Intelligent Rapid Detection Techniques for Low-Content Components in Fruits and Vegetables: A Comprehensive Review
Abstract
1. Introduction
2. Low-Content Components in Fruits and Vegetables
2.1. Nutrients in Fruits and Vegetables
2.2. Ingredients Required for the Growth of Fruits and Vegetables
2.3. Toxic Residues in Fruits and Vegetables
3. Rapid and Intelligent Detection Technology for Low-Content Components
3.1. Near-Infrared Spectroscopy (NIR)
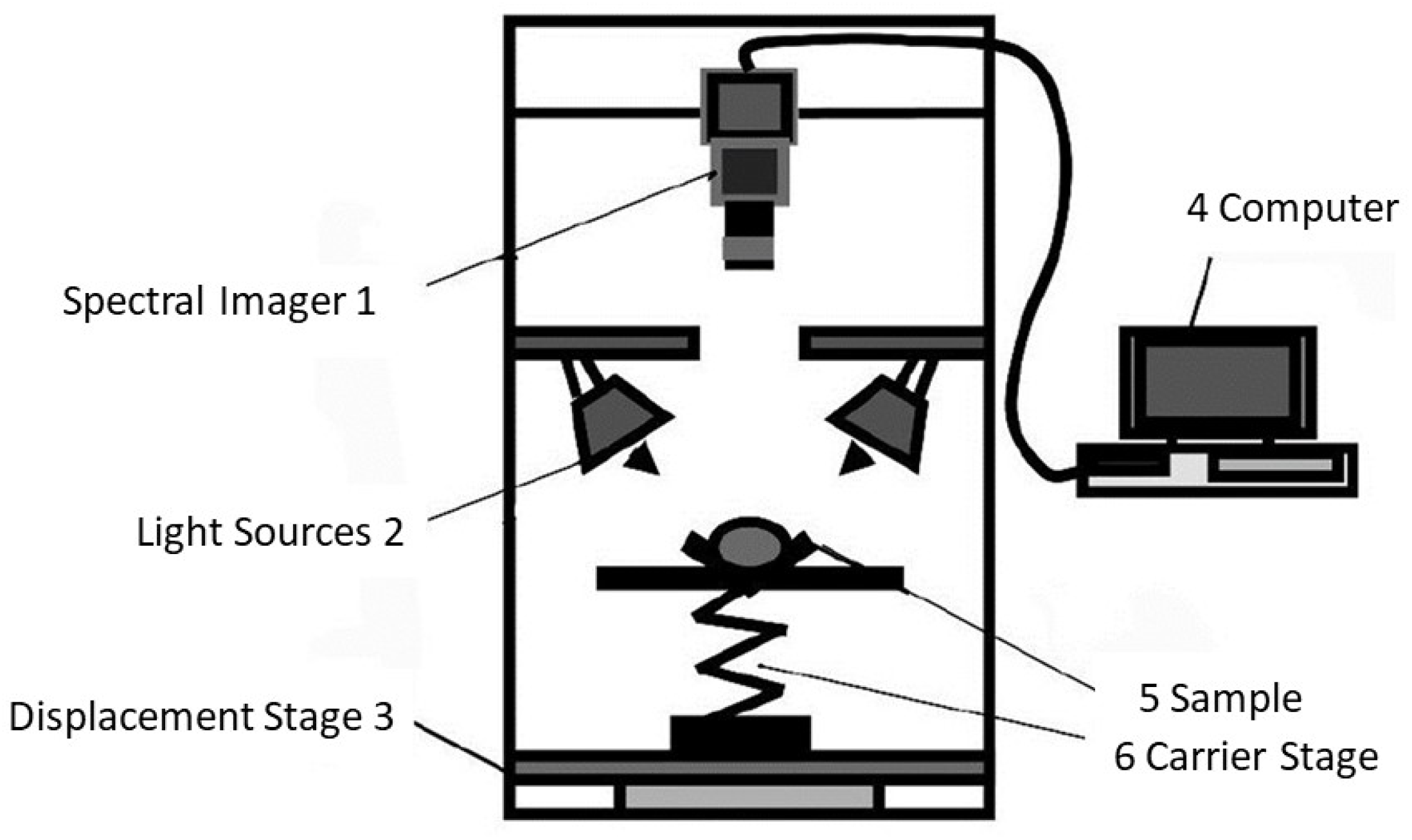
3.2. Hyperspectral Imaging (HSI)
3.3. Raman Spectroscopy
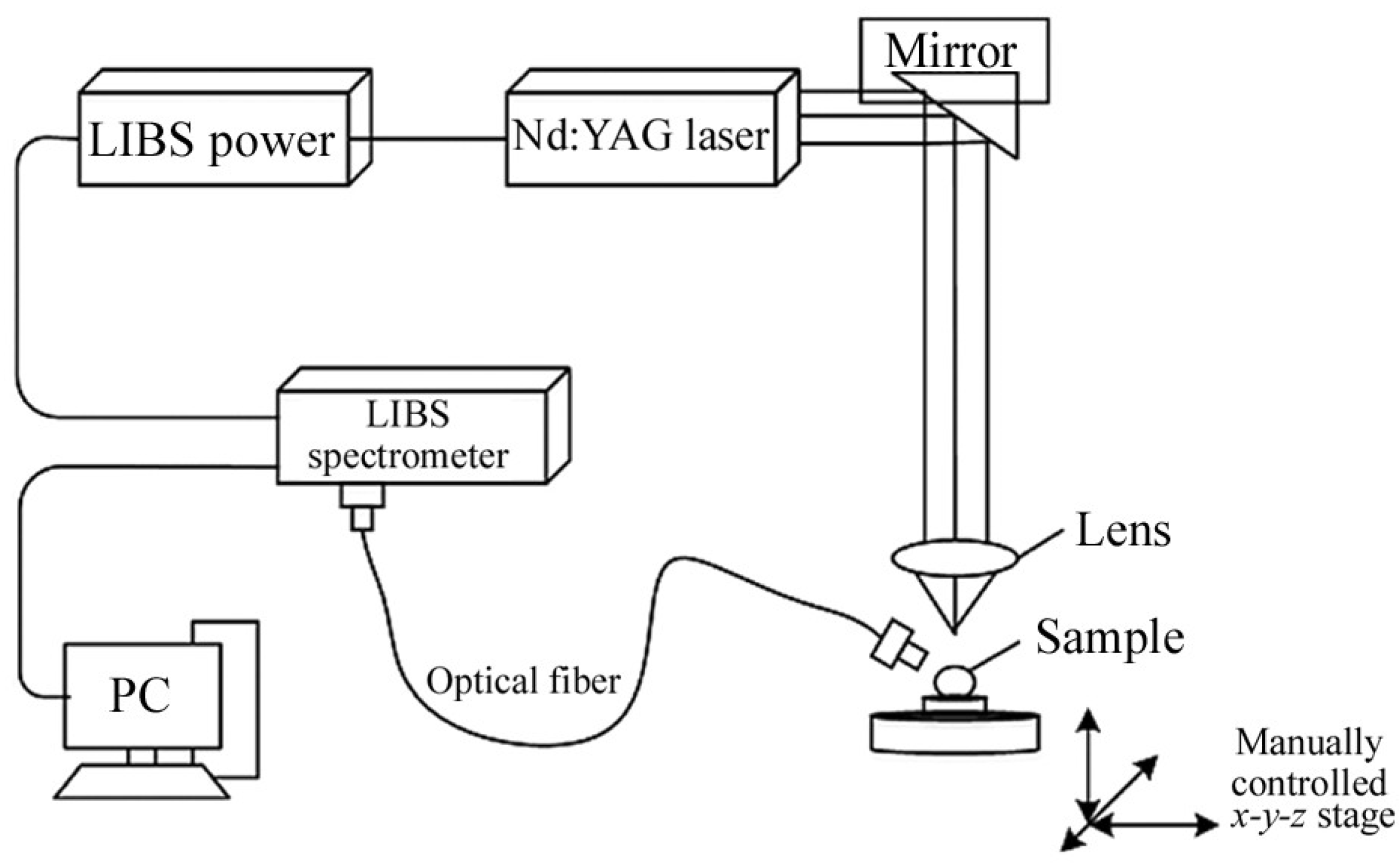
3.4. Laser Induced Breakdown Spectroscopy (LIBS)
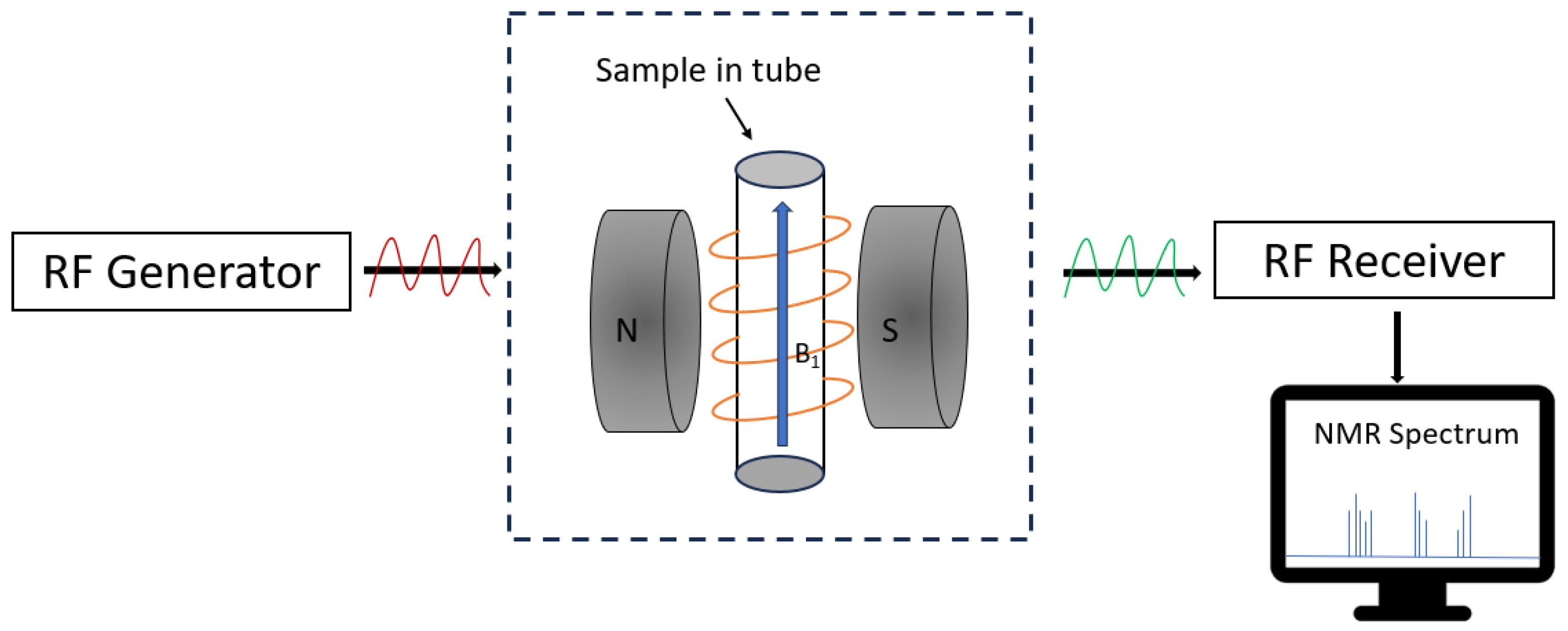
3.5. Nuclear Magnetic Resonance (NMR)
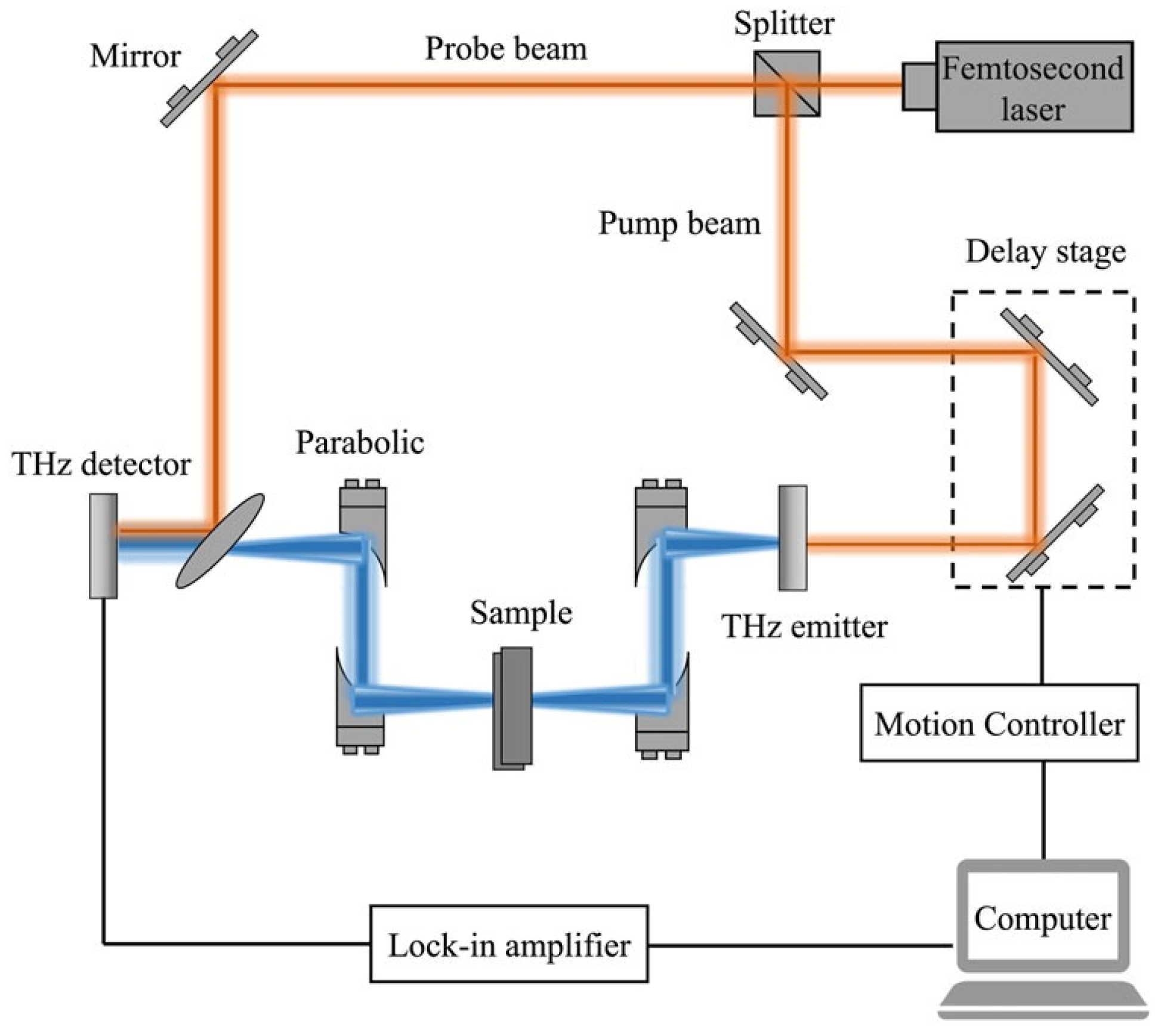
3.6. Terahertz Spectroscopy (THz)
4. Application of Rapid and Intelligent Detection Technology
4.1. Near-Infrared Spectroscopy (NIR)
4.1.1. Health-Promoting Components
4.1.2. Harmful Components
4.1.3. Components Required for Fruit and Vegetable Growth
4.2. Hyperspectral Imaging (HSI)
4.2.1. Health-Promoting Components
4.2.2. Chlorophyll
4.2.3. Heavy Metal
4.3. Raman Spectroscopy
