Nondestructive Determination of Tocopherol and Tocotrienol in Vitamin E Powder Using Near- and Mid-Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. NIR and MIR Spectroscopic Analysis
2.4. Vitamin E Determination
2.5. Data Analysis
3. Results and Discussion
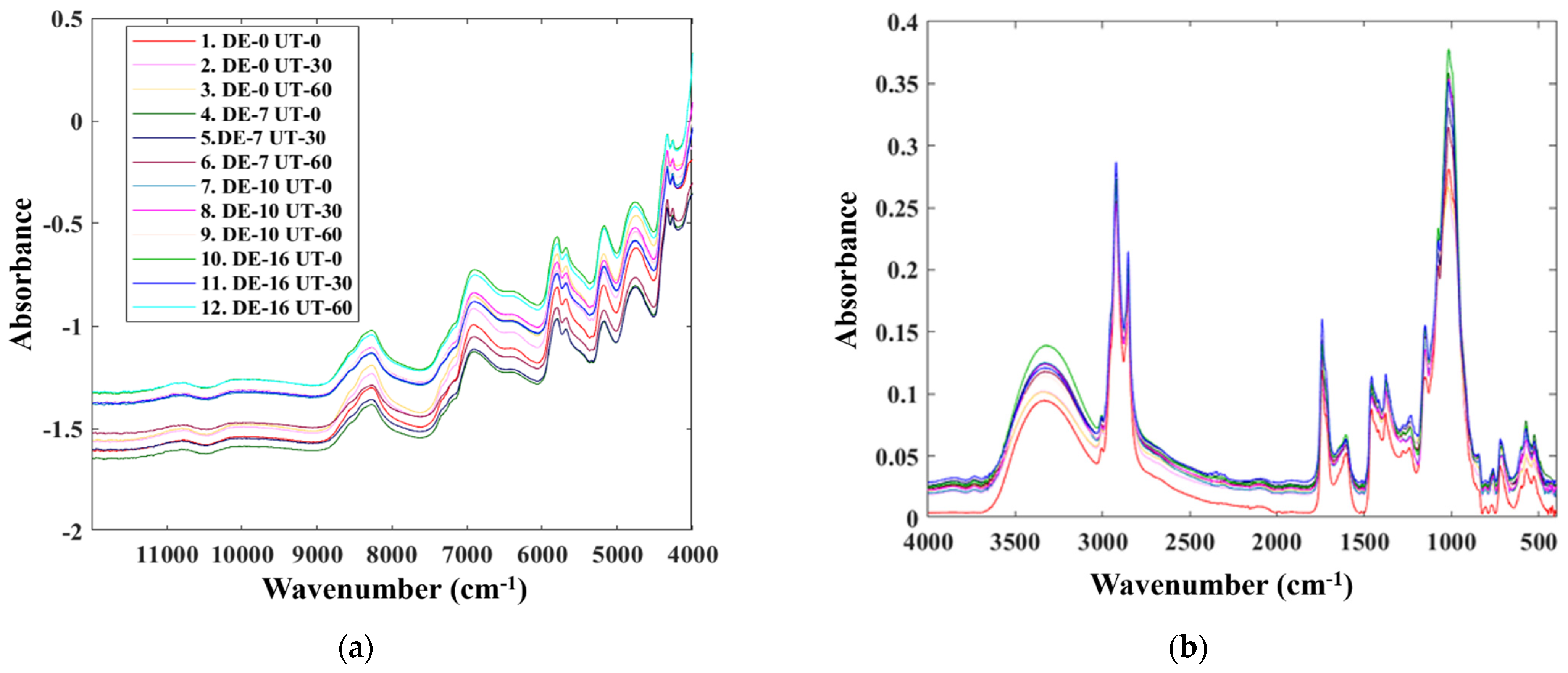
3.1. Characterization of NIR and MIR Spectral Data
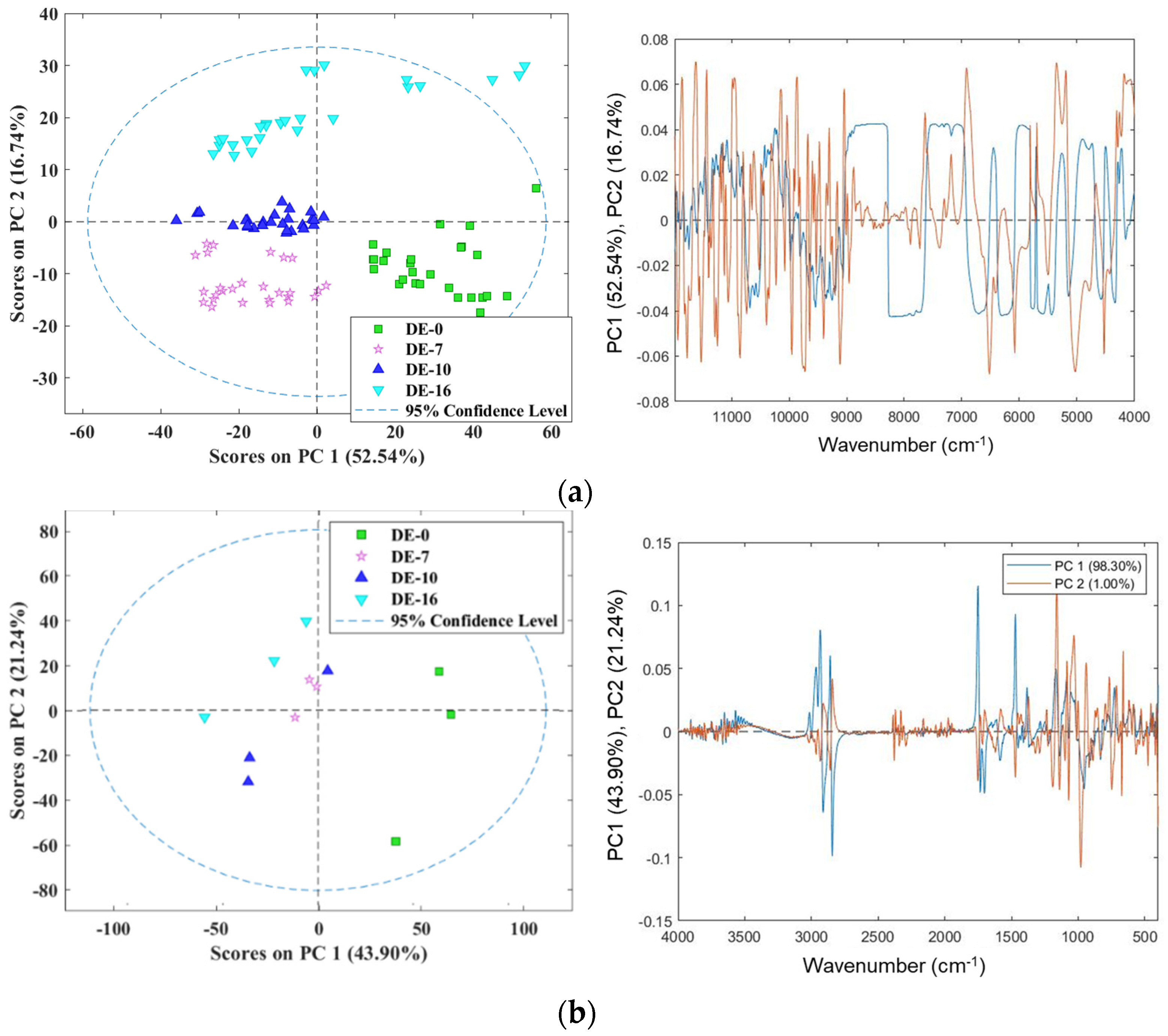
3.2. Exploratory Data Analysis of NIR and MIR Spectra Using PCA
3.3. Distribution of Calibration and Validation Reference Data for MIR and NIR Prediction Models
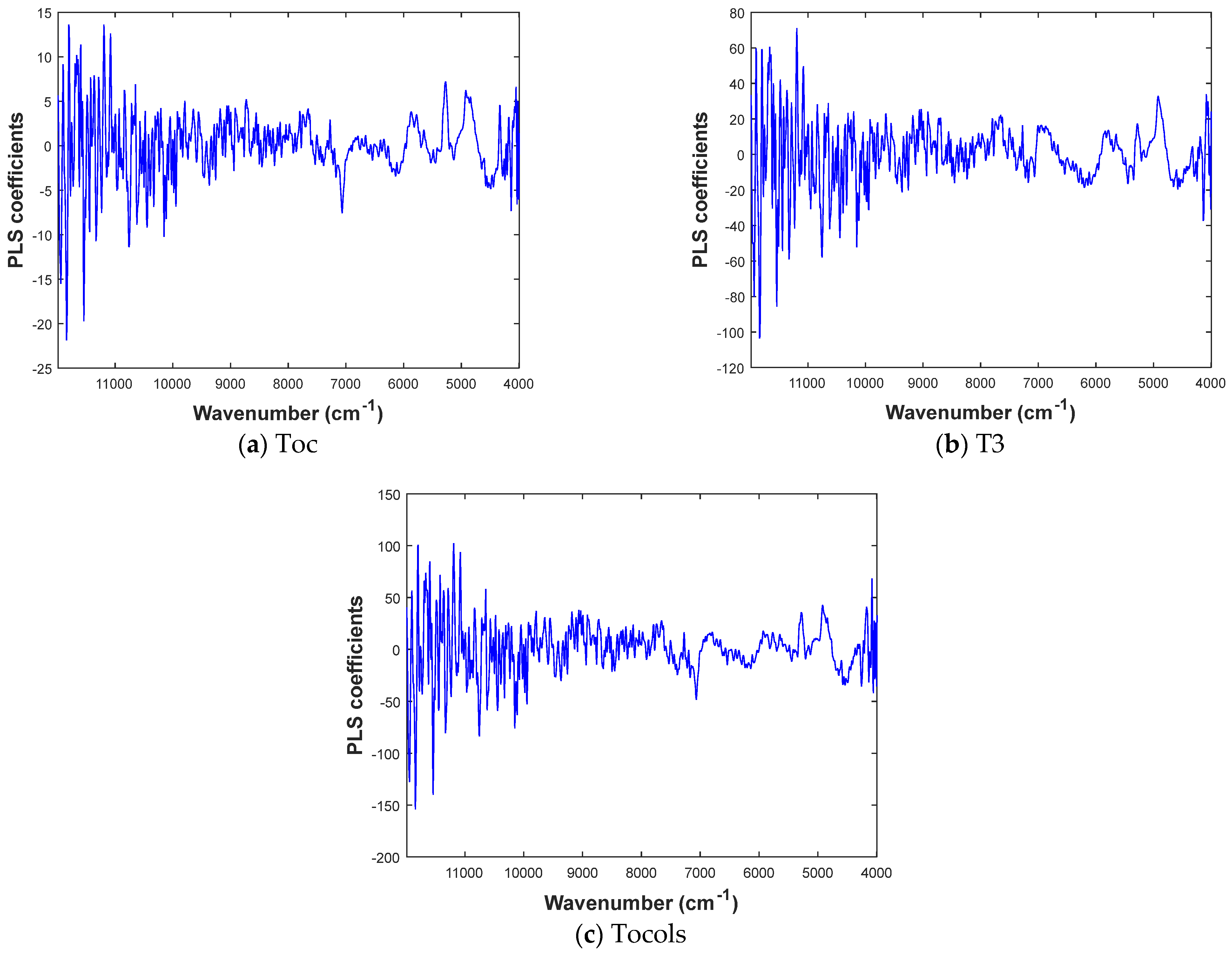
3.4. Quantitative Analysis of Encapsulated Vitamin E Using PLS Regression
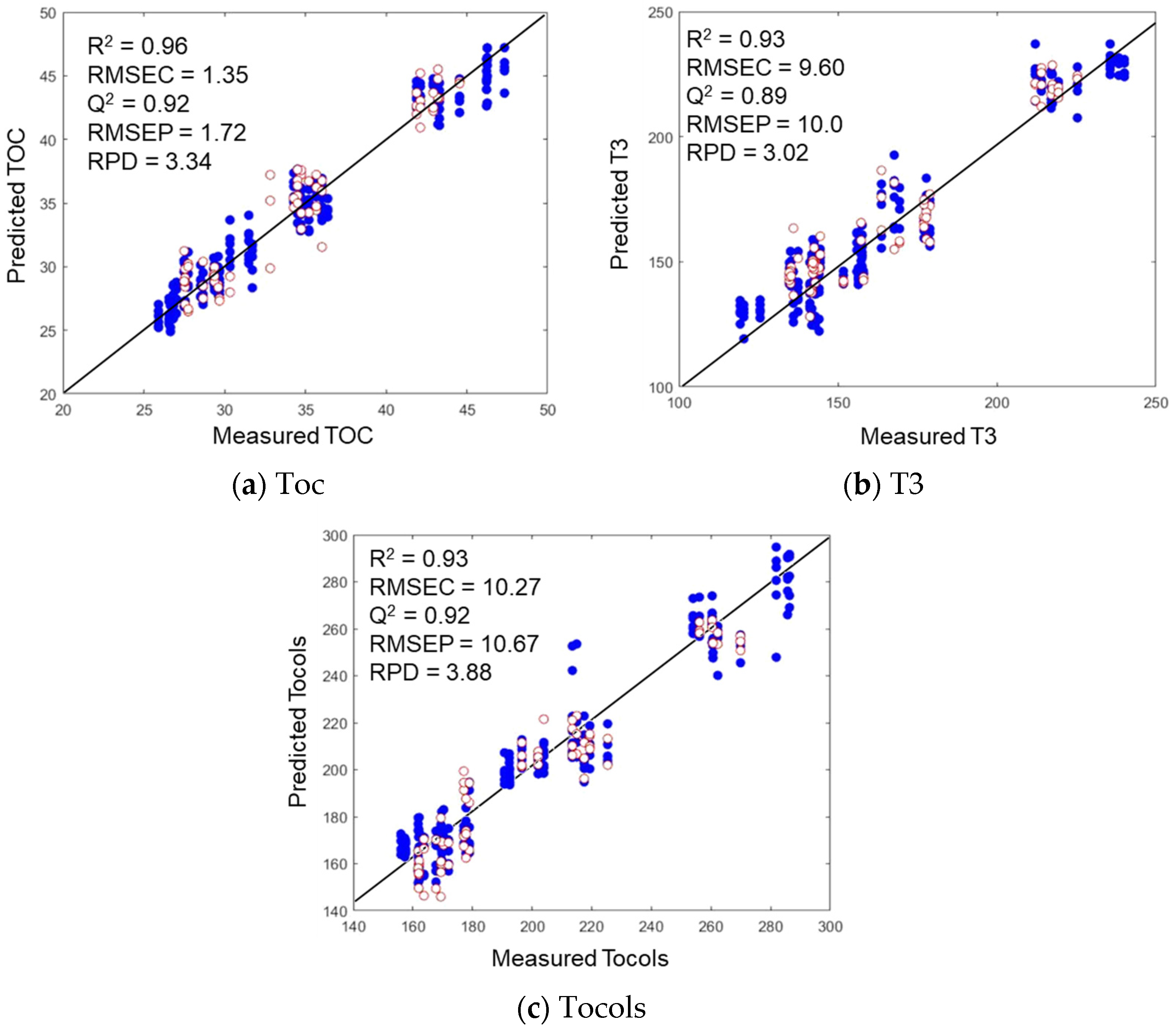
3.4.1. Cross-Validation of MIR and NIR Predictions
3.4.2. Test Set Validation of NIR Predictions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niki, E.; Abe, K. Vitamin E: Structure, Properties and Functions. In Vitamin E: Chemistry and Nutritional Benefits; The Royal Society of Chemistry: Tokyo, Japan, 2019. [Google Scholar] [CrossRef]
- Ye, Z.; Shi, B.; Huang, Y.; Ma, T.; Xiang, Z.; Hu, B.; Kuang, Z.; Huang, M.; Lin, X.; Tian, Z.; et al. Revolution of vitamin E production by starting from microbial fermented farnesene to isophytol. Innovation 2022, 3, 100228. [Google Scholar] [CrossRef]
- Sawadikiat, P.; Setwipattanachai, P.; Chaiseri, S.; Hongsprabhas, P. Rice phytochemicals concentrated by molecular distillation process and their use as co-surfactant in water dispersion. J. Food Sci. Technol. 2015, 52, 8014–8022. [Google Scholar] [CrossRef]
- Ko, S.; Lee, S.; Kim, I. The concentration of tocols from rice bran oil deodorizer distillate using solvent. Eur. J. Lipid Sci. Technol. 2008, 110, 914–919. [Google Scholar] [CrossRef]
