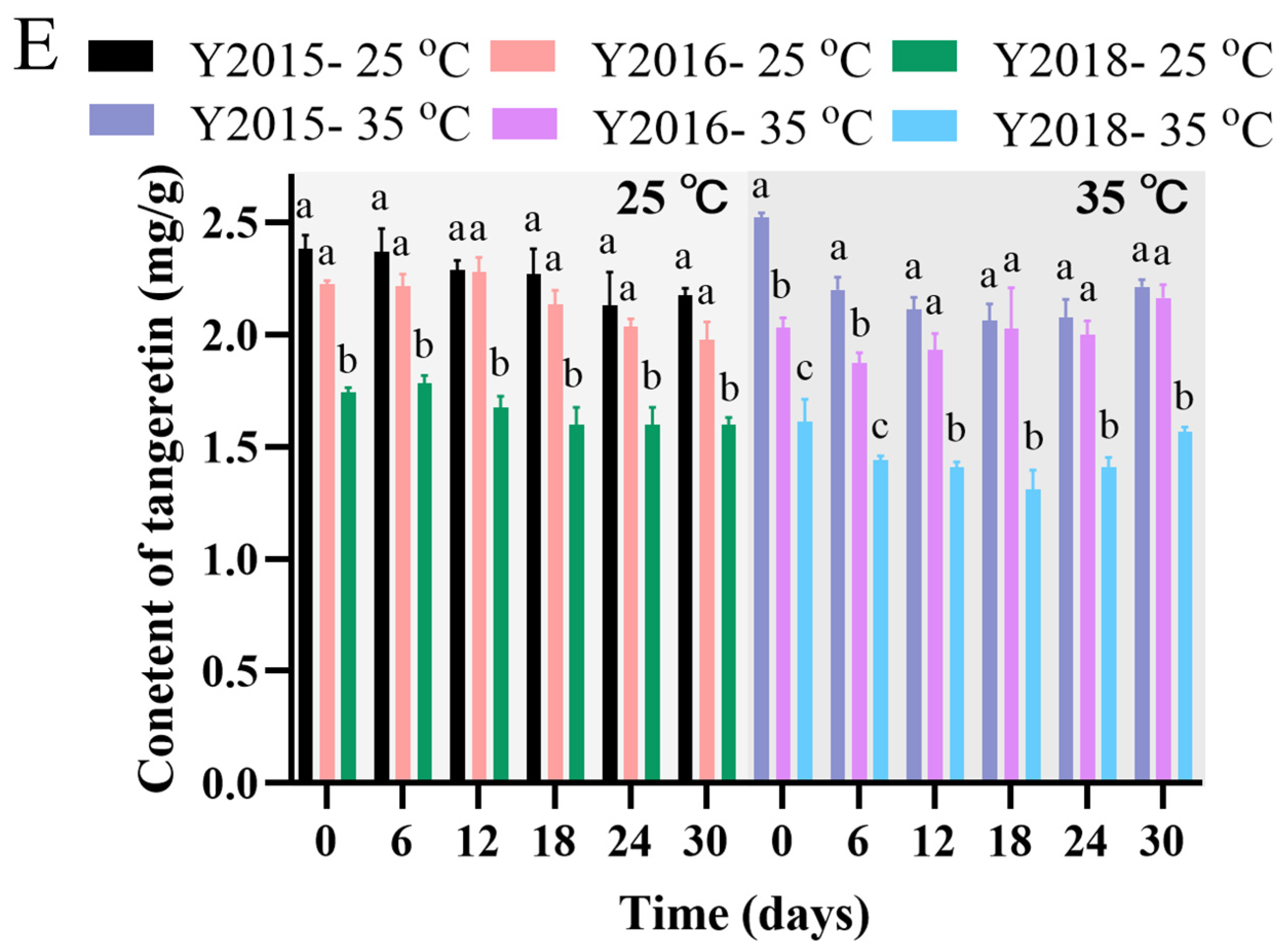
3.2.3. Changes in Flavonoid Levels During Storage and Accelerated Spoilage
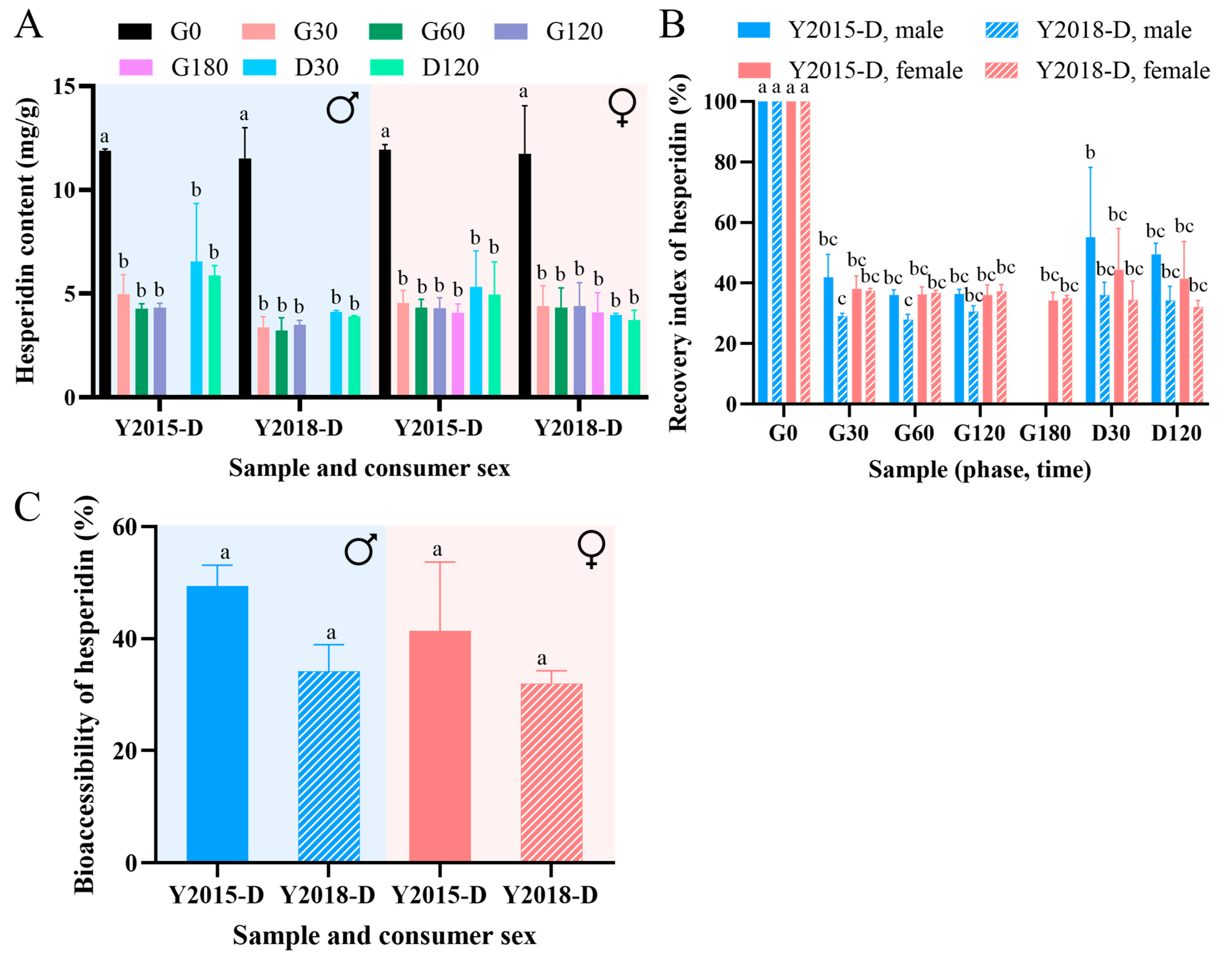
3.3. Evaluation of In Vitro Digestive Performance of GCP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Yu, X.; Sun, S.; Guo, Y.; Liu, Y.; Yang, D.; Li, G.; Lü, S. Citri Reticulatae Pericarpium (Chenpi): Botany, Ethnopharmacology, Phytochemistry, and Pharmacology of a Frequently Used Traditional Chinese Medicine. J. Ethnopharmacol. 2018, 220, 265–282. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Lv, W.; Lin, T.; Ren, Z.; Jiang, Y.; Zhang, J.; Bi, F.; Gu, L.; Hou, H.; He, J. Rapid Discrimination of Citrus reticulata ‘Chachi’ by Headspace-Gas Chromatography-Ion Mobility Spectrometry Fingerprints Combined with Principal Component Analysis. Food Res. Int. 2020, 131, 108985. [Google Scholar] [CrossRef]
- Duan, L.; Guo, L.; Dou, L.L.; Zhou, C.L.; Xu, F.G.; Zheng, G.D.; Li, P.; Liu, E.H. Discrimination of Citrus reticulata Blanco and Citrus reticulata ‘Chachi’ by Gas Chromatograph-Mass Spectrometry Based Metabolomics Approach. Food Chem. 2016, 212, 123–127. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Zheng, Y.; Yang, F.; Jiang, L.; Liu, X.; Ding, S.; Shan, Y. A Novel Method for the Nondestructive Classification of Different-Age Citri Reticulatae Pericarpium Based on Data Combination Technique. Food Sci. Nutr. 2021, 9, 943–951. [Google Scholar] [CrossRef]
- Fu, M.; An, K.; Xu, Y.; Chen, Y.; Wu, J.; Yu, Y.; Zou, B.; Xiao, G.; Ti, H. Effects of Different Temperature and Humidity on Bioactive Flavonoids and Antioxidant Activity in Pericarpium Citri Reticulata (Citrus reticulata ‘Chachi’). LWT 2018, 93, 167–173. [Google Scholar] [CrossRef]
- Kang, W.; Lin, H.; Jiang, H.; Yao-Say Solomon Adade, S.; Xue, Z.; Chen, Q. Advanced Applications of Chemo-Responsive Dyes Based Odor Imaging Technology for Fast Sensing Food Quality and Safety: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5145–5172. [Google Scholar] [CrossRef]
- Jiang, K.; Xu, K.; Wang, J.; Meng, F.; Wang, B. Based on HS-SPME-GC-MS Combined with GC-O-MS to Analyze the Changes of Aroma Compounds in the Aging Process of Citri Reticulatae Pericarpium. Food Biosci. 2023, 54, 102798. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Li, Y.; Li, L. Effect of Drying Methods on Volatile Compounds of Citrus reticulata Ponkan and Chachi Peels as Characterized by GC-MS and GC-IMS. Foods 2022, 11, 2662. [Google Scholar] [CrossRef]
