Influence of the Different Maturation Conditions of Cocoa Beans on the Chemical Profile of Craft Chocolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Extraction and Identification of Volatile Organic Compound
2.3. Extraction and Paper Spray Mass Spectrometry Analysis
3. Results
3.1. Volatile Organic Compound Profile
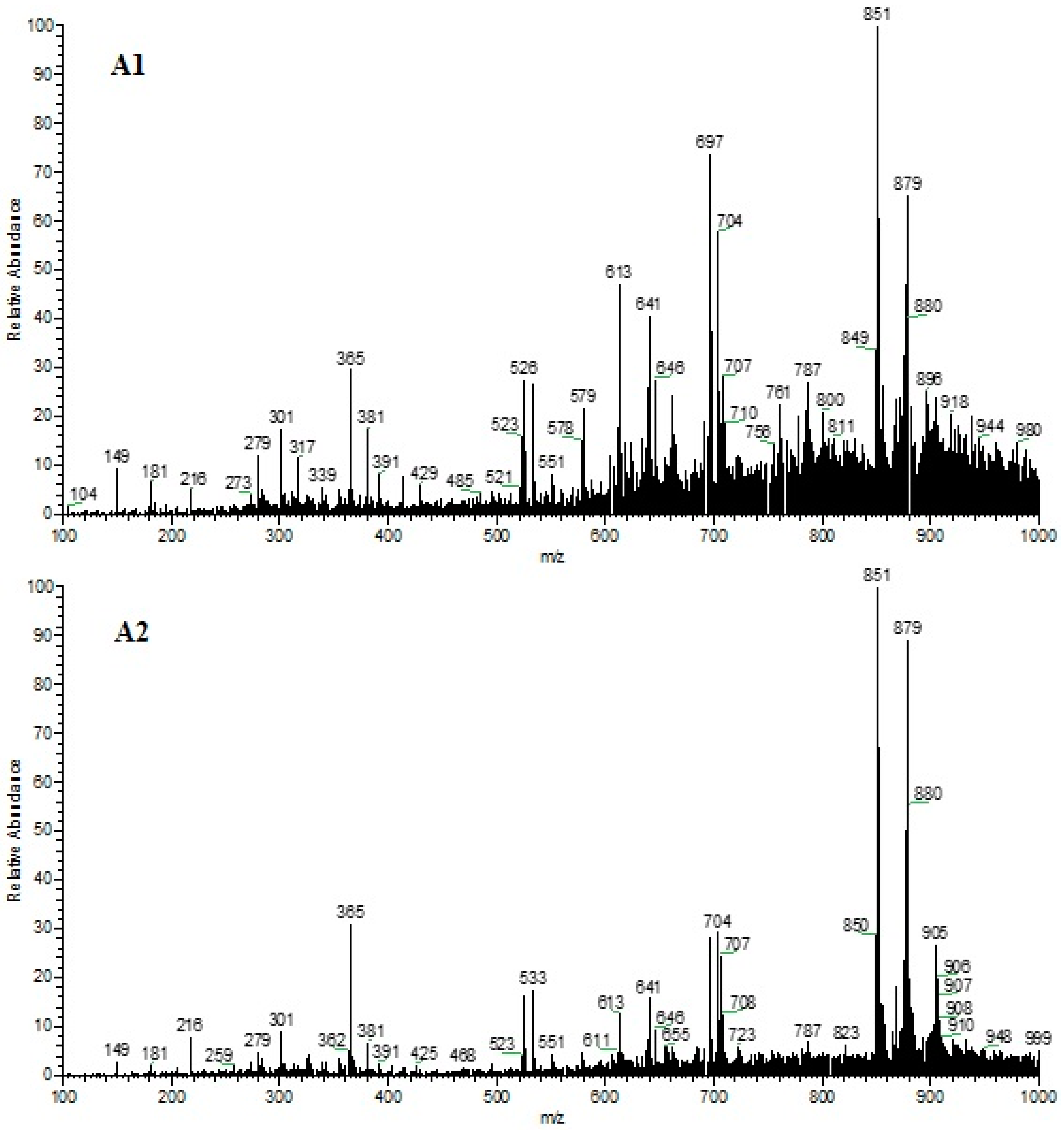
3.2. Paper Spray Mass Spectrometry Analysis
4. Discussion
4.1. Volatile Organic Compound Profile
4.2. Paper Spray Mass Spectrometry Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, A.C. Consumos e Benefícios Do Cacau. Rev. Biodiversidade 2022, 21, 89. [Google Scholar]
- Caligiani, A.; Marseglia, A.; Prandi, B.; Palla, G.; Sforza, S. Influence of Fermentation Level and Geographical Origin on Cocoa Bean Oligopeptide Pattern. Food Chem. 2016, 211, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Lopes, U.V.; Monteiro, W.R.; Pires, J.L.; Clement, D.; Yamada, M.M.; Gramacho, K.P. Cacao Breeding in Bahia, Brazil: Strategies and Results. Crop Breed. Appl. Biotechnol. 2011, 11, 73–81. [Google Scholar] [CrossRef]
- De Oliveira Júnior, A.H.; Ramos, A.L.C.C.; Guedes, M.N.S.; Fagundes, M.C.P.; Augusti, R.; Melo, J.O.F. Chemical Profile and Bioprospecting of Cocoa Beans Analyzed by Paper Spray Mass Spectrometry. Res. Soc. Dev. 2020, 9, e975986882. [Google Scholar] [CrossRef]
- Soares, T.F.; Oliveira, M.B.P.P. Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects. Molecules 2022, 27, 1625. [Google Scholar] [CrossRef]
- Cerri, M.; Reale, L.; Zadra, C. Metabolite Storage in Theobroma cacao L. Seed: Cyto-Histological and Phytochemical Analyses. Front. Plant Sci. 2019, 10, 1599. [Google Scholar] [CrossRef]
- Tennhardt, L.; Lazzarini, G.; Weisshaidinger, R.; Schader, C. Do Environmentally-Friendly Cocoa Farms Yield Social and Economic Co-Benefits? Ecol. Econ. 2022, 197, 107428. [Google Scholar] [CrossRef]
- Da Silva Freitas, R.V.; da Silva, F.L.H.; de Assis Cavalcante, J.; de Souza Costa, I.I.; Sarmento, D.H.A.; Braga, R.C.; da Silva, F.S.; Barbosa, M.C.F.; de Almeida Rodrigues, E. Avaliação Da Composição Nutricional, Caracterização e Correlação Dos Parâmetros de Qualidade Da Polpa Do Cacau. Res. Soc. Dev. 2022, 11, e52511326677. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Zhu, F. Changes in the Composition of Methylxanthines, Polyphenols, and Volatiles and Sensory Profiles of Cocoa Beans from the Sul 1 Genotype Affected by Fermentation. J. Agric. Food Chem. 2020, 68, 8658–8675. [Google Scholar] [CrossRef]
- Borges, E.M.E.S.; da Silva, F.L.H.; de Oliveira Ferreira, A.L.; de Medeiros, L.L. Cocoa Pulp (Theobroma cacao L.) as a Substrate in the Preparation of Potentially Probiotic. Res. Soc. Dev. 2021, 10, e01101119002. [Google Scholar] [CrossRef]
- Martins, L.M.; de Santana, L.R.R.; Maciel, L.F.; Soares, S.E.; Ferreira, A.C.R.; Biasoto, A.C.T.; da Silva Bispo, E. Phenolic Compounds, Methylxanthines, and Preference Drivers of Dark Chocolate Made with Hybrid Cocoa Beans. Res. Soc. Dev. 2023, 12, e22912440782. [Google Scholar] [CrossRef]