- Jaiswal, S.G.; Pradhan, S.; Patel, M.; Naik, M.; Naik, S. Rice Bran Oil Distillate, a Choice for Gamma-Oryzanol: Separation and Oxidative Stability Study. J. Food Res. 2014, 4, p36. [Google Scholar] [CrossRef]
- Fan, C.; Feng, T.; Wang, X.; Xia, S.; Swing, C.J. Liposomes for encapsulation of liposoluble vitamins (A, D, E and K): Comparation of loading ability, storage stability and bilayer dynamics. Food Res. Int. 2022, 163, 112264. [Google Scholar] [CrossRef] [PubMed]
- Rayhani, Z.; Kurniasih, E.; Savia; Fadhilah, R. Classification of dextrose equivalent analysis maltodextrin starch seeds through enzymatic hydrolysis reaction. IOP Conf. Ser. Mater. Sci. Eng. 2018, 420, 012072. [Google Scholar] [CrossRef]
- Sringarm, C.; Numthuam, S.; Jiamyangyuen, S.; Kittiwachana, S.; Kielar, F.; Wongsaipun, S.; Rungchang, S. Quantitative and qualitative evaluation of maltodextrin products in the industry using near-infrared spectroscopy. Int. J. Food Sci. Technol. 2024, 59, 7391–7402. [Google Scholar] [CrossRef]
- Sringarm, C.; Numthuam, S.; Jiamyangyuen, S.; Kittiwachana, S.; Saeys, W.; Rungchang, S. Classification of industrial tapioca starch hydrolysis products based on their Brix and dextrose equivalent values using near-infrared spectroscopy. J. Sci. Food Agric. 2024, 104, 7249–7257. [Google Scholar] [CrossRef] [PubMed]
- Gamna, F.; Spriano, S. Vitamin E: A Review of Its Application and Methods of Detection When Combined with Implant Biomaterials. Materials 2021, 14, 3691. [Google Scholar] [CrossRef]
- Katuwal, S.; Knadel, M.; Moldrup, P.; Norgaard, T.; Greve, M.H.; de Jonge, L.W. Visible–Near-Infrared Spectroscopy can predict Mass Transport of Dissolved Chemicals through Intact Soil. Sci. Rep. 2018, 8, 11188. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.L.d.S.; Lima, A.F.; Gonçalves, M.C.; Godoy, H.T.; Barbin, D.F. Portable near-infrared (NIR) spectrometer and chemometrics for rapid identification of butter cheese adulteration. Food Chem. 2023, 425, 136461. [Google Scholar] [CrossRef] [PubMed]
