Tailoring the Techno-Functional Properties of Fava Bean Protein Isolates: A Comparative Evaluation of Ultrasonication and Pulsed Electric Field Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fava Bean Protein Isolate
2.3. Ultrasonication (US) Treatment of FBPI
2.4. PEF Treatment of FBPI
2.5. Determination of Functional Properties
2.5.1. Protein Solubility
2.5.2. Surface Hydrophobicity and Reactive Sulfhydryl (SHr) Content
2.5.3. Emulsifying Properties
2.5.4. Foaming Properties
2.6. Characterization of FBPI Powder
2.6.1. Particle Size and Zeta Potential
2.6.2. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6.4. Circular Dichroism (CD) Spectroscopy
2.7. Thermal Properties
2.8. X-ray Diffraction (X-RD)
2.9. Statistical Analysis
3. Results
3.1. Effect of US and PEF Treatment on the Functional Properties of FBPI
3.1.1. Solubility
3.1.2. Surface Hydrophobicity and SHr Content
3.1.3. Emulsifying Properties
3.1.4. Foaming Properties
3.2. Characterization of FBPI as Affected by Varying US and PEF Treatments
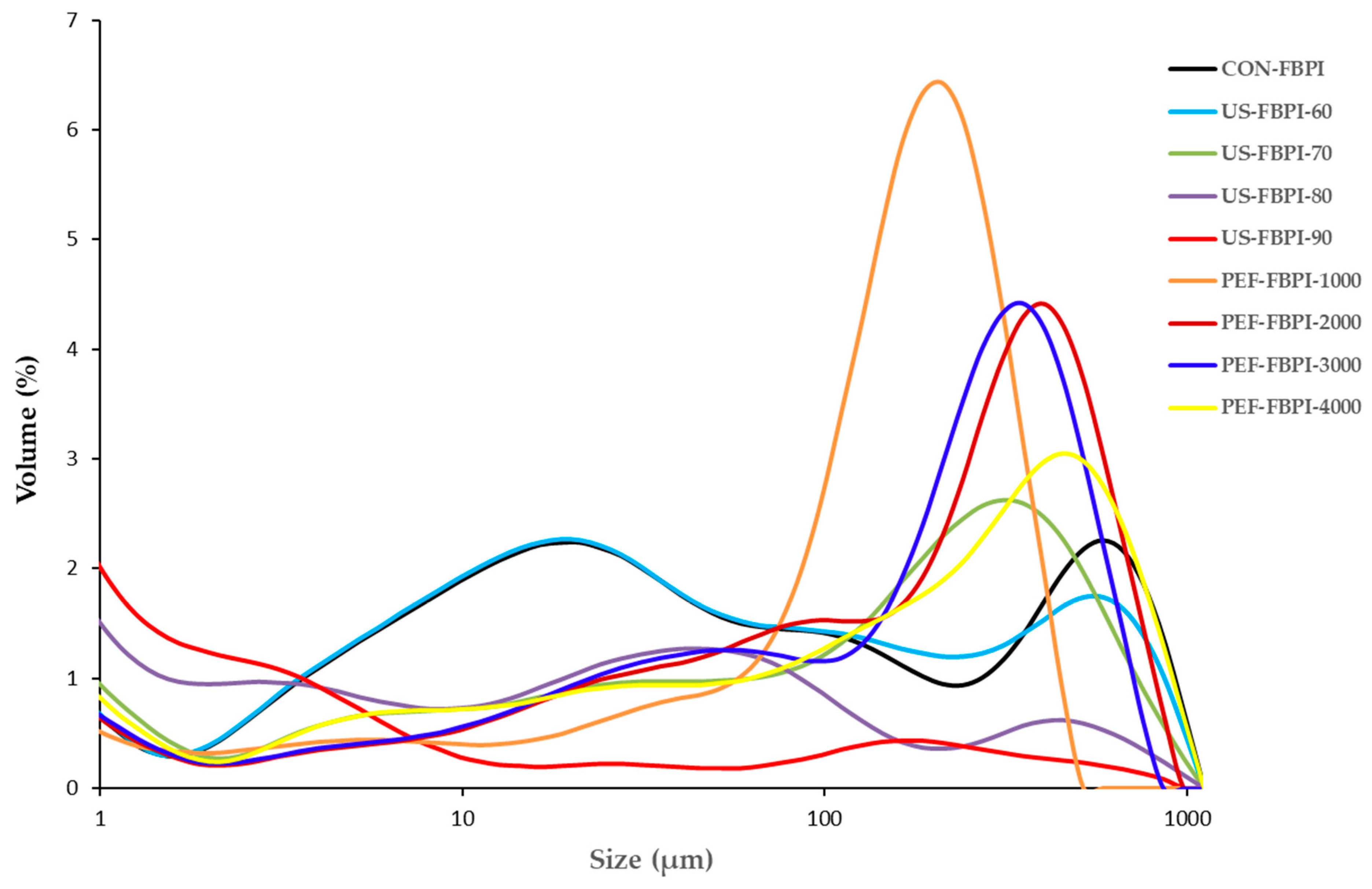