- Salles, B.P.A.; David, A.M.S.S.; Figueiredo, J.C.; Maia, V.M.; dos Santos Prudêncio, J.R.; Pereira, K.K.G. Viabilidade de Sementes de Cacau e Limitações No Armazenamento. Rev. Ciências Agrárias 2019, 42, 1010–1014. [Google Scholar] [CrossRef]
- Paixão, M.V.S.; Demuner, F.M.; de Sousa Rodrigues, P.; de Faria Junior, H.P.; Bozetti, M. Pre Germinating Treatments on Germination of Cocoa Seeds. Int. J. Adv. Eng. Res. Sci. 2019, 6, 130–134. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento. Ceplac—Cultivares. Available online: https://www.gov.br/agricultura/pt-br/assuntos/ceplac/publicacoes/cultivares/bannerclones-def2.pdf/view (accessed on 16 February 2023).
- Afoakwa, E.; Ofosu-Ansah, E.; Budu, A.; Mensah-Brown, H.; Takrama, J. Changes in Some Biochemical Qualities during Drying of Pulp Pre-Conditioned and Fermented Cocoa (Theobroma cacao) Beans. African J. Food, Agric. Nutr. Dev. 2015, 15, 9651–9670. [Google Scholar] [CrossRef]
- Kitani, Y.; Putri, S.P.; Fukusaki, E. Investigation of the Effect of Processing on the Component Changes of Single-Origin Chocolate during the Bean-to-Bar Process. J. Biosci. Bioeng. 2022, 134, 138–143. [Google Scholar] [CrossRef]
- Beegum, P.P.S.; Pandiselvam, R.; Ramesh, S.V.; Sugatha, P.; Nooh, A.; Neenu, S.; Gupta, A.; Varghese, E.; Balasubramanian, D.; Apshara, E.S.; et al. Sensorial, Textural, and Nutritional Attributes of Coconut Sugar and Cocoa Solids Based “Bean-to-bar” Dark Chocolate. J. Texture Stud. 2022, 53, 870–882. [Google Scholar] [CrossRef]
- Giller, M. Bean-to-Bar Chocolate: America’s Craft Chocolate Revolution: The Origins, the Makers, and the Mind-Blowing Flavors, 1st ed.; Storey Publishing, LLC: Hachette, UK, 2017; ISBN 978-1612128214. [Google Scholar]
- Castro, M.C.; Silvello, G.C.; Corniani, L.S.; Acevedo, M.S.M.S.F.; de Andrade Marcondes Pereira, A.; Alcarde, A.R. Maturation-Related Phenolic Compounds in Cachaça Aged in Oak Barrels: Influence of Reuses. Wood Sci. Technol. 2023, 57, 781–795. [Google Scholar] [CrossRef]
- Cemin, P.; Reis Ribeiro, S.; de Candido de Oliveira, F.; Leal Leães, F.; Regina dos Santos Nunes, M.; Wagner, R.; Sant’Anna, V. Chocolates with Brazilian Cocoa: Tracking Volatile Compounds According to Consumers’ Preference. Food Res. Int. 2022, 159, 111618. [Google Scholar] [CrossRef]
- Bickel Haase, T.; Schweiggert-Weisz, U.; Ortner, E.; Zorn, H.; Naumann, S. Aroma Properties of Cocoa Fruit Pulp from Different Origins. Molecules 2021, 26, 7618. [Google Scholar] [CrossRef]
- Rottiers, H.; Tzompa Sosa, D.A.; De Winne, A.; Ruales, J.; De Clippeleer, J.; De Leersnyder, I.; De Wever, J.; Everaert, H.; Messens, K.; Dewettinck, K. Dynamics of Volatile Compounds and Flavor Precursors during Spontaneous Fermentation of Fine Flavor Trinitario Cocoa Beans. Eur. Food Res. Technol. 2019, 245, 1917–1937. [Google Scholar] [CrossRef]
- Mariano, A.P.X.; Ramos, A.L.C.C.; de Oliveira Júnior, A.H.; García, Y.M.; de Paula, A.C.C.F.F.; Silva, M.R.; Augusti, R.; de Araújo, R.L.B.; Melo, J.O.F. Optimization of Extraction Conditions and Characterization of Volatile Organic Compounds of Eugenia klotzschiana O. Berg Fruit Pulp. Molecules 2022, 27, 935. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, M.C.; Tarazona-Díaz, M.P.; Meneses-Marentes, N.A.; González-Sarmiento, G.; Pineda-Guerrero, A.S.; Gómez-Uribe, G.E. Development of Color Guides to Evaluate the Maturity of Cacao Clones by Digital Image Processing. Pesqui. Agropecuária Trop. 2021, 51, e69621. [Google Scholar] [CrossRef]