- Sringarm, C.; Numthuam, S.; Jiamyangyuen, S.; Klangpetch, W.; Wongsaipun, S.; Kittiwachana, S.; Saeys, W.; Rungchang, S. Quantification of individual sugars in tapioca syrups with near-infrared spectroscopy. J. Food Compos. Anal. 2023, 125, 105852. [Google Scholar] [CrossRef]
- Huck, C.W. Advances of vibrational spectroscopic methods in phytomics and bioanalysis. J. Pharm. Biomed. Anal. 2014, 87, 26–35. [Google Scholar] [CrossRef]
- Nunes, M.A.; Páscoa, R.N.; Alves, R.C.; Costa, A.S.; Bessada, S.; Oliveira, M.B.P. Fourier transform near infrared spectroscopy as a tool to discriminate olive wastes: The case of monocultivar pomaces. Waste Manag. 2020, 103, 378–387. [Google Scholar] [CrossRef]
- Cayuela, J.A.; García, J.F. Sorting olive oil based on alpha-tocopherol and total tocopherol content using near-infra-red spectroscopy (NIRS) analysis. J. Food Eng. 2017, 202, 79–88. [Google Scholar] [CrossRef]
- Díaz, E.O.; Kawamura, S.; Matsuo, M.; Kato, M.; Koseki, S. Combined analysis of near-infrared spectra, colour, and physicochemical information of brown rice to develop accurate calibration models for determining amylose content. Food Chem. 2019, 286, 297–306. [Google Scholar] [CrossRef]
- Fernández-Espinosa, A.J. Combining PLS regression with portable NIR spectroscopy to on-line monitor quality parameters in intact olives for determining optimal harvesting time. Talanta 2016, 148, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Buvé, C.; Saeys, W.; Rasmussen, M.A.; Neckebroeck, B.; Hendrickx, M.; Grauwet, T.; Van Loey, A. Application of multivariate data analysis for food quality investigations: An example-based review. Food Res. Int. 2022, 151, 110878. [Google Scholar] [CrossRef]
