Bioactive Compounds, Antioxidant Properties, and Antimicrobial Profiling of a Range of West Algerian Honeys: In Vitro Comparative Screening Prior to Therapeutic Purpose
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Honey Samples and Physico-Chemical Characterization
2.3. Preparation of Honey Solutions
2.4. Quantification of Bioactive Compound
2.4.1. Determination of Total Phenolic Content (TPC)
2.4.2. Determination of Total Flavonoids Content (TFC)
2.5. Determination of Antioxidant Activity
2.5.1. Diphenyl-2-Picrylhydrazyl (DPPH) Radical Scavenging Activity
- Abscontrol is the absorbance of the control;
- Abssample is the absorbance of the honey sample solution.
2.5.2. Ferric Reducing Antioxidant Power (FRAP)
2.5.3. The Antiradical Activity of ABTS
- Abs0 is the absorbance of the control;
- Abs1 is the absorbance of the test sample.
2.5.4. β-Carotene-Linoleic Acid Emulsion Method
- A0 is the initial absorbance of the emulsion at time 0;
- At is the absorbance at time t;
- t is the time in minutes.
- Rcontrol is the bleaching rate of the β-carotene emulsion without an antioxidant;
- Rsample is the bleaching rate of the β-carotene emulsion with the honey sample.
2.6. Determination of Antimicrobial Activity
2.6.1. Microbial Strains and Cultures Conditions
2.6.2. Well-Diffusion Assay
2.6.3. Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs) Determination
2.7. Statistical Analysis
3. Results
3.1. Characterization of Honey Samples
3.2. Quantification of Bioactive Compounds
3.3. Assessment of Antioxidant Properties
3.4. Evaluation of Antimicrobial Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sindi, A.; Chawn, M.V.B.; Hernandez, M.E.; Green, K.; Islam, M.K.; Locher, C.; Hammer, K. Anti-Biofilm Effects and Characterisation of the Hydrogen Peroxide Activity of a Range of Western Australian Honeys Compared to Manuka and Multifloral Honeys. Sci. Rep. 2019, 9, 17666. [Google Scholar] [CrossRef] [PubMed]
- Owayss, A.A.; Elbanna, K.; Iqbal, J.; Abulreesh, H.H.; Organji, S.R.; Raweh, H.S.A.; Alqarni, A.S. In Vitro Antimicrobial Activities of Saudi Honeys Originating from Ziziphus Spina-christi L. and Acacia Gerrardii Benth. Trees. Food Sci. Nutr. 2020, 8, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison With Important Commercial Honeys. Front. Microbiol. 2019, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Laallam, H.; Boughediri, L.; Bissati, S.; Menasria, T.; Mouzaoui, M.S.; Hadjadj, S.; Hammoudi, R.; Chenchouni, H. Modeling the Synergistic Antibacterial Effects of Honey Characteristics of Different Botanical Origins from the Sahara Desert of Algeria. Front. Microbiol. 2015, 6, 1239. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Hau-Yama, N.E.; Magaña-Ortiz, D.; Oliva, A.I.; Ortiz-Vázquez, E. Antifungal Activity of Honey from Stingless Bee Melipona beecheii Against Candida albicans. J. Apic. Res. 2020, 59, 12–18. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, M.; Tit, D.M.; Behl, T.; Hegheduș-Mîndru, R.C.; Zaha, D.C.; Ursu, F.; Popa, M.; Glevitzky, I.; Bungău, S. The Antimicrobial Activity of Honey and Propolis Extracts from the Central Region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Luca, L.; Pauliuc, D.; Oroian, M. Honey Microbiota, Methods for Determining the Microbiological Composition and the Antimicrobial Effect of Honey—A Review. Food Chem. X 2024, 23, 101524. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.E.; Jenkins, R.E. Honey: A Sweet Solution to the Growing Problem of Antimicrobial Resistance? Future Microbiol. 2013, 8, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.S.; Salom, K.; Butler, G.; Al Ghamdi, A.A. Honey and Microbial Infections: A Review Supporting the Use of Honey for Microbial Control. J. Med. Food 2011, 14, 1079–1096. [Google Scholar] [CrossRef]