- Da Rocha, R.F.J.; da Silva Araújo, Í.M.; de Freitas, S.M.; dos Santos Garruti, D. Optimization of Headspace Solid Phase Micro-Extraction of Volatile Compounds from Papaya Fruit Assisted by GC–Olfactometry. J. Food Sci. Technol. 2017, 54, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
- Montagna, M.T.; Diella, G.; Triggiano, F.; Caponio, G.R.; De Giglio, O.; Caggiano, G.; Di Ciaula, A.; Portincasa, P. Chocolate, “Food of the Gods”: History, Science, and Human Health. Int. J. Environ. Res. Public Health 2019, 16, 4960. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.; Meyer-Gerspach, A.C.; Wendebourg, M.J.; Gruber, M.; Heinrich, H.; Sauter, M.; Woelnerhanssen, B.; Koeberle, D.; Juengling, F. Effect of Cocoa on the Brain and Gut in Healthy Subjects: A Randomised Controlled Trial. Br. J. Nutr. 2019, 121, 654–661. [Google Scholar] [CrossRef]
- Herrera-Rocha, F.; Fernández-Niño, M.; Cala, M.P.; Duitama, J.; Barrios, A.F.G. Omics Approaches to Understand Cocoa Processing and Chocolate Flavor Development: A Review. Food Res. Int. 2023, 165, 112555. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, B.; Derewiaka, D.; Lenart, A.; Kowalska, J. Changes in the Composition and Content of Polyphenols in Chocolate Resulting from Pre-Treatment Method of Cocoa Beans and Technological Process. Eur. Food Res. Technol. 2019, 245, 2101–2112. [Google Scholar] [CrossRef]
- Ramos, A.L.C.C.; Mendes, D.D.; Silva, M.R.; Augusti, R.; Melo, J.O.F.; de Araújo, R.L.B.; Lacerda, I.C.A. Chemical Profile of Eugenia brasiliensis (Grumixama) Pulp by PS/MS Paper Spray and SPME-GC/MS Solid-Phase Microextraction. Res. Soc. Dev. 2020, 9, e318974008. [Google Scholar] [CrossRef]
- Correia, V.T.V.; Silva, V.D.M.; Mendonça, H.O.P.; Ramos, A.L.C.C.; Silva, M.R.; Augusti, R.; de Paula, A.C.C.F.F.; Ferreira, R.M.S.B.; Melo, J.O.F.; Fante, C.A. Efficiency of Different Solvents in the Extraction of Bioactive Compounds from Plinia cauliflora and Syzygium cumini Fruits as Evaluated by Paper Spray Mass Spectrometry. Molecules 2023, 28, 2359. [Google Scholar] [CrossRef]
- Ramos, A.L.C.C.; Minighin, E.C.; Soares, I.I.C.; Ferreira, B.d.S.; de Sousa, I.M.N.; Augusti, R.; Labanca, R.A.; de Araújo, R.L.B.; Melo, J.O.F. Evaluation of the Total Phenolic Content, Antioxidative Capacity, and Chemical Fingerprint of Annona crassiflora Mart. Bioaccessible Molecules. Food Res. Int. 2023, 165, 112514. [Google Scholar] [CrossRef]
- Minighin, E.C.; de Souza, K.F.; Valenzuela, V.d.C.T.; de Oliveira Couto e Silva, N.; Anastácio, L.R.; Labanca, R.A. Effect of in Vitro Gastrointestinal Digestion on the Mineral Content, Phenolic Compounds, and Antioxidant Capacity of Commercial Pulps of Purple and White Açaí (Euterpe oleracea Mart.). J. Food Sci. Technol. 2020, 57, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, Y.G.; Bueno, F.C.; de Oliveira Júnior, A.H.; do Carmo Mazzinghy, A.C.; Mendonça, H.D.O.P.; de Oliveira, A.F.; de Melo, A.C.; Reina, L.D.C.B.; Augusti, R.; Melo, J.O.F. Profile of the Volatile Organic Compounds of Pink Pepper and Black Pepper. Sci. Electron. Arch. 2021, 14. [Google Scholar] [CrossRef]