- Yort, L.; Singanusong, R.; Yuenyong, J.; Sookwong, P.; Jiamyangyuen, S. Optimization of Vitamin E Extraction from Rice Bran Oil Deodorizer Distillate using Response Surface Methodology. Curr. Res. Nutr. Food Sci. J. 2022, 10, 1150–1160. [Google Scholar] [CrossRef]
- Sahlan, M.; Fadhan, A.M.; Pratami, D.K.; Lischer, K.; Wijanarko, A.; Hermansyah, H.; Mahira, K.F. Encapsulation of Agarwood Essential Oil with Maltodextrin and Gum Arabic. Int. J. Technol. 2019, 10, 1541–1547. [Google Scholar] [CrossRef]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef] [PubMed]
- Funsueb, S.; Thanavanich, C.; Theanjumpol, P.; Kittiwachana, S. Development of new fruit quality indices through aggregation of fruit quality parameters and their predictions using near-infrared spectroscopy. Postharvest Biol. Technol. 2023, 204, 112438. [Google Scholar] [CrossRef]
- Sringarm, C.; Numthuam, S.; Singanusong, R.; Jiamyangyuen, S.; Kittiwatchana, S.; Funsueb, S.; Rungchang, S. Quantitative determination of quality control parameters using near infrared spectroscopy and chemometrics in process monitoring of tapioca sweetener production. LWT 2022, 167, 113876. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer-aided Design of Experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Marrubini, G.; Papetti, A.; Genorini, E.; Ulrici, A. Determination of the Sugar Content in Commercial Plant Milks by Near Infrared Spectroscopy and Luff-Schoorl Total Glucose Titration. Food Anal. Methods 2016, 10, 1556–1567. [Google Scholar] [CrossRef]
- Fathi, M.; Nasrabadi, M.N.; Varshosaz, J. Characteristics of vitamin E-loaded nanofibres from dextran. Int. J. Food Prop. 2017, 20, 2665–2674. [Google Scholar] [CrossRef]