- Luo, M.; Luo, H.; Hu, P.; Yang, Y.; Wu, B.; Zheng, G. Evaluation of Chemical Components in Citri Reticulatae Pericarpium of Different Cultivars Collected from Different Regions by GC–MS and HPLC. Food Sci. Nutr. 2018, 6, 400–416. [Google Scholar] [CrossRef]
- Li, J.; Zou, S.; Yang, W.; Peng, M.; Chen, B.; Deng, J.; Wei, M.; Zheng, G. Identification of Volatile and Nonvolatile Compounds in Citri Reticulatae Pericarpium Viride Using GC–MS, UPLC-Q-Exactive Orbitrap-MS, and HPLC-PDA. Food Sci. Nutr. 2023, 11, 1415–1425. [Google Scholar] [CrossRef]
- Yu, L.; Li, X.; Liu, S.; Xu, G.; Liang, Y. Comparative Analysis of Volatile Constituents in Citrus reticulata Blanco Using GC–MS and Alternative Moving Window Factor Analysis. J. Sep. Sci. 2009, 32, 3457–3465. [Google Scholar] [CrossRef]
- Liu, H.; Wen, J.; Xu, Y.; Wu, J.; Yu, Y.; Yang, J.; Liu, H.; Fu, M. Evaluation of Dynamic Changes and Formation Regularity in Volatile Flavor Compounds in Citrus reticulata ‘Chachi’ Peel at Different Collection Periods Using Gas Chromatography-Ion Mobility Spectrometry. LWT 2022, 171, 114126. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Pinazo, A.; Heredia, A.; Andrés, A. Evaluation Studies of Persimmon Plant (Diospyros kaki) for Physiological Benefits and Bioaccessibility of Antioxidants by in Vitro Simulated Gastrointestinal Digestion. Food Chem. 2017, 214, 478–485. [Google Scholar] [CrossRef]
- Lajterer, C.; Shani Levi, C.; Lesmes, U. An in Vitro Digestion Model Accounting for Sex Differences in Gastro-Intestinal Functions and Its Application to Study Differential Protein Digestibility. Food Hydrocoll. 2022, 132, 107850. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.G.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of Gastrointestinal Tract Variability on Oral Drug Absorption and Pharmacokinetics: An UNGAP Review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef]
- Hu, Y.; Kou, G.; Chen, Q.; Li, Y.; Zhou, Z. Protection and Delivery of Mandarin (Citrus reticulata Blanco) Peel Extracts by Encapsulation of Whey Protein Concentrate Nanoparticles. LWT 2019, 99, 24–33. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Impact of in Vitro Digestion Phases on the Stability and Bioaccessibility of Carotenoids and Their Esters in Mandarin Pulps. Food Funct. 2017, 8, 3951–3963. [Google Scholar] [CrossRef]