- Ramos, A.L.C.C.; Silva, M.R.; Mendonça, H.D.O.P.; do Carmo Mazzinghy, A.C.; Silva, V.D.M.; Botelho, B.G.; Augusti, R.; Ferreira, R.M.D.S.B.; de Sousa, I.M.N.; Batista-Santos, P.; et al. Use of Pulp, Peel, and Seed of Annona crassiflora Mart. in Elaborating Extracts for Fingerprint Analysis Using Paper Spray Mass Spectrometry. Food Res. Int. 2022, 160, 111687. [Google Scholar] [CrossRef] [PubMed]
- García, Y.M.; Ramos, A.L.C.C.; de Oliveira Júnior, A.H.; de Paula, A.C.C.F.F.; de Melo, A.C.; Andrino, M.A.; Silva, M.R.; Augusti, R.; de Araújo, R.L.B.; de Lemos, E.E.P.; et al. Physicochemical Characterization and Paper Spray Mass Spectrometry Analysis of Myrciaria floribunda (H. West Ex Willd.) O. Berg Accessions. Molecules 2021, 26, 7206. [Google Scholar] [CrossRef]
- Silva, M.; Freitas, L.; Mendonça, H.; Souza, A.; Pereira, H.; Augusti, R.; Lacerda, I.; Melo, J.; Araújo, R. Determination of Chemical Profile of Eugenia dysenterica Ice Cream Using PS-MS AND HS-SPME/GC-MS. Quim. Nova 2021, 44, 129–136. [Google Scholar] [CrossRef]
- Marseglia, A.; Musci, M.; Rinaldi, M.; Palla, G.; Caligiani, A. Volatile fingerprint of unroasted and roasted cocoa beans (Theobroma cacao L.) from different geographical origins. Food Res. Int. 2020, 132, 109101. [Google Scholar] [CrossRef]
- Tuenter, E.; Delbaere, C.; De Winne, A.; Bijttebier, S.; Custers, D.; Foubert, K.; Pieters, L. Non-volatile and volatile composition of West African bulk and Ecuadorian fine-flavor cocoa liquor and chocolate. Food Res. Int. 2020, 130, 108943. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.O.; Rúales, F.H.; Perdomo, D.A.; Jimenez Mora, J.P. Avaliação do método de extração SPME-GC-MS para a análise de compostos orgânicos voláteis em licor de cacau do Nariño-Colômbia. Rev. ION 2022, 35, 103–116. [Google Scholar] [CrossRef]
- Greño, M.; Plaza, M.; Marina, M.L.; Puyana, M.C. Untargeted HPLC-MS-based metabolomics approach to reveal cocoa powder adulterations. Food Chem. 2023, 402, 134209. [Google Scholar] [CrossRef]
- Lavorgna, M.; Pacifico, S.; Nugnes, R.; Russo, C.; Orlo, E.; Piccolella, S.; Isidori, M. Theobroma cacao criollo var. Beans: Biological properties and chemical profile. Foods 2021, 10, 571. [Google Scholar] [CrossRef]
- Patras, M.A.; Milev, B.P.; Vrancken, G.; Kuhnert, N. Identification of novel cocoa flavonoids from raw fermented cocoa beans by HPLC–MSn. Food Res. Int. 2014, 63, 353–359. [Google Scholar] [CrossRef]
- Guillén-Casla, V.; Rosales-Conrado, N.; León-González, M.E.; Pérez-Arribas, L.V.; Polo-Díez, L. Determination of serotonin and its precursors in chocolate samples by capillary liquid chromatography with mass spectrometry detection. J. Chromatograp. 2012, 1232, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, H.S.; de Camargo, V.B.; Camargo, G.; Garcia, C.V.; Fuentefria, A.M.; Mendez, A.S. Ecdysteroids in Sida tuberculata R.E. Fries (Malvaceae): Chemical composition by LC-ESI-MS and selective anti-Candida krusei activity. Food Chem. 2015, 182, 193–199. [Google Scholar] [CrossRef]
