Valorization of Spent Grains from Beer Production through β-Glucan Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of β-glucan from BSG
2.3. Functional Properties Analysis of the Extract
2.3.1. Water Holding Capacity (WHC)
2.3.2. Viscosity
2.3.3. Emulsion Stabilizing Capacity
2.4. Beverage Preparation and Characterization
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Temperature and Time on β-Glucan Extraction
3.2. Comparison of the Functional Properties of BSG β-glucan with Other Commercial Stabilizers
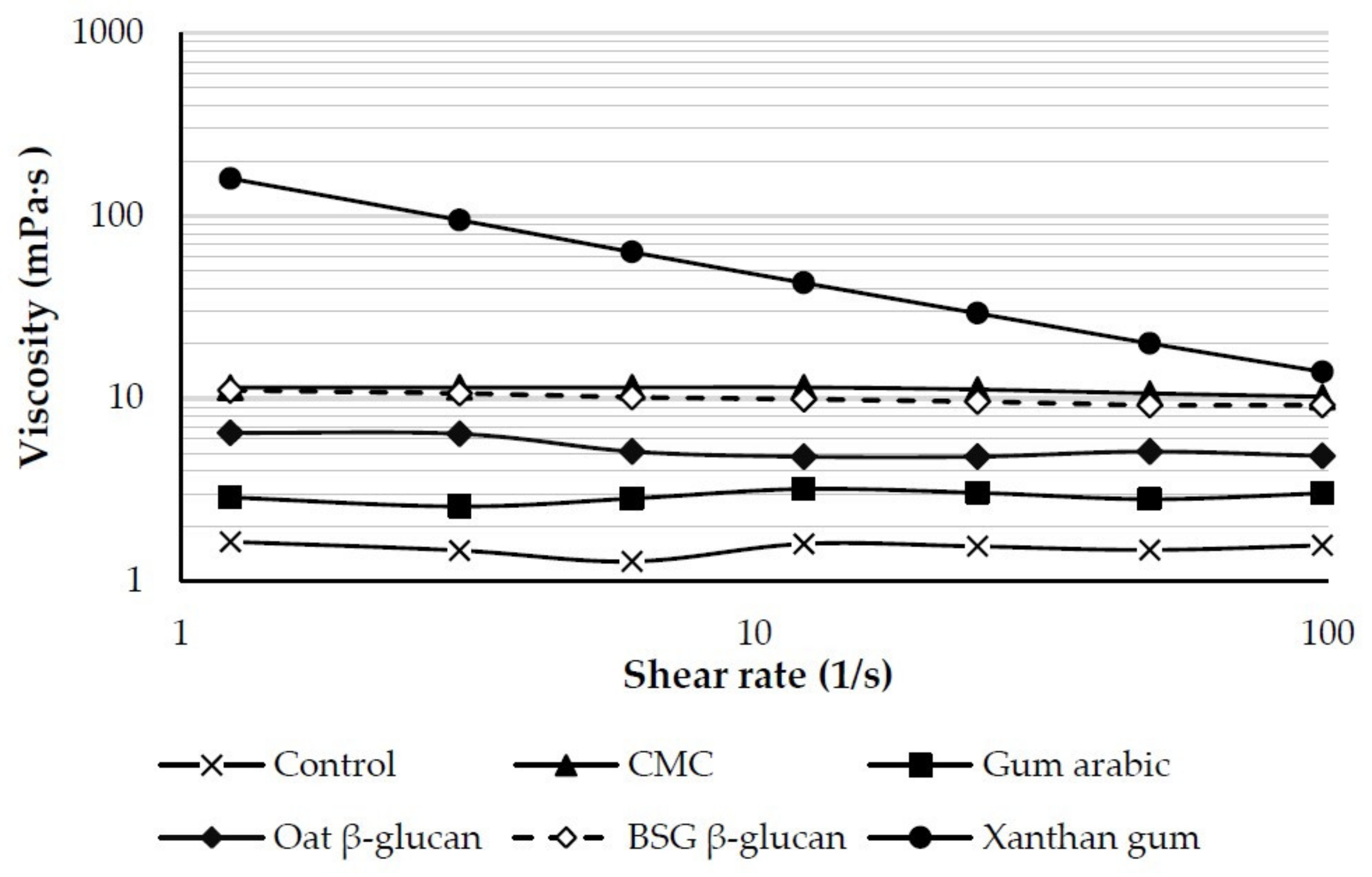
3.2.1. Apparent Viscosity
3.2.2. Water Holding Capacity (WHC)
3.2.3. Emulsion Stabilizing Capacity
3.3. Application of BSG β-Glucan in Orange Juice Beverage
3.3.1. Viscosity
3.3.2. Color
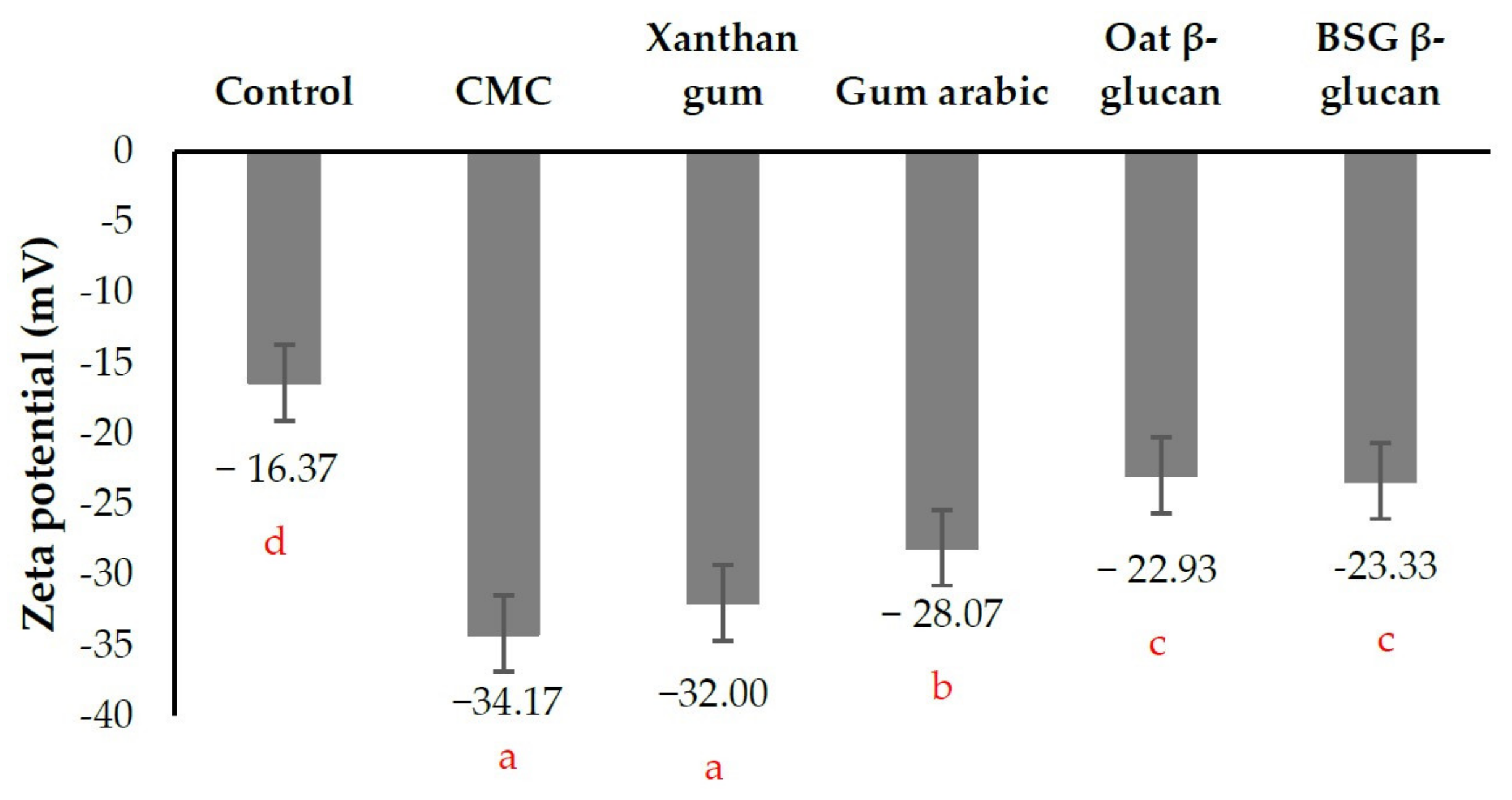
3.3.3. Zeta Potential of Beverage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Nirmala Prasadi, V.P.; Joye, I.J. Dietary fibre from whole grains and their benefits on metabolic health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Steiner, J.; Kupetz, M.; Becker, T. Influence of hydrothermal treatment of brewer’s spent grain on the concentration and molecular weight distribution of 1,3-1,4-β-D-glucan and arabinoxylan. Foods 2023, 12, 3778. [Google Scholar] [CrossRef] [PubMed]
- Lante, A.; Canazza, E.; Tessari, P. Beta-glucans of cereals: Functional and technological properties. Nutrients 2023, 15, 2124. [Google Scholar] [CrossRef] [PubMed]
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal β-glucans: The impact of processing and how it affects physiological responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Din, A. Physicochemical and functional properties of barley β-glucan as affected by different extraction procedures. Int. J. Food Sci. Technol. 2009, 44, 181–187. [Google Scholar] [CrossRef]