- Hou, F.; Hu, K.; Gong, Y.; Xu, J.; Wu, Y.; Zhang, M. Effects of in Vitro Simulated Digestion on the Flavonoid Content and Antioxidant Activity of Aged and Fresh Dried Tangerine Peel. J. Food Process. Preserv. 2018, 42, e13532. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, Z.; Chen, Y.; Song, Y.; Zhou, A.; Cao, Y.; Xiao, J.; Xiao, H.; Song, M. Review of Recent Advances on Health Benefits, Microbial Transformations, and Authenticity Identification of Citri Reticulatae Pericarpium Bioactive Compounds. Crit. Rev. Food Sci. Nutr. 2023, 64, 10332–10360. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Pomeranz, Y.; Meloan, C.E. Food Analysis: Theory and Practice, 3rd ed.; CBS Publishers & Distributors Pvt. Ltd.: New Delhi, India, 1994. [Google Scholar]
- Dar, S.U.; Wu, Z.; Zhang, L.; Yu, P.; Qin, Y.; Shen, Y.; Zou, Y.; Poh, L.; Eichen, Y.; Achmon, Y. On the Quest for Novel Bio-Degradable Plastics for Agricultural Field Mulching. Front. Bioeng. Biotechnol. 2022, 10, 922974. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Lin, Y.; Liu, X.; Zou, Y.; Yu, P.; Zeng, Y.; Wang, X.; Wang, Y.; Van Horne, C.; et al. Assessment of Using Solid Residues of Fish for Treating Soil by the Biosolarization Technique as an Alternative to Soil Fumigation. J. Clean. Prod. 2022, 357, 131886. [Google Scholar] [CrossRef]
- Zeng, S.L.; Li, S.Z.; Lai, C.J.S.; Wei, M.Y.; Chen, B.Z.; Li, P.; Zheng, G.D.; Liu, E.H. Evaluation of Anti-Lipase Activity and Bioactive Flavonoids in the Citri Reticulatae Pericarpium from Different Harvest Time. Phytomedicine 2018, 43, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Shani-Levi, C.; Levi-Tal, S.; Lesmes, U. Comparative Performance of Milk Proteins and Their Emulsions under Dynamic in Vitro Adult and Infant Gastric Digestion. Food Hydrocoll. 2013, 32, 349–357. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Ortega, N.; Macià, A.; Romero, M.P.; Reguant, J.; Motilva, M.J. Matrix Composition Effect on the Digestibility of Carob Flour Phenols by an In-Vitro Digestion Model. Food Chem. 2011, 124, 65–71. [Google Scholar] [CrossRef]
