Changes in Cooking Characteristics, Structural Properties and Bioactive Components of Wheat Flour Noodles Partially Substituted with Whole-Grain Hulled Tartary Buckwheat Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Whole-Grain HTBF
2.3. Preparation of TBNs
2.4. Cooking Characteristics of TBNs
2.5. Texture Properties of Cooked TBNs
2.6. Sensory Evaluation
2.7. Observation of Surface Morphology and Scanning Electron Microscope (SEM)
2.8. X-ray Diffraction (XRD) Analysis
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
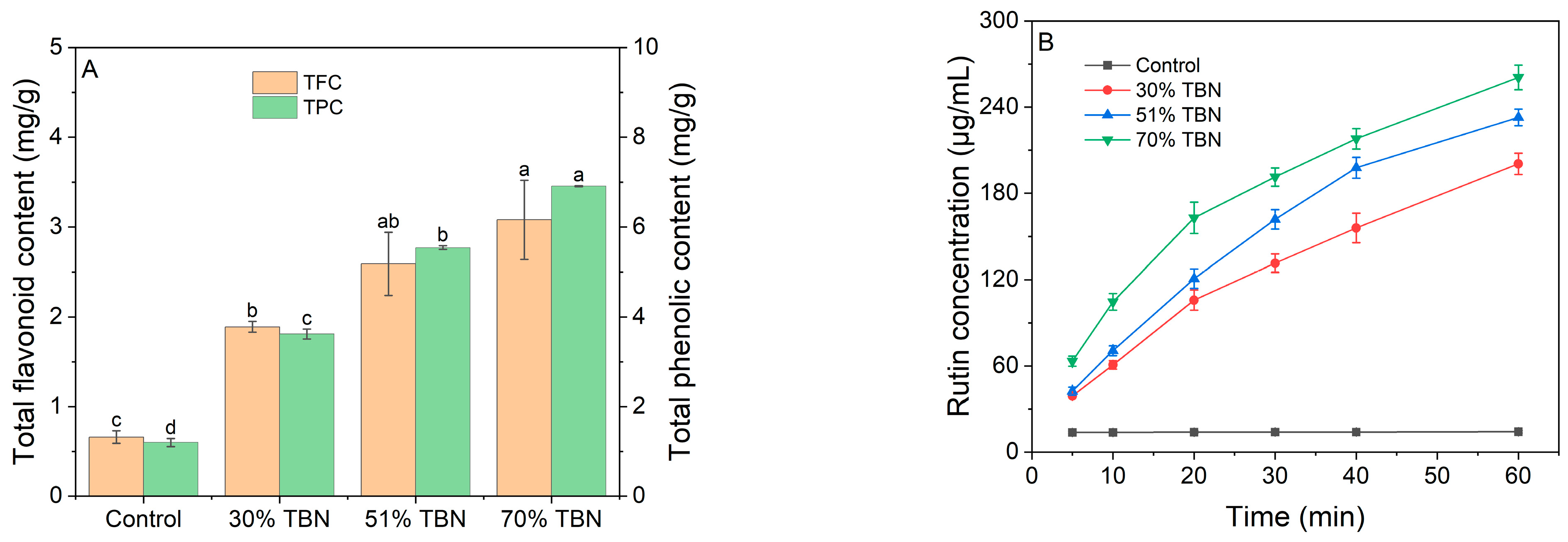
2.10. Rutin Determination Assay
2.11. Total Flavonoid Content (TFC) and Total Phenolic Content (TPC) Assay
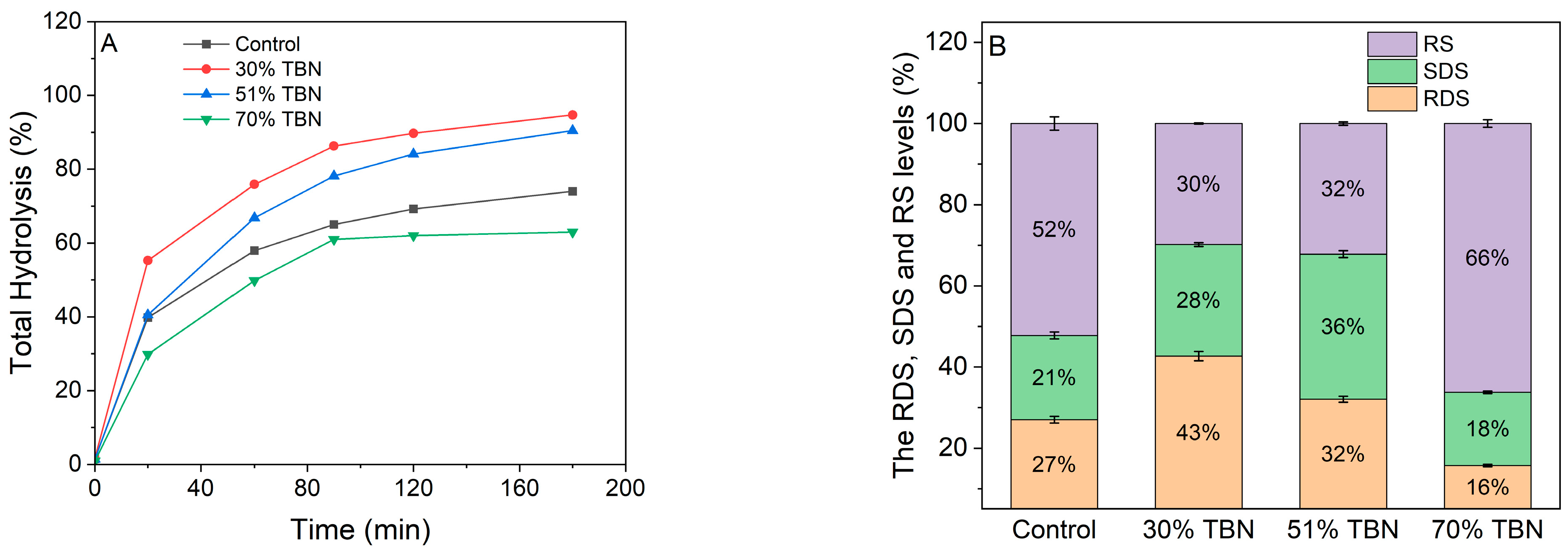
2.12. In Vitro Starch Digestibility
2.13. Statistical Analysis
3. Results and Discussion
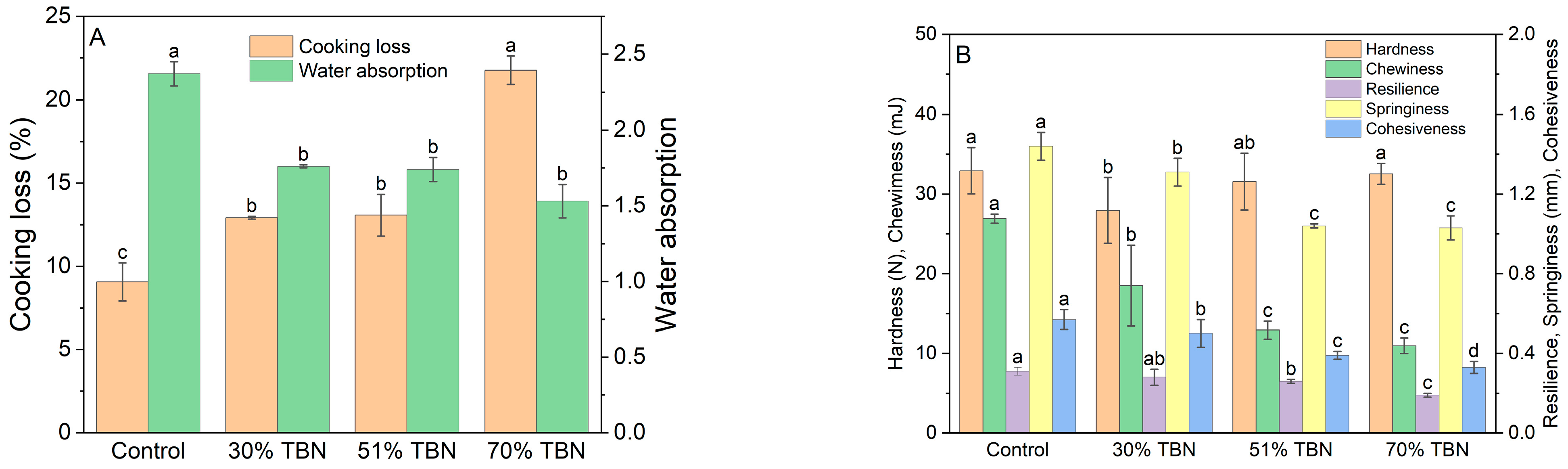
3.1. Cooking Characteristics and Texture Properties of Noodles