- Zielke, C.; Teixeira, C.; Ding, H.; Cui, S.; Nyman, M.; Nilsson, L. Analysis of β-glucan molar mass from barley malt and brewer’s spent grain with asymmetric flow field-flow fractionation (AF4) and their association to proteins. Carbohydr. Polym. 2017, 157, 541–549. [Google Scholar] [CrossRef]
- Kobelev, K.V.; Gribkova, I.N.; Kharlamova, L.N.; Danilyan, A.V.; Zakharov, M.A.; Lazareva, I.V.; Kozlov, V.I.; Borisenko, O.A. Study of brewer’s spent grain environmentally friendly processing ways. Molecules 2023, 28, 4553. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Ahmed, Z. Extraction and characterization of β-D-glucan from oat for industrial utilization. Int. J. Biol. Macromol. 2010, 46, 304–309. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemist (AOAC): Washington, DC, USA, 1993. [Google Scholar]
- AOAC. Official Methods of Analysis, 21st ed.; Association of Official Analytical Chemists (AOAC): Washington, DC, USA, 2019. [Google Scholar]
- Papageorgiou, M.; Lakhdara, N.; Lazaridou, A.; Biliaderis, C.G.; Izydorczyk, M.S. Water extractable (1→3,1→4)-β-D-glucans from barley and oats: An intervarietal study on their structural features and rheological behaviour. J. Cereal Sci. 2005, 42, 213–224. [Google Scholar] [CrossRef]