- Xu, M.; Tian, G.; Zhao, C.; Ahmad, A.; Zhang, H.; Bi, J.; Xiao, H.; Zheng, J. Infrared Drying as a Quick Preparation Method for Dried Tangerine Peel. Int. J. Anal. Chem. 2017, 2017, 6254793. [Google Scholar] [CrossRef]
- Nei, D.; Uchino, T.; Sakai, N.; Tanaka, S. ichirou Prediction of Sugar Consumption in Shredded Cabbage Using a Respiratory Model. Postharvest Biol. Technol. 2006, 41, 56–61. [Google Scholar] [CrossRef]
- Vageesan, V.; Chakravarthi, M.K.; Kumar, V.B.; Charan, G. Anoxic Microbial Methanogenic Detection for Food Safety Sustentation. In Proceedings of the 2021 6th International Conference on Recent Trends on Electronics, Information, Communication and Technology, (RTEICT 2021), Bengaluru, India,, 27–28 August 2021; pp. 495–498. [Google Scholar] [CrossRef]
- Raudienė, E.; Rušinskas, D.; Balčiūnas, G.; Juodeikienė, G.; Gailius, D. Carbon Dioxide Respiration Rates in Wheat at Various Temperatures and Moisture Contents. Mapan J. Metrol. Soc. India 2017, 32, 51–58. [Google Scholar] [CrossRef]
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a Food Spoilage Indicator for Monitoring Freshness of Skinless Chicken Breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef]
- Kumar, A. An Arduino Sensor-Based Approach for Detecting the Food Spoilage. Int. J. Eng. Appl. Sci. Technol. 2020, 5, 596–599. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, W.; Huang, K.E.; Li, D.X.; Chen, W.; Yu, X.Q.; Ke, X.H. Discrimination of Citrus reticulata Blanco and Citrus reticulata ‘Chachi’ as Well as the Citrus reticulata ‘Chachi’ within Different Storage Years Using Ultra High Performance Liquid Chromatography Quadrupole/Time-of-Flight Mass Spectrometry Based Metabolomics Approach. J. Pharm. Biomed. Anal. 2019, 171, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jiang, Z.; Wen, M.; Wu, Z.; Zha, M.; Xu, W.; Zhang, L. Chemical Variation of Chenpi (Citrus Peels) and Corresponding Correlated Bioactive Compounds by LC-MS Metabolomics and Multibioassay Analysis. Front. Nutr. 2022, 9, 825381. [Google Scholar] [CrossRef] [PubMed]
- Peña-Vázquez, G.I.; Dominguez-Fernández, M.T.; Camacho-Zamora, B.D.; Hernandez-Salazar, M.; Urías-Orona, V.; De Peña, M.P.; de la Garza, A.L. In Vitro Simulated Gastrointestinal Digestion Impacts Bioaccessibility and Bioactivity of Sweet Orange (Citrus sinensis) Phenolic Compounds. J. Funct. Foods 2022, 88, 104891. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, W.; Huang, H.; Ye, X.; Sun, P. Flavonoids, Phenolic Acids, Carotenoids and Antioxidant Activity of Fresh Eating Citrus Fruits, Using the Coupled in Vitro Digestion and Human Intestinal HepG2 Cells Model. Food Chem. 2019, 279, 321–327. [Google Scholar] [CrossRef]