4.3.1. Mycotoxin
4.3.2. Pesticide Residue
4.4. Laser-Induced Breakdown Spectroscopy (LIBS)
4.4.1. Health-Promoting Components
4.4.2. Pesticide Residue
4.4.3. Heavy Metal
4.5. Nuclear Magnetic Resonance (NMR)
4.6. Terahertz Spectroscopy (THz)
5. Conclusions
5.1. Existing Problems
- Sensor stability problems are hindering the accurate detection of low-content components in fruits and vegetables. Despite being crucial components of detection devices, sensors face challenges relating to their stability. Firstly, environmental factors significantly affect sensor performance. For instance, temperature fluctuations lead to temperature drift errors, resulting in inconsistent detection results at different temperatures. Secondly, traditional sensor materials have limited response capabilities, thus impacting detection sensitivity. While using new materials can mitigate this issue, the complex preparation process and high cost associated with these materials hamper their commercial implementation. Moreover, large-scale production and utilization of these new materials pose additional obstacles. As a result, addressing these challenges requires innovative approaches to improve sensor stability and overcome the limitations of traditional materials.
- Sample storage and handling methods in the field of rapid detection technology and chemometrics lack standardization, resulting in potential discrepancies in the data obtained from the same sample in different studies. While intelligent rapid detection technology combined with chemometrics has improved detection efficiency and minimized environmental and sample interference, the results are still influenced by sample characteristics such as skin thickness, surface irregularities causing scattering, and internal moisture of fruits and vegetables. Consequently, a significant amount of extraneous information is present in the results, affecting the accuracy of the test results for fruits and vegetables that differ in origin, variety, and transportation conditions. It is crucial to explore methods to mitigate the impact of these characteristics and external conditions. Additionally, when detecting components present in low concentrations, the instrument’s sensitivity and service life can contribute to errors. Addressing this technical challenge necessitates effective improvement in the instrument’s structure to accommodate the detection of low-content components.