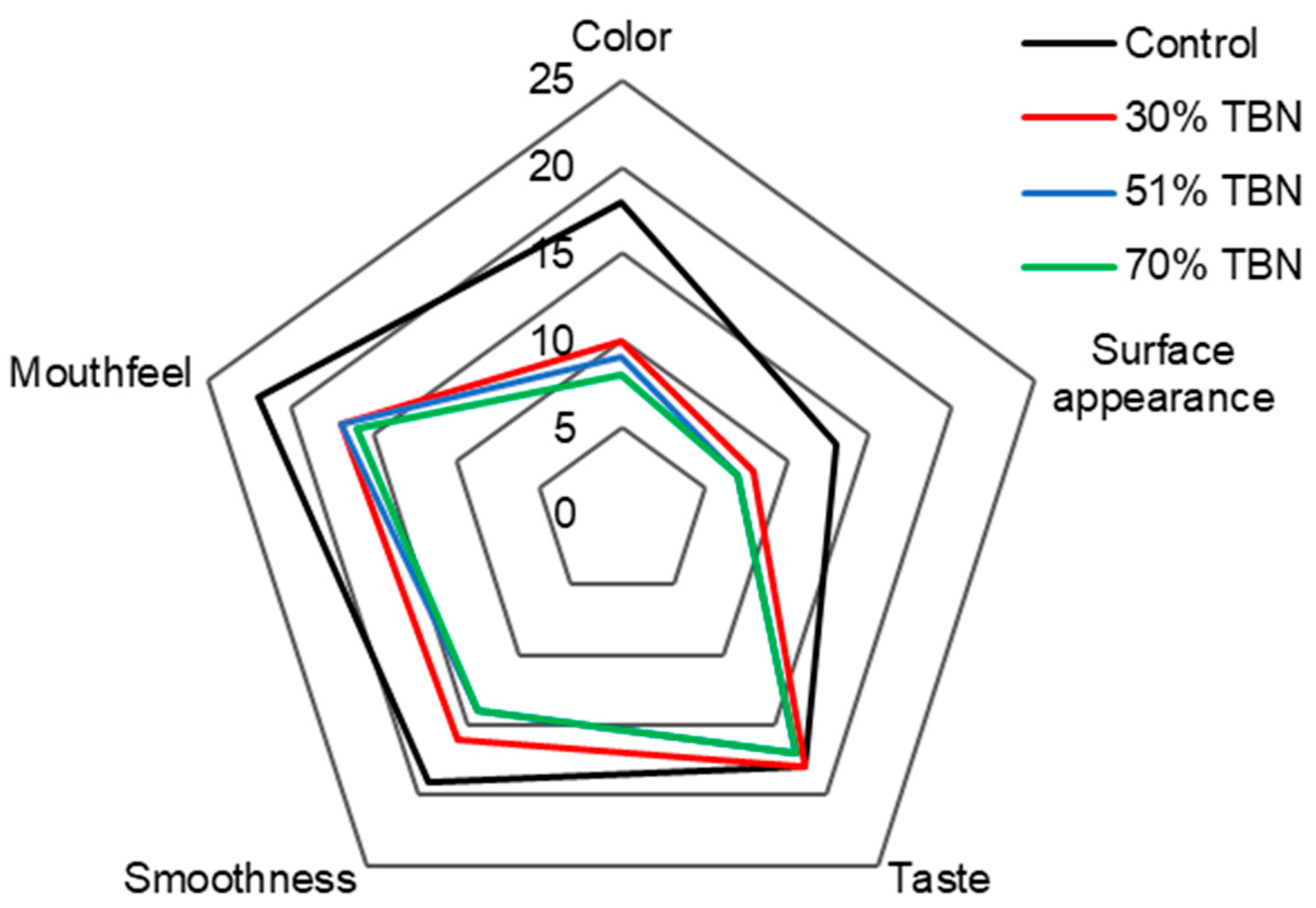
3.2. Sensory Analysis
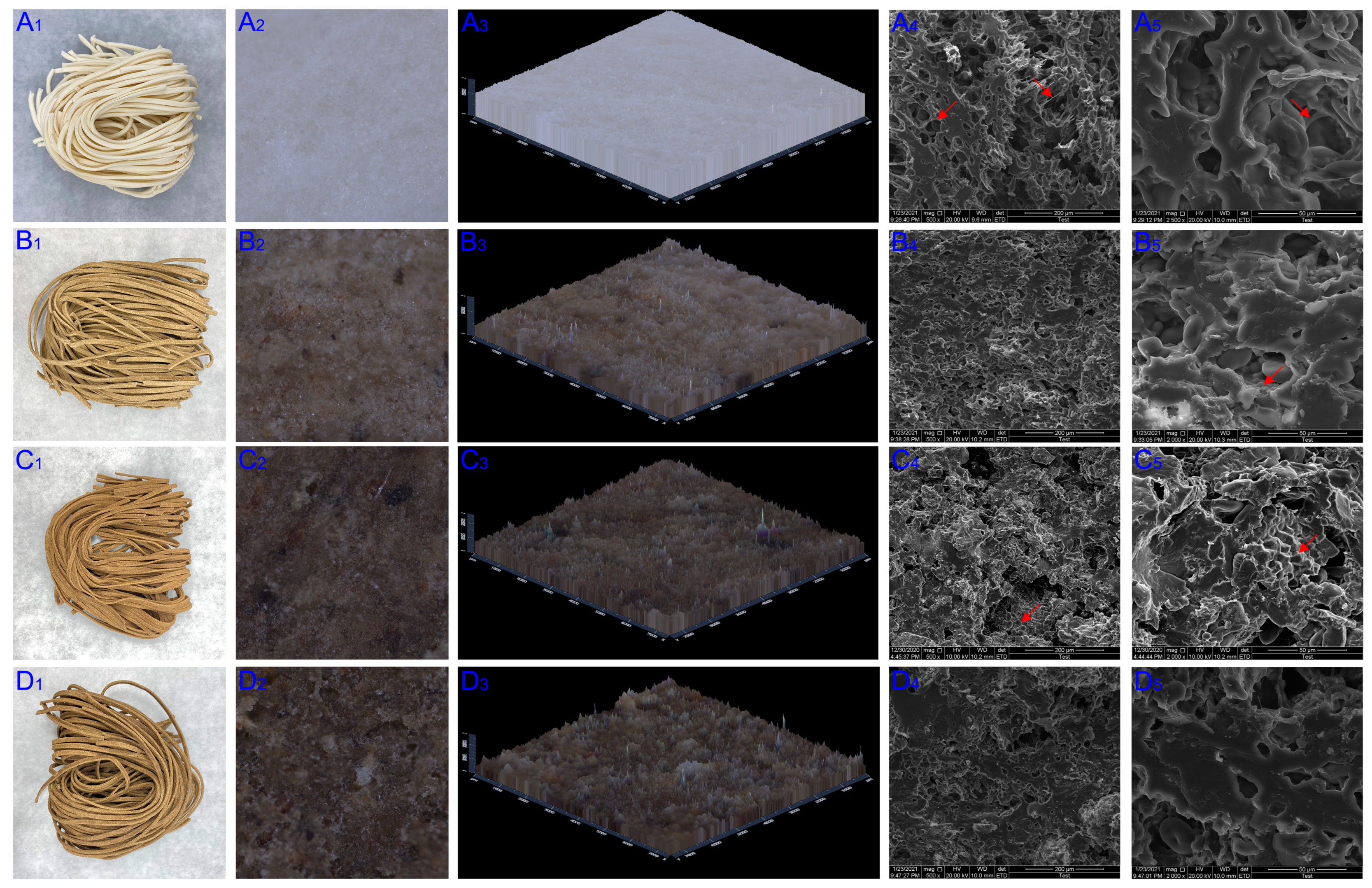
3.3. Surface Morphology and SEM
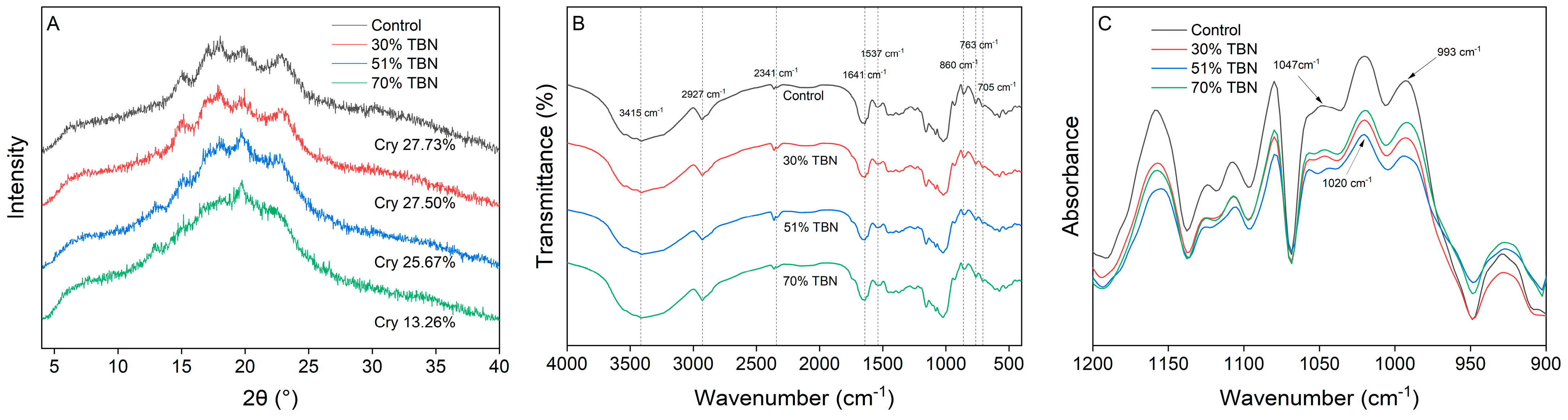
3.4. XRD Analysis
3.5. FTIR Analysis
3.6. Content of Bioactive Compounds
3.7. In Vitro Starch Digestibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakac, M.; Torbica, A.; Sedej, I.; Hadnadev, M. Influence of breadmaking on antioxidant capacity of gluten free breads based on rice and buckwheat flours. Food Res. Int. 2011, 44, 2806–2813. [Google Scholar] [CrossRef]
- Puligundla, P.; Lim, S. Buckwheat noodles: Processing and quality enhancement. Food Sci. Biotechnol. 2021, 30, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Merendino, N.; Molinari, R.; Costantini, L.; Mazzucato, A.; Pucci, A.; Bonafaccia, F.; Esti, M.; Ceccantoni, B.; Papeschi, C.; Bonafaccia, G. A new “functional” pasta containing