- Cayuela-Sánchez, J.A.; Palarea-Albaladejo, J.; García-Martín, J.F.; Pérez-Camino, M. del C. Olive Oil Nutritional Labeling by Using Vis/NIR Spectroscopy and Compositional Statistical Methods. Innov. Food Sci. Emerg. Technol. 2019, 51, 139–147. [Google Scholar] [CrossRef]
- Oliveira-Folador, G.; Bicudo, M.d.O.; de Andrade, E.F.; Renard, C.M.-G.C.; Bureau, S.; de Castilhos, F. Quality traits prediction of the passion fruit pulp using NIR and MIR spectroscopy. LWT 2018, 95, 172–178. [Google Scholar] [CrossRef]
- De Oliveira, G.A.; de Castilhos, F.; Renard, C.M.G.C.; Bureau, S. Comparison of NIR and MIR Spectroscopic Methods for Determination of Individual Sugars, Organic Acids and Carotenoids in Passion Fruit. Food Res. 2014, 60, 154–162. [Google Scholar] [CrossRef]
- Bázár, G.; Romvári, R.; Szabó, A.; Somogyi, T.; Éles, V.; Tsenkova, R. NIR detection of honey adulteration reveals differences in water spectral pattern. Food Chem. 2016, 194, 873–880. [Google Scholar] [CrossRef] [PubMed]
- López, M.G.; García-González, A.S.; Franco-Robles, E. Carbohydrate Analysis by NIRS-Chemometrics. Dev. Near-Infrared Spectrosc. 2017, 10, 67208. [Google Scholar]
- Henn, R.; Schwab, A.; Huck, C.W. Evaluation of benchtop versus portable near-infrared spectroscopic method combined with multivariate approaches for the fast and simultaneous quantitative analysis of main sugars in syrup formulations. Food Control. 2016, 68, 97–104. [Google Scholar] [CrossRef]
- Masithoh, R.E.; Amanah, H.Z.; Yoon, W.S.; Joshi, R.; Cho, B.K. Determination of Protein and Glucose of Tuber and Root Flours Using NIR and MIR Spectroscopy. Infrared Phys. Technol. 2021, 113, 103577. [Google Scholar] [CrossRef]
- Borghi, F.T.; Santos, P.C.; Santos, F.D.; Nascimento, M.H.; Corrêa, T.; Cesconetto, M.; Pires, A.A.; Ribeiro, A.V.; Lacerda, V.; Romão, W.; et al. Quantification and classification of vegetable oils in extra virgin olive oil samples using a portable near-infrared spectrometer associated with chemometrics. Microchem. J. 2020, 159, 105544. [Google Scholar] [CrossRef]