3.2.1. Particle Size and Zeta Potential
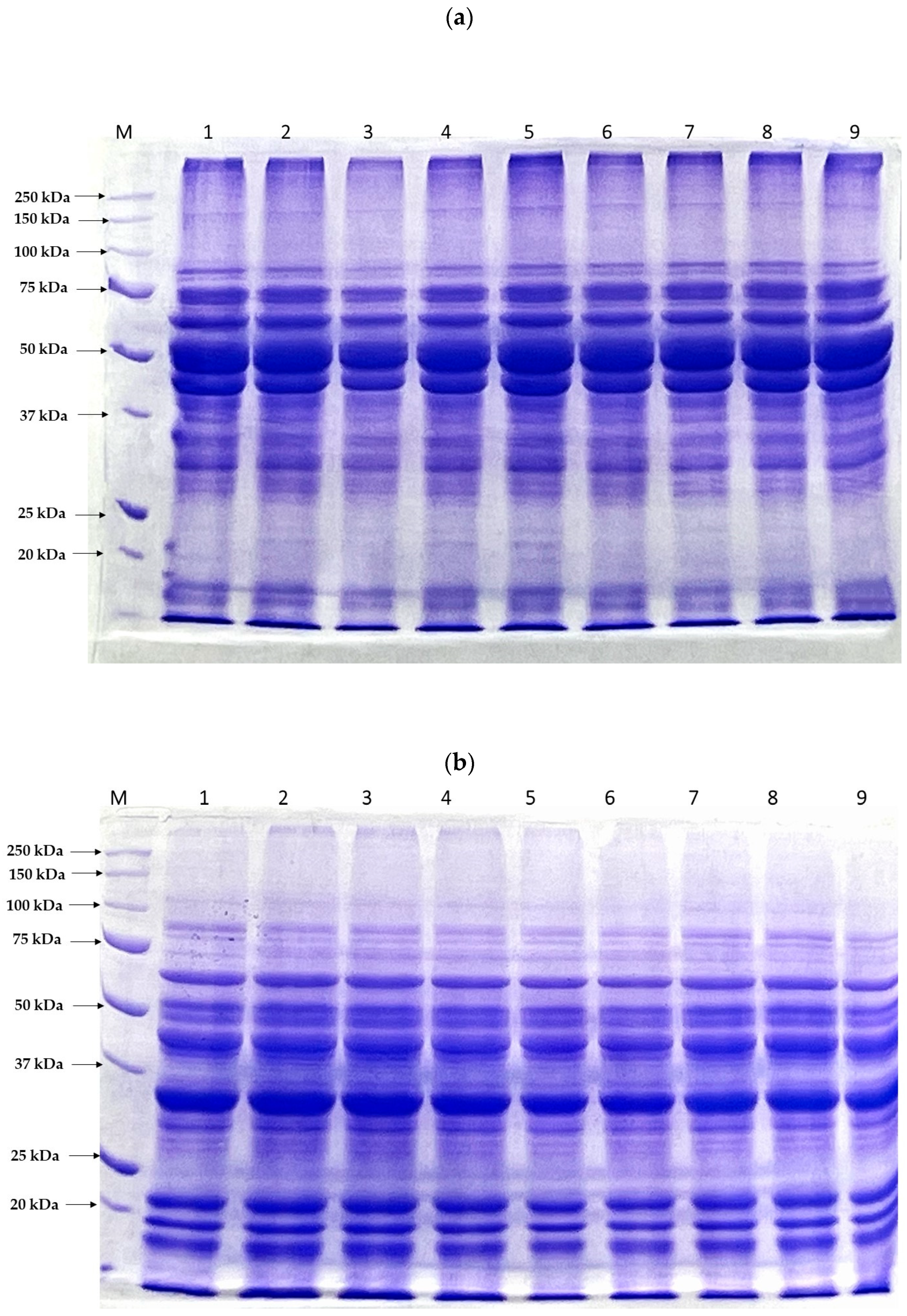
3.2.2. SDS-PAGE Protein Patterns
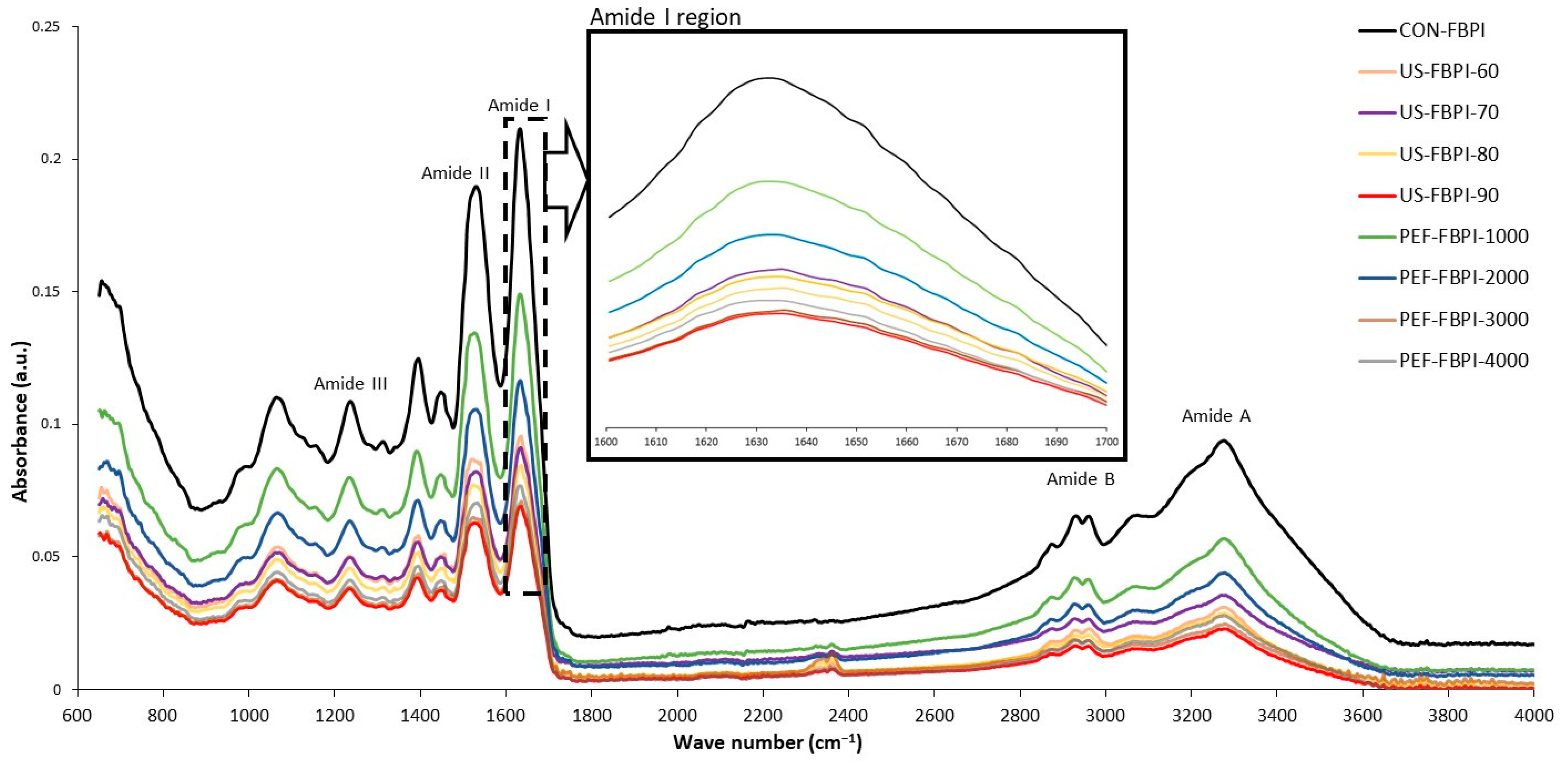
3.2.3. Fourier-Transform Infrared (FTIR) Spectra
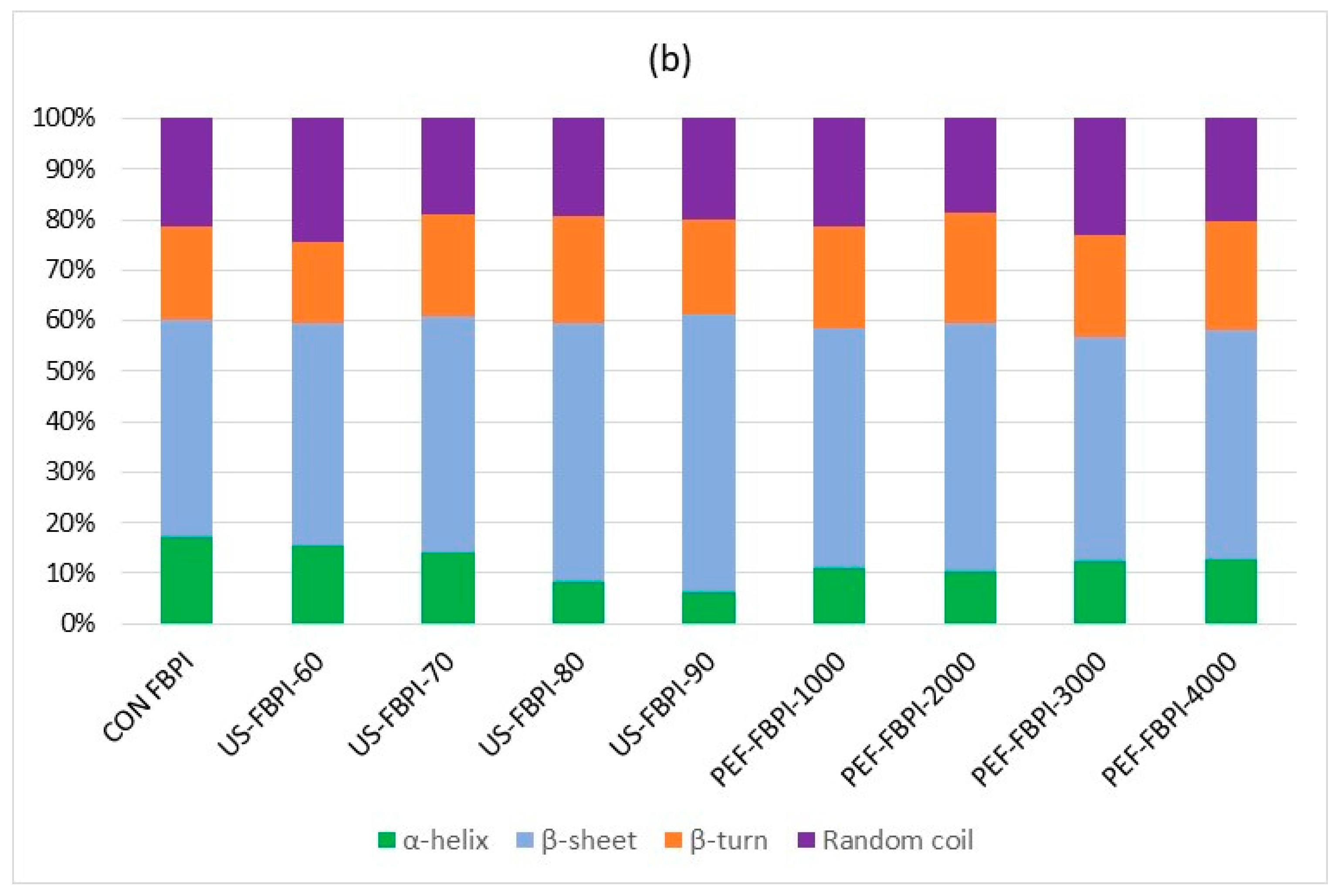
3.2.4. Circular Dichroism (CD) Spectra
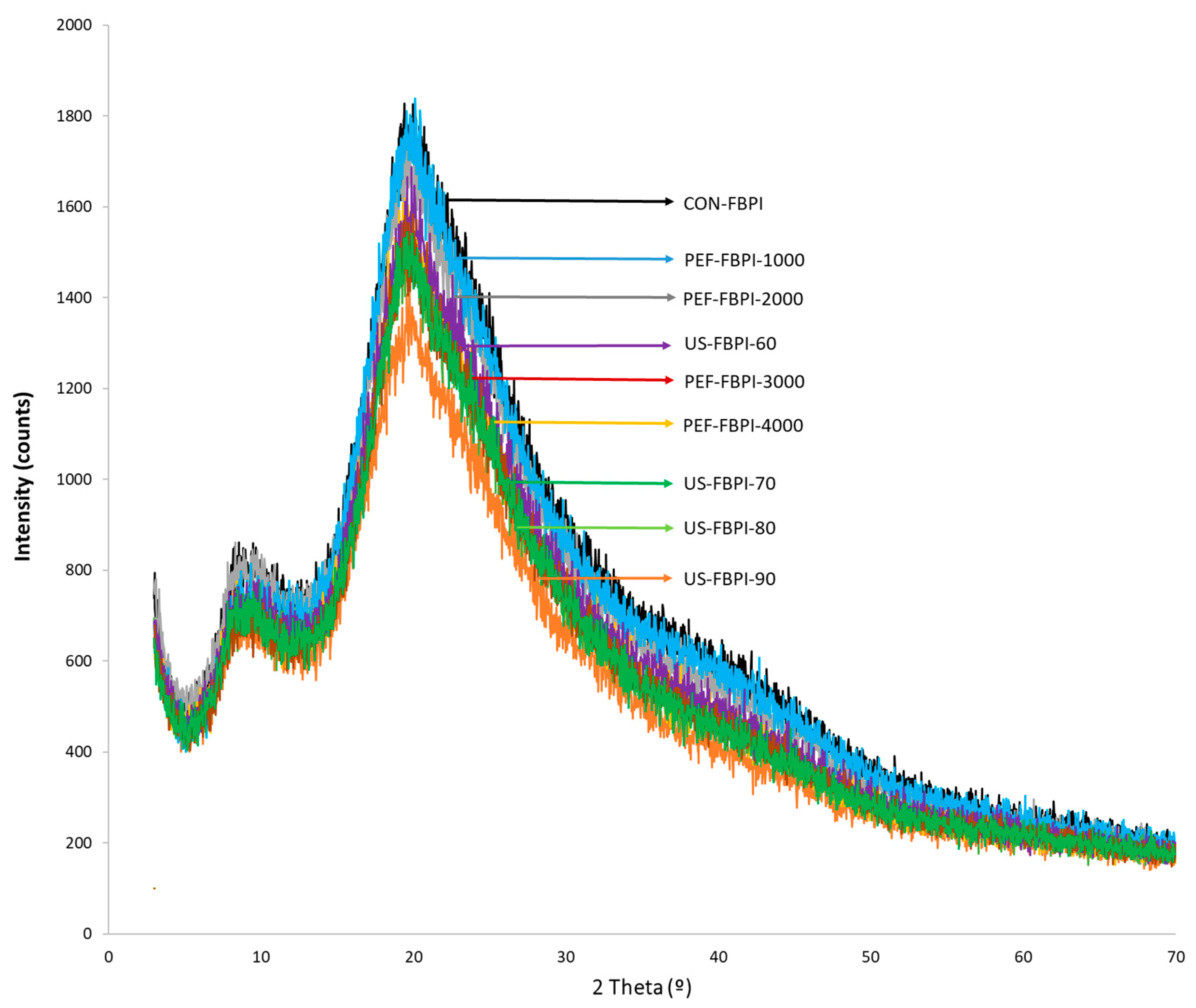
3.3. X-ray Diffraction (XRD)
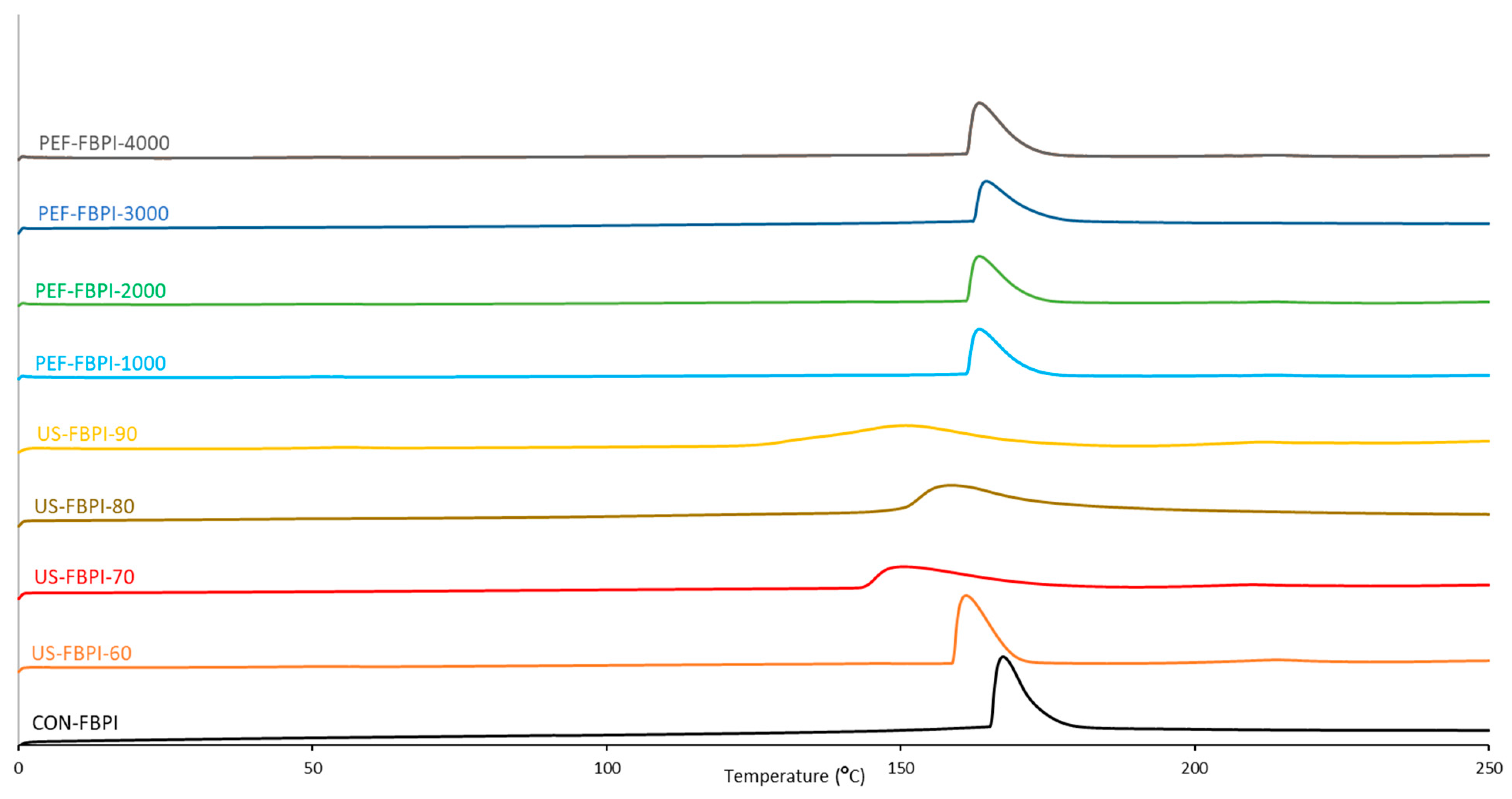
3.4. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, L. Faba Beans Global Market Report 2021 Featuring Prairie Fava, Unigrain, Roland Beans, Aviip Group, Alberta Pulse Growers, Stamp Seeds, Riddell Seed, and Pawnee Buttes Seed; Business Research Company: London, UK, 2021. [Google Scholar]
- Day, L. Proteins from Land Plants–Potential Resources for Human Nutrition and Food Security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Transparency Market Research Plant-Based Protein Market Forecast 2021–2031. Available online: https://www.transparencymarketresearch.com/plantbased-protein-market.html (accessed on 15 December 2023).