- Martín, R.A.P.; Hortigüela, L.V.; Lozano, P.L.; Cortina, M.D.R.; Carretero, C.D.L. In Vitro Antioxidant and Antimicrobial Activities of Spanish Honeys. Int. J. Food Prop. 2008, 11, 727–737. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Bammou, M.; Sellam, K.; Ramchoun, M.; Benlyas, M.; Alem, C.; Zegzouti, Y. Evaluation of Antioxidant, Antibacterial and Antifungal Activities of Eleven Monofloral Honey Samples Collected from Morocco. J. Chem. Pharm. Res. 2016, 8, 299–306. [Google Scholar]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2019, 67, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Ouchemoukh, S.; Louaileche, H.; Schweitzer, P. Physico-Chemical Characteristics and Pollen Spectrum of Some Algerian Honey. Food Control 2007, 18, 52–58. [Google Scholar] [CrossRef]
- Nair, S.; Maghraoui, N.B. Physicochemical Properties of Honeys Produced in North-West of Algeria. Adv. Food Sci. Eng. 2017, 1, 123–128. [Google Scholar] [CrossRef]
- Bereksi-Reguig, D.; Bouchentouf, S.; Allali, H.; Adamczuk, A.; Kowalska, G.; Kowalski, R. Trace Elements and Heavy Metal Contents in West Algerian Natural Honey. J. Anal. Methods Chem. 2022, 2022, 7890856. [Google Scholar] [CrossRef] [PubMed]
- Homrani, M.; Escuredo, O.; Rodríguez-Flores, M.S.; Fatiha, D.; Mohammed, B.; Homrani, A.; Seijo, M.C. Botanical Origin, Pollen Profile, and Physicochemical Properties of Algerian Honey from Different Bioclimatic Areas. Foods 2020, 9, 938. [Google Scholar] [CrossRef] [PubMed]
- Zerrouk, S.; Seijo, M.C.; Escuredo, O.; Rodríguez-Flores, M.S. Characterization of Ziziphus lotus (Jujube) Honey Produced in Algeria. J. Apic. Res. 2018, 57, 166–174. [Google Scholar] [CrossRef]
- Makhloufi, C.; Kerkvliet, J.; Schweitzer, P. Characterisation of Some Monofloral Algerian Honeys by Pollen Analysis. Grana 2015, 54, 156–166. [Google Scholar] [CrossRef]
- Haderbache, L.; Mouna, B.; Mohammedi, A. Ziziphus lotus and Euphorbia Bupleuroides Algerian Honeys. World Appl. Sci. J. 2013, 24, 1536–1543. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Gómez-Romero, M.; Aboud, F.; Giuseppe, A.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Characterisation of Phenolic Compounds in Algerian Honeys by RP-HPLC Coupled to Electrospray Time-of-Flight Mass Spectrometry. LWT Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; Von Der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.-G.; Marcazzan, G.L.; Piro, R.; et al. Honey Quality and International Regulatory Standards: Review by the International Honey Commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Bogdanov, S.; Martin, P. Honey Authenticity: A Review. Mitt. Lebensm. Hyg. 2002, 93, 232–254. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant Activity and Total Phenolic Content of Some Brazilian Species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Karagözler, A.A.; Erdağ, B.; Emek, Y.Ç.; Uygun, D.A. Antioxidant Activity and Proline Content of Leaf Extracts from Dorystoechas Hastata. Food Chem. 2008, 111, 400–407. [Google Scholar] [CrossRef]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; da Silva, W.P.; Zanusso, J.T.; Dutra, I. Antibacterial and Antioxidant Activity of Honeys from the State of Rio Grande Do Sul, Brazil. LWT Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef]
- Silva, T.M.S.; dos Santos, F.P.; Evangelista-Rodrigues, A.; da Silva, E.M.S.; da Silva, G.S.; de Novais, J.S.; de Assis Ribeiro dos Santos, F.; Camara, C.A. Phenolic Compounds, Melissopalynological, Physicochemical Analysis and Antioxidant Activity of Jandaíra (Melipona subnitida) Honey. J. Food Compost. Anal. 2013, 29, 10–18. [Google Scholar] [CrossRef]
- Ghramh, H.; Khan, K.; Alshehri, A. Antibacterial Potential of Some Saudi Honeys from Asir Region against Selected Pathogenic Bacteria. Saudi J. Biol. Sci. 2018, 26, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Küçük, M.; Kolaylı, S.; Karaoğlu, Ş.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological Activities and Chemical Composition of Three Honeys of Different Types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 26th Edition, Wayne. References Scientific Research Publishing. 2016. Available online: https://www.scirp.org/reference/referencespapers?referenceid=2196113 (accessed on 5 November 2024).
