Bifidobacterium Relieved Fluoride-Induced Hepatic and Ileal Toxicity via Inflammatory Response and Bile Acid Transporters in Mice
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Treatments
2.2. Organ Coefficient Analysis
2.3. Bone Fluoride Determination
2.4. Grading of Dental Damage
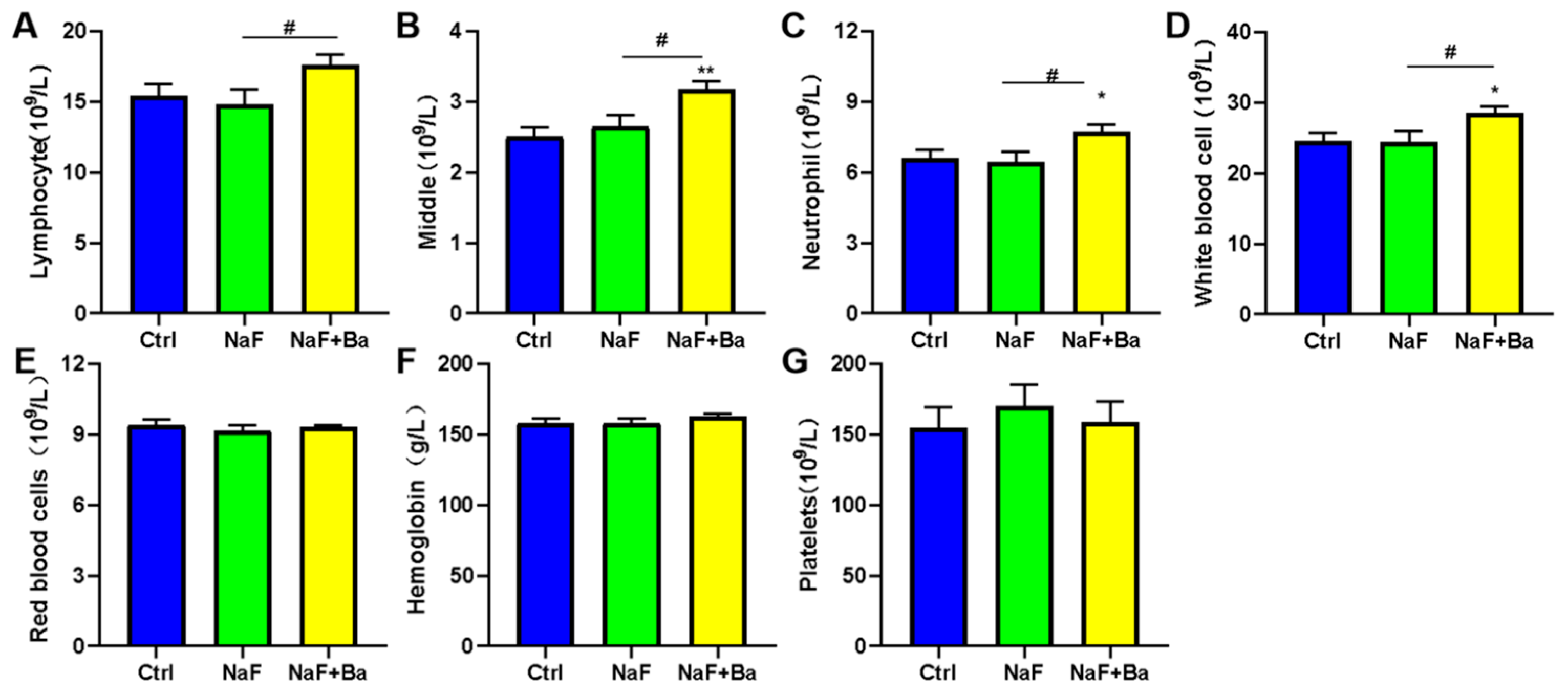
2.5. Blood Routine
2.6. Histopathological Examination
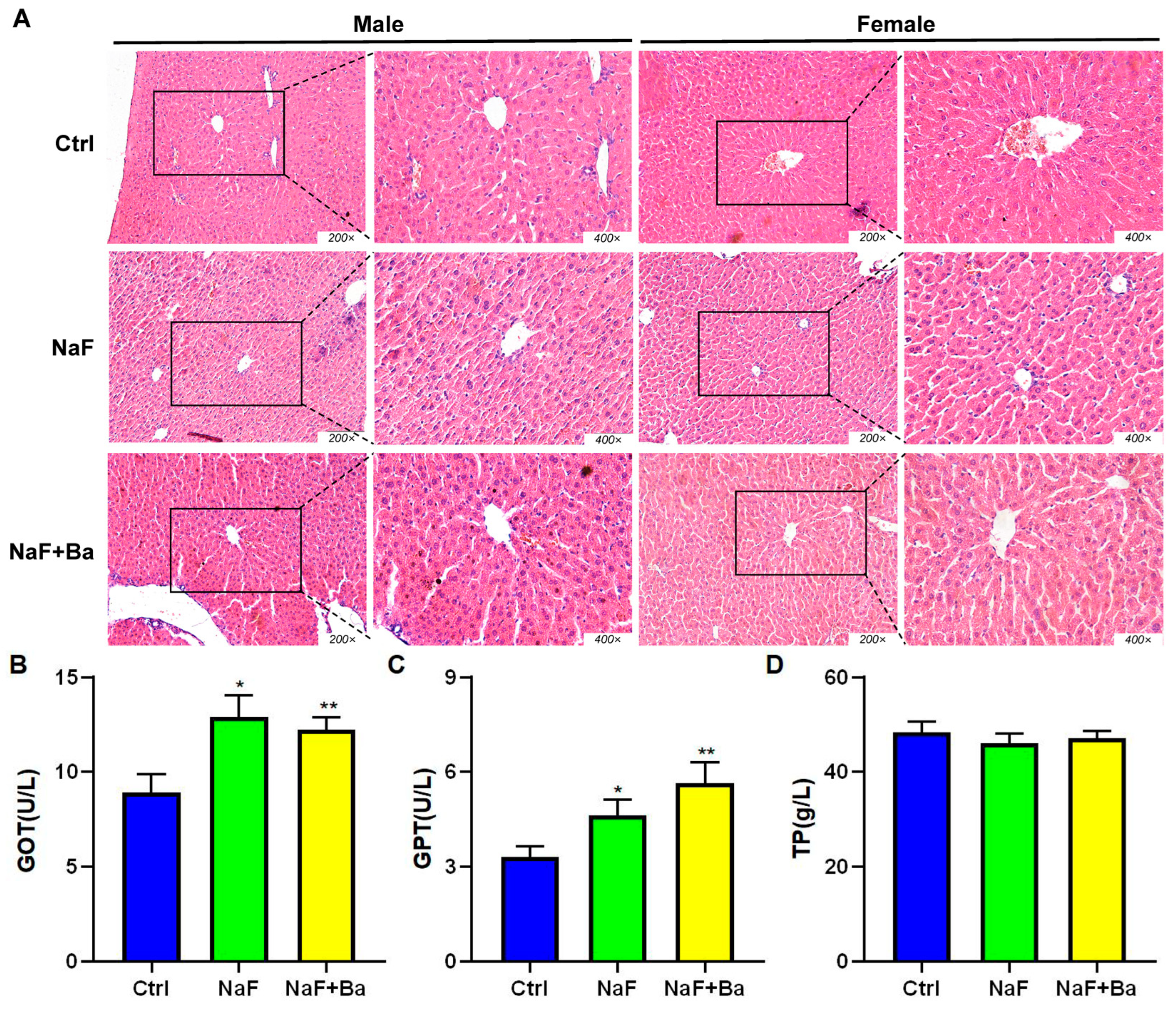
2.7. Biochemical Assays
2.8. ELISA Analysis
2.9. Real-Time PCR
2.10. Statistical Analysis
3. Results
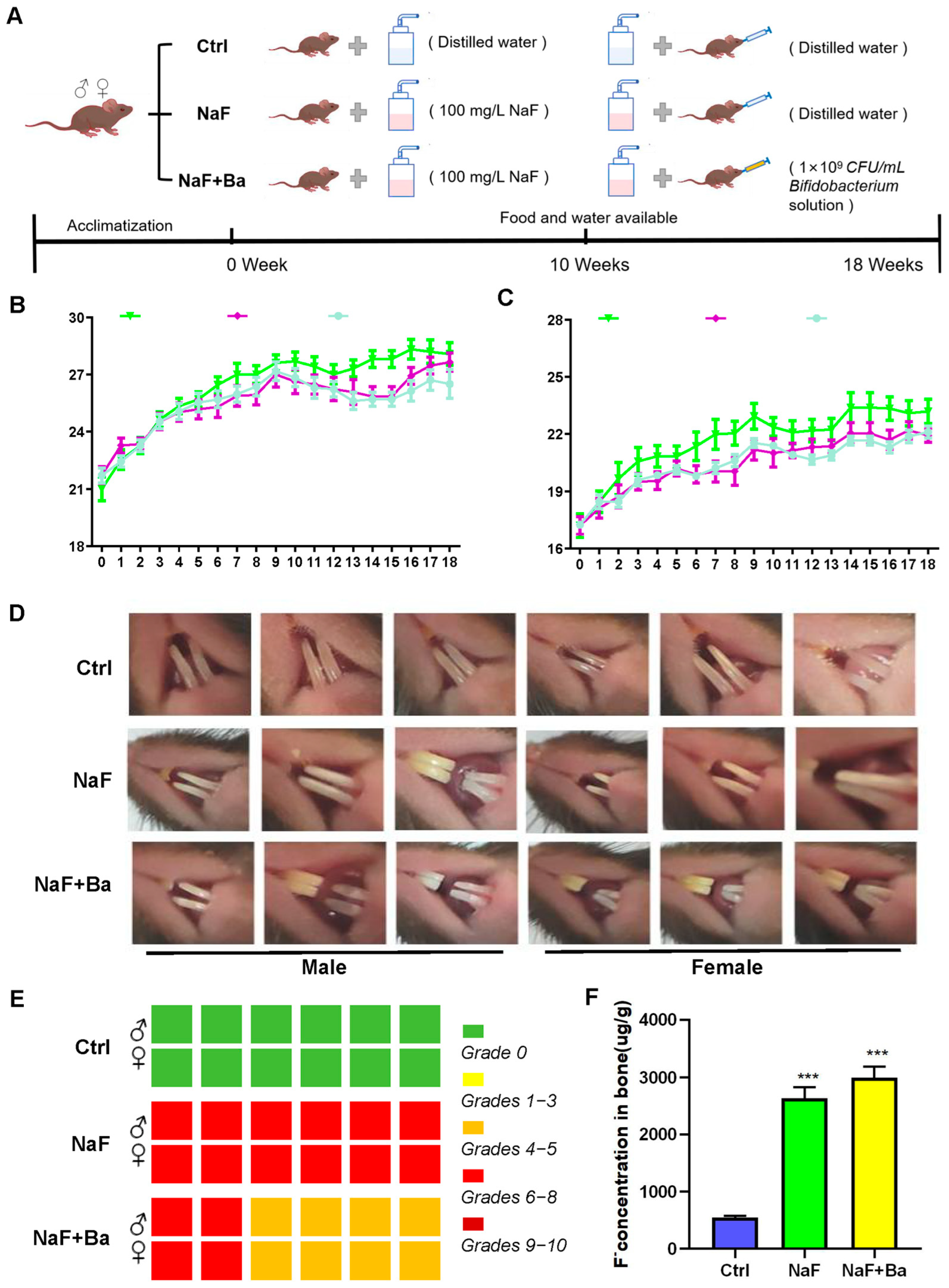
3.1. Mouse Model Design and Primary Evaluation of Fluoride Exposure and Bifidobacterium Intervention
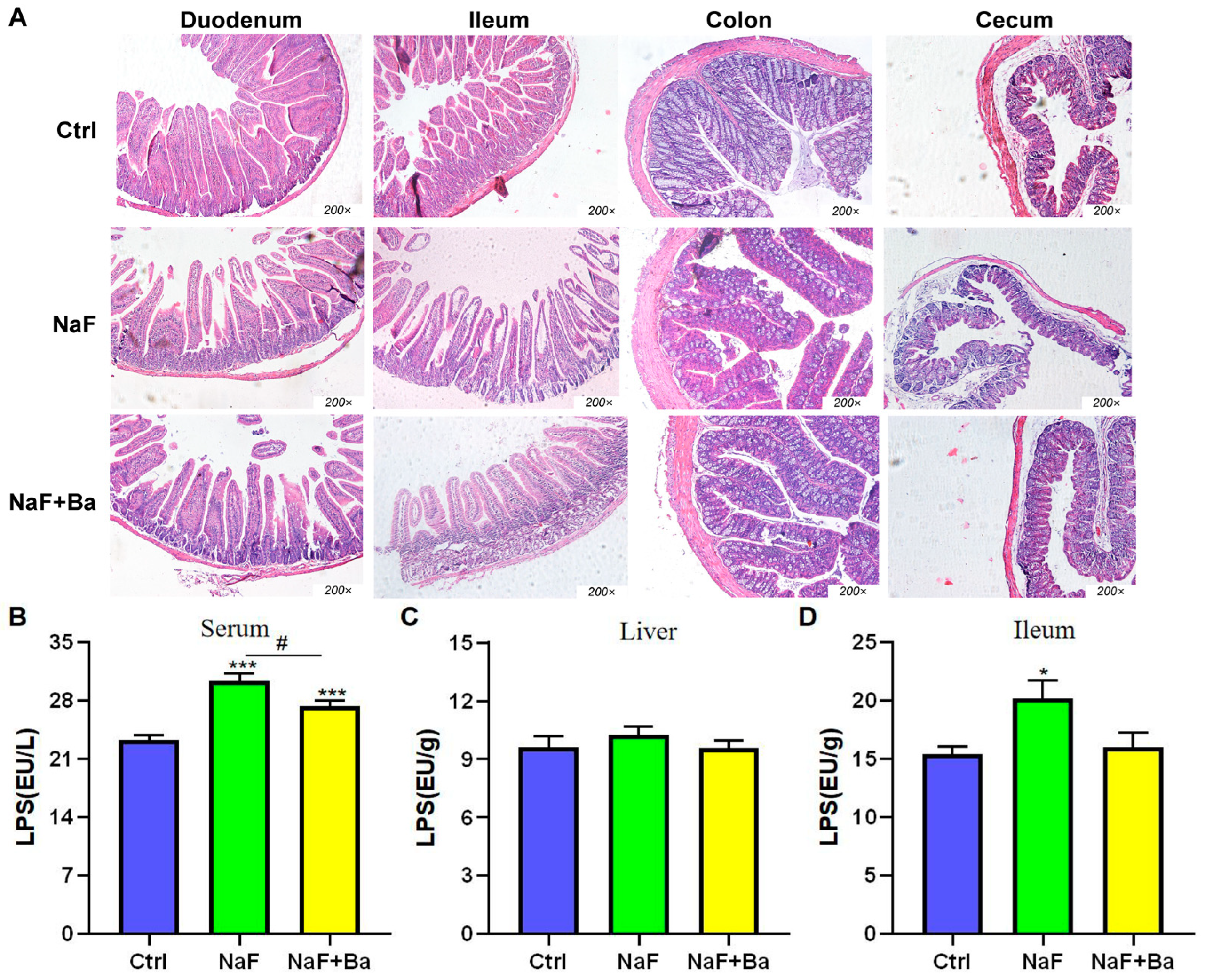
3.2. Bifidobacterium Alleviates Fluoride-Induced Liver and Ileal Inflammation
3.3. Bifidobacterium Restored the Alteration of Morphology of Liver Induced by Fluoride
3.4. Bifidobacterium Intervention Alleviates Fluoride-Induced Intestinal Injury in Mice
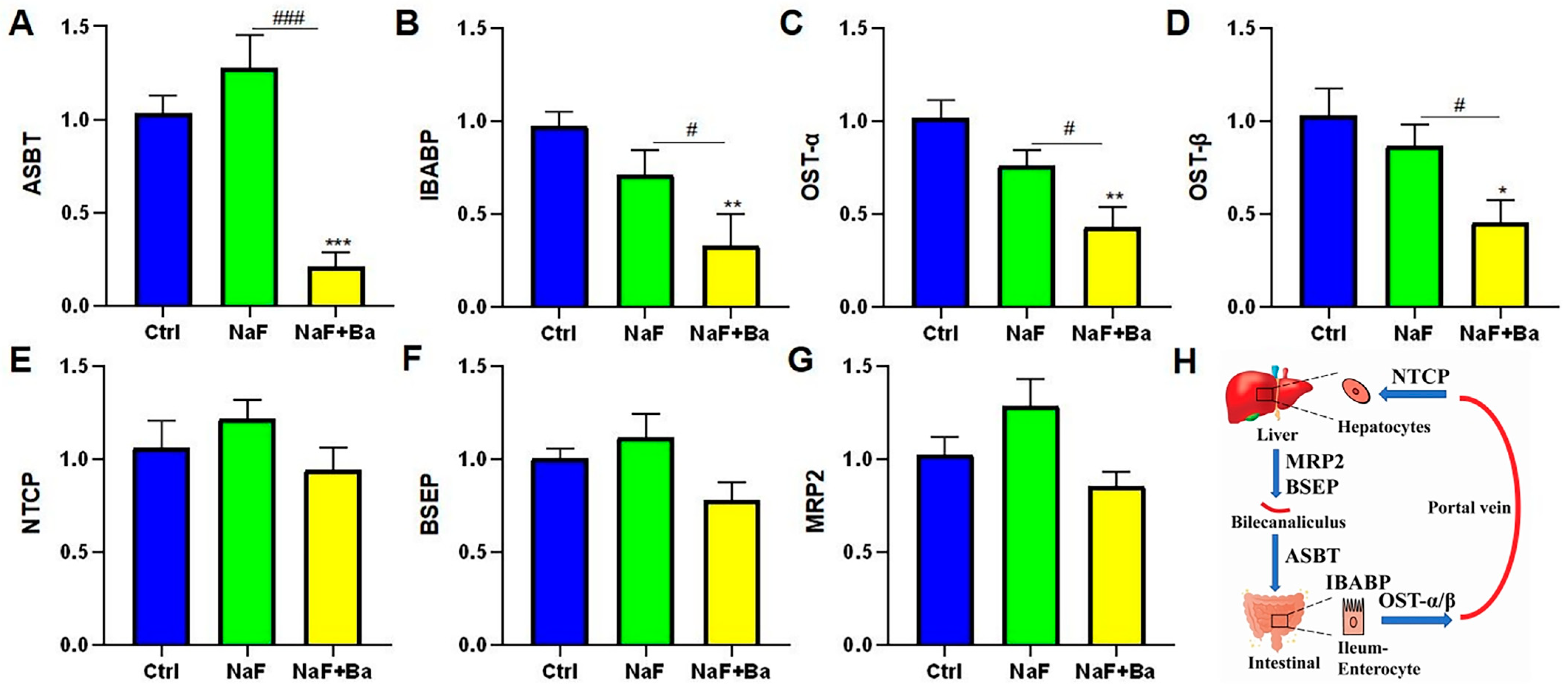
3.5. Bifidobacterium Regulated Bile Acid Transporters in Enterohepatic Circulation
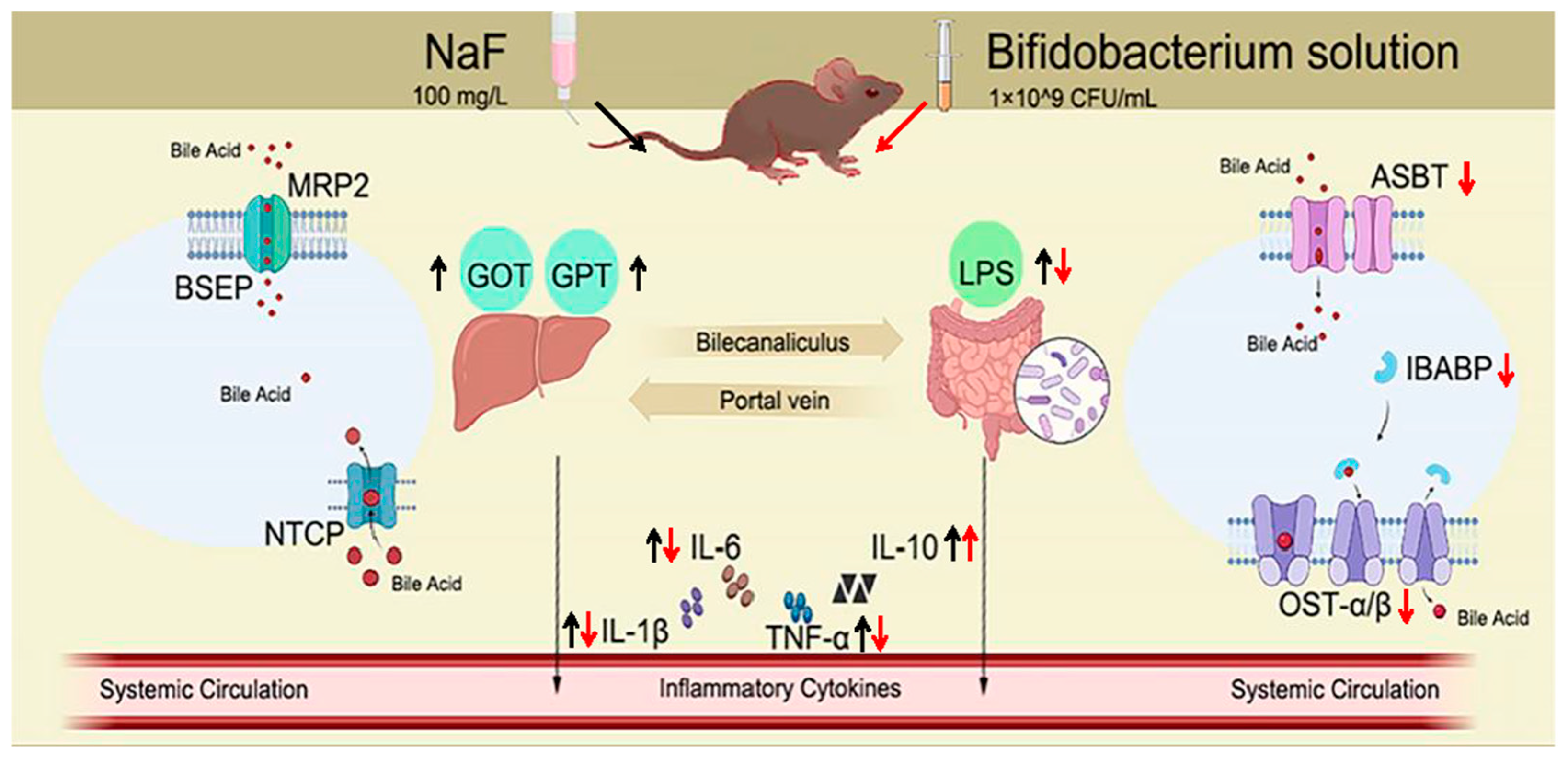
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wei, W.; Pang, S.; Sun, D. The pathogenesis of endemic fluorosis: Research progress in the last 5 years. J. Cell. Mol. Med. 2019, 23, 2333–2342. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global analysis and prediction of fluoride in groundwater. Nat. Commun. 2022, 13, 4232. [Google Scholar] [CrossRef] [PubMed]
