Glycated Walnut Meal Peptide–Calcium Chelates: Preparation, Characterization, and Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Walnut Meal Protein Hydrolysates
2.3. Preparation and Process Optimization of WMPHs–COS
2.3.1. Preparation of WMPHs–COS
2.3.2. Single-Factor Experiment
2.3.3. Response Surface Methodology (RSM) Optimization
2.4. The Structure and Processing Characteristics of WMPHs–COS
2.4.1. SDS–PAGE
2.4.2. Amino Acid Composition
2.4.3. Circular Dichroism (CD) Spectroscopy
2.4.4. Protein Solubility
2.4.5. Emulsifying Ability and Emulsion Stability
2.5. Preparation and Process Optimization of WMPHs–COS–Ca
2.6. Structure Characterization
2.6.1. Scanning Electron Microscopy (SEM)
2.6.2. UV–Vis Spectroscopy
2.6.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.7. Stability under Different Temperatures, pH Values, and Simulated Digestion Conditions In Vitro
2.7.1. Temperature Stability
2.7.2. pH Stability
2.7.3. Simulated Digestion In Vitro
2.8. Statistical Analysis
3. Results
3.1. Preparation and Process Optimization of WMPHs–COS
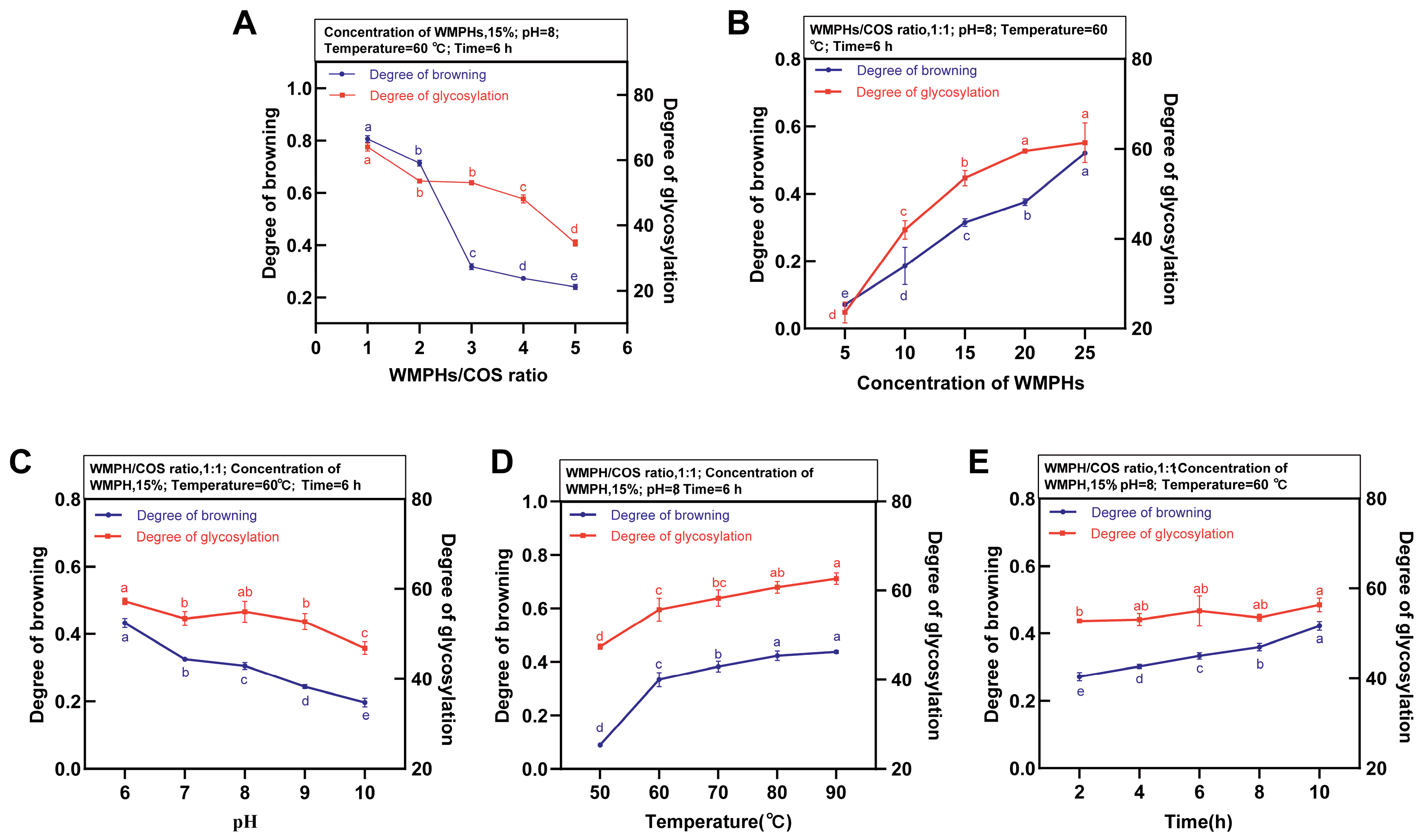
3.1.1. Optimization of the Extraction Process Parameters in the One-Factor Experiment
3.1.2. Optimization of Glycation Conditions by RSM
− 3.59AC − 7.98AD + 2.22BC − 1.64BD − 1.77CD − 4.50A2
− 4.27B2 − 4.31C2 − 10.11D2
3.2. Structure and Processing Characteristics of the WMPHs and WMPH–COSs
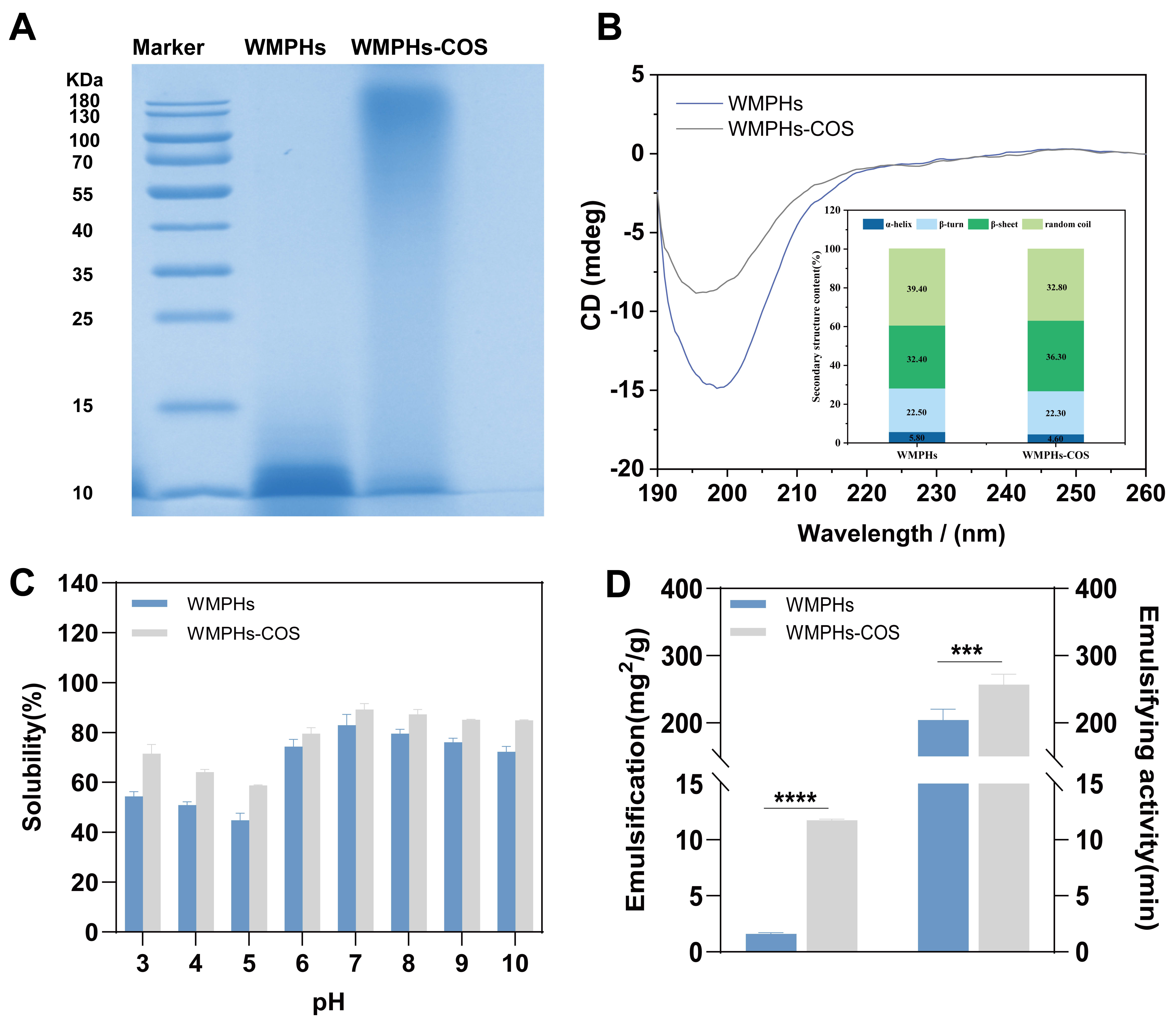
3.2.1. SDS–PAGE
3.2.2. Circular Dichroism (CD) Spectroscopy
3.2.3. Amino Acid Composition
3.2.4. Protein Solubility of the WMPHs and WMPHs–COS
3.2.5. Emulsifying Ability and Emulsion Stability of WMPHs and WMPHs–COS
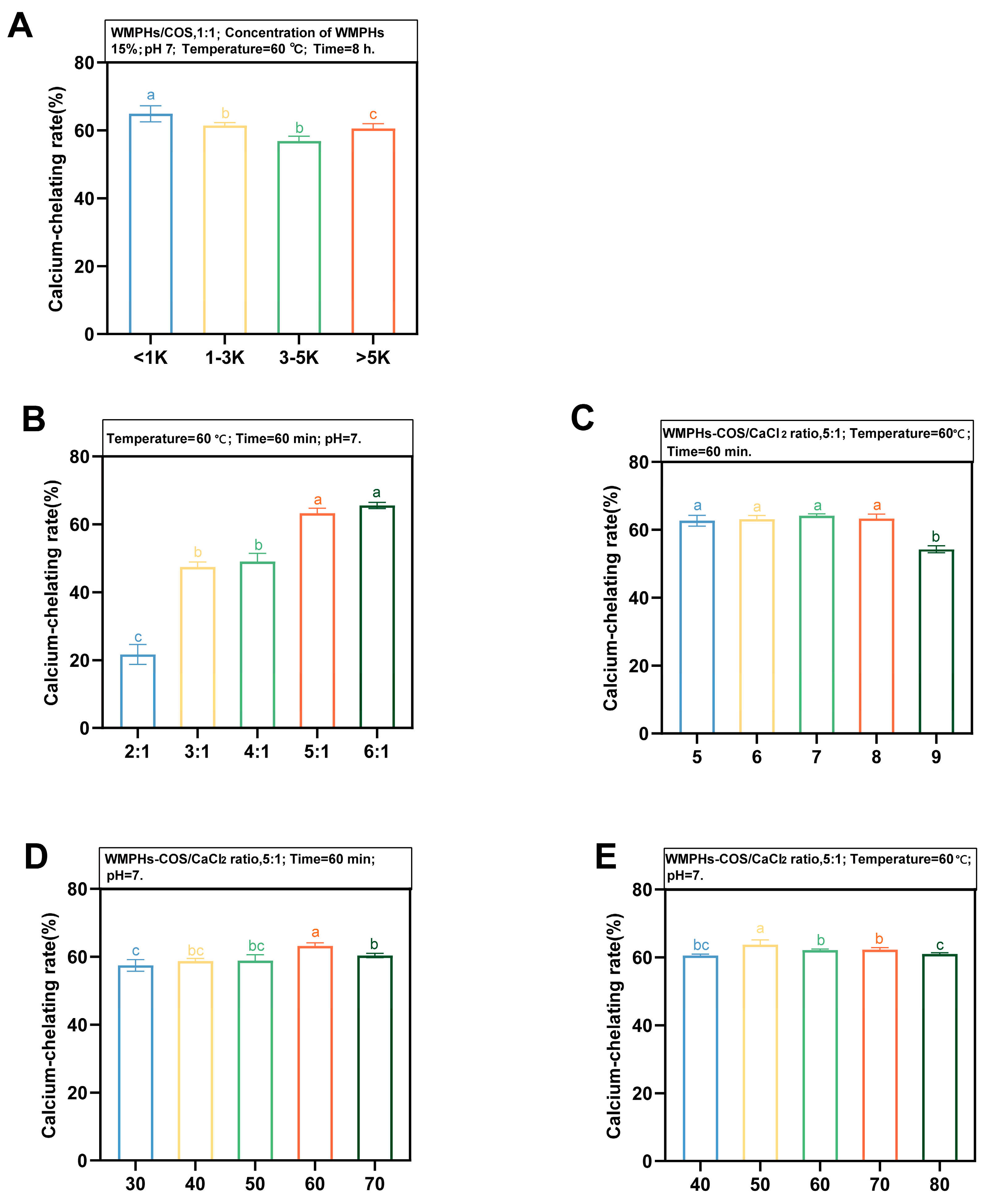
3.3. Preparation of WMPHs–COS–Ca
3.4. Structural Characterization
3.4.1. UV–Vis Spectroscopy
3.4.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.4.3. Scanning Electron Microscopy (SEM)
3.5. Stability under Different Temperatures, pH Values, and Simulated Digestion Conditions In Vitro
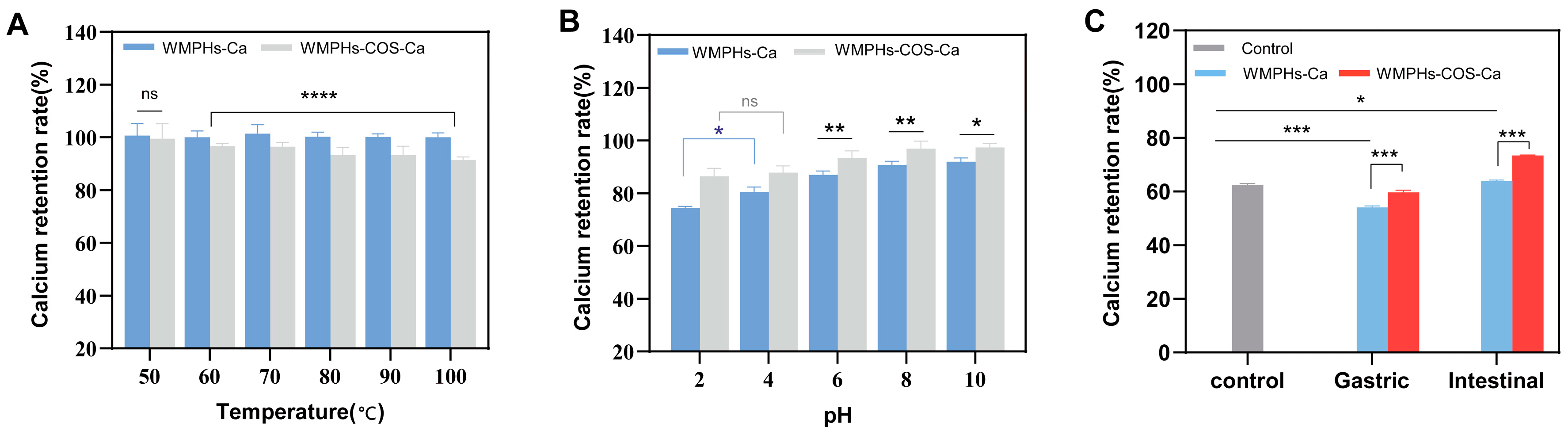
3.5.1. Temperature Stability
3.5.2. pH Stability
3.5.3. Simulated Digestion In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, X.; Wang, W.; Zheng, C.; Liu, C.; Huang, Y.; Zhao, W.; Du, J. Quality Evaluation of Walnuts from Different Regions in China. Foods 2023, 12, 4123. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, M.V.; Tsantili, E. Oil composition in stored walnut cultivars—Quality and nutritional value. J. Eur. J. Lipid Sci. Technol. 2015, 117, 338–348. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, M.; Lin, L.; Wang, J.; Sun-Waterhouse, D.; Dong, Y.; Zhuang, M.; Su, G. Identification of antioxidative peptides from defatted walnut meal hydrolysate with potential for improving learning and memory. Food Res. Int. 2015, 78, 216–223. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Liu, C.; Fang, L.; Min, W. Protein hydrolyzates from Changbai mountain Walnut (Juglans mandshurica Maxim.) boost mouse immune system and exhibit immunoregulatory activities. Evid.-Based Complement. Altern. Med. 2018, 2018, 4576561. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.; Tao, G.; Chen, J.; Zheng, Z. Investigating the inhibitory activity and mechanism differences between norartocarpetin and luteolin for tyrosinase: A combinatory kinetic study and computational simulation analysis. Food Chem. 2017, 223, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Hou, H.; Zhang, K.; Li, B. Effect of calcium-binding peptide from Pacific cod (Gadus macrocephalus) bone on calcium bioavailability in rats. Food Chem. 2017, 221, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.W.; Abrams, S.A.; Belizán, J.M.; Boy, E.; Cormick, G.; Quijano, C.D.; Gibson, S.; Gomes, F.; Hofmeyr, G.J.; Humphrey, J.; et al. Interventions to improve calcium intake through foods in populations with low intake. Ann. New York Acad. Sci. 2022, 1511, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, R. Vitamin D and the dual processes of intestinal calcium absorption. J. Nutr. 2004, 134, 3137–3139. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mu, X.; Huang, H.; Nie, R.; Liu, Z.; Zeng, M. Isolation of a calcium-binding peptide from tilapia scale protein hydrolysate and its calcium bioavailability in rats. J. Funct. Foods 2014, 6, 575–584. [Google Scholar] [CrossRef]
- Sun, N.; Wu, H.; Du, M.; Tang, Y.; Liu, H.; Fu, Y.; Zhu, B. Food protein-derived calcium chelating peptides: A review. Trends Food Sci. Technol. 2016, 58, 140–148. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, X.; Hao, G.; Zhang, M.; Weng, W. Preparation and bioavailability of calcium-chelating peptide complex from tilapia skin hydrolysates. J. Sci. Food Agric. 2017, 97, 4898–4903. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Xu, H.; Li, X.; Hao, X. Preparation of sheep bone collagen peptide–calcium chelate using enzymolysis-fermentation methodology and its structural characterization and stability analysis. RSC Adv. 2020, 10, 11624–11633. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, H.; Tian, L.; Shi, C.; Zheng, Y.; Wang, J.; Tan, Y.; Luo, Y.; Hong, H. Gut microbiota and metabolic profile as affected by Maillard reaction products derived from bighead carp meat hydrolysates with galactose and galacto-oligosaccharides during in vitro pig fecal fermentation. Food Chem. 2023, 398, 133905. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.C.; dos Reis Coimbra, J.S.; de Oliveira, E.B.; Giraldo Zuñiga, A.D.; Garcia Rojas, E.E. Food protein-polysaccharide conjugates obtained via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Tang, M.; Zhao, N.; Dong, S.; Wu, H. Effects of fish protein with glycation extent on gut microbiota and colonic barrier function in mice fed a high-fat diet. J. Funct. Foods 2021, 85, 104636. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; Gao, Y.; McClements, D.J. Food-grade covalent complexes and their application as nutraceutical delivery systems: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wen, B.; Xie, H.; Zhang, C.; Bai, Y.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Advances in the preparation and assessment of the biological activities of chitosan oligosaccharides with different structural characteristics. Food Funct. 2021, 12, 926–951. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Gu, F.; Liu, Y.; Yang, M.; Wu, X.; Sheng, J.; Tian, Y. Preparation of antioxidant peptides from Moringa oleifera leaves and their protection against oxidative damage in HepG2 cells. Front. Nutr. 2022, 9, 1062671. [Google Scholar] [CrossRef]
- Zhao, N.; Hu, J.; Hou, T.; Ma, Z.; Wang, C.; He, H. Effects of desalted duck egg white peptides and their products on calcium absorption in rats. J. Funct. Foods 2014, 8, 234–242. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, L.; Wu, W.; Wang, Y. Biological activities and physicochemical properties of Maillard reaction products in sugar–bovine casein peptide model systems. Food Chem. 2013, 141, 3837–3845. [Google Scholar] [CrossRef]
- Ajandouz, E.; Tchiakpe, L.; Ore, F.; Benajiba, A.; Puigserver, A. Effects of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Hung, B.P.; Harvestine, J.N.; Saiz, A.M.; Gonzalez-Fernandez, T.; Sahar, D.E.; Weiss, M.L.; Leach, J.K. Defining hydrogel properties to instruct lineage-and cell-specific mesenchymal differentiation. Biomaterials 2019, 189, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Bi, C.; Ding, M.; Xie, J.; Xu, C.; Qiao, R.; Wang, X.; Zhong, J. Polymorphism and stability of nanostructures of three types of collagens from bovine flexor tendon, rat tail, and tilapia skin. Food Hydrocoll. 2019, 93, 253–260. [Google Scholar] [CrossRef]
- Rodríguez-Ambriz, S.; Martínez-Ayala, A.L.; Millán, F.; Dávila-Ortíz, G. Composition and functional properties of Lupinus campestris protein isolates. Plant Foods Hum. Nutr. 2005, 60, 99–107. [Google Scholar] [CrossRef]
- Molina, E.; Papadopoulou, A.; Ledward, D. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- Liao, W.; Liu, S.; Liu, X.; Duan, S.; Xiao, S.; Yang, Z.; Cao, Y.; Miao, J. The purification, identification and bioactivity study of a novel calcium-binding peptide from casein hydrolysate. Food Funct. 2019, 10, 7724–7732. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, L.; Liang, Y.; Yue, L.; Peng, W.; Jin, G.; Ma, M. Preparation process optimization of pig bone collagen peptide-calcium chelate using response surface methodology and its structural characterization and stability analysis. Food Chem. 2019, 284, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, A.; Tang, Y.; Li, D.; Yang, P.; Cheng, H. Bound phenolics release from dried bamboo shoots prepared by different processes during in vitro gastrointestinal digestion: Bioaccessibility and bioactivity. Int. J. Food Sci. Technol. 2022, 57, 7047–7056. [Google Scholar] [CrossRef]
- Zha, F.; Wei, B.; Chen, S.; Dong, S.; Zeng, M.; Liu, Z. The Maillard reaction of a shrimp by-product protein hydrolysate: Chemical changes and inhibiting effects of reactive oxygen species in human HepG2 cells. Food Funct. 2015, 6, 1919–1927. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Wei, X.; Liu, F.; Xu, Z.; Chen, M. Synergistic effects of pH, temperature and glycosylation on the functional properties of rice protein. Int. J. Food Sci. Technol. 2021, 56, 5286–5295. [Google Scholar] [CrossRef]
- Saha, B.C.; Zeikus, J. Novel highly thermostable pullulanase from thermophiles. Trends Biotechnol. 1989, 7, 234–239. [Google Scholar] [CrossRef]
- Riciputi, Y.; Diaz-de-Cerio, E.; Akyol, H.; Capanoglu, E.; Cerretani, L.; Caboni, M.F.; Verardo, V. Establishment of ultrasound-assisted extraction of phenolic compounds from industrial potato by-products using response surface methodology. Food Chem. 2018, 269, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, F.; Tan, J.; Faudzi, S.M.M.; Rahman, M.B.A.; Ashari, S.E. Ultrasound-assisted extraction conditions optimisation using response surface methodology from Mitragyna speciosa (Korth.) Havil leaves. Ultrason. Sonochem. 2021, 81, 105851. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Wang, Z.; Chen, L.; Zhang, Q.; Xin, N. Enzymolysis technology optimization for production of antioxidant peptides from goat milk casein. Acta Universitatis Cibiniensis. Ser. E Food Technol. 2017, 21, 51–60. [Google Scholar] [CrossRef]
- Bause, E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem. J. 1983, 209, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Cui, Q.; Yu, Z.; Wang, X.; Zhao, X. Effects of transglutaminase glycosylated soy protein isolate on its structure and interfacial properties. J. Sci. Food Agric. 2021, 101, 5097–5105. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, H.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Modification of soy protein hydrolysates by Maillard reaction: Effects of carbohydrate chain length on structural and interfacial properties. Colloids Surf. B Biointerfaces 2016, 138, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Drickamer, K.; Hendrickson, W.A. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nat. Protoc. 1992, 360, 127–134. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, W.; Xu, Z.; Wen, L.; Chen, M. Structure and functional properties of rice protein–dextran conjugates prepared by the Maillard reaction. Int. J. Food Sci. Technol. 2018, 53, 372–380. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Zhu, X.; Wang, L.; Pan, S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 2013, 51, 490–495. [Google Scholar] [CrossRef]