- Khalil, M.I.; Moniruzzaman, M.; Boukraâ, L.; Benhanifia, M.; Islam, M.A.; Islam, M.N.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Algerian Honey. Molecules 2012, 17, 11199–11215. [Google Scholar] [CrossRef]
- Ibrahimi, H.; Hajdari, A. Phenolic and Flavonoid Content, and Antioxidant Activity of Honey from Kosovo. J. Apic. Res. 2020, 59, 452–457. [Google Scholar] [CrossRef]
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsì, F.; Ferrari, G.; Hamdi, S. Physicochemical and Bioactive Properties of Six Honey Samples from Various Floral Origins from Tunisia. Arab. J. Chem. 2018, 11, 265–274. [Google Scholar] [CrossRef]
- El-Haskoury, R.; Kriaa, W.; Lyoussi, B.; Makni, M. Ceratonia siliqua Honeys from Morocco: Physicochemical Properties, Mineral Contents, and Antioxidant Activities. J. Food Drug Anal. 2018, 26, 67–73. [Google Scholar] [CrossRef]
- Dżugan, M.; Grabek-Lejko, D.; Swacha, S.; Tomczyk, M.; Bednarska, S.; Kapusta, I. Physicochemical Quality Parameters, Antibacterial Properties and Cellular Antioxidant Activity of Polish Buckwheat Honey. Food Biosci. 2020, 34, 100538. [Google Scholar] [CrossRef]
- Mouhoubi-Tafinine, Z.; Ouchemoukh, S.; Tamendjari, A. Antioxydant Activity of Some Algerian Honey and Propolis. Ind. Crops Prod. 2016, 88, 85–90. [Google Scholar] [CrossRef]
- Perna, A.; Simonetti, A.; Intaglietta, I.; Sofo, A.; Gambacorta, E. Metal Content of Southern Italy Honey of Different Botanical Origins and Its Correlation with Polyphenol Content and Antioxidant Activity. Int. J. Food Sci. Tech. 2012, 47, 1909–1917. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Antunes, D.; Miguel, M.G. Physicochemical Characterization and Antioxidant Activity of 17 Commercial Moroccan Honeys. Int. J. Food Sci. Nutr. 2014, 65, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Galhardo, D.; Garcia, R.C.; Schneider, C.R.; Braga, G.C.; Chambó, E.D.; de França, D.L.B.; Ströher, S.M. Physicochemical, Bioactive Properties and Antioxidant of Apis mellifera L. Honey from Western Paraná, Southern Brazil. Food Sci. Technol. 2020, 41, 247–253. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant Activity of Portuguese Honey Samples: Different Contributions of the Entire Honey and Phenolic Extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Gül, A.; Pehlivan, T. Antioxidant Activities of Some Monofloral Honey Types Produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Physicochemical Characterization and Antioxidant Activity of Palestinian Honey Samples. Food Sci. Nutr. 2018, 6, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.S.; Donovan, J.L.; Pearson, D.A.; Waterhouse, A.L.; Frankel, E.N. Fruit Hydroxycinnamic