- Limberger-Bayer, V.M.; De Francisco, A.; Chan, A.; Oro, T.; Ogliari, P.J.; Barreto, P.L.M. Barley β-glucans extraction and partial characterization. Food Chem. 2014, 154, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Lyly, M.; Salmenkallio-Marttila, M.; Suortti, T.; Autio, K.; Poutanen, K.; Lähteenmäki, L. Influence of oat β-glucan preparations on the perception of mouthfeel and on rheological properties in beverage prototypes. Cereal Chem. 2003, 80, 536–541. [Google Scholar] [CrossRef]
- Zhang, Z.; Smith, C.; Li, W. Extraction and modification technology of arabinoxylans from cereal by-products: A critical review. Food Res. Int. 2014, 65, 423–436. [Google Scholar] [CrossRef]
- Arzami, A.N.; Ho, T.M.; Mikkonen, K.S. Mikkonen, Valorization of cereal by-product hemicelluloses: Fractionation and purity considerations. Food Res. Int. 2022, 151, 110818. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852), reduction of post-prandial glycaemic responses (ID 821, 824), and “digestive function” (ID 850) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2207. [Google Scholar] [CrossRef]
- Sharafbafi, N.; Tosh, S.; Alexander, M.; Corredig, M. Phase behaviour, rheological properties, and microstructure of oat β-glucan-milk mixtures. Food Hydrocolloids 2014, 41, 274–280. [Google Scholar] [CrossRef]
- Barone Lumaga, R.; Azzali, D.; Fogliano, V.; Scalfi, L.; Vitaglione, P. Sugar and dietary fibre composition influence, by different hormonal response, the satiating capacity of a fruit-based and a β-glucan-enriched beverage. Food Funct. 2012, 3, 67–75. [Google Scholar] [CrossRef]
- GRAS Associates, LLC. GRAS Assessment of Oat Beta-Glucan-B-CAN TM Food Usage Conditions for General Recognition of Safety. 2012. Available online: https://wayback.archive-it.org/7993/20171031044318/https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM316626.pdf (accessed on 30 December 2023).