- Ortega, N.; Romero, M.P.; Macià, A.; Reguant, J.; Anglès, N.; Morelló, J.R.; Motilva, M.J. Obtention and characterization of phenolic extracts from different cocoa sources. J. Agric. Food Chem. 2008, 56, 9621–9627. [Google Scholar] [CrossRef] [PubMed]
- Calderon, A.I.; Wright, B.J.; Hurst, W.J.; Van Breemen, R.B. Screening antioxidants using LC-MS: Case study with cocoa. J. Ag. Food Chem. 2009, 57, 5693–5699. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Waehrens, S.S.; Zhang, S.; Hedelund, P.I.; Petersen, M.A.; Byrne, D.V. Application of the fast sensory method ‘Rate-All-That-Apply’ in chocolate Quality Control compared with DHS-GC-MS. Int. J. Food Sci. Technol. 2016, 51, 1877–1887. [Google Scholar] [CrossRef]
- Kouassi, A.D.D.; Koné, K.M.; Assi-Clair, B.J.; Lebrun, M.; Maraval, I.; Boulanger, R.; Fontana, A.; Guehi, T.S. Effect of spontaneous fermentation location on the fingerprint of volatile compound precursors of cocoa and the sensory perceptions of the end-chocolate. J. Food Sci. Technol. 2022, 59, 4466–4478. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Tessieri, C.; Pistelli, L. From the raw seed to chocolate: Volatile profile of Blanco de Criollo in different phases of the processing chain. Microchem. J. 2017, 133, 474–479. [Google Scholar] [CrossRef]
- Chagas Junior GC, A.; Ferreira, N.R.; Andrade EH, D.A.; Nascimento LD, D.; Siqueira FC, D.; Lopes, A.S. Profile of volatile compounds of on-farm fermented and dried cocoa beans inoculated with Saccharomyces cerevisiae KY794742 and Pichia kudriavzevii KY794725. Molecules 2021, 26, 344. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Calva-Estrada, S.J.; Utrilla-Vázquez, M.; Vallejo-Cardona, A.; Roblero-Pérez, D.B.; Lugo-Cervantes, E. Thermal properties and volatile compounds profile of commercial dark-chocolates from different genotypes of cocoa beans (Theobroma cacao L.) from Latin America. Food Res. Int. 2020, 136, 109594. [Google Scholar] [CrossRef]
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Montet, D. Impacto da viragem, armazenamento da vagem e tempo de fermentação na ecologia microbiana e na composição volátil dos grãos de cacau. Food Res. Int. 2019, 119, 477–491. [Google Scholar] [CrossRef]
- Valle-Epquinalle, M.G. O processo de torrefação e o local de cultivo influenciam a impressão digital volátil do cacau crioulo do Amazonas, Peru. Agric. Sci. 2020, 11, 599–610. [Google Scholar]
- Ramos-Escudero, F.; Casimiro-Gonzales, S.; Fernández-Prior, Á.; Chávez, K.C.; Gómez-Mendoza, J.; de la Fuente-Carmelino, L.; Muñoz, A.M. Colour, fatty acids, bioactive compounds, and total antioxidant capacity in commercial cocoa beans (Theobroma cacao L.). LWT 2021, 147, 111629. [Google Scholar] [CrossRef]
- Melo, C.W.B.D.; Bandeira, M.D.J.; Maciel, L.F.; Bispo, E.D.S.; Souza, C.O.D.; Soares, S.E. Chemical composition and fatty acids profile of chocolates produced with different cocoa (Theobroma cacao L.) cultivars. Food Sci. Tec. 2020, 40, 326–333. [Google Scholar] [CrossRef]