- Malin, A.J.; Lesseur, C.; Busgang, S.A.; Curtin, P.; Wright, R.O.; Sanders, A.P. Fluoride exposure and kidney and liver function among adolescents in the United States: NHANES, 2013–2016. Environ. Int. 2019, 132, 105012. [Google Scholar] [CrossRef] [PubMed]
- Dec, K.; Łukomska, A.; Baranowska-Bosiacka, I.; Pilutin, A.; Maciejewska, D.; Skonieczna-Żydecka, K.; Derkacz, R.; Goschorska, M.; Wąsik, A.; Rębacz-Maron, E.; et al. Pre-and postnatal exposition to fluorides induce changes in rats liver morphology by impairment of antioxidant defense mechanisms and COX induction. Chemosphere 2018, 211, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, R.; Yao, Q.; Liang, Z.; Wu, M.; Wang, H. Effects of fluoride on the histology, lipid metabolism, and bile acid secretion in liver of Bufo gargarizans larvae. Environ. Pollut. 2019, 254 Pt B, 113052. [Google Scholar] [CrossRef]
- Aydin, Y.; Orta-Yilmaz, B. Synergistic effects of arsenic and fluoride on oxidative stress and apoptotic pathway in Leydig and Sertoli cells. Toxicology 2022, 475, 153241. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Iqbal, M.; Mehmood, K.; Li, Y.; Tang, Z.; Zhang, H. Challenges of fluoride pollution in environment: Mechanisms and pathological significance of toxicity—A review. Environ. Pollut. 2022, 304, 119241. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Chen, L.; Kong, M.; Qiu, L.; Lü, P.; Wu, P.; Yang, Y.; Chen, K. Toxic effects of fluoride on organisms. Life Sci. 2018, 198, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Cao, Q.; Li, R.; Fu, R.; Zhang, X.; Yue, B.; Wang, J.; Sun, Z.; Niu, R. Intestinal fungal dysbiosis in mice induced by fluoride. Chemosphere 2020, 245, 125617. [Google Scholar] [CrossRef]
- Nabwera, H.M.; Espinoza, J.L.; Worwui, A.; Betts, M.; Okoi, C.; Sesay, A.K.; Bancroft, R.; Agbla, S.C.; Jarju, S.; Bradbury, R.S.; et al. Interactions between fecal gut microbiome, enteric pathogens, and energy regulating hormones among acutely malnourished rural Gambian children. EBioMedicine 2021, 73, 103644. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.W.; Lin, L.; Miao, C.Y.; Zhang, Y.; Zhou, B.H. Intestinal barrier damage involved in intestinal microflora changes in fluoride-induced mice. Chemosphere 2019, 234, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, M.; Li, Y.; Liu, H.; Hou, C.; Zeng, Q.; Li, P.; Zhao, Q.; Dong, L.; Yu, X.; et al. Low-to-moderate fluoride exposure in relation to overweight and obesity among school-age children in China. Ecotoxicol. Environ. Saf. 2019, 183, 109558. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Niu, R.; Li, R.; Yue, B.; Zhang, X.; Cao, Q.; Wang, J.; Sun, Z. Fluoride-Induced alteration in the diversity and composition of bacterial microbiota in mice colon. Biol. Trace Elem. Res. 2020, 196, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Chopyk, D.M.; Grakoui, A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology 2020, 159, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Paroni Sterbini, F.; Petito, V.; et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. 2020, 20, 357. [Google Scholar] [CrossRef]
- Wang, L.; Jiao, T.; Yu, Q.; Wang, J.; Wang, L.; Wang, G.; Zhang, H.; Zhao, J.; Chen, W. Bifidobacterium bifidum Shows More Diversified Ways of Relieving Non-Alcoholic Fatty Liver Compared with Bifidobacterium adolescentis. Biomedicines 2021, 10, 84. [Google Scholar] [CrossRef]
- Fontana, L.; Plaza-Díaz, J.; Robles-Bolívar, P.; Valente-Godínez, H.; Sáez-Lara, M.J.; Abadía-Molina, F.; Gómez-Llorented, C.; Gil, Á.; Álvarez-Mercado, A.I. Bifidobacterium breve CNCM I-4035, Lactobacillus paracasei CNCM I-4034 and Lactobacillus rhamnosus CNCM I-4036 Modulate Macrophage Gene Expression and Ameliorate Damage Markers in the Liver of Zucker-Leprfa/fa Rats. Nutrients 2021, 13, 202. [Google Scholar] [CrossRef]
- Linhares, D.; Camarinho, R.; Garcia, P.V.; Rodrigues, A.D.S. Mus musculus bone fluoride concentration as a useful biomarker for risk assessment of skeletal fluorosis in volcanic areas. Chemosphere 2018, 205, 540–544. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Liang, C.; Yang, W.; Zhu, Q.; Luo, H.; Liu, X.; Wang, J.; Zhang, J. Potential protective effect of riboflavin against pathological changes in the main organs of male mice induced by fluoride exposure. Biol. Trace Elem. Res. 2022, 200, 1262–1273. [Google Scholar] [CrossRef]