| Parameter | Stock (g/L) | SGF (mL) | SDF (mL) |
|---|---|---|---|
| KCl | 46.72 | 11 | 10.8 |
| KH2PO4 | 68 | 1.8 | 1.6 |
| NaCl | 120 | 35.2 | 60.1 |
| MgCl2(H2O)6 | 30 | 0.8 | 2.2 |
| (NH4)2CO3 | 48 | 1 | - |
| DW | - | To be completed up to 1L after pH correction | |
| pH | - | 3 | 7 |
| Parameter | Y2015 | Y2016 | Y2018 |
|---|---|---|---|
| Moisture content (g water/g DS) | 0.16 ± 0.01 a | 0.11 ± 0.01 a | 0.13 ± 0.01 a |
| Volatile solids content (%DS) | 96.17 ± 0.45 a | 96.42 ± 0.64 a | 96.89 ± 0.90 a |
| Ash content (g/g DS) | 0.038 ± 0.004 a | 0.036 ± 0.006 a | 0.031 ± 0.009 a |
| Lipid content (g/100 g) | 3.75 ± 0.77 a | 3.71 ± 0.38 a | 2.64 ± 0.53 b |
| Crude protein content (g/100 g) | 9.91 ± 0.13 a | 8.62 ± 0.02 a | 9.33 ± 0.16 a |
| Total sugar content (mg/gDW) | 420.66 ± 37.21 b | 449.88 ± 14.95 a | 451.39 ± 6.39 a |
| P content (g/kg) | 1.10 ± 0.02 a | 1.06 ± 0.10 a | 0.86 ± 0.16 a |
| C content (g/kg) | 347.09 ± 13.92 b | 341.36 ± 12.92 b | 441.27 ± 6.44 a |
| N content (g/kg) | 16.95 ± 0.52 a | 15.77 ± 0.18 a | 15.35 ± 0.14 a |
| B content (mg/kg) | 28.94 ± 2.48 a | 25.46 ± 1.94 a | 20.58 ± 2.91 a |
| Cr content (mg/kg) | 5.33 ± 0.18 a | 5.78 ± 0.27 a | 5.28 ± 0.05 a |
| Co content (mg/kg) | 0.08 ± 0.003 a | 0.10 ± 0.002 a | 0.09 ± 0.0007 a |
| Ni content (mg/kg) | 1.43 ± 0.02 a | 1.73 ± 0.02 a | 1.64 ± 0.05 a |
| Cu content (mg/kg) | 6.33 ± 0.31 a | 5.85 ± 0.39 a | 6.19 a |
| Mn content (mg/kg) | 12.65 ± 0.36 a | 14.04 ± 2.64 a | 15.54 ± 0.27 a |
| As content (mg/kg) | 0.071 ± 0.005 a | 0.067 ± 0.003 a | 0.065 ± 0.005 a |
| Se content (mg/kg) | 1.56 ± 0.14 a | 1.92 ± 0.17 a | 1.69 ± 0.05 a |
| Sr content (mg/kg) | 26.31 ± 0.25 a | 28.01 ± 1.85 a | 22.99 ± 0.53 a |
| Mo content (mg/kg) | 0.11 ± 0.002 a | 0.12 ± 0.00008 a | 0.11 ± 0.006 a |
| Cd content (μg/kg) | 6.46 ± 0.61 a | 15.81 ± 2.12 a | 15.54 ± 0.89 a |
| Sn content (μg/kg) | 67.16 ± 1.46 a | 61.17 ± 7.21 ab | 51.75 ± 5.29 b |
| Ba content (mg/kg) | 22.67 ± 0.11 a | 16.32 ± 0.30 a | 16.11 ± 0.08 a |
| Zn content (mg/kg) | 10.97 ± 0.17 a | 10.39 ± 0.53 a | 7.68 ± 0.04 a |