tartary buckwheat sprouts as an ingredient improves the oxidative status and normalizes some blood pressure parameters in spontaneously hypertensive rats. Food Funct. 2014, 5, 1017–1026. [Google Scholar] [CrossRef]
- Lv, L.J.; Xia, Y.; Zou, D.Z.; Han, H.R.; Wang, Y.L.; Fang, H.Y.; Li, M.H. Fagopyrum tataricum (L.) Gaertn.: A review on its traditional uses, phytochemical and pharmacology. Food Sci. Technol. Res. 2017, 23, 1–7. [Google Scholar] [CrossRef]
- Gelencsér, T.; Gál, V.; Salgó, A. Effects of applied process on the in vitro digestibility and resistant starch content of pasta products. Food Bioprocess Technol. 2010, 3, 491–497. [Google Scholar] [CrossRef]
- Sinkovič, L.; Sinkovič, D.K.; Meglič, V. Milling fractions composition of common (Fagopyrum esculentum Moench) and Tartary (Fagopyrum tataricum (L.) Gaertn.) buckwheat. Food Chem. 2021, 365, 130459. [Google Scholar] [CrossRef]
- Zhu, K.X.; Dai, X.; Guo, X.; Peng, W.; Zhou, H.M. Retarding effects of organic acids, hydrocolloids and microwave treatment on the discoloration of green tea fresh noodles. LWT—Food Sci. Technol. 2014, 55, 176–182. [Google Scholar] [CrossRef]
- Wang, R.B.; Li, M.; Chen, S.Q.; Ying, H.; Tang, A.X.; Wei, Y.M. Effects of flour dynamic viscosity on the quality properties of buckwheat noodles. Carbohydr. Polym. 2019, 207, 815–823. [Google Scholar] [CrossRef]
- Javornik, B.; Kreft, I. Characterization of buckwheat protein. Fagopyrum 1984, 4, 30–38. [Google Scholar]
- Krkošková, B.; Mrázová, Z. Prophylactic components of buckwheat. Food Res. Int. 2005, 38, 561–568. [Google Scholar] [CrossRef]
- Bastida, J.; Zielinski, H. Buckwheat as a functional food and its effects on health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.P.; Zhou, H.M.; Zhu, K.R.; Li, Q. Effect of thermal treatment on the physicochemical, ultrastructural and nutritional characteristics of whole grain highland barley. Food Chem. 2021, 346, 128657. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.P.; Zhou, H.M. Impact of aqueous ozone mixing on microbiological, quality and physicochemical characteristics of semi-dried buckwheat noodles. Food Chem. 2021, 336, 127709. [Google Scholar] [CrossRef]
- Fu, M.X.; Sun, X.Y.; Wu, D.; Meng, L.H.; Feng, X.; Cheng, W.W.; Gao, C.C.; Yang, Y.L.; Shen, X.C.; Tang, X.Z. Effect of partial substitution of buckwheat on cooking characteristics, nutritional composition, and in vitro starch digestibility of extruded gluten-free rice noodles. LWT—Food Sci. Technol. 2020, 126, 109332. [Google Scholar] [CrossRef]
- GB/T 35875-2018; Grain and Oil Inspection, Quality Evaluation of Wheat Flour Noodles. National Grain and Oil Standardization Technical Committee: Beijing, China, 2018.