- Nagy, K.; Tiuca, I.D. Importance of fatty acids in physiopathology of human body. In Fatty Acids; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Jokić, S.; Gagić, T.; Knez, Ž.; Šubarić, D.; Škerget, M. Separation of active compounds from food by-product (cocoa shell) using subcritical water extraction. Molecules 2018, 23, 1408. [Google Scholar] [CrossRef]
- Wang, L.; Nägele, T.; Doerfler, H.; Fragner, L.; Chaturvedi, P.; Nukarinen, E.; Weckwerth, W. System level analysis of cacao seed ripening reveals a sequential interplay of primary and secondary metabolism leading to polyphenol accumulation and preparation of stress resistance. TPJ. 2016, 87, 318–332. [Google Scholar] [CrossRef]
- Sezini, A.M.; do Coutto Gil, C.S.G. Nutrientes e depressão. Vita Sanitas 2014, 8, 39–57. [Google Scholar]
- De Barros, A.R.; Ferreira, F.C.R.G.; Ferreira, J.P.; de Azevedo Pacheco, P.M.; dos Santos Ambrosoli, S.; Pinheiro, A.M.D.S.G. Chocolate e emoções: A relação entre o consumo de chocolate teor 70% cacau e ansiedade. RECIMA 2022, 3, e3122419. [Google Scholar] [CrossRef]
- Seem, S.A.; Yuan, Y.V.; Tou, J.C. Chocolate and chocolate constituents influence bone health and osteoporosis risk. Nutrition 2019, 65, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Walker, K.M.; Dyer, K.A.; Bryan, J. Estimation of daily intake of flavonoids and major food sources in middle-aged Australian men and women. Nutr. Res. 2019, 61, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Reyes, C.N.; Aguilar-Méndez, M.Á. Continuous ultrasound and pulsed ultrasound: Selective extraction tools to obtain enriched antioxidants extracts from cocoa beans (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2022, 80, 103095. [Google Scholar] [CrossRef]
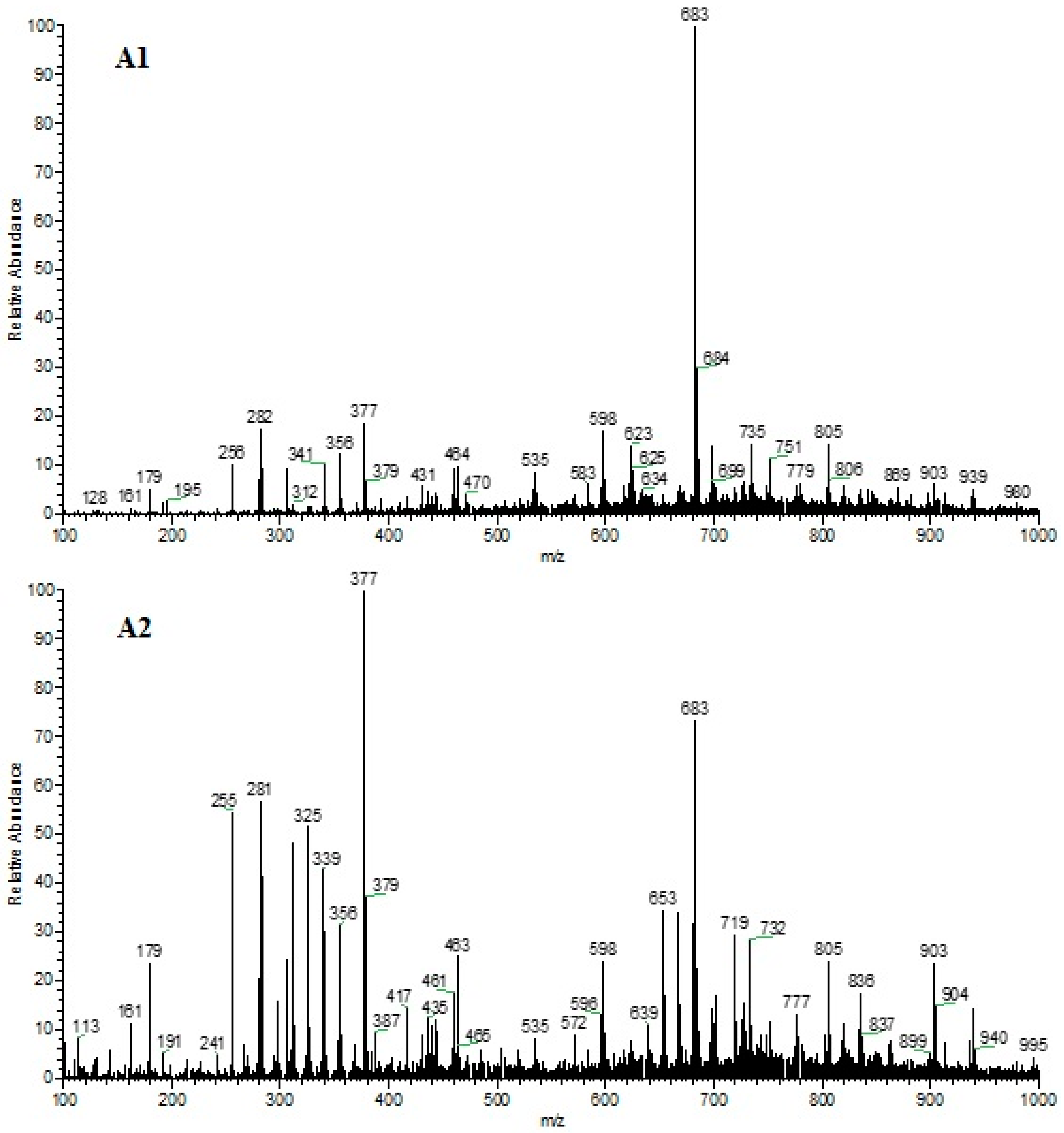
| N° | Compound | Formula | CAS | Sample 1 | (%) Area | Sample 2 | (%) Area | Reference |
|---|---|---|---|---|---|---|---|---|
| Fatty acid | ||||||||
| 1 | 9-octadecenoic acid | C18H34O2 | 112-79-8 | X | 1.72 | X | 5.70 | [38] |
| 2 | Decanoic acid | C10H20O2 | 334-48-5 | ND | X | 3.51 | [20] | |
| 3 | 3-hydroxydecanoic acid | C12H24O3 | 1883-13-2 | ND | X | 0.73 | [20] | |
| 4 | Dodecanoic acid | C12H24O2 | 143-07-7 | ND | X | 0.65 | [20] | |
| 5 | Cyclopropanetetradecanoic acid | C26H50O2 | 27198-62-5 | X | 1.19 | ND | [20] | |
| 6 | Eicosanoic acid | C20H40O2 | 506-30-9 | X | 0.79 | ND | [20] | |
| 7 | Oleic acid | C18H34O2 | 112-80-1 | X | 4.46 | ND | [20] | |
| 8 | Hexadecanoic acid ethyl ester | C18H36NO2 | 658-97-7 | ND | X | 10.61 | [20] | |
| Organic acid | ||||||||
| 9 | Acetic acid | C2H4O2 | 64-19-7 | X | 25.68 | X | 39.23 | - |
| 10 | Benzene acetic acid | C8H8O2 | 103-82-2 | X | 12.33 | X | 3.58 | - |
| 11 | Benzoic acid | C7H6O2 | 65-85-0 | X | 4.35 | X | 1.50 | - |
| 12 | 3-methyl-butanoic acid | C5H10O2 | 503-74-2 | X | 3.38 | ND | - | |
| 13 | Nonanoic acid | C9H18O2 | 112-05-0 | X | 2.24 | X | 4.43 | - |
| 14 | Phthalic acid | C8H6O4 | 88-99-3 | X | 3.03 | ND | - | |
| 15 | Propanoic acid | C3H6O2 | 79-09-4 | X | 0.77 | X | 0.68 | - |
| Alcohol | ||||||||