- Algorithmic problems arise in the field of intelligent rapid detection technology combined with chemometrics, particularly when establishing qualitative and quantitative analysis models for the rapid and intelligent detection of low-content components in fruits and vegetables. One major challenge is the reliance on a large amount of sample data to improve and optimize the model, which can lead to information overload and negatively impact detection efficiency. Thus, it is crucial to effectively mine the data in the detection results and establish optimal detection models. Moreover, the current models lack versatility for databases of different samples, necessitating the establishment of separate databases for each type of sample. This can increase the workload of the model and potentially affect its stability and repeatability. Therefore, it is necessary to address the issue of developing a versatile and stable model for fruit and vegetable composition detection, which remains a prominent concern in this field.
5.2. Prospects
- To enhance the stability of the sensor, various approaches can be considered. Firstly, it is crucial to conduct research on new sensor materials that have minimal environmental impact but can still improve sensitivity. This can significantly contribute to the overall stability of the sensor. Additionally, the implementation of multi-sensor fusion technology can further enhance system stability and improve detection accuracy. Through the collaborative effect of different sensors, the overall performance of the system can be greatly improved. To address the influence of environmental factors on the sensor, it is advisable to integrate advanced environmental compensation technology. Temperature compensation algorithms can be employed to reduce the impact of temperature variations on the sensor’s performance. This would extend its applicability across diverse environmental conditions. To ensure reliable performance, regular testing and maintenance of the sensor are necessary. This practice helps to identify and rectify any issues promptly, ensuring continued stable operation.