- De Boer, J.; Schösler, H.; Aiking, H. Towards a Reduced Meat Diet: Mindset and Motivation of Young Vegetarians, Low, Medium and High Meat-Eaters. Appetite 2017, 113, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Mehr, H.M.; Koocheki, A. Physicochemical Properties of Grass Pea (Lathyrus sativus L.) Protein Nanoparticles Fabricated by Cold Atmospheric-Pressure Plasma. Food Hydrocoll. 2021, 112, 106328. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural Aspects of Legume Proteins and Nutraceutical Properties. Food Res. Int. 2015, 76, 19–30. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, H.K.; Saini, C.S. High Intensity Ultrasound Treatment of Protein Isolate Extracted from Dephenolized Sunflower Meal: Effect on Physicochemical and Functional Properties. Ultrason. Sonochem. 2017, 39, 511–519. [Google Scholar] [CrossRef]
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional Performance of Plant Proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef]
- Gulzar, S.; Tagrida, M.; Patil, U.; Ma, L.; Zhang, B.; Benjakul, S. Mung Bean Protein Isolate Treated with High-Intensity Pulsed Electric Field: Characteristics and Its Use for Encapsulation of Asian Seabass Oil. J. Microencapsul. 2023, 40, 330–344. [Google Scholar] [CrossRef]
- Yang, J.; Liu, G.; Zeng, H.; Chen, L. Effects of High Pressure Homogenization on Faba Bean Protein Aggregation in Relation to Solubility and Interfacial Properties. Food Hydrocoll. 2018, 83, 275–286. [Google Scholar] [CrossRef]
- Eckert, E.; Han, J.; Swallow, K.; Tian, Z.; Jarpa-Parra, M.; Chen, L. Effects of Enzymatic Hydrolysis and Ultrafiltration on Physicochemical and Functional Properties of Faba Bean Protein. Cereal Chem. 2019, 96, 725–741. [Google Scholar] [CrossRef]
- Liu, C.; Damodaran, S.; Heinonen, M. Effects of Microbial Transglutaminase Treatment on Physiochemical Properties and Emulsifying Functionality of Faba Bean Protein Isolate. LWT 2019, 99, 396–403. [Google Scholar] [CrossRef]
- Nivala, O.; Mäkinen, O.E.; Kruus, K.; Nordlund, E.; Ercili-Cura, D. Structuring Colloidal Oat and Faba Bean Protein Particles via Enzymatic Modification. Food Chem. 2017, 231, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Karaca, A.C. Modification of Legume Proteins for Improved Functionality. In Grain and Seed Proteins Functionality; IntechOpen: London, UK, 2021; ISBN 1839685913. [Google Scholar]
- Elez-Martínez, P.; Odriozola-Serrano, I.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Effects of Pulsed Electric Fields Processing Strategies on Health-Related Compounds of Plant-Based Foods. Food Eng. Rev. 2017, 9, 213–225. [Google Scholar] [CrossRef]
- Paniwnyk, L. Applications of Ultrasound in Processing of Liquid Foods: A Review. Ultrason. Sonochem. 2017, 38, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wu, J.; Li-Chan, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of Ultrasound on Structural and Physical Properties of Soy Protein Isolate (SPI) Dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Taha, A.; Casanova, F.; Šimonis, P.; Stankevič, V.; Gomaa, M.A.E.; Stirkė, A. Pulsed Electric Field: Fundamentals and Effects on the Structural and Techno-Functional Properties of Dairy and Plant Proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Vanga, S.K.; Wang, J.; Jayaram, S.; Raghavan, V. Effects of Pulsed Electric Fields and Ultrasound Processing on Proteins and Enzymes: A Review. Processes 2021, 9, 722. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, X.; Liu, W.; Liu, Q.; Zhang, L.; Hu, H. Effect of High-Intensity Ultrasound on the Structural, Rheological, Emulsifying and Gelling Properties of Insoluble Potato Protein Isolates. Ultrason. Sonochem. 2022, 85, 105969. [Google Scholar] [CrossRef]
- Alavi, F.; Chen, L.; Emam-Djomeh, Z. Effect of Ultrasound-Assisted Alkaline Treatment on Functional Property Modifications of Faba Bean Protein. Food Chem. 2021, 354, 129494. [Google Scholar] [CrossRef]
- Bekard, I.; Dunstan, D.E. Electric Field Induced Changes in Protein Conformation. Soft Matter 2014, 10, 431–437. [Google Scholar] [CrossRef]