- Ke, C.; Li, L. Influence mechanism of polysaccharides induced Maillard reaction on plant proteins structure and functional properties: A review. Carbohydr. Polym. 2023, 302, 120430. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Ruiz, J.A.; Pacheco-Aguilar, R.; Ramírez-Suárez, J.C.; Lugo-Sánchez, M.E.; García-Orozco, K.D.; Sotelo-Mundo, R.R.; Peña-Ramos, A. Conformational changes in proteins recovered from jumbo squid (Dosidicus gigas) muscle through pH shift washing treatments. Food Chem. 2016, 196, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; McClements, D.C.; Liu, X.; Liu, F. Design principles of oil-in-water emulsions with functionalized interfaces: Mixed, multilayer, and covalent complex structures. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3159–3190. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, G. Improving method, properties and application of polysaccharide as emulsifier. Food Chem. 2022, 376, 131937. [Google Scholar] [CrossRef] [PubMed]
- Koutina, G.; Knudsen, J.C.; Anderen, U.; Skibsted, L.H. Temperature effect on calcium and phosphorus equilibria in relation to gel formation during acidification of skim milk. Int. Dairy J. 2014, 36, 65–73. [Google Scholar] [CrossRef]
- Xue, C.; You, J.; Zhang, H.; Xiong, S.; Yin, T.; Huang, Q. Capacity of myofibrillar protein to adsorb characteristic fishy-odor compounds: Effects of concentration, temperature, ionic strength, pH and yeast glucan addition. Food Chem. 2021, 363, 130304. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhao, L.; Cai, X.; Wang, S.; Huang, Y.; Hong, J.; Rao, P. Purification and characterisation of a glutamic acid-containing peptide with calcium-binding capacity from whey protein hydrolysate. J. Dairy Res. 2015, 82, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cai, X.; Tang, M.; Wang, S. Preparation and evaluation of the chelating nanocomposite fabricated with marine algae Schizochytrium sp. protein hydrolysate and calcium. J. Agric. Food Chem. 2015, 63, 9704–9714. [Google Scholar] [CrossRef]