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.D.; Ma, Y.J.; Parry, J.; Gao, J.M.; Yu, L.L.; Wang, M. Phenolics content and antioxidant activity of Tartary buckwheat from different locations. Molecules 2011, 16, 9850–9867. [Google Scholar] [CrossRef]
- Sun, X.Y.; Yu, C.; Fu, M.X.; Wu, D.; Gao, C.C.; Feng, X.; Cheng, W.W.; Shen, X.C.; Tang, X.Z. Extruded whole buckwheat noodles: Effects of processing variables on the degree of starch gelatinization, changes of nutritional components, cooking characteristics and in vitro starch digestibility. Food Funct. 2019, 10, 6362–6373. [Google Scholar] [CrossRef]
- Zhang, M.L.; Ma, M.; Yang, T.B.; Li, M.; Sun, Q.J. Dynamic distribution and transition of gluten proteins during noodle processing. Food Hydrocoll. 2022, 123, 107114. [Google Scholar] [CrossRef]
- Han, C.; Ma, M.; Li, M.; Sun, Q. Further interpretation of the underlying causes of the strengthening effect of alkali on gluten and noodle quality: Studies on gluten, gliadin, and glutenin. Food Hydrocoll. 2020, 103, 105661. [Google Scholar] [CrossRef]
- Kang, S.; Nie, L.X.; Zheng, Y.G.; Zuo, T.T.; Wang, Y.; Shi, J.; Ma, S.C. Micro-morphological identification study on Cordyceps sinensis (Berk.) Sacc. and its adulterants based on stereo microscope and desktop scanning electron microscope. Microsc. Res. Tech. 2021, 84, 1936–1946. [Google Scholar] [CrossRef]
- Wang, R.; Li, M.; Wei, Y.; Guo, B.; Brennan, M.; Brennan, C.S. Quality differences between fresh and dried buckwheat noodles associated with water status and inner structure. Foods 2021, 10, 187. [Google Scholar] [CrossRef]
- Cheetham, N.; Tao, L.P. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Wang, X.J.; Ullah, N.; Sun, X.C.; Guo, Y.; Chen, L.; Li, Z.X.; Feng, X.C. Development and characterization of bacterial cellulose reinforced biocomposite films based on protein from buckwheat distiller’s dried grains. Int. J. Biol. Macromol. 2017, 96, 353–360. [Google Scholar] [CrossRef]
- Dar, M.Z.; Deepika, K.; Jan, K.; Swer, T.L.; Kumar, P.; Verma, R.; Bashir, K. Modification of structure and physicochemical properties of buckwheat and oat starch by γ-irradiation. Int. J. Biol. Macromol. 2018, 108, 1348–1356. [Google Scholar] [CrossRef]
- Long, G.H.; Ji, Y.; Pan, H.B.; Sun, Z.W.; Li, Y.T.; Qin, G.X. Characterization of thermal denaturation structure and morphology of soy glycinin by FTIR and SEM. Int. J. Food Prop. 2015, 18, 763–774. [Google Scholar] [CrossRef]
- Liu, T.T.; Duan, H.P.; Mao, X.H.; Yu, X.Z. Influence of flaxseed flour as a partial replacement for wheat flour on the characteristics of Chinese steamed bread. RSC Adv. 2020, 10, 28114–28120. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Jiang, B.; Zhang, T.; Jin, Z.Y. Impact of mild acid hydrolysis on structure and digestion properties of waxy maize starch. Food Chem. 2011, 126, 506–513. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Capron, I.; Robert, P.; Colonna, P.; Brogly, M.; Planchot, V. Starch in rubbery and glassy states by FTIR spectroscopy. Carbohydr. Polym. 2007, 68, 249–259. [Google Scholar] [CrossRef]