- Benjakul, S.; Morrissey, M.T. Protein Hydrolysates from Pacific Whiting Solid Wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Beveridge, T.; Toma, S.J.; Nakai, S. Determination of SH-and SS-groups in Some Food Proteins Using Ellman’s Reagent. J. Food Sci. 1974, 39, 49–51. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying Properties of Proteins: Evaluation of a Turbidimetric Technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Shahidi, F.; Han, X.-Q.; Synowiecki, J. Production and Characteristics of Protein Hydrolysates from Capelin (Mallotus villosus). Food Chem. 1995, 53, 285–293. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Nakai, S. Relationships of Hydrophobicity and Net Charge to the Solubility of Milk and Soy Proteins. J. Food Sci. 1985, 50, 486–491. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of Ultrasound on the Technological Properties and Bioactivity of Food: A Review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Mozafarpour, R.; Koocheki, A.; Nicolai, T. Modification of Grass Pea Protein Isolate (Lathyrus sativus L.) Using High Intensity Ultrasound Treatment: Structure and Functional Properties. Food Res. Int. 2022, 158, 111520. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, C.; Wang, B.; Yagoub, A.A.; Ma, H.; Zhang, X.; Wu, M. Study of Ultrasonic Cavitation during Extraction of the Peanut Oil at Varying Frequencies. Ultrason. Sonochem. 2017, 37, 106–113. [Google Scholar] [CrossRef]
- Sharma, N.; Madhumita, M.; Kumar, Y.; Prabhakar, P.K. Ultrasonic Modulated Rice Bran Protein Concentrate: Induced Effects on Morphological, Functional, Rheological, and Thermal Characteristics. Innov. Food Sci. Emerg. Technol. 2023, 85, 103332. [Google Scholar] [CrossRef]
- Rahman, M.M.; Byanju, B.; Grewell, D.; Lamsal, B.P. High-Power Sonication of Soy Proteins: Hydroxyl Radicals and Their Effects on Protein Structure. Ultrason. Sonochem. 2020, 64, 105019. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Mo, H. Effects of Pulsed Electric Fields on Physicochemical Properties of Soybean Protein Isolates. LWT Food Sci. Technol. 2007, 40, 1167–1175. [Google Scholar] [CrossRef]
- Giteru, S.G.; Oey, I.; Ali, M.A. Feasibility of Using Pulsed Electric Fields to Modify Biomacromolecules: A Review. Trends Food Sci. Technol. 2018, 72, 91–113. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Laurindo, J.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Influence of Emerging Technologies on the Utilization of Plant Proteins. Front. Nutr. 2022, 9, 809058. [Google Scholar] [CrossRef] [PubMed]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative Study of High Intensity Ultrasound Effects on Food Proteins Functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Hu, H.; Li-Chan, E.C.Y.; Wan, L.; Tian, M.; Pan, S. The Effect of High Intensity Ultrasonic Pre-Treatment on the Properties of Soybean Protein Isolate Gel Induced by Calcium Sulfate. Food Hydrocoll. 2013, 32, 303–311. [Google Scholar] [CrossRef]
- Lee, H.; Yildiz, G.; Dos Santos, L.C.; Jiang, S.; Andrade, J.E.; Engeseth, N.J.; Feng, H. Soy Protein Nano-Aggregates with Improved Functional Properties Prepared by Sequential PH Treatment and Ultrasonication. Food Hydrocoll. 2016, 55, 200–209. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, J.; Zhou, Y.; Huang, X.; Liu, F.; Pan, S.; Hu, H. Effect of High Intensity Ultrasound on Physicochemical and Functional Properties of Soybean Glycinin at Different Ionic Strengths. Innov. Food Sci. Emerg. Technol. 2016, 34, 205–213. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of High Intensity Ultrasound on Structure and Foaming Properties of Pea Protein Isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of Ultrasound on the Structure and Physical Properties of Black Bean Protein Isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of Ultrasound on the Thermal and Structural Characteristics of Proteins in Reconstituted Whey Protein Concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef]
- Yanjun, S.; Jianhang, C.; Shuwen, Z.; Hongjuan, L.; Jing, L.; Lu, L.; Uluko, H.; Yanling, S.; Wenming, C.; Wupeng, G. Effect of Power Ultrasound Pre-Treatment on the Physical and Functional Properties of Reconstituted Milk Protein Concentrate. J. Food Eng. 2014, 124, 11–18. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.; Cheng, J.-H.; Sun, D.-W. Effects of Electric Fields and Electromagnetic Wave on Food Protein Structure and Functionality: A Review. Trends Food Sci. Technol. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Xiang, B.Y.; Ngadi, M.O.; Ochoa-Martinez, L.A.; Simpson, M. V Pulsed Electric Field-Induced Structural Modification of Whey Protein Isolate. Food Bioprocess Technol. 2011, 4, 1341–1348. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, T.; Feng, S.; Xu, Q.; Zheng, T.; Zhou, M.; Chu, X.; Huang, X.; Lu, X.; Pan, S. Effect of High Intensity Ultrasound on Transglutaminase-Catalyzed Soy Protein Isolate Cold Set Gel. Ultrason. Sonochem. 2016, 29, 380–387. [Google Scholar] [CrossRef]
- Jiang, S.; Ding, J.; Andrade, J.; Rababah, T.M.; Almajwal, A.; Abulmeaty, M.M.; Feng, H. Modifying the Physicochemical Properties of Pea Protein by PH-Shifting and Ultrasound Combined Treatments. Ultrason. Sonochem. 2017, 38, 835–842. [Google Scholar] [CrossRef]
- Huang, H.; Kwok, K.-C.; Liang, H.-H. Inhibitory Activity and Conformation Changes of Soybean Trypsin Inhibitors Induced by Ultrasound. Ultrason. Sonochem. 2008, 15, 724–730. [Google Scholar] [CrossRef]