| 16 | 1-hexanol-2-ethyl | C8H18O | 104-76-7 | X | 0.86 | ND | [20] | |
| 17 | Butane-2,3-diol | C4H10O2 | 513-85-9 | X | 7.94 | X | 4.95 | [20] |
| 18 | 2-dodecanol | C12H26O | 10203-28-8 | X | 1.95 | ND | [20] | |
| 19 | Phenylethyl alcohol | C8H10O | 60-12-8 | X | 1.84 | X | 1.00 | [20] |
| Aldehyde | ||||||||
| 20 | 5-methyl-2-phenyl-2-hexenal | C13H16O | 21834-92-4 | ND | X | 0.61 | [38] | |
| 21 | Benzene acetaldehyde | C8H8O | 122-78-1 | X | 1.19 | ND | [38] | |
| 22 | Benzaldehyde | C7H6O | 100-52-7 | ND | X | 1.52 | [38] | |
| 23 | Nonanal | C9H18O | 124-19-6 | X | 13.16 | X | 13.89 | [38] |
| 24 | 3-methyl hexanal | C7H14O | 19269-28-4 | ND | X | 0.94 | [38] | |
| Ketone | ||||||||
| 25 | Acetoin | C4H8O2 | 513-86-0 | X | 1.35 | ND | [20] | |
| 26 | Ethanone | C2H2O | X | 1.66 | X | 0.62 | [20] | |
| Benzoic acid derivatives | ||||||||
| 27 | 1-2-4-enzenetricarboxylic acid | C9H6O6 | 528-44-9 | X | 1.27 | ND | [38] | |
| 28 | 2-5-dihydroxybenzaldehyde | C7H6O3 | 1194-98-5 | X | 0.65 | ND | [38] | |
| Ester | ||||||||
| 29 | Acetic acid-2-phenylethyl-ester | C10H12O2 | 103-45-7 | X | 2.68 | X | 1.30 | [39] |
| 30 | Hexadecanoic acid methyl ester | C17H34O2 | 112-39-0 | ND | X | 0.98 | [39] | |
| Phenylpropanoid | ||||||||
| 31 | N-benzyl-2-aminociannamate | C17H17NO2 | 18429-69-1 | X | 2.20 | ND | - | |
| Pyrazine | ||||||||
| 32 | Pyrazine tetramethyl | C8H12N2 | 1124-11-4 | X | 0.91 | ND | [40] | |
| 33 | Pyrazine | C4H4N2 | 290-37-9 | ND | X | 3.58 | [40] | |
| Others | ||||||||
| 34 | 4H-pyran-4-2-3 dihydro 3,5-dihydroxy-6-methyl | C5H6O2 | 28564-83-2 | X | 1.93 | ND | - | |
| 35 | 3H-pyrazol-3-one, 2,4-dihydro-5-methyl-2-phenyl- | C10H10N2O | 89-25-8 | X | 0.48 | ND | - | |
| N° | Identification Attempt | m/z | MS/MS | ID | Sample 1 | Sample 2 | Reference |
|---|---|---|---|---|---|---|---|
| Fatty acids | |||||||
| 1 | Hydroxy octadecenedioic | 327 | 171, 211, 229 | [M–H]− | ND | X | [41] |
| Sugar | |||||||
| 2 | Disaccharide | 341 | 341, 236, 198 | [M–H]− | ND | X | [42] |
| 3 | Glucose | 179 | 161, 113, 89 | [M–H]− | ND | X | [43] |
| 4 | Hexitol | 181 | 181, 113, 101 | [M–H]− | X | ND | [42] |
| 5 | β-D-xylopyranosyl-α-L-rhamnopyranosyl-D-fucose | 441 | - | [M–H]− | ND | X | [42] |
| Amino acids and derivaties | |||||||
| 6 | l-tryptophan | 205 | 146, 188, 184 | [M+H]+ | ND | X | [44] |
| 7 | Serotonin | 149 | 132, 136, 118 | [M+H]+ | X | X | [44] |
| Benzoic acid derivatives | |||||||
| 8 | Vanillic acid diglucoside | 461 | 353, 353, 123 | [M–H]− | ND | X | [4] |
| 9 | 20-hydroxyecdysone-3-O-β-d-xylose | 579 | - | [M+H]+ | X | X | [45] |
| Phenylpropanoid | |||||||
| 10 | Caffeoyl tyrosine | 354 | 342, 298, 256 | [M–H]− | ND | X | [43] |
| 11 | Dideoxyclovamide | 342 | 147, 119, 120 | [M–H]− | ND | X | [43] |
| 12 | Epigallocatechin | 305 | 289, 151, 169 | [M–H]− | ND | X | [46,47] |
| 13 | 12-hydroxy-jasmonic acid | 225 | - | [M–H]− | ND | X | [41] |
| Flavonoids | |||||||
| 14 | Apigenin-7-O-glucoside | 578 | 577, 269 | [M–H]− | X | ND | [48] |
| 15 | Apigenin-8-C-glucoside | 432 | 431, 341, 311 | [M–H]− | ND | X | [48] |
| 16 | Dimethyl-O-EC-EC-ECG trimer | 909 | - | [M–H]− | ND | X | [48] |