Acids Inhibit Human Low-Density Lipoprotein Oxidation in Vitro. J. Agric. Food Chem. 1998, 46, 1783–1787. [Google Scholar] [CrossRef]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Maffei Facino, R. Standardization of Antioxidant Properties of Honey by a Combination of Spectrophotometric/Fluorimetric Assays and Chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Taormina, P.J.; Niemira, B.A.; Beuchat, L.R. Inhibitory Activity of Honey against Foodborne Pathogens as Influenced by the Presence of Hydrogen Peroxide and Level of Antioxidant Power. Int. J. Food Microbiol. 2001, 69, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Matzen, R.D.; Zinck Leth-Espensen, J.; Jansson, T.; Nielsen, D.S.; Lund, M.N.; Matzen, S. The Antibacterial Effect In Vitro of Honey Derived from Various Danish Flora. Dermatol. Res. Pract. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Romário-Silva, D.; Alencar, S.M.; Bueno-Silva, B.; Sardi, J.C.O.; Franchin, M.; Carvalho, R.D.P.; Ferreira, T.E.S.A.; Rosalen, P.L. Antimicrobial Activity of Honey against Oral Microorganisms: Current Reality, Methodological Challenges, and Solutions. Microorganisms 2022, 10, 2325. [Google Scholar] [CrossRef]
- Sagdic, O.; Silici, S.; Ekici, L. Evaluation of the Phenolic Content, Antiradical, Antioxidant, and Antimicrobial Activity of Different Floral Sources of Honey. Int. J. Food Prop. 2013, 16, 658–666. [Google Scholar] [CrossRef]
- Bouacha, M.; Ayed, H.; Grara, N. Honey Bee as Alternative Medicine to Treat Eleven Multidrug-Resistant Bacteria Causing Urinary Tract Infection during Pregnancy. Sci. Pharm. 2018, 86, 14. [Google Scholar] [CrossRef] [PubMed]
- Osés, S.M.; Pascual-Maté, A.; de la Fuente, D.; de Pablo, A.; Fernández-Muiño, M.A.; Sancho, M.T. Comparison of Methods to Determine Antibacterial Activity of Honeys against Staphylococcus aureus. NJAS Wagen. J. Life Sci. 2016, 78, 29–33. [Google Scholar] [CrossRef]
- Hussain, M.B.; Kamel, Y.M.; Ullah, Z.; Jiman-Fatani, A.A.M.; Ahmad, A.S. In Vitro Evaluation of Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Susceptibility to Saudi Honeys. BMC Complement Altern. Med. 2019, 19, 185. [Google Scholar] [CrossRef] [PubMed]

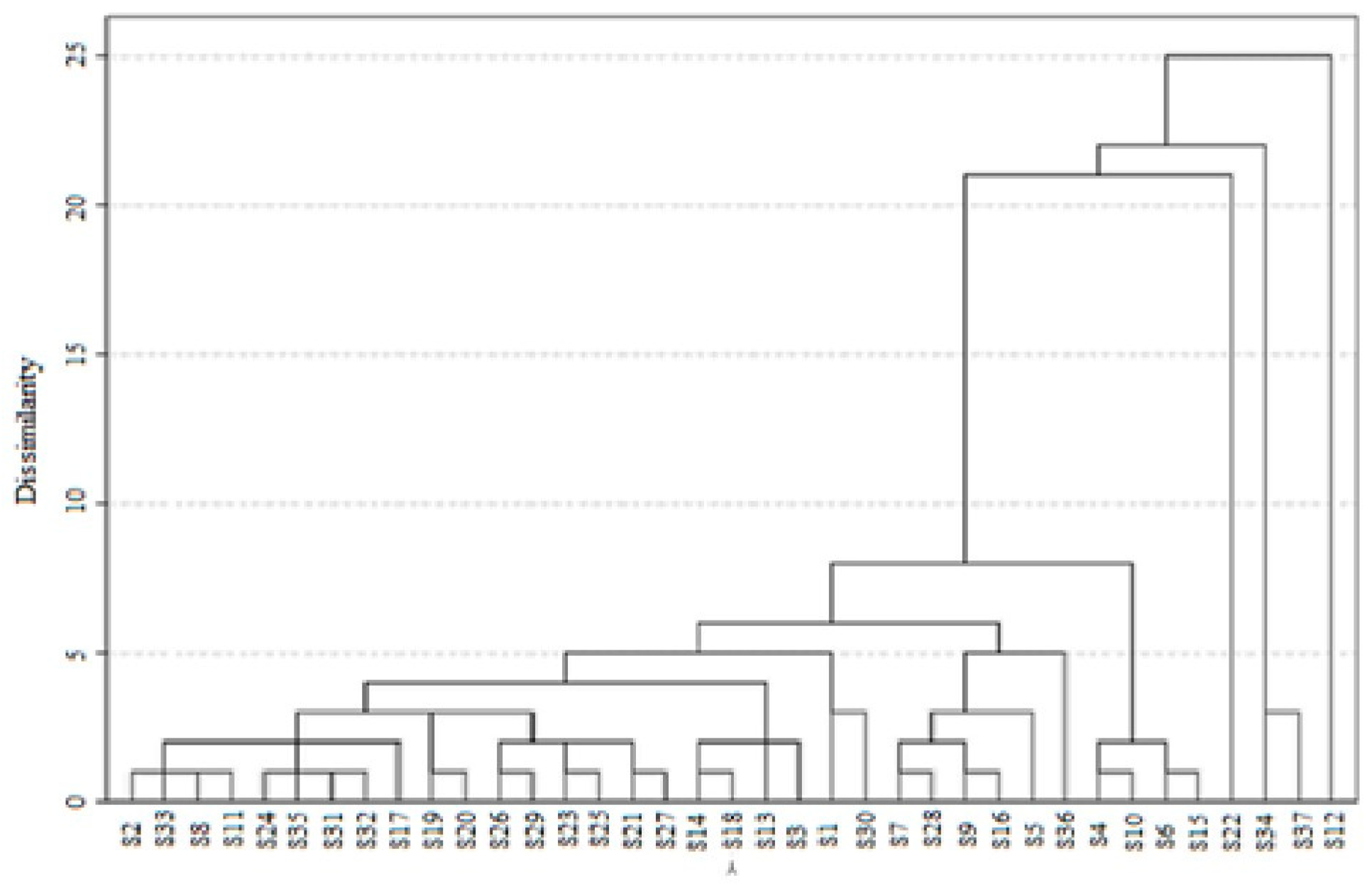
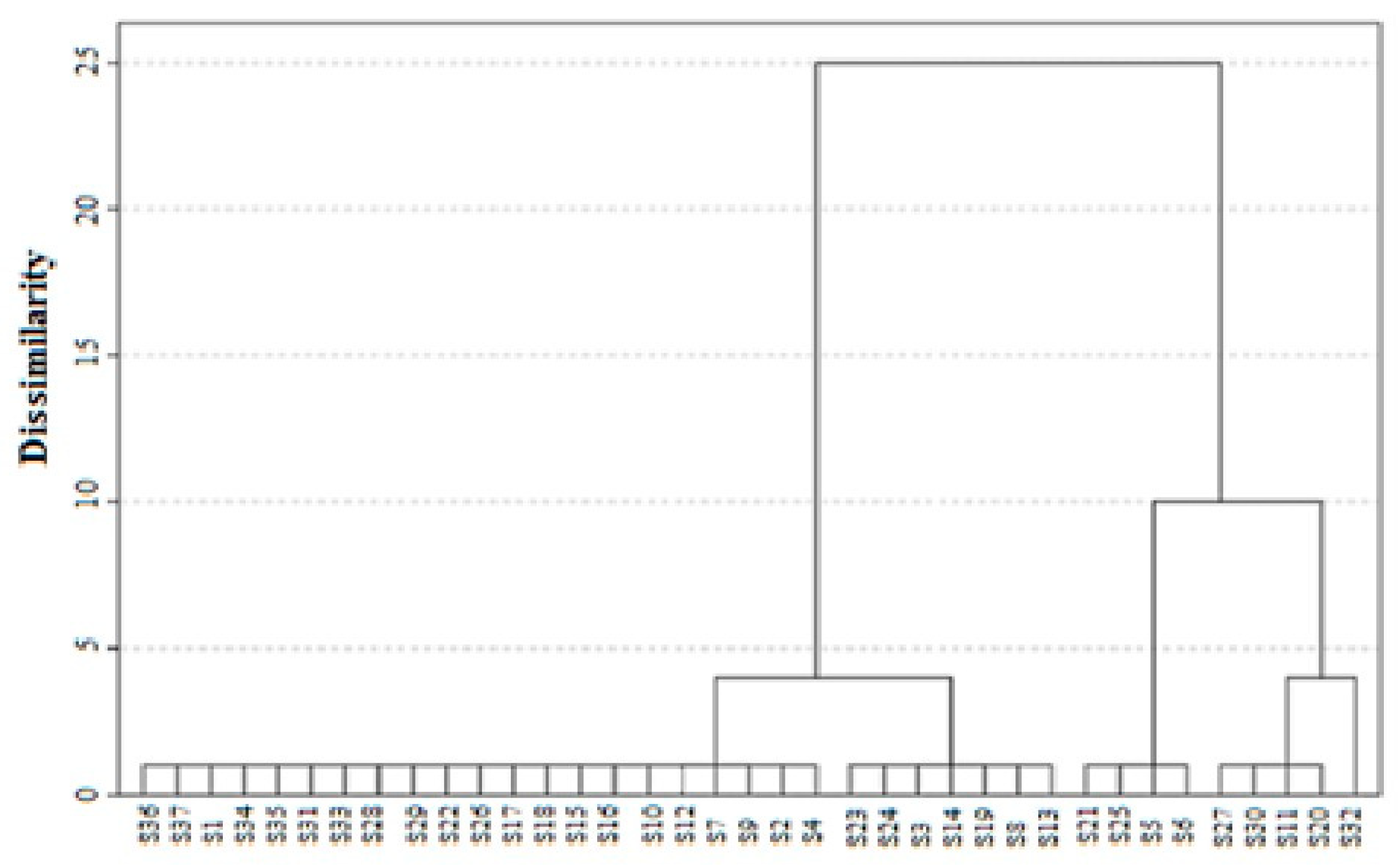
| N° | TPC * (mg GAE/100 g) | TFC * (mg QE/100 g) | pH * | EC * (mS/cm) | DPPH Scavenging Activity * IC50 (mg/mL) | ABTS Assay * IC50 (mg/mL) | β-Carotene * IC50 (mg/mL) | FRAP Value * (mg AAE/100 g Honey) |
|---|---|---|---|---|---|---|---|---|
| S1 | 24.17 ± 1.38 | 0.07 ± 0.01 | 3.61 ± 0.03 | 0.445 ± 0.006 | 33.05 ± 0.61 | 64.86 ± 2.94 | 56.50 ± 0.96 | 6.69 ± 0.22 |