- Fernandez-Diaz, M.D.; Barsotti, L.; Dumay, E.; Cheftel, J.C. Effects of Pulsed Electric Fields on Ovalbumin Solutions and Dialyzed Egg White. J. Agric. Food Chem. 2000, 48, 2332–2339. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, W.; Yang, R.; Yan, W.; Sun, Q. Aggregation of Egg White Proteins with Pulsed Electric Fields and Thermal Processes. J. Sci. Food Agric. 2016, 96, 3334–3341. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Kołoczek, H.; Tomasik, P. Effect of the External Electric Field on Selected Tripeptides. Amino Acids 2015, 47, 1399–1408. [Google Scholar] [CrossRef]
- Panyam, D.; Kilara, A. Enhancing the Functionality of Food Proteins by Enzymatic Modification. Trends Food Sci. Technol. 1996, 7, 120–125. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Handa, C.L.; Xu, J. Effects of Ultrasound Pre-Treatment on the Structure of β-Conglycinin and Glycinin and the Antioxidant Activity of Their Hydrolysates. Food Chem. 2017, 218, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Velasco, A.; Lobato-Calleros, C.; Hernández-Rodríguez, B.E.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. High Intensity Ultrasound Treatment of Faba Bean (Vicia Faba L.) Protein: Effect on Surface Properties, Foaming Ability and Structural Changes. Ultrason. Sonochem. 2018, 44, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Roginski, H.; Williams, R.P.W.; Versteeg, C.; Wan, J. Effect of Pulsed Electric Field and Thermal Treatment on the Physicochemical and Functional Properties of Whey Protein Isolate. Int. Dairy J. 2011, 21, 206–213. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.-J.; Jiang, W.; Qian, J.-Y. Effect of Pulsed Electric Field on Functional and Structural Properties of Canola Protein by Pretreating Seeds to Elevate Oil Yield. LWT 2017, 84, 73–81. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Herceg, Z.; Herceg, I.L. Effect of Ultrasound Treatment on Solubility and Foaming Properties of Whey Protein Suspensions. J. Food Eng. 2008, 86, 281–287. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, K.; Bai, Y.; Li, B.; Xu, W. Effect of High Intensity Ultrasound on Physicochemical, Interfacial and Gel Properties of Chickpea Protein Isolate. LWT 2020, 129, 109563. [Google Scholar] [CrossRef]
- Ofir, E.; Oren, Y.; Adin, A. Electroflocculation: The Effect of Zeta-Potential on Particle Size. Desalination 2007, 204, 33–38. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K. Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef]
- Sharan, S.; Zanghelini, G.; Zotzel, J.; Bonerz, D.; Aschoff, J.; Saint-Eve, A.; Maillard, M. Fava Bean (Vicia faba L.) for Food Applications: From Seed to Ingredient Processing and Its Effect on Functional Properties, Antinutritional Factors, Flavor, and Color. Compr. Rev. Food Sci. Food Saf. 2021, 20, 401–428. [Google Scholar] [CrossRef]
- Żmudziński, D.; Goik, U.; Ptaszek, P. Functional and Rheological Properties of Vicia faba L. Protein Isolates. Biomolecules 2021, 11, 178. [Google Scholar] [CrossRef]
- Carbonaro, M.; Nucara, A. Secondary Structure of Food Proteins by Fourier Transform Spectroscopy in the Mid-Infrared Region. Amino Acids 2010, 38, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Benjakul, S. Characteristics of Gelatin from the Skin of Unicorn Leatherjacket (Aluterus monoceros) as Influenced by Acid Pretreatment and Extraction Time. Food Hydrocoll. 2011, 25, 381–388. [Google Scholar] [CrossRef]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-Y.; Cai, L.-H.; Cai, X.-L.; Wang, Y.-J.; Li, Y.-Q. Structure Characterization of Protein Fractions from Lotus (Nelumbo nucifera) Seed. J. Mol. Struct. 2011, 1001, 139–144. [Google Scholar] [CrossRef]
- Gorinstein, S.; Zemser, M.; Friedman, M.; Chang, S. Simultaneous Differential Scanning Calorimetry, X-ray Diffraction and FTIR Spectrometry in Studies of Ovalbumin Denaturation. Int. J. Pept. Protein Res. 1995, 45, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Anderle, G.; Mendelsohn, R. Thermal Denaturation of Globular Proteins. Fourier Transform-Infrared Studies of the Amide III Spectral Region. Biophys. J. 1987, 52, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Ji, Y.; Pan, H.; Sun, Z.; Li, Y.; Qin, G. Characterization of Thermal Denaturation Structure and Morphology of Soy Glycinin by FTIR and SEM. Int. J. food Prop. 2015, 18, 763–774. [Google Scholar] [CrossRef]
- Stathopulos, P.B.; Scholz, G.A.; Hwang, Y.; Rumfeldt, J.A.O.; Lepock, J.R.; Meiering, E.M. Sonication of Proteins Causes Formation of Aggregates That Resemble Amyloid. Protein Sci. 2004, 13, 3017–3027. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, H. Study on Crystal Structures of Enzyme-Hydrolyzed Cellulosic Materials by X-Ray Diffraction. Enzyme Microb. Technol. 2005, 36, 314–317. [Google Scholar] [CrossRef]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.F.; Kasapis, S. Physicochemical and Functional Properties of Lentil Protein Isolates Prepared by Different Drying Methods. Food Chem. 2011, 129, 1513–1522. [Google Scholar] [CrossRef]