- Armas, A.; Sonois, V.; Mothes, E.; Mazarguil, H.; Faller, P. Zinc (II) binds to the neuroprotective peptide humanin. J. Inorg. Biochem. 2006, 100, 1672–1678. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Q.; Huang, S.; Lin, J.; Wang, S.; Huang, Y.; Hong, J.; Rao, P. Novel peptide with a specific calcium-binding capacity from whey protein hydrolysate and the possible chelating mode. J. Agric. Food Chem. 2014, 62, 10274–10282. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhao, L.; Wang, S.; Rao, P. Fabrication and characterization of the nano-composite of whey protein hydrolysate chelated with calcium. Food Funct. 2015, 6, 816–823. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Lu, W.; Liu, J.; Zhang, W.; Wang, Y.; Ma, M.; Cao, X.; Guo, Y. Cell proliferation stimulation ability and osteogenic activity of low molecular weight peptides derived from bovine gelatin hydrolysates. J. Agric. Food Chem. 2020, 68, 7630–7640. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Shen, Q.; Qi, L.; Jiang, S.; Guo, Y.; Zhang, C.; Richel, A. Preparation of cattle bone collagen peptides-calcium chelate and its structural characterization and stability. LWT-Food Sci. Technol. 2021, 144, 111264. [Google Scholar] [CrossRef]
- Mohan, N.H.; Choudhury, M.; Ammayappan, L.; Pathak, P.; Chakraborty, S.; Thomas, R.; Debnath, S.; Paul, M.; Sarma, D.K. Characterization of secondary structure of pig hair fiber using fourier-transform infrared spectroscopy. J. Nat. Fibers 2022, 19, 4223–4235. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Y.; Bao, Z.; Cui, P.; Wang, S.; Lin, S. Calcium binding to herring egg phosphopeptides: Binding characteristics, conformational structure and intermolecular forces. Food Chem. 2020, 310, 125867. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Huai, H.; Hou, W.; Qi, Y.; Min, W. Purification, identification, chelation mechanism, and calcium absorption activity of a novel calcium-binding peptide from peanut (Arachis hypogaea) protein hydrolysate. J. Agric. Food Chem. 2023, 71, 11970–11981. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Tong, P.; Mao, X. Zinc-binding capacity of yak casein hydrolysate and the zinc-releasing characteristics of casein hydrolysate-zinc complexes. J. Dairy Sci. 2011, 94, 2731–2740. [Google Scholar] [CrossRef]
- Huang, M.; Liu, P.; Song, S.; Zhang, X.; Hayat, K.; Xia, S.; Jia, C.; Gu, F. Contribution of sulfur-containing compounds to the colour-inhibiting effect and improved antioxidant activity of Maillard reaction products of soybean protein hydrolysates. J. Sci. Food Agric. 2011, 91, 710–720. [Google Scholar] [CrossRef]
- Benjakul, S.; Lertittikul, W.; Bauer, F. Antioxidant activity of Maillard reaction products from a porcine plasma protein–sugar model system. Food Chem. 2005, 93, 189–196. [Google Scholar] [CrossRef]
- Kathuria, D.; Hamid; Gautam, S.; Thakur, A. Maillard reaction in different food products: Effect on product quality, human health and mitigation strategies. Food Control 2023, 153, 109911. [Google Scholar] [CrossRef]