- Zhang, W.L.; Yang, Q.H.; Xia, M.J.; Bai, W.M.; Wang, P.K.; Gao, X.L.; Gao, J. Effects of nitrogen level on the physicochemical properties of tartary buckwheat (Fagopyrum tataricum (l.) gaertn.) starch. Int. J. Biol. Macromol. 2019, 129, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Gidley, M.J.; Dhital, S. High-amylose starches to bridge the “fiber gap”: Development, structure, and nutritional functionality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 362–379. [Google Scholar] [CrossRef] [PubMed]
| Noodles | Addition Amount | |||
|---|---|---|---|---|
| Wheat Flour g/100 g | Salt g/100 g | Water/mL | Buckwheat Flour g/100 g | |
| Control | 100 | 1.5 | 30.0 | 0 |
| 30% TBNs | 70 | 1.5 | 36.0 | 30 |
| 51% TBNs | 49 | 1.5 | 44.0 | 51 |
| 70% TBNs | 30 | 1.5 | 47.5 | 70 |
| Evaluation Index | Scoring Criteria | Score (Points) |
|---|---|---|
| Color (20 points) | Bright white or bright yellow noodles, uniform color and glossy | 16–20 |
| Noodles with average or slightly dark brightness | 8–15 | |
| Noodles with dull color and poor brightness | 2–7 | |
| Surface appearance (15 points) | Smooth surface with a translucent appearance | 12–15 |
| Relatively smooth surface with less noticeable translucent appearance | 8–11 | |
| Rough and uneven surface without translucent appearance | 3–7 | |
| Taste (20 points) | Pure and authentic flavor with a sweet cereal aroma | 17–20 |
| No off-flavors or a mild, sweet cereal aroma | 12–16 | |
| Unpleasant or off-putting odor | 7–11 | |
| Smoothness (20 points) | Smooth and nonsticky texture | 17–20 |
| Relatively smooth with slight stickiness | 13–16 | |
| Not smooth and sticky | 8–12 | |
| Mouthfeel (25 points) | Moderate firmness with chewiness and elasticity | 21–25 |
| Slightly soft or firm with less noticeable chewiness and slightly weak elasticity | 16–20 | |
| Very soft or very firm with no chewiness and poor elasticity | 10–15 |
| Samples | Short-Range Ordered Structure | |
|---|---|---|
| R1047/1020 | R1020/993 | |
| Control | 0.75 ± 0.02 a | 1.11 ± 0.01 b |
| 30% TBNs | 0.75 ± 0.01 a | 1.11 ± 0.04 b |
| 51% TBNs | 0.75 ± 0.00 a | 1.20 ± 0.03 ab |
| 70% TBNs | 0.74 ± 0.02 a | 1.22 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Chen, Z. Changes in Cooking Characteristics, Structural Properties and Bioactive Components of Wheat Flour Noodles Partially Substituted with Whole-Grain Hulled Tartary Buckwheat Flour. Foods 2024, 13, 395. https://doi.org/10.3390/foods13030395
Zhang M, Chen Z. Changes in Cooking Characteristics, Structural Properties and Bioactive Components of Wheat Flour Noodles Partially Substituted with Whole-Grain Hulled Tartary Buckwheat Flour. Foods. 2024; 13(3):395. https://doi.org/10.3390/foods13030395
Chicago/Turabian StyleZhang, Mengna, and Zhigang Chen. 2024. "Changes in Cooking Characteristics, Structural Properties and Bioactive Components of Wheat Flour Noodles Partially Substituted with Whole-Grain Hulled Tartary Buckwheat Flour" Foods 13, no. 3: 395. https://doi.org/10.3390/foods13030395
APA StyleZhang, M., & Chen, Z. (2024). Changes in Cooking Characteristics, Structural Properties and Bioactive Components of Wheat Flour Noodles Partially Substituted with Whole-Grain Hulled Tartary Buckwheat Flour. Foods, 13(3), 395. https://doi.org/10.3390/foods13030395