- Yang, X.H.; Zhu, W.L. Viscosity properties of sodium carboxymethylcellulose solutions. Cellulose 2007, 14, 409–417. [Google Scholar] [CrossRef]
- Karp, S.; Wyrwisz, J.; Kurek, M.A. Comparative analysis of the physical properties of o/w emulsions stabilised by cereal β-glucan and other stabilisers. Int. J. Biol. Macromol. 2019, 132, 236–243. [Google Scholar] [CrossRef]
- Mothé, C.G.; Rao, M.A. Rheological behaviour of aqueous dispersions of cashew gum and gum arabic: Effect of concentration and blending. Food Hydrocolloids 1999, 13, 501–506. [Google Scholar] [CrossRef]
- Becker, J.M.; Caldwell, G.A.; Zachgo, E.A. Exercise 13—Protein Assays. In Biotechnology, 2nd ed.; Becker, J.M., Caldwell, G.A., Zachgo, E.A., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 119–124. [Google Scholar] [CrossRef]
- Hematian Sourki, A.; Koocheki, A.; Elahi, M. Ultrasound-assisted extraction of β-D-glucan from hull-less barley: Assessment of physicochemical and functional properties. Int. J. Biol. Macromol. 2017, 95, 462–475. [Google Scholar] [CrossRef]
- Clogston, J.; Patri, A. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Clifton, N.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 697, pp. 63–70. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids and emulsion stability. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Boca Raton, FL, USA, 2009; pp. 23–49. ISBN 978-1-84569-414-2. [Google Scholar]