| Pb content (μg/kg) | 253.35 ± 18.23 b | 322.72 ± 11.45 a | 332.65 ± 2.70 a |
| K content (g/kg) | 10.19 ± 0.03 a | 11.27 ± 0.74 a | 6.37 ± 0.01 a |
| Mg content (g/kg) | 0.57 ± 0.01 a | 0.56 ± 0.02 a | 0.56 ± 0.01 a |
| Fe content (mg/kg) | 112.99 ± 1.95 a | 104.98 ± 13.98 a | 74.64 ± 0.05 b |
| Na content (g/kg) | 0.016 ± 0.003 a | 0.031 ± 0.01 a | 0.021 ± 0.01 a |
| Ca content (g/kg) | 3.97 ± 0.17 a | 3.10 ± 0.14 a | 3.30 ± 0.11 a |
| Al content (mg/kg) | 72.69 ± 0.88 b | 70.07 ± 1.76 b | 116.28 ± 6.77 a |
| V content (μg/kg) | 128.69 ± 1.88 a | 130.37 ± 1.25 a | 73.11 ± 1.19 b |
| Tl content (μg/kg) | 4.83 ± 0.05 a | 8.05 ± 1.91 a | 8.79 ± 1.61 a |
| No. | m/z | Protonated Chemical Formula a | Tentative Identification b | Tentative Structure c | Refs. |
|---|---|---|---|---|---|
| A1 | 32 | (CH5N)H+ fragment; O2+ fragment | Not identified; Not identified |  | - |
| A2 | 81 | (C6H8)H+ fragment | 1,4-Cyclohexadiene fragment */Bicyclo[3.1.0]hex-2-ene fragment * |  | [12] |
| A3 | 95 | (C5H6N2)H+ | Methylpyrazine | 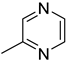 | [3] |
| A4 | 137 | (C10H16)H+; (C8H8O2)H+ | D-Limonene/α-Pinene/β-Pinene/Limonene/Myrcene/α-Phellandrene/β-Phellandrene/α-Terpinene/γ-Terpinene/Sabinene/Terpinolene/Camphene/13,7-dimethyl-3,6-Octatriene/Ocimene/1-Methyl-4-(1-methylethylidene) cyclohexene/α-Thujene/3-Carene/4-Carene/trans-1,2-Bis(1-methylethenyl)-cyclobutene/4-Methyl-1-(1-methylethyl) bicyclo[3.1.0]hexane didehydro deriv; Benzoic acid methyl ester | 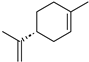 | [1,3,9,10,11,13] |
| No. | m/z | VIP | Protonated Chemical Formula a | Tentative Identification b | Tentative Structure c | Refs. |
|---|---|---|---|---|---|---|
| B1 | 75 | 2.29 | (C3H6O2)H+; (C4H10O)H+ | Hydroxyacetone/Propionic acid/Methyl acetate; 2-Methylpropanol/2-Butanol/n-Butanol | 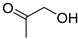 | [3,9,13] |
| B2 | 47 | 2.18 | (C2H6O)H+ | Ethanol |  | [13] |
| B3 | 61 | 2.15 | (C3H8O)H+ | 1-Propanol |  | [9] |
| B4 | 101 | 1.69 | (C6H12O)H+; (C5H8O2)H+ | (3Z)-Hex-3-en-1-ol/Hexanal/2-Hexanone/2-Hexenol/2-Hexen-1-ol; 2,3-Pentadione |  | [3,9,13] |
| B5 | 73 | 1.54 | (C4H8O)H+ | Butanone/Butanal/Tetrahydrofuran | 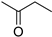 | [3,9,13] |
| B6 | 179 | 1.33 | (C14H10)H+ | Phenanthrene/Anthracene | 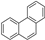 | [10] |