| S2 | 39.14 ± 2.81 | 6.11 ± 0.72 | 3.38 ± 0.06 | 0.259 ± 0.001 | 26.05 ± 0.79 | 33.47 ± 0.49 | 38.70 ± 0.48 | 14.07 ± 0.44 |
| S3 | 57.64 ± 1.20 | 2.47 ± 0.45 | 3.88 ± 0.03 | 0.209 ± 0.001 | 28.09 ± 1.67 | 39.86 ± 1.49 | 25.23 ± 0.16 | 16.08 ± 0.17 |
| S4 | 82.49 ± 2.06 | 26.49 ± 3.04 | 4.96 ± 0.02 | 0.276 ± 0.005 | 32.85 ± 1.54 | 31.93 ± 2.58 | 34.67 ± 0.20 | 18.49 ± 0.37 |
| S5 | 42.91 ± 0.71 | 9.35 ± 1.65 | 5.62 ± 0.04 | 0.250 ± 0.002 | 61.60 ± 1.23 | 71.58 ± 7.79 | 69.10 ± 6.28 | 16.72 ± 0.53 |
| S6 | 71.18 ± 5.65 | 17.26 ± 2.38 | 3.99 ± 0.05 | 0.553 ± 0.001 | 22.44 ± 0.54 | 17.97 ± 0.72 | 36.37 ± 0.40 | 15.94 ± 1.06 |
| S7 | 57.45 ± 3.18 | 1.63 ± 0.83 | 4.30 ± 0.01 | 0.293 ± 0.001 | 49.17 ± 4.42 | 49.54 ± 2.03 | 56.47 ± 1.26 | 26.06 ± 0.70 |
| S8 | 54.82 ± 4.43 | 4.85 ± 0.57 | 4.28 ± 0.06 | 0.470 ± 0.006 | 29.22 ± 2.06 | 34.59 ± 0.61 | 42.91 ± 0.20 | 17.99 ± 0.92 |
| S9 | 40.19 ± 5.47 | 9.08 ± 0.75 | 4.64 ± 0.01 | 0.254 ± 0.028 | 60.39 ± 1.96 | 48.27 ± 1.25 | 68.86 ± 0.68 | 18.00 ± 0.62 |
| S10 | 77.77 ± 3.64 | 19.10 ± 1.41 | 4.50 ± 0.01 | 0.415 ± 0.018 | 34.66 ± 0.38 | 24.17 ± 0.80 | 37.50 ± 0.31 | 29.01 ± 1.18 |
| S11 | 44.25 ± 4.02 | 5.76 ± 0.80 | 4.60 ± 0.01 | 0.445 ± 0.008 | 35.23 ± 1.24 | 36.14 ± 0.20 | 47.65 ± 0.16 | 18.05 ± 0.44 |
| S12 | 122.15 ± 3.55 | 33.49 ± 4.90 | 4.36 ± 0.01 | 0.725 ± 0.013 | 27.25 ± 0.81 | 27.12 ± 0.70 | 31.92 ± 0.41 | 34.40 ± 0.61 |
| S13 | 52.23 ± 0.98 | 13.37 ± 2.27 | 4.11 ± 0.02 | 0.910 ± 0.008 | 17.73 ± 1.53 | 57.39 ± 2.52 | 32.38 ± 0.16 | 39.79 ± 0.86 |
| S14 | 56.55 ± 2.62 | 9.54 ± 1.21 | 4.63 ± 0.05 | 0.518 ± 0.008 | 27.14 ± 0.44 | 37.82 ± 0.40 | 34.04 ± 0.14 | 34.59 ± 1.26 |
| S15 | 57.23 ± 2.75 | 19.15 ± 2.51 | 4.28 ± 0.01 | 0.380 ± 0.005 | 22.91 ± 0.91 | 24.51 ± 0.57 | 29.20 ± 0.13 | 13.78 ± 0.27 |
| S16 | 38.25 ± 1.64 | 2.40 ± 0.35 | 4.28 ± 0.02 | 0.165 ± 0.017 | 59.29 ± 3.52 | 35.92 ± 0.81 | 55.07 ± 0.79 | 14.53 ± 0.18 |
| S17 | 48.90 ± 2.95 | 2.22 ± 0.50 | 4.62 ± 0.05 | 0.421 ± 0.002 | 44.14 ± 0.76 | 48.90 ± 1.22 | 38.17 ± 0.29 | 14.47 ± 0.26 |
| S18 | 67.22 ± 4.44 | 4.79 ± 0.73 | 3.77 ± 0.10 | 0.321 ± 0.002 | 29.11 ± 2.37 | 49.20 ± 1.69 | 32.84 ± 0.13 | 34.65 ± 0.50 |
| S19 | 39.62 ± 1.06 | 5.04 ± 0.11 | 5.12 ± 0.08 | 1.196 ± 0.021 | 25.94 ± 0.41 | 37.75 ± 0.57 | 37.66 ± 0.12 | 31.90 ± 2.32 |
| S20 | 32.87 ± 2.83 | 5.53 ± 0.76 | 4.40 ± 0.00 | 1.040 ± 0.002 | 37.93 ± 2.69 | 28.40 ± 0.12 | 27.24 ± 0.55 | 35.06 ± 0.65 |
| S21 | 61.57 ± 3.48 | 7.11 ± 0.94 | 4.19 ± 0.01 | 0.498 ± 0.001 | 38.58 ± 0.86 | 33.28 ± 0.79 | 44.88 ± 0.70 | 39.27 ± 0.75 |
| S22 | 55.04 ± 1.49 | 8.57 ± 1.33 | 4.51 ± 0.65 | 0.236 ± 0.005 | 98.58 ± 6.29 | 39.15 ± 0.61 | 91.80 ± 1.69 | 14.57 ± 0.58 |