- Genovese, D.B.; Lozano, J.E. The effect of hydrocolloids on the stability and viscosity of cloudy apple juices. Food Hydrocolloids 2001, 15, 1–7. [Google Scholar] [CrossRef]
- Gama, A.P.; Hung, Y.-C.; Adhikari, K. Optimization of emulsifier and stabilizer concentrations in a model peanut-based beverage system: A mixture design approach. Foods 2019, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S. Modeling the relationship between the main emulsion components and stability, viscosity, fluid behaviour, ζ-potential, and electrophoretic mobility of orange beverage emulsion using response surface methodology. J. Agric. Food Chem. 2007, 55, 7659–7666. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Xu, B. Development of an orange juice beverage formulated with oat beta-glucan and whey protein isolate. J. Sci. Food Agric. 2018, 98, 4685–4691. [Google Scholar] [CrossRef] [PubMed]
- Temelli, F.; Bansema, C.; Stobbe, K. Development of an orange-flavored barley β-glucan beverage. Cereal Chem. 2004, 81, 499–503. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S. Effect of Arabic gum, xanthan gum and orange oil contents on ζ-potential, conductivity, stability, size index and pH of orange beverage emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 47–56. [Google Scholar] [CrossRef]
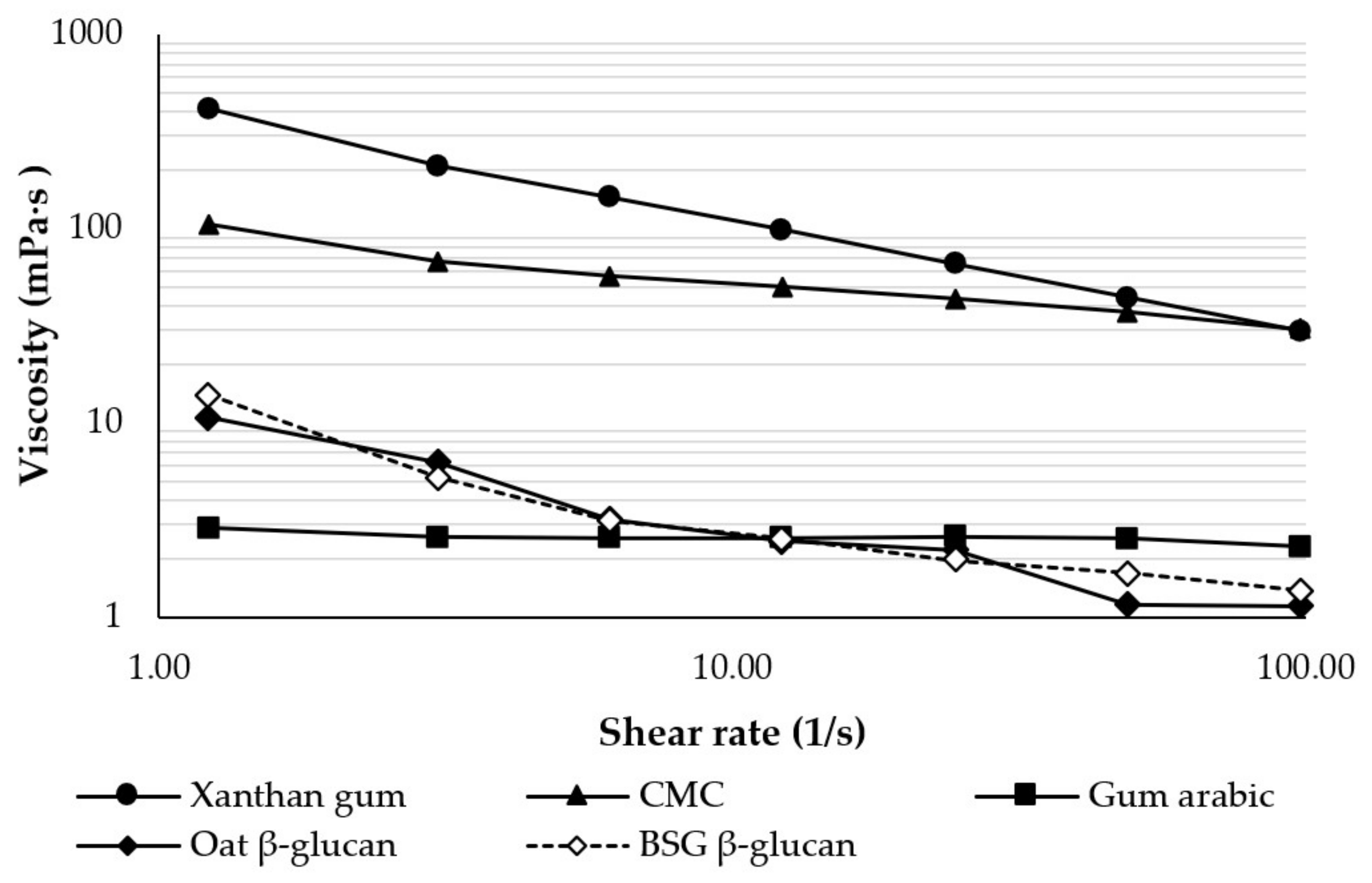
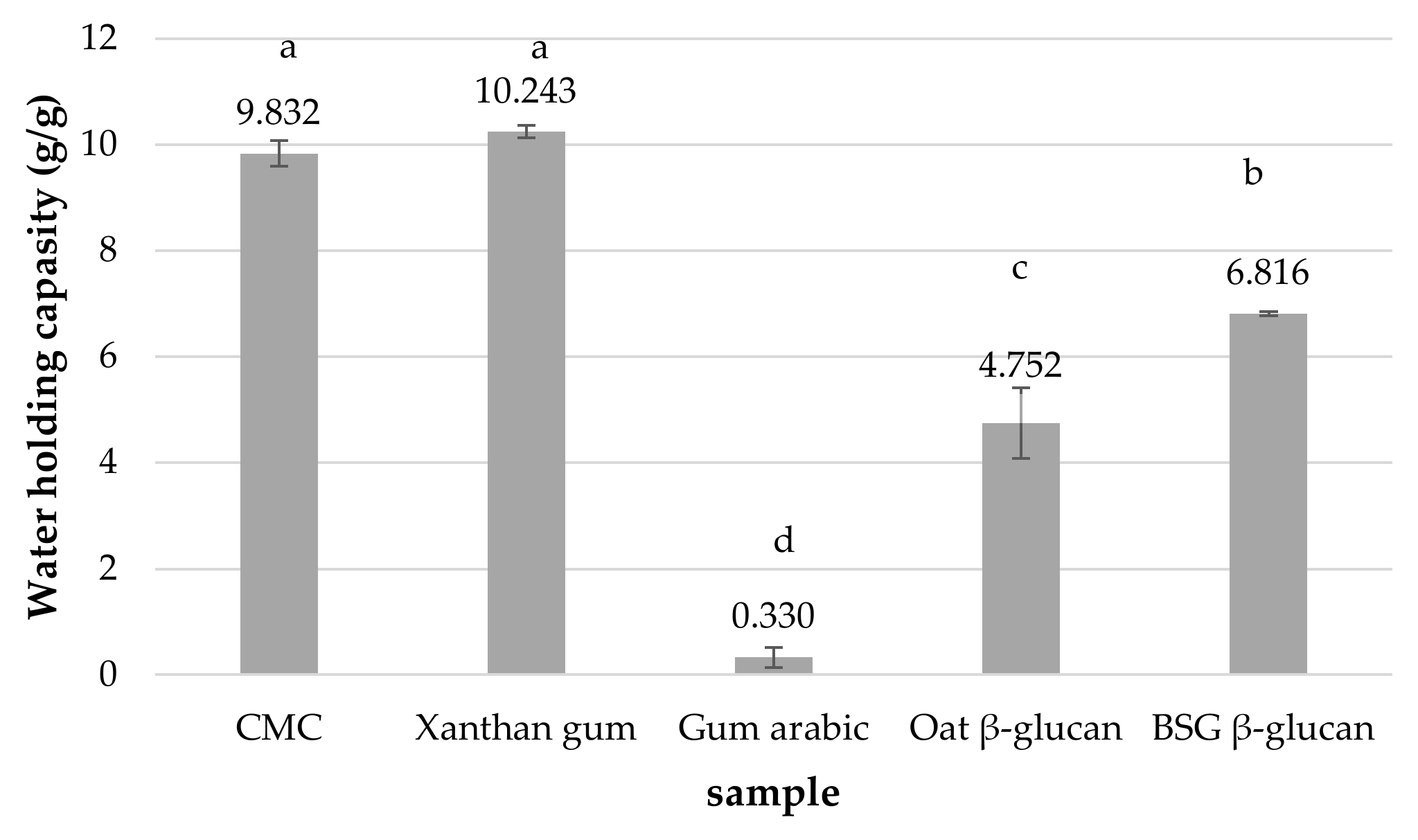
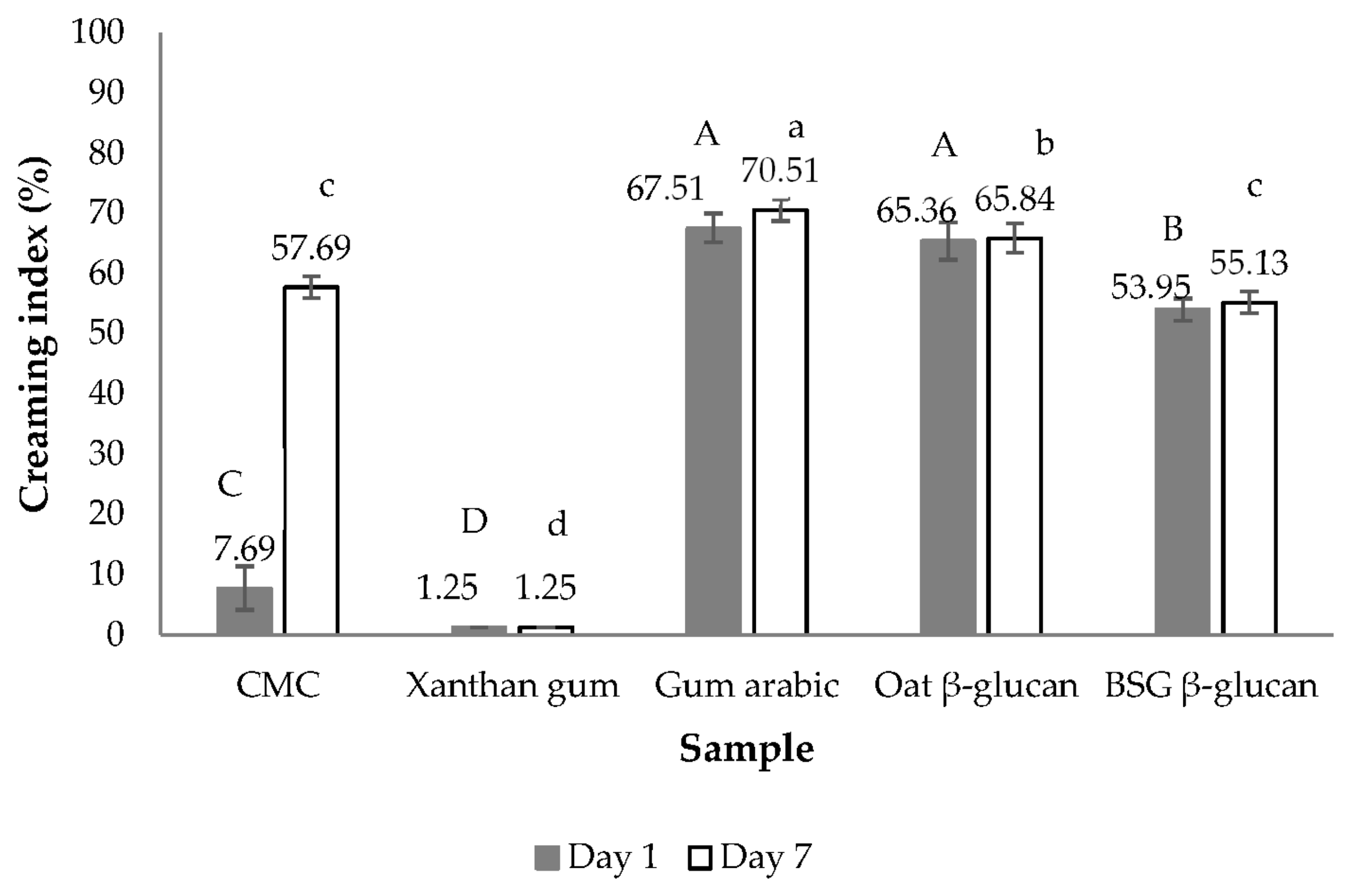

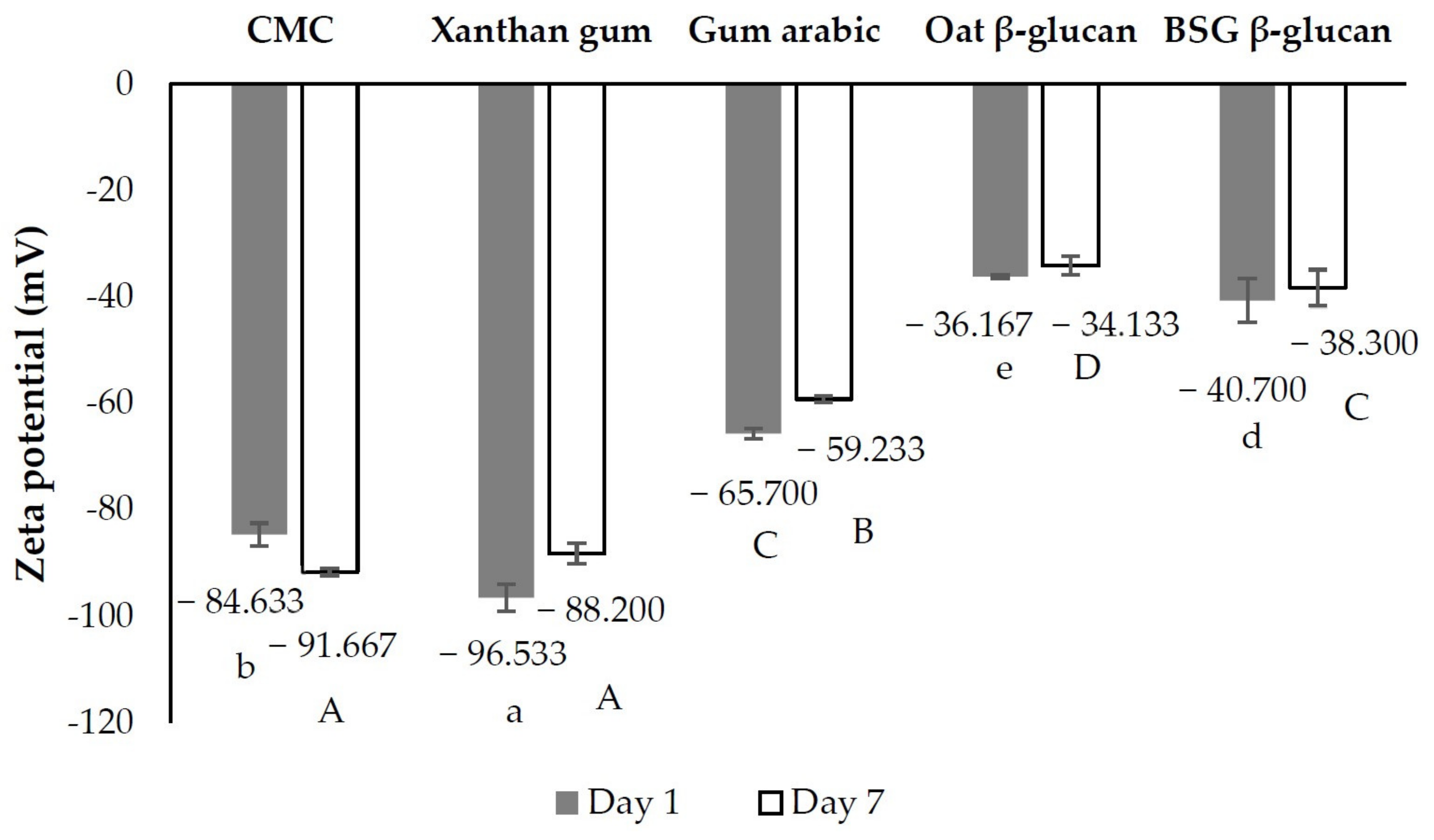

| Condition | Yield of the Extract (%, w/w) | β-Glucan Content of the Extract (%, w/w) | Extraction Yield of β-Glucan (%, w/w) | Recovery Yield of β-Glucan (%, w/w) | |
|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | ||||
| 45 | 30 | 0.087 ± 0.004 fgh | 48.305 ± 9.524 bcd | 0.042 ± 0.010 abc | 9.490 ± 2.305 abc |
| 60 | 0.088 ± 0.045 cd | 55.419 ± 3.123 ab | 0.050 ± 0.027 abc | 11.160 ± 6.166 abc | |
| 90 | 0.135 ± 0.008 ab | 42.203 ± 1.628 def | 0.057 ± 0.001 ab | 12.830 ± 0.269 ab | |
| 120 | 0.148 ± 0.004 a | 34.658 ± 0.221 f | 0.051 ± 0.001 abc | 11.500 ± 0.212 abc | |
| 60 | 30 | 0.085 ± 0.002 fgh | 37.744 ± 0.153 ef | 0.032 ± 0.001 c | 7.220 ± 0.141 c |
| 60 | 0.081 ± 0.012 gh | 39.331 ± 1.964 ef | 0.032 ± 0.006 c | 7.200 ± 1.428 c | |
| 90 | 0.096 ± 0.001 defgh | 59.837 ± 0.054 a | 0.057 ± 0.001 a | 12.855 ± 0.177 a | |
| 120 | 0.090 ± 0.015 efgh | 53.359 ± 4.248 abc | 0.048 ± 0.012 abc | 10.830 ± 2.673 abc | |
| 75 | 30 | 0.134 ± 0.002 ab | 41.664 ± 2.918 def | 0.056 ± 0.005 abc | 12.535 ± 1.068 abc |