| 17 | Dimethyl-O-EC-ECG dimer | 621 | - | [M–H]− | ND | X | [48] |
| 18 | Naringenin-7-O-neohesperidoside | 580 | 579, 459, 271 | [M–H]− | X | ND | [48] |
| 19 | Quercetin-3-O-arabinoside | 433 | 383, 301, 139 | [M–H]− | ND | X | [4] |
| 20 | Quercetin-3-O-galactoside | 464 | 463, 301 | [M–H]− | ND | X | [48] |
| 21 | Quercetina-3-O-β-d-glucopyranosside | 463 | 107, 121, 151 | [M–H]− | ND | X | [48] |
| Tannins and precursors | |||||||
| 22 | Procyanidin A-type pentamer arabinoside | 785 | 591, 547, 439 | [M–H]− | X | ND | [43] |
| 23 | Procyanidin 2A-type trimer | 861 | 575, 425, 289 | [M–H]− | X | X | [48] |
| 24 | Procyanidin A hexoside; | 737 | 611, 585, 539 | [M–H]− | ND | X | [48] |
| 25 | Procyanidin A pentoside | 707 | 581, 539, 449 | [M–H]− | X | X | [48] |
| 26 | Procyanidin A-type pentamer | 719 | - | [M–H]− | ND | X | [4] |
| 27 | Procyanidin A-type tetramer arabinoside | 641 | - | [M–H]− | ND | X | [48] |
| 28 | Procyanidin A-type tetramer hexoside | 656 | - | [M–H]− | ND | X | [48] |
| 29 | Procyanidin A-type trimer | 865 | 739, 713, 695 | [M–H]− | ND | X | [48] |
| 30 | Procyanidin B dimer | 577 | 425, 407, 289 | [M–H]− | ND | X | [48] |
| 31 | Procyanidin A-type hexamer | 864 | - | [M–H]+ | X | ND | [43] |
| 32 | Procyanidin A-type trimer | 863 | - | [M–H]− | X | ND | [43] |
| 33 | Dimethyl-O-procyanidin B trime | 893 | - | [M–H]− | X | ND | [4] |
| 34 | Procyanidin A-type hexamer arabinoside | 929 | 739, 713, 695 | [M–H]− | X | ND | [4] |
| 35 | Procyanidin trimer | 944 | 695, 577, 425 | [M–H]− | X | ND | [4] |
| Terpenoids | |||||||
| 36 | Soyasaponin B I | 941 | 615, 733, 879 | [M–H]− | X | X | [41] |
| 37 | Soyasaponin B II | 911 | 695, 577, 425 | [M–H]− | X | ND | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Carmo Mazzinghy, A.C.; Silva, V.D.M.; Ramos, A.L.C.C.; de Oliveira, C.P.; de Oliveira, G.B.; Augusti, R.; de Araújo, R.L.B.; Melo, J.O.F. Influence of the Different Maturation Conditions of Cocoa Beans on the Chemical Profile of Craft Chocolates. Foods 2024, 13, 1031. https://doi.org/10.3390/foods13071031
do Carmo Mazzinghy AC, Silva VDM, Ramos ALCC, de Oliveira CP, de Oliveira GB, Augusti R, de Araújo RLB, Melo JOF. Influence of the Different Maturation Conditions of Cocoa Beans on the Chemical Profile of Craft Chocolates. Foods. 2024; 13(7):1031. https://doi.org/10.3390/foods13071031
Chicago/Turabian Styledo Carmo Mazzinghy, Ana Carolina, Viviane Dias Medeiros Silva, Ana Luiza Coeli Cruz Ramos, Carla Patrícia de Oliveira, Gabriel Barbosa de Oliveira, Rodinei Augusti, Raquel Linhares Bello de Araújo, and Júlio Onésio Ferreira Melo. 2024. "Influence of the Different Maturation Conditions of Cocoa Beans on the Chemical Profile of Craft Chocolates" Foods 13, no. 7: 1031. https://doi.org/10.3390/foods13071031
APA Styledo Carmo Mazzinghy, A. C., Silva, V. D. M., Ramos, A. L. C. C., de Oliveira, C. P., de Oliveira, G. B., Augusti, R., de Araújo, R. L. B., & Melo, J. O. F. (2024). Influence of the Different Maturation Conditions of Cocoa Beans on the Chemical Profile of Craft Chocolates. Foods, 13(7), 1031. https://doi.org/10.3390/foods13071031