- Ando, M.; Tadano, M.; Asanuma, S.; Tamura, K.; Matsushima, S.; Watanabe, T.; Kondo, T.; Sakurai, S.; Ji, R.; Liang, C.; et al. Health effects of indoor fluoride pollution from coal burning in China. Environ. Health Perspect. 1998, 106, 239–244. [Google Scholar] [CrossRef]
- Yadav, K.K.; Kumar, S.; Pham, Q.B.; Gupta, N.; Rezania, S.; Kamyab, H.; Yadav, S.; Vymazal, J.; Kumar, V.; Tri, D.Q.; et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review. Ecotoxicol. Environ. Saf. 2019, 182, 109362. [Google Scholar] [CrossRef]
- Rasool, A.; Farooqi, A.; Xiao, T.; Ali, W.; Noor, S.; Abiola, O.; Ali, S.; Nasim, W. A review of global outlook on fluoride contamination in groundwater with prominence on the Pakistan current situation. Environ. Geochem. Health 2018, 40, 1265–1281. [Google Scholar] [CrossRef]
- Lim, J.K.; Renaldo, G.J.; Chapman, P. LD50 of SnF2, NaF, and Na2PO3F in the mouse compared to the rat. Caries Res. 1978, 12, 177–179. [Google Scholar] [CrossRef]
- Luo, H.; Liu, R.; Lang, Y.; Zhao, J.; Zhuang, C.; Wang, J.; Liang, C.; Zhang, J. Melatonin alleviated fluoride-induced impairment of spermatogenesis and sperm maturation process via Interleukin-17A. Food Chem. Toxicol. 2023, 178, 113867. [Google Scholar] [CrossRef]