- Xu, J.; Nwafor, C.C.; Shah, N.; Zhou, Y.; Zhang, C. Identification of genetic variation in Brassica napus seeds for tocopherol content and composition using near-infrared spectroscopy technique. Plant Breed. 2019, 138, 624–634. [Google Scholar] [CrossRef]
- Pu, Y.-Y.; O’donnell, C.; Tobin, J.; O’shea, N. Review of near-infrared spectroscopy as a process analytical technology for real-time product monitoring in dairy processing. Int. Dairy J. 2019, 103, 104623. [Google Scholar] [CrossRef]
- Okere, E.E.; Arendse, E.; Nieuwoudt, H.; Perold, W.J.; Opara, U.L. Non-destructive Evaluation of the Quality Characteristics of Pomegranate Kernel Oil by Fourier Transform Near-Infrared and Mid-Infrared Spectroscopy. Front. Plant Sci. 2022, 13, 867555. [Google Scholar] [CrossRef]
- Kahrıman, F.; Onaç, I.; Türk, F.M.; Öner, F.; Egesel, C. Determination of carotenoid and tocopherol content in maize flour and oil samples using near-infrared spectroscopy. Spectrosc. Lett. 2019, 52, 473–481. [Google Scholar] [CrossRef]
- Páscoa, R.N.M.J.; Nunes, M.A.; Reszczyński, F.; Costa, A.S.G.; Oliveira, M.B.P.P.; Alves, R.C. Near Infrared (NIR) Spectroscopy as a Tool to Assess Blends Composition and Discriminate Antioxidant Activity of Olive Pomace Cultivars. Waste Biomass Valorization 2021, 12, 4901–4913. [Google Scholar] [CrossRef]
| Parameter | Cross-Validation (MIR: 12 Samples; NIR: 108 Samples) | Test Set Validation (NIR) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calibration Set (n = 72) | Validation Set (n = 36) | |||||||||||
| Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| α-Toc (mg/g) | 12.59 | 2.70 | 9.54 | 16.74 | 12.68 | 2.57 | 9.54 | 16.74 | 12.68 | 2.20 | 9.68 | 16.51 |