| B7 | 89 | 1.30 | (C4H8O2)H+; (C5H12O)H+ | Ethyl acetate/3-Hydroxy-2-butanone/Butanoic acid/1,4-Dioxane; 2-Methyl-1-butanol/3-Methyl-1-butanol | 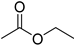 | [9,13] |
| B8 | 180 | 1.25 | (C10H13NO2)H+ | Benzoic acid, 2-(methylamino)-, ethyl ester | 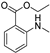 | [11] |
| B9 | 87 | 1.23 | (C5H10O)H+; (C4H6O2)H+ | 2-Methylbutanal/Pentanal/3-Methylbutanal/1-Penten-3-ol/3-Pentanone/2-Pentanone; 2,3-Butanedione |  | [9,13] |
| B10 | 223 | 1.20 | (C15H26O)H+ | Patchouli alcohol/Elemol/α-Cadinol/Nerolidol/(−)-Globulol/α-Eudesmol/T-muurolol/Cyclohexanemethanol, 4-ethenyl-a,a,4-tri-methyl-3-(1-methylethenyl)-, [1R-(1a,3a,4a)]- |  | [1,10,11,12] |
| B11 | 151 | 1.14 | (C9H10O2)H+; (C10H14O)H+ | p-Vinylguaiacol/2-Methoxy-4-vinylphenol; p-Cymen-7-ol/2-Methyl-5-(1-methylethyl)-phenol/5-Methyl-2-(1-methylethyl)-phenol/Carvacrol/Perilla aldehyde/Carvone/Thymol/4-(1-Methylethenyl)-1-cyclohexene-1-carboxaldehyde/3-Methyl-4-isopropylphenol/2-(4-Methylphenyl)propan-2-ol/Perillene/D-carvone/Piperitenone/5-isopropyl-2-methylphenol/1-Cyclohexene-1-carboxaldehyde, 4-(1-methyl-ethenyl)- | 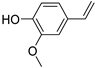 | [1,4,8,9,10,11,12] |
| B12 | 85 | 1.07 | (C5H8O)H+ | (E)-2-Pentenal/3-Methyl-2-butenal/1-Penten-3-one/3-Methyl butynol | 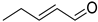 | [8,9,13] |
| B13 | 152 | 1.04 | (C8H9NO2)H+ | Methyl 2-aminobenzoate | 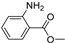 | [10] |
| B14 | 97 | 1.03 | (C5H4O2)H+ | Furfural/2-Furan-methyl aldehyde | 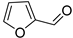 | [1,8,9,12,13] |
| B15 | 95 | 1.03 | (C5H6N2)H+ | Methylpyrazine | 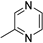 | [3] |
| B16 | 114 | 1.03 | (C7H14O)+ | Heptanal |  | [9,13] |
| B17 | 157 | 1.02 | (C10H20O)H+ | Decanal/(R)-(+)-β-Citronellol/1-Menthol/Citronellol |  | [1,3,4,8,9,10,13] |
| B18 | 161 | 1.02 | (C12H16)H+ fragment | Not identified |  | - |
| No. | m/z | VIP | Protonated Chemical Formula a | Tentative Identification b | Tentative Structure c | Refs. |
|---|---|---|---|---|---|---|
| C1 | 143 | 1.19 | (C9H18O)H+ | Nonanal |  | [1,3,8,9,10,12] |
| C2 | 165 | 1.15 | (C10H12O2)H+; (C11H16O)H+ | Phenylacetic acid ethyl ester/Eugenol/Ethyl phenylacetate/Benzene,4-ethenyl-1,2-dimethoxy-; 2-Isopropyl-5-methylanisole/2-Isopropyl-4-methylanisole | 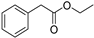 | [8,9,10,11,13] |