| 60 | 0.079 ± 0.012 h | 41.536 ± 3.644 def | 0.033 ± 0.008 bc | 7.435 ± 1.789 bc | |
| 90 | 0.099 ± 0.011 defg | 44.941 ± 0.764 def | 0.045 ± 0.006 abc | 10.020 ± 1.315 abc | |
| 120 | 0.128 ± 0.003 bc | 41.254 ± 2.376 def | 0.053 ± 0.002 abc | 11.845 ± 0.375 abc | |
| 90 | 30 | 0.107 ± 0.031 ab | 45.807 ± 3.391 cde | 0.050 ± 0.018 abc | 11.165 ± 3.953 abc |
| 60 | 0.084 ± 0.006 fgh | 48.319 ± 0.897 bcd | 0.041 ± 0.002 abc | 9.090 ± 0.509 abc | |
| 90 | 0.107 ± 0.003 de | 38.500 ± 5.640 ef | 0.041 ± 0.007 abc | 9.270 ± 1.626 abc | |
| 120 | 0.103 ± 0.001 def | 45.706 ± 1.590 cde | 0.047 ± 0.002 acb | 10.600 ± 0.509 abc | |
| Viscosity in Water | Creaming Index | Zeta Potential | |
|---|---|---|---|
| Viscosity in water | 1 | ||
| Creaming index | −0.818 | 1 | |
| Zeta potential | 0.79 | −0.855 | 1 |
| Sample | L* | a* | b* |
|---|---|---|---|
| Control | 52.82 ± 0.78 d | 15.54 ± 0.42 d | 51.59 ± 0.60 c |
| Xanthan gum | 60.09 ± 0.46 c | 16.60 ± 0.05 bc | 53.72 ± 1.41 bc |
| CMC | 67.31 ± 1.17 b | 16.55 ± 0.08 bc | 52.64 ± 1.58 bc |
| Gum arabic | 61.82 ± 0.78 c | 16.13 ± 0.10 c | 48.93 ± 1.01 d |
| Oat β-glucan | 60.08 ± 0.79 c | 16.88 ± 0.01 ab | 55.13 ± 0.95 b |
| BSG β-glucan | 70.09 ± 0.55 a | 17.18 ± 0.11 a | 58.71 ± 0.04 a |
| Sample | L* | a* | b* |
|---|---|---|---|