- He, X.; Hao, P.; Wang, Y.; Wu, C.; Yin, W.; Shahid, M.A.; Wu, S.; Nawaz, S.; Du, W.; Xu, Y.; et al. Swertia bimaculata moderated liver damage in mice by regulating intestine microbiota. Ecotoxicol. Environ. Saf. 2023, 263, 115223. [Google Scholar] [CrossRef]
- Xin, J.; Wang, H.; Sun, N.; Bughio, S.; Zeng, D.; Li, L.; Wang, Y.; Khalique, A.; Zeng, Y.; Pan, K.; et al. Probiotic alleviate fluoride-induced memory impairment by reconstructing gut microbiota in mice. Ecotoxicol. Environ. Saf. 2021, 215, 112108. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Jin, L.; Gao, Y.; Ding, Y.; Wen, H.; Qian, Z.; Zhang, C.; Hong, L.; Yang, H.; Zhang, J.; et al. Artificial-enzymes-armed Bifidobacterium longum probiotics for alleviating intestinal inflammation and microbiota dysbiosis. Nat. Nanotechnol. 2023, 18, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Li, Y.; Yang, Y.; Fan, C.; Li, Y.; Du, X.; Sun, G.; Cui, Y. Human health risk assessment in aluminium smelting site: Soil fluoride bioaccessibility and relevant mechanism in simulated gastrointestinal tract. J. Hazard. Mater. 2021, 416, 125899. [Google Scholar] [CrossRef]
- Dasarathy, S.; Das, T.K.; Gupta, I.P.; Susheela, A.K.; Tandon, R.K. Gastroduodenal manifestations in patients with skeletal fluorosis. J. Gastroenterol. 1996, 31, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; von der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef]