- Peng, X.; Xu, Y.; Liu, T.; Tang, C. Molecular mechanism for improving emulsification efficiency of soy glycinin by glycation with soy soluble polysaccharide. J. Agric. Food Chem. 2018, 66, 12316–12326. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Al-Asbahy, W.M.; Afzal, M.; Arjmand, F. Synthesis, characterization and interaction studies of copper based drug with human serum albumin (HSA): Spectroscopic and molecular docking investigations. J. Photochem. Photobiol. B Biol. 2012, 114, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Chaud, M.V.; Izumi, C.; Nahaal, Z.; Shuhama, T.; de Lourdes Pires Bianchi, M.; de Freitas, O. Iron derivatives from casein hydrolysates as a potential source in the treatment of iron deficiency. J. Agric. Food Chem. 2002, 50, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Sudha, T.B.; Thanikaivelan, P.; Ashokkumar, M.; Chandrasekaran, B. Structural and thermal investigations of biomimetically grown casein–soy hybrid protein fibers. Appl. Biochem. Biotechnol. 2011, 163, 247–257. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, D. Characterization of the complex of human-like collagen with calcium. Biol. Trace Elem. Res. 2012, 145, 33–38. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, H.; Wang, S.; Wang, R.; Wang, Z. Casein phosphopeptide-calcium chelate: Preparation, calcium holding capacity and simulated digestion in vitro. Food Chem. 2023, 401, 134218. [Google Scholar] [CrossRef]


| Level | Factors | |||
|---|---|---|---|---|
| A Time (h) | B Concentration of WMPHs (mg/mL) | C pH | D Temperature (°C) | |
| −1 | 4 | 10 | 7 | 70 |
| 0 | 6 | 15 | 8 | 80 |
| 1 | 8 | 20 | 9 | 90 |
| Number | A Time (h) | B Concentration (mg/mL) | C pH | D Temperature (°C) | Y Glycation Degree (%) |
|---|---|---|---|---|---|
| 1 | 6 | 10 | 8 | 70 | 37.75 ± 0.54 |
| 2 | 6 | 10 | 7 | 80 | 50.97 ± 2.47 |
| 3 | 4 | 15 | 8 | 70 | 40.64 ± 0.59 |
| 4 | 8 | 15 | 8 | 70 | 53.31 ± 0.26 |
| 5 | 6 | 15 | 8 | 80 | 65.75 ± 1.34 |
| 6 | 6 | 20 | 8 | 70 | 49.30 ± 0.44 |
| 7 | 6 | 15 | 9 | 90 | 48.06 ± 1.16 |
| 8 | 6 | 20 | 8 | 90 | 55.37 ± 0.74 |
| 9 | 4 | 15 | 9 | 80 | 56.19 ± 0.96 |
| 10 | 4 | 15 | 8 | 90 | 56.84 ± 0.12 |
| 11 | 8 | 15 | 8 | 90 | 37.61 ± 1.54 |
| 12 | 6 | 20 | 7 | 80 | 57.48 ± 0.17 |
| 13 | 6 | 10 | 8 | 90 | 50.38 ± 0.59 |
| 14 | 4 | 15 | 7 | 80 | 47.85 ± 1.76 |
| 15 | 6 | 15 | 8 | 80 | 62.27 ± 0.13 |
| 16 | 6 | 15 | 8 | 80 | 65.12 ± 0.43 |
| 17 | 8 | 15 | 7 | 80 | 58.53 ± 0.44 |
| 18 | 4 | 10 | 8 | 80 | 56.68 ± 1.30 |
| 19 | 4 | 20 | 8 | 80 | 52.33 ± 0.34 |
| 20 | 6 | 15 | 7 | 90 | 56.29 ± 0.42 |
| 21 | 6 | 15 | 8 | 80 | 60.35 ± 2.10 |
| 22 | 8 | 15 | 9 | 80 | 52.50 ± 0.38 |
| 23 | 6 | 20 | 9 | 80 | 59.71 ± 1.01 |
| 24 | 6 | 10 | 9 | 80 | 44.34 ± 0.36 |
| 25 | 6 | 15 | 7 | 70 | 47.00 ± 1.78 |
| 26 | 6 | 15 | 8 | 80 | 59.92 ± 0.15 |
| 27 | 8 | 10 | 8 | 80 | 45.67 ± 1.83 |
| 28 | 6 | 15 | 9 | 70 | 45.86 ± 0.74 |