| Xanthan gum | 60.09 ± 0.46 b | 0.27 ± 0.09 b | 1.99 ± 0.11 b |
| CMC | 61.31 ± 1.17 b | 0.05 ± 0.02 c | 0.85 ± 0.07 d |
| Gum arabic | 61.80 ± 0.76 b | 0.12 ± 0.03 bc | 0.98 ± 0.04 d |
| Oat β-glucan | 57.08 ± 0.79 c | 0.32 ± 0.12 b | 1.27 ± 0.07 c |
| BSG β-glucan | 65.94 ± 0.34 a | 0.72 ± 0.06 a | 3.23 ± 0.18 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantason, N.; Suphantharika, M.; Wipatanawin, A.; Chansong, S.; Payongsri, P. Valorization of Spent Grains from Beer Production through β-Glucan Extraction. Foods 2024, 13, 440. https://doi.org/10.3390/foods13030440
Jantason N, Suphantharika M, Wipatanawin A, Chansong S, Payongsri P. Valorization of Spent Grains from Beer Production through β-Glucan Extraction. Foods. 2024; 13(3):440. https://doi.org/10.3390/foods13030440
Chicago/Turabian StyleJantason, Natcha, Manop Suphantharika, Angkana Wipatanawin, Suwan Chansong, and Panwajee Payongsri. 2024. "Valorization of Spent Grains from Beer Production through β-Glucan Extraction" Foods 13, no. 3: 440. https://doi.org/10.3390/foods13030440
APA StyleJantason, N., Suphantharika, M., Wipatanawin, A., Chansong, S., & Payongsri, P. (2024). Valorization of Spent Grains from Beer Production through β-Glucan Extraction. Foods, 13(3), 440. https://doi.org/10.3390/foods13030440