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut microbiota dysbiosis and increased plasma LPS and TMAO levels in patients with preeclampsia. Front. Cell. Infect. Microbiol. 2019, 9, 409. [Google Scholar] [CrossRef]

| Gene Name | Primer Sequences | Accession No. | Product Sizes (bp) |
|---|---|---|---|
| β-actin | F:TACCCAGGCATTGCTGACAG R:ACTCCTGCTTGCTGATCCAC | NM_007393.5 | 98 |
| NTCP | F:ATGCCTTTCACTGGCTTCC R:CTGTTTCCATGCTGATGGTG | NM_011387.2 | 91 |
| BSEP | F:GGACAATGATGTGCTTGTGG R:AGGGCCATTCTGAGATGTTG | NM_021022.3 | 80 |
| MRP2 | F:AAGCAGGTGTTCGTTGTGTG R:ACAGGAGGAATTGTGGCTTG | NM_013806.2 | 95 |
| ASBT | F:CATGACCACTTGCTCCACAC R:AATCGTTCCCGAGTCAACC | NM_011388.3 | 91 |
| IBABP | F:GTCTTCCAGGAGACGTGATTG R:CCGAAGTCTGGTGATAGTTGG | NM_008375.2 | 114 |
| OST-α | F:TGCATCTGGGTGAACAGAAC R:AGCGATCTGCCCACTGTTAG | NM_145932.3 | 116 |
| OST-β | F:GACCTGCATCTTGATGACTCC R:GGCCAAGTCTGGTTTCTCTG | NM_178933.2 | 90 |
| Male | Liver | Kidney | Spleen | Testis | Left Epididymis (10−2) | Brain |
|---|---|---|---|---|---|---|
| Crtl | 3.53 ± 0.20 | 1.33 ± 0.05 | 0.21 ± 0.07 | 0.82 ± 0.03 | 17.52 ± 1.28 | 1.75 ± 0.10 |
| NaF | 3.56 ± 0.16 | 1.33 ± 0.04 | 0.24 ± 0.01 | 0.72 ± 0.10 | 15.37 ± 1.43 | 1.77 ± 0.12 |
| NaF + Ba | 3.59 ± 0.06 | 1.24 ± 0.04 | 0.25 ± 0.01 | 0.82 ± 0.03 | 17.44 ± 1.96 | 1.84 ± 0.05 |
| Female | Liver | Kidney | Spleen | Ovary (10−2) | Uterus | Brain |
|---|---|---|---|---|---|---|
| Crtl | 3.55 ± 0.16 | 1.26 ± 0.05 | 0.27 ± 0.03 | 5.49 ± 0.82 | 0.23 ± 0.07 | 2.14 ± 0.12 |