| β-Toc (mg/g) | 2.05 | 0.53 | 1.15 | 2.82 | 2.03 | 0.49 | 1.15 | 2.82 | 2.05 | 0.41 | 1.32 | 2.73 |
| γ-Toc (mg/g) | 17.11 | 3.53 | 12.77 | 24.29 | 17.11 | 3.43 | 12.41 | 24.85 | 17.01 | 2.86 | 13.25 | 24.27 |
| δ-Toc (mg/g) | 2.24 | 0.38 | 1.63 | 2.80 | 2.25 | 0.37 | 1.55 | 2.93 | 2.23 | 0.34 | 1.67 | 2.81 |
| Toc (mg/g) | 33.99 | 6.94 | 26.38 | 46.60 | 33.99 | 6.81 | 25.91 | 47.33 | 34.52 | 5.71 | 27.50 | 44.55 |
| α-T3 (mg/g) | 1.27 | 0.39 | 0.78 | 2.04 | 1.26 | 0.40 | 0.78 | 2.04 | 1.30 | 0.34 | 0.81 | 1.70 |
| γ-T3 (mg/g) | 157.71 | 35.44 | 115.53 | 224.15 | 158.30 | 35.23 | 115.53 | 224.15 | 156.79 | 27.70 | 126.50 | 212.43 |
| δ-T3 (mg/g) | 8.62 | 2.06 | 5.43 | 12.36 | 8.60 | 2.01 | 5.32 | 12.46 | 8.81 | 1.59 | 5.48 | 12.37 |
| T3 (mg/g) | 167.60 | 37.62 | 121.74 | 238.01 | 167.24 | 36.86 | 119.25 | 240.08 | 167.41 | 30.21 | 134.73 | 225.32 |
| Tocols (mg/g) | 201.59 | 44.42 | 148.12 | 284.61 | 199.27 | 44.65 | 148.12 | 284.61 | 199.34 | 41.38 | 161.76 | 269.87 |
| Parameter | Cross-Validation | Test Set Validation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NIR | MIR | NIR | |||||||||
| RMSECV | Q2 | RMSECV | Q2 | Preprocessing | LVs | RMSEC | R2 | RMSEP | Q2 | RPD | |
| α-Toc (mg/g) | 0.37 | 0.98 | 1.48 | 0.70 | 1st derivative | 9 | 0.55 | 0.96 | 0.56 | 0.94 | 3.92 |
| β-Toc (mg/g) | 0.14 | 0.93 | 0.38 | 0.52 | 1st derivative | 7 | 0.15 | 0.90 | 0.16 | 0.86 | 2.56 |
| γ-Toc (mg/g) | 0.90 | 0.93 | 2.20 | 0.58 | 1st derivative | 7 | 1.10 | 0.89 | 1.16 | 0.86 | 2.60 |
| δ-Toc (mg/g) | 0.10 | 0.92 | 0.34 | 0.35 | 1st derivative + SNV | 7 | 0.13 | 0.87 | 0.14 | 0.82 | 2.43 |
| Toc (mg/g) | 1.26 | 0.96 | 4.41 | 0.64 | 1st derivative + SNV | 8 | 1.35 | 0.96 | 1.72 | 0.92 | 3.34 |
| α-T3 (mg/g) | 0.25 | 0.58 | 0.40 | 0.20 | 1st derivative | 8 | 0.20 | 0.71 | 0.22 | 0.66 | 1.54 |