- To optimize the structure of testing instruments, it is crucial to focus on the core components that directly influence testing accuracy. By enhancing the sensitivity of sensors, stabilizing the signal processing system, and increasing the brightness of the light source, the detection accuracy can be significantly improved. These optimization strategies aim to minimize interference factors generated by the instrument, enhance electrical signals captured during the detection process, and ultimately achieve precise detection of low-content components in fruits and vegetables.
- Optimization of artificial intelligence algorithms: In the process of detecting fruit and vegetable ingredients, artificial intelligence algorithms play a vital role through data processing, feature variable selection, and modeling analysis. The optimization of these artificial intelligence algorithms can better reduce data errors and interference, and improve the accuracy of fruit and vegetable composition detection. The optimization of AI algorithms can start by reducing the redundant information in the data, reducing the data dimension; combining the cross-validation method to verify the model, selecting the best combination of parameters to improve the accuracy of the model; and adopting more complex neural network structure and training strategy to improve the model’s explanatory and accuracy. These optimization directions will make the algorithm process the data more efficiently, and thus improve the reliability and accuracy of fruit and vegetable composition detection.
- Utilize intelligent rapid detection technology and integrate it into various applications, such as a mobile app, to enable real-time transmission and sharing of data. This would allow users to conveniently view and share test results on their cell phones, providing a timely solution for the detection of fruits and vegetables with low ingredient content. Combining the advantages of intelligent detection technology and mobile app functionality, it offers a more convenient, intelligent, and comprehensive approach to testing the quality of produce.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolber, F.M.; Beck, K.L.; Conlon, C.A.; Kruger, M.C. Chapter Thirteen—Kiwifruit and Mineral Nutrition. In Advances in Food and Nutrition Research; Boland, M., Moughan, P.J., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 68, pp. 233–256. [Google Scholar]
- Pál, É.; Ungvári, Z.; Benyó, Z.; Várbíró, S. Role of Vitamin D Deficiency in the Pathogenesis of Cardiovascular and Cerebrovascular Diseases. Nutrients 2023, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.C.; Chang, Q.Y.; Li, H.; Liang, S.X.; Zhe, Z.; Shen, S.G.; Pang, G.F. Risk assessment of carbofuran residues in fruits and vegetables at the Chinese market: A 7-year survey. Ecotoxicol. Environ. Saf. 2022, 239, 113667. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Pan, W.J.; Liang, S.Y.; Li, N.; Zeng, L.X.; Zhang, Q.Y.; Luo, J.W. Assessment of relative bioavailability of heavy metals in soil using in vivo mouse model and its implication for risk assessment compared with bioaccessibility using in vitro assay. Environ. Geochem. Health 2016, 38, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S. Metal ligands in micronutrient acquisition and homeostasis. Plant Cell Environ. 2019, 42, 2902–2912. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Pierzak, M.; Krecisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Sun, B.L.; Tan, B.B.; Zhang, P.X.; Zhu, L.L.; Wei, H.M.; Huang, T.; Li, C.; Yang, W.E. Iron deficiency anemia: A critical review on iron absorption, supplementation and its influence on gut microbiota. Food Funct. 2024, 15, 1144–1157. [Google Scholar] [CrossRef]