- Zhang, M.; Song, F.; Wang, X.-L.; Wang, Y.-Z. Development of Soy Protein Isolate/Waterborne Polyurethane Blend Films with Improved Properties. Colloids Surf. B Biointerfaces 2012, 100, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, M.; Dejmek, P. Thermal Denaturation of Whey Proteins in Mixtures with Caseins Studied by Differential Scanning Calorimetry. J. Dairy Sci. 1990, 73, 590–600. [Google Scholar] [CrossRef]
- Farber, P.; Darmawan, H.; Sprules, T.; Mittermaier, A. Analyzing Protein Folding Cooperativity by Differential Scanning Calorimetry and NMR Spectroscopy. J. Am. Chem. Soc. 2010, 132, 6214–6222. [Google Scholar] [CrossRef] [PubMed]
- Nazari, B.; Mohammadifar, M.A.; Shojaee-Aliabadi, S.; Feizollahi, E.; Mirmoghtadaie, L. Effect of Ultrasound Treatments on Functional Properties and Structure of Millet Protein Concentrate. Ultrason. Sonochem. 2018, 41, 382–388. [Google Scholar] [CrossRef]

| Sample | Solubility (%) | Surface Hydrophobicity | Free Sulfhydryl Content (µmol/g Protein) | EAI (m2/g Protein) | ESI (min) | Foaming Ability (%) | Foaming Stability (%) |
|---|---|---|---|---|---|---|---|
| CON-FBPI | 74.38 ± 2.16 c | 5625 ± 191 e | 10.36 ± 0.34 f | 34.90 ± 3.18 d | 39.47 ± 5.26 c | 74.44 ± 9.26 c | 52.19 ± 3.62 e |
| US-FBPI-60 | 87.63 ± 2.89 a | 7923 ± 204 b | 11.76 ± 0.41 e | 47.75 ± 4.42 c | 43.30 ± 3.98 bc | 119.97 ± 12.59 bc | 78.83 ± 6.18 bc |
| US-FBPI-70 | 90.98 ± 2.67 a | 9459 ± 336 a | 17.08 ± 0.28 a | 69.14 ± 4.34 a | 55.13 ± 4.27 a | 198.96 ± 11.75 a | 102.74 ± 7.21 a |
| US-FBPI-80 | 80.53 ± 2.36 b | 7422 ± 223 c | 15.91 ± 0.27 b | 58.65 ± 5.16 b | 47.88 ± 2.97 b | 135.33 ± 10.87 b | 83.33 ± 8.92 b |
| US-FBPI-90 | 78.91 ± 2.81 bc | 7589 ± 195 bc | 15.71 ± 0.31 b | 54.23 ± 3.89 bc | 42.23 ± 5.21 bc | 132.63 ± 8.21 b | 82.61 ± 6.31 b |
| PEF-FBPI-1000 | 81.82 ± 2.98 b | 7386 ± 208 c | 13.51 ± 0.22 c | 52.78 ± 6.67 bc | 42.92 ± 2.25 bc | 103.36 ± 15.78 | 63.34 ± 4.12 d |
| PEF-FBPI-2000 | 82.12 ± 1.99 b | 7894 ± 301 b | 13.56 ± 0.28 c | 54.65 ± 3.27 bc | 45.55 ± 3.64 bc | 138.89 ± 13.34 b | 72.72 ± 5.33 bcd |
| PEF-FBPI-3000 | 79.15 ± 2.25 b | 6691 ± 197 d | 11.34 ± 0.33 e | 54.30 ± 6.91 bc | 42.98 ± 4.18 bc | 121.35 ± 6.63 bc | 79.63 ± 7.43 bc |
| PEF-FBPI-4000 | 78.86 ± 2.37 bc | 6568 ± 257 d | 12.75 ± 0.19 d | 53.95 ± 6.53 bc | 43.66 ± 4.21 bc | 122.58 ± 9.98 bc | 68.16 ± 5.28 cd |
| Sample | Particle Size Parameters (µm) | Zeta Potential | |
|---|---|---|---|
| (mV) | |||
| D[4,3] | D[3,2] | ||
| CON-FBPI | 72.6 ± 5.1 d | 1.20 ± 0.02 a | −26.27 ± 0.54 a |
| US-FBPI-60 | 70.9 ± 6.1 d | 1.13 ± 0.06 a | −24.27 ± 0.39 b |
| US-FBPI-70 | 61.2 ± 4.9 d | 0.69 ± 0.04 c | −22.47 ± 0.17 c |
| US-FBPI-80 | 44.7 ± 2.8 e | 0.5 ± 0.02 d | −22.40 ± 0.33 c |
| US-FBPI-90 | 25.65 ± 2.7 f | 0.39 ± 0.01 e | −20.10 ± 0.86 d |
| PEF-FBPI-1000 | 144.1 ± 7.9 b | 1.17 ± 0.16 a | −25.73 ± 1.27 a |
| PEF-FBPI-2000 | 162.3 ± 8.7 a | 0.92 ± 0.04 b | −25.07 ± 0.66 ab |
| PEF-FBPI-3000 | 164.3 ± 8.9 a | 0.83 ± 0.02 b | −25.87 ± 0.53 a |
| PEF-FBPI-4000 | 120.6 ± 7.2 c | 0.81 ± 0.02 b | −25.57 ± 0.78 a |
| Sample | Onset (°C) | Peak (°C) | Endset (°C) | Enthalpy (J/g) |
|---|---|---|---|---|
| CON-FBPI | 164.25 | 166.63 | 179.17 | 191.86 |
| US-FBPI-60 | 158.67 | 160.15 | 168.97 | 179.87 |
| US-FBPI-70 | 143.85 | 150.09 | 173.17 | 119.40 |
| US-FBPI-80 | 148.66 | 159.41 | 177.66 | 120.66 |
| US-FBPI-90 | 128.89 | 150.48 | 172.09 | 112.75 |
| PEF-FBPI-1000 | 160.31 | 162.85 | 173.01 | 147.71 |
| PEF-FBPI-2000 | 161.89 | 164.84 | 174.58 | 152.50 |
| PEF-FBPI-3000 | 163.20 | 165.10 | 178.92 | 144.97 |
| PEF-FBPI-4000 | 161.16 | 162.57 | 171.45 | 145.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulzar, S.; Martín-Belloso, O.; Soliva-Fortuny, R. Tailoring the Techno-Functional Properties of Fava Bean Protein Isolates: A Comparative Evaluation of Ultrasonication and Pulsed Electric Field Treatments. Foods 2024, 13, 376. https://doi.org/10.3390/foods13030376
Gulzar S, Martín-Belloso O, Soliva-Fortuny R. Tailoring the Techno-Functional Properties of Fava Bean Protein Isolates: A Comparative Evaluation of Ultrasonication and Pulsed Electric Field Treatments. Foods. 2024; 13(3):376. https://doi.org/10.3390/foods13030376
Chicago/Turabian StyleGulzar, Saqib, Olga Martín-Belloso, and Robert Soliva-Fortuny. 2024. "Tailoring the Techno-Functional Properties of Fava Bean Protein Isolates: A Comparative Evaluation of Ultrasonication and Pulsed Electric Field Treatments" Foods 13, no. 3: 376. https://doi.org/10.3390/foods13030376