| γ-T3 (mg/g) | 6.85 | 0.96 | 22.89 | 0.56 | 1st derivative | 7 | 9.31 | 0.93 | 10.36 | 0.87 | 2.67 |
| δ-T3 (mg/g) | 0.54 | 0.93 | 0.90 | 0.79 | 1st derivative | 7 | 0.71 | 0.88 | 0.84 | 0.73 | 1.89 |
| T3 (mg/g) | 7.41 | 0.96 | 23.68 | 0.58 | 1st derivative | 7 | 9.60 | 0.93 | 10.0 | 0.89 | 3.02 |
| Tocols (mg/g) | 8.44 | 0.96 | 28.20 | 0.57 | 1st derivative + SNV | 8 | 10.27 | 0.93 | 10.67 | 0.92 | 3.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rungchang, S.; Kittiwachana, S.; Funsueb, S.; Rachtanapun, C.; Tantala, J.; Sookwong, P.; Yort, L.; Sringarm, C.; Jiamyangyuen, S. Nondestructive Determination of Tocopherol and Tocotrienol in Vitamin E Powder Using Near- and Mid-Infrared Spectroscopy. Foods 2024, 13, 4079. https://doi.org/10.3390/foods13244079
Rungchang S, Kittiwachana S, Funsueb S, Rachtanapun C, Tantala J, Sookwong P, Yort L, Sringarm C, Jiamyangyuen S. Nondestructive Determination of Tocopherol and Tocotrienol in Vitamin E Powder Using Near- and Mid-Infrared Spectroscopy. Foods. 2024; 13(24):4079. https://doi.org/10.3390/foods13244079
Chicago/Turabian StyleRungchang, Saowaluk, Sila Kittiwachana, Sujitra Funsueb, Chitsiri Rachtanapun, Juthamas Tantala, Phumon Sookwong, Laichheang Yort, Chayanid Sringarm, and Sudarat Jiamyangyuen. 2024. "Nondestructive Determination of Tocopherol and Tocotrienol in Vitamin E Powder Using Near- and Mid-Infrared Spectroscopy" Foods 13, no. 24: 4079. https://doi.org/10.3390/foods13244079
APA StyleRungchang, S., Kittiwachana, S., Funsueb, S., Rachtanapun, C., Tantala, J., Sookwong, P., Yort, L., Sringarm, C., & Jiamyangyuen, S. (2024). Nondestructive Determination of Tocopherol and Tocotrienol in Vitamin E Powder Using Near- and Mid-Infrared Spectroscopy. Foods, 13(24), 4079. https://doi.org/10.3390/foods13244079