| C3 | 87 | 1.23 | (C5H10O)H+; (C4H6O2)H+ | 2-Methylbutanal/Pentanal/3-Methylbutanal/1-Penten-3-ol/3-Pentanone/2-Pentanone; 2,3-Butanedione |  | [9,13] |
| C4 | 107 | 1.12 | (C7H6O)H+ | Benzaldehyde | 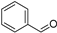 | [8,9] |
| C5 | 71 | 1.11 | (C4H6O)H+ | 2-Methyl-2-propenal | 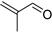 | [13] |
| C6 | 149 | 1.11 | (C10H12O)H+ | Cuminaldehyde |  | [8] |
| C7 | 59 | 1.11 | (C3H6O)H+ | 2-Propanone | 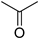 | [9,13] |
| C8 | 205 | 1.10 | (C15H24)H+ | α-Cubebene/Copaene/Ylangene/β-Cubebene/β-Caryophyllene/Caryophyllene/Humulene/Germacrene D/α-Farnesene/(+)-δ-Cadinene/1,2,3,4,6,8a-Hexahydro-1-isopropyl-4,7-dimethylnaphthalene/α-Caryophyllene/(+)-Aromadendrene/Elemene/Bicyclosesquiphellandrene/α-Gurjunene/Valencene/Aristolene/Longifolene/δ-Elemene/β-Elemene/Eremophilene/α-Serinene/β-Cadinene/γ-Elemene/β-Guaiene/2,4-Diisopropenyl-/Gemacrene B/4,7-Dimethyl-1-isopropyl naphthalene |  | [1,4,8,10,11,13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Zeng, Y.; Li, C.; Qiu, B.; Shi, Y.; He, Q.; Lesmes, U.; Achmon, Y. Quality Change of Citri Reticulatae Pericarpium (Pericarps of Citrus reticulata ‘Chachi’) During Storage and Its Sex-Based In Vitro Digestive Performance. Foods 2024, 13, 3671. https://doi.org/10.3390/foods13223671
Yu P, Zeng Y, Li C, Qiu B, Shi Y, He Q, Lesmes U, Achmon Y. Quality Change of Citri Reticulatae Pericarpium (Pericarps of Citrus reticulata ‘Chachi’) During Storage and Its Sex-Based In Vitro Digestive Performance. Foods. 2024; 13(22):3671. https://doi.org/10.3390/foods13223671
Chicago/Turabian StyleYu, Peirong, Yuying Zeng, Chunyu Li, Bixia Qiu, Yuan Shi, Qixi He, Uri Lesmes, and Yigal Achmon. 2024. "Quality Change of Citri Reticulatae Pericarpium (Pericarps of Citrus reticulata ‘Chachi’) During Storage and Its Sex-Based In Vitro Digestive Performance" Foods 13, no. 22: 3671. https://doi.org/10.3390/foods13223671
APA StyleYu, P., Zeng, Y., Li, C., Qiu, B., Shi, Y., He, Q., Lesmes, U., & Achmon, Y. (2024). Quality Change of Citri Reticulatae Pericarpium (Pericarps of Citrus reticulata ‘Chachi’) During Storage and Its Sex-Based In Vitro Digestive Performance. Foods, 13(22), 3671. https://doi.org/10.3390/foods13223671