| 29 | 8 | 20 | 8 | 80 | 65.14 ± 2.03 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 1574.87 | 14 | 112.49 | 11.76 | <0.0001 | Significant |
| A–Time | 0.41 | 1 | 0.41 | 0.043 | 0.8392 | |
| B–Concentration of WMPHs | 238.89 | 1 | 238.89 | 24.98 | 0.0002 | |
| C–pH | 10.97 | 1 | 10.97 | 1.15 | 0.3023 | |
| D–Temperature | 78.46 | 1 | 78.46 | 8.2 | 0.0125 | |
| AB | 141.84 | 1 | 141.84 | 14.83 | 0.0018 | |
| AC | 51.59 | 1 | 51.59 | 5.39 | 0.0358 | |
| AD | 254.47 | 1 | 254.47 | 26.61 | 0.0001 | |
| BC | 19.64 | 1 | 19.64 | 2.05 | 0.1738 | |
| BD | 10.74 | 1 | 10.74 | 1.12 | 0.3072 | |
| CD | 12.55 | 1 | 12.55 | 1.31 | 0.2712 | |
| A2 | 131.49 | 1 | 131.49 | 13.75 | 0.0023 | |
| B2 | 118.45 | 1 | 118.45 | 12.38 | 0.0034 | |
| C2 | 120.76 | 1 | 120.76 | 12.63 | 0.0032 | |
| D2 | 663.14 | 1 | 663.14 | 69.33 | <0.0001 | |
| Residual | 133.9 | 14 | 9.56 | |||
| Lack of Fit | 105.31 | 10 | 10.53 | 1.47 | 0.3779 | not significant |
| Pure Error | 28.59 | 4 | 7.15 | |||
| Cor Total | 1708.77 | 28 |
| Amino Acid | Relative Contents | |
|---|---|---|
| WMPHs | WMPHs–COS | |
| Asp | 8.11 ± 0.16 | 6.04 ± 0.16 |
| Thr | 2.52 ± 0.32 | 1.82 ± 0.19 |
| Ser | 4.53 ± 0.15 | 3.17 ± 0.06 |
| Glu | 17.82 ± 0.93 | 13.47 ± 0.98 |
| Gly | 3.63 ± 0.65 | 4.00 ± 0.12 |
| Ala | 3.01 ± 0.01 | 2.90 ± 0.13 |
| Cys | 0.47 ± 0.01 | 0.65 ± 0.08 |
| Val | 5.59 ± 0.53 | 5.91 ± 0.28 |
| Met | 1.86 ± 0.06 | 4.31 ± 0.41 |
| Ile | 3.27 ± 0.32 | 3.79 ± 0.57 |
| Leu | 6.78 ± 0.26 | 4.83 ± 0.81 |
| Tyr | 3.34 ± 0.46 | 0.18 ± 0.01 |
| Phe | 4.31 ± 0.59 | 24.61 ± 0.59 |
| His | 2.05 ± 0.05 | 1.75 ± 0.28 |
| Lys | 2.03 ± 0.01 | 1.57 ± 0.27 |
| Arg | 2.95 ± 0.07 | 1.89 ± 0.44 |
| Pro | 8.19 ± 0.24 | 6.07 ± 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhao, Y.; Yang, M.; Wang, Y.; Wang, Y.; Shi, C.; Dai, T.; Wang, Y.; Tao, L.; Tian, Y. Glycated Walnut Meal Peptide–Calcium Chelates: Preparation, Characterization, and Stability. Foods 2024, 13, 1109. https://doi.org/10.3390/foods13071109
Wang Z, Zhao Y, Yang M, Wang Y, Wang Y, Shi C, Dai T, Wang Y, Tao L, Tian Y. Glycated Walnut Meal Peptide–Calcium Chelates: Preparation, Characterization, and Stability. Foods. 2024; 13(7):1109. https://doi.org/10.3390/foods13071109
Chicago/Turabian StyleWang, Zilin, Ye Zhao, Min Yang, Yuanli Wang, Yue Wang, Chongying Shi, Tianyi Dai, Yifan Wang, Liang Tao, and Yang Tian. 2024. "Glycated Walnut Meal Peptide–Calcium Chelates: Preparation, Characterization, and Stability" Foods 13, no. 7: 1109. https://doi.org/10.3390/foods13071109
APA StyleWang, Z., Zhao, Y., Yang, M., Wang, Y., Wang, Y., Shi, C., Dai, T., Wang, Y., Tao, L., & Tian, Y. (2024). Glycated Walnut Meal Peptide–Calcium Chelates: Preparation, Characterization, and Stability. Foods, 13(7), 1109. https://doi.org/10.3390/foods13071109