| S23 | 48.69 ± 1.86 | 13.37 ± 0.78 | 4.27 ± 0.01 | 0.436 ± 0.006 | 50.36 ± 1.01 | 36.75 ± 1.22 | 50.43 ± 0.31 | 26.32 ± 1.04 |
| S24 | 33.50 ± 2.29 | 4.40 ± 1.22 | 3.88 ± 0.01 | 0.163 ± 0.002 | 40.47 ± 0.13 | 33.30 ± 0.82 | 40.06 ± 0.06 | 17.36 ± 1.13 |
| S25 | 43.34 ± 2.31 | 9.40 ± 1.25 | 4.10 ± 0.01 | 0.320 ± 0.002 | 49.36 ± 0.68 | 41.21 ± 0.68 | 42.43 ± 0.25 | 29.23 ± 0.99 |
| S26 | 28.37 ± 0.60 | 6.57 ± 0.39 | 4.70 ± 0.03 | 0.317 ± 0.003 | 40.76 ± 1.78 | 45.94 ± 0.83 | 45.77 ± 0.66 | 40.74 ± 0.48 |
| S27 | 46.53 ± 2.73 | 10.84 ± 1.55 | 4.29 ± 0.04 | 0.575 ± 0.010 | 43.62 ± 1.25 | 32.29 ± 0.24 | 47.44 ± 0.77 | 42.36 ± 1.14 |
| S28 | 41.95 ± 0.96 | 1.79 ± 0.27 | 4.65 ± 0.02 | 0.210 ± 0.001 | 47.34 ± 1.26 | 58.69 ± 0.77 | 59.76 ± 1.07 | 18.81 ± 0.79 |
| S29 | 36.52 ± 1.12 | 2.94 ± 0.61 | 4.19 ± 0.02 | 0.249 ± 0.001 | 47.45 ± 0.63 | 49.37 ± 0.42 | 49.28 ± 0.79 | 36.89 ± 0.61 |
| S30 | 36.37 ± 1.10 | 13.37 ± 1.90 | 4.00 ± 0.02 | 0.363 ± 0.001 | 25.88 ± 1.80 | 52.60 ± 2.58 | 41.58 ± 0.48 | 14.30 ± 0.20 |
| S31 | 48.47 ± 3.13 | 13.34 ± 1.91 | 3.94 ± 0.01 | 0.447 ± 0.004 | 45.13 ± 0.62 | 24.02 ± 0.50 | 46.41 ± 0.56 | 16.58 ± 0.41 |
| S32 | 40.27 ± 2.58 | 12.65 ± 1.80 | 4.69 ± 0.02 | 0.248 ± 0.018 | 54.80 ± 0.77 | 34.28 ± 0.38 | 43.53 ± 3.78 | 15.28 ± 0.53 |
| S33 | 42.22 ± 1.73 | 7.33 ± 0.91 | 5.31 ± 0.01 | 0.226 ± 0.002 | 33.75 ± 2.59 | 30.07 ± 0.22 | 36.96 ± 4.15 | 11.08 ± 0.84 |
| S34 | 42.09 ± 1.81 | 11.27 ± 1.21 | 4.56 ± 0.05 | 0.202 ± 0.001 | 49.27 ± 0.23 | 100.62 ± 9.13 | 69.34 ± 0.80 | 13.10 ± 0.42 |
| S35 | 36.43 ± 1.16 | 9.64 ± 0.66 | 4.99 ± 0.01 | 0.236 ± 0.001 | 46.51 ± 2.20 | 29.23 ± 0.29 | 33.64 ± 0.54 | 11.02 ± 0.58 |
| S36 | 32.23 ± 1.66 | 3.35 ± 0.91 | 5.01 ± 0.06 | 0.464 ± 0.006 | 67.49 ± 0.68 | 73.33 ± 1.71 | 41.11 ± 1.03 | 22.44 ± 0.88 |
| S37 | 49.85 ± 3.12 | 4.01 ± 0.42 | 4.44 ± 0.01 | 0.249 ± 0.001 | 58.53 ± 1.64 | 118.12 ± 10.22 | 85.22 ± 0.65 | 12.45 ± 0.35 |
| TPC | TFC | pH | EC | DPPH | ABTS | β-Carotene | FRAP Value | |
|---|---|---|---|---|---|---|---|---|
| TPC | 1 | |||||||
| TFC | 0.734 ** | 1 | ||||||
| pH | −0.046 | 0.093 | 1 | |||||
| EC | 0.152 | 0.179 | 0.034 | 1 | ||||
| DPPH | −0.269 | −0.260 | 0.328 * | −0.415 * | 1 | |||
| ABTS | −0.286 | −0.344 * | 0.141 | −0.214 | 0.330 * | 1 | ||
| β-Carotene | −0.229 | −0.262 | 0.165 | −0.392 * | 0.766 ** | 0.600 ** | 1 | |
| FRAP value | 0.229 | 0.163 | −0.062 | 0.532 ** | −0.218 | −0.207 | −0.321 | 1 |
| Gram-Positive | Gram-Negative | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 43300 | Staphylococcus aureus ATCC 25923 | Pseudomonas aeruginosa ATCC 27853 | |||||||||||||
| DIZ a | Sig.b | MIC c | MBC d | DIZ | Sig. | MIC | MBC | DIZ | Sig. | MIC | MBC | ||||