| NaF | 3.60 ± 0.12 | 1.23 ± 0.04 | 0.30 ± 0.04 | 4.68 ± 1.62 | 0.26 ± 0.11 | 2.14 ± 0.11 |
| NaF + Ba | 3.41 ± 0.19 | 1.21 ± 0.04 | 0.25 ± 0.03 | 5.30 ± 1.12 | 0.23 ± 0.07 | 2.19 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Cheng, A.; Wang, Y.; Zhu, Q.; Ren, X.; Lu, Y.; Shi, E.; Zhuang, C.; Wang, J.; Liang, C.; et al. Bifidobacterium Relieved Fluoride-Induced Hepatic and Ileal Toxicity via Inflammatory Response and Bile Acid Transporters in Mice. Foods 2024, 13, 1011. https://doi.org/10.3390/foods13071011
Wu Y, Cheng A, Wang Y, Zhu Q, Ren X, Lu Y, Shi E, Zhuang C, Wang J, Liang C, et al. Bifidobacterium Relieved Fluoride-Induced Hepatic and Ileal Toxicity via Inflammatory Response and Bile Acid Transporters in Mice. Foods. 2024; 13(7):1011. https://doi.org/10.3390/foods13071011
Chicago/Turabian StyleWu, Yue, Ao Cheng, Yu Wang, Qianlong Zhu, Xuting Ren, Yiguang Lu, Erbao Shi, Cuicui Zhuang, Jundong Wang, Chen Liang, and et al. 2024. "Bifidobacterium Relieved Fluoride-Induced Hepatic and Ileal Toxicity via Inflammatory Response and Bile Acid Transporters in Mice" Foods 13, no. 7: 1011. https://doi.org/10.3390/foods13071011
APA StyleWu, Y., Cheng, A., Wang, Y., Zhu, Q., Ren, X., Lu, Y., Shi, E., Zhuang, C., Wang, J., Liang, C., & Zhang, J. (2024). Bifidobacterium Relieved Fluoride-Induced Hepatic and Ileal Toxicity via Inflammatory Response and Bile Acid Transporters in Mice. Foods, 13(7), 1011. https://doi.org/10.3390/foods13071011