- Moore, S.S.; Costa, A.; Pozza, M.; Weaver, C.M.; De Marchi, M. Nutritional scores of milk and plant-based alternatives and their difference in contribution to human nutrition. LWT-Food Sci. Technol. 2024, 191, 115688. [Google Scholar] [CrossRef]
- Xu, T.; Wan, S.T.; Shi, J.X.; Xu, T.C.; Wang, L.R.; Guan, Y.R.; Luo, J.J.; Luo, Y.T.; Sun, M.Y.; An, P.; et al. Antioxidant Minerals Modified the Association between Iron and Type 2 Diabetes in a Chinese Population. Nutrients 2024, 16, 335. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Catoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Food Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Galasso, C.; Gentile, A.; Orefice, I.; Ianora, A.; Bruno, A.; Noonan, D.M.; Sansone, C.; Albini, A.; Brunet, C. Microalgal Derivatives as Potential Nutraceutical and Food Supplements for Human Health: A Focus on Cancer Prevention and Interception. Nutrients 2019, 11, 1226. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- BRIAN, P.W. Role of gibberellin-like hormones in regulation of plant growth & flowering. Nature 1958, 181, 1122–1123. [Google Scholar] [CrossRef]
- Jukes, T.H. Mineral nutrition of plants. Photosynth. Res. 1995, 46, 13–15. [Google Scholar] [CrossRef]
- Barbosa, H.Z.; Batista, K.; Gimenes, F.; Gerdes, L.; Giacomini, A.A.; de Mattos, W.T.; Barbosa, C. Yield responses of Macrotyloma axillare (family Fabaceae) to combinations of doses of phosphorus and calcium. Semin.-Cienc. Agrar. 2019, 40, 2561–2570. [Google Scholar] [CrossRef]
- Ramadori, G.P. Organophosphorus Poisoning: Acute Respiratory Distress Syndrome (ARDS) and Cardiac Failure as Cause of Death in Hospitalized Patients. Int. J. Mol. Sci. 2023, 24, 6658. [Google Scholar] [CrossRef]
- Miao, S.; Wei, Y.; Pan, Y.; Wang, Y.; Wei, X. Detection methods, migration patterns, and health effects of pesticide residues in tea. Compr. Rev. Food. Sci. Food Saf. 2023, 22, 2945–2976. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Mahmood, D.; Elkhalifa, A.E.O.; Siddiqui, A.J.; Khan, M.I.; Ashfaq, F.; Patel, M.; Snoussi, M.; Kieliszek, M.; Adnan, M. Exposure to pesticide residues in honey and its potential cancer risk assessment. Food. Chem. Toxicol. 2023, 180, 114014. [Google Scholar] [CrossRef]
- de Andrade, J.C.; Galvan, D.; Effting, L.; Tessaro, L.; Aquino, A.; Conte-Junior, C.A. Multiclass pesticide residues in fruits and vegetables from Brazil: A systematic review of sample preparation until post-harvest. Crit. Rev. Anal. Chem. 2023, 53, 1174–1196. [Google Scholar] [CrossRef]
- Zheng, K.M.; Wu, X.P.; Chen, J.N.; Chen, J.X.; Lian, W.H.; Su, J.F.; Shi, L.H. Establishment of an LC-MS/MS Method for the Determination of 45 Pesticide Residues in Fruits and Vegetables from Fujian, China. Molecules 2022, 27, 8674. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Bai, X.; Xiao, Q.; Liu, F.; Zhou, L.; Zhang, C. Detection of adulteration in food based on nondestructive analysis techniques: A review. Crit. Rev. Food. Sci. Nutr. 2021, 61, 2351–2371. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef]
- Zheng, K.; Zeng, Z.; Tian, Q.; Huang, J.; Zhong, Q.; Huo, X. Epidemiological evidence for the effect of environmental heavy metal exposure on the immune system in children. Sci. Total Environ. 2023, 868, 161691. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Beghi, R.; Spinardi, A.; Bodria, L.; Mignani, I.; Guidetti, R. Apples Nutraceutic Properties Evaluation through a Visible and Near-Infrared Portable System. Food Bioprocess Technol. 2013, 6, 2547–2554. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Nieuwoudt, H.; Cronje, P.; Saeys, W.; Nicolaï, B. NIR Spectroscopy Applications for Internal and External Quality Analysis of Citrus Fruit-A Review. Food Bioprocess Technol. 