| N° | 80% (w/v) e | 50% (w/v) | [%] | [%] | 80% (w/v) | 50% (w/v) | [%] | [%] | 80% (w/v) | 50% (w/v) | [%] | [%] | |||
| S1 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S2 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S3 | / | / | - | / | / | 12.33 ± 0.58 | 9.33 ± 0.58 | * | 60.00 ± 0.00 | 60.00 ± 0.00 | / | / | - | / | / |
| S4 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S5 | 13.33 ± 1.15 | 10.00 ± 2.00 | NS | 80.00 ± 0.00 | 80.00 ± 0.00 | 28.33 ± 0.58 | 25.00 ± 0.00 | ** | 100.00 ± 0.00 | 100.00 ± 0.00 | / | / | - | / | / |
| S6 | 14.33 ± 0.58 | 11.67 ± 0.58 | * | 60.00 ± 0.00 | 60.00 ± 0.00 | 14.00 ± 1.73 | 11.33 ± 1.15 | NS | 60.00 ± 0.00 | 60.00 ± 0.00 | / | / | - | / | / |
| S7 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S8 | / | / | - | / | / | 13.33 ± 0.58 | 11.67 ± 1.15 | NS | 80.00 ± 0.00 | 100.00 ± 0.00 | / | / | - | / | / |
| S9 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S10 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S11 | / | / | - | / | / | 10.33 ± 1.53 | 8.67 ± 1.53 | NS | 80.00 ± 0.00 | 80.00 ± 0.00 | 8.00 ± 0.00 | / | - | 60.00 ± 0.00 | 60.00 ± 0.00 |
| S12 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S13 | / | / | - | / | / | 15.67 ± 0.58 | 13.33 ± 1.15 | * | 80.00 ± 0.00 | 100.00 ± 0.00 | / | / | - | / | / |
| S14 | / | / | - | / | / | 9.00 ± 0.00 | / | - | 60.00 ± 0.00 | 60.00 ± 0.00 | / | / | - | / | / |
| S15 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S16 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S17 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S18 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S19 | / | / | - | / | / | 12.33 ± 0.58 | 9.33 ± 0.58 | * | 80.00 ± 0.00 | 80.00 ± 0.00 | / | / | - | / | / |
| S20 | / | / | - | / | / | 13.00 ± 1.00 | 11.33 ± 0.58 | NS | 80.00 ± 0.00 | 80.00 ± 0.00 | 8.00 ± 0.00 | / | - | 80.00 ± 0.00 | 80.00 ± 0.00 |
| S21 | 14.33 ± 0.57 | 8.33 ± 1.53 | * | 80.00 ± 0.00 | 80.00 ± 0.00 | 14.33 ± 0.58 | 12.67 ± 0.58 | * | 80.00 ± 0.00 | 80.00 ± 0.00 | / | / | - | / | / |
| S22 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S23 | / | / | - | / | / | 30.00 ± 0.00 | 25.00 ± 0.00 | - | 80.00 ± 0.00 | 100.00 ± 0.00 | / | / | - | / | / |
| S24 | / | / | - | / | / | 17.67 ± 1.15 | 14.67 ± 1.15 | * | 80.00 ± 0.00 | 80.00 ± 0.00 | / | / | - | / | / |
| S25 | 14.33 ± 0.57 | 10.00 ± 1.00 | * | 100.00 ± 0.00 | 100.00 ± 0.00 | 12.33 ± 0.58 | 10.00 ± 1.73 | NS | 80.00 ± 0.00 | 80.00 ± 0.00 | / | / | - | / | / |
| S26 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S27 | / | / | - | / | / | 10.33 ± 1.53 | 8.33 ± 1.53 | NS | 60.00 ± 0.00 | 60.00 ± 0.00 | 8.00 ± 0.00 | / | - | 80.00 ± 0.00 | 80.00 ± 0.00 |
| S28 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S29 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S30 | / | / | - | / | / | 13.33 ± 1.53 | 10.33 ± 1.53 | NS | 80.00 ± 0.00 | 100.00 ± 0.00 | 8.67 ± 0.58 | / | - | 80.00 ± 0.00 | 80.00 ± 0.00 |
| S31 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S32 | 11.33 ± 1.53 | 8.67 ± 1.53 | * | 60.00 ± 0.00 | 60.00 ± 0.00 | 12.33 ± 0.58 | 9.33 ± 0.58 | * | 100.00 ± 0.00 | 100.00 ± 0.00 | 8.33 ± 0.58 | / | - | 60.00 ± 0.00 | 60.00 ± 0.00 |
| S33 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S34 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S35 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S36 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| S37 | / | / | - | / | / | / | / | - | / | / | / | / | - | / | / |
| Microorganisms | Antibiotics a | |
|---|---|---|
| DIZ b | ||
| Gent a (10 µg) | AMB a (100 µg) | |
| Gram-positive | ||
| Staphylococcus aureus ATCC43300 | 25.00 ± 0.00 | / |
| Staphylococcus aureus ATCC25923 | 27.00 ± 0.00 | / |
| Gram-negative | ||
| Pseudomonas aeruginosa ATCC 27853 | 21.00 ± 0.00 | / |
| Yeast | ||
| Candida albicans ATCC10231 | / | 27.50 ± 3.54 |
| Candida albicans ATCC26790 | / | 31.00 ± 0.33 |
| Candida albicans IPP444 | / | 26.00 ± 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bereksi-Reguig, D.; Allali, H.; Taib, N.; Aissaoui, N.; Wlodarczyk-Stasiak, M.; Kowalski, R. Bioactive Compounds, Antioxidant Properties, and Antimicrobial Profiling of a Range of West Algerian Honeys: In Vitro Comparative Screening Prior to Therapeutic Purpose. Foods 2024, 13, 4120. https://doi.org/10.3390/foods13244120
Bereksi-Reguig D, Allali H, Taib N, Aissaoui N, Wlodarczyk-Stasiak M, Kowalski R. Bioactive Compounds, Antioxidant Properties, and Antimicrobial Profiling of a Range of West Algerian Honeys: In Vitro Comparative Screening Prior to Therapeutic Purpose. Foods. 2024; 13(24):4120. https://doi.org/10.3390/foods13244120
Chicago/Turabian StyleBereksi-Reguig, Dalila, Hocine Allali, Nadjat Taib, Nadia Aissaoui, Marzena Wlodarczyk-Stasiak, and Radoslaw Kowalski. 2024. "Bioactive Compounds, Antioxidant Properties, and Antimicrobial Profiling of a Range of West Algerian Honeys: In Vitro Comparative Screening Prior to Therapeutic Purpose" Foods 13, no. 24: 4120. https://doi.org/10.3390/foods13244120
APA StyleBereksi-Reguig, D., Allali, H., Taib, N., Aissaoui, N., Wlodarczyk-Stasiak, M., & Kowalski, R. (2024). Bioactive Compounds, Antioxidant Properties, and Antimicrobial Profiling of a Range of West Algerian Honeys: In Vitro Comparative Screening Prior to Therapeutic Purpose. Foods, 13(24), 4120. https://doi.org/10.3390/foods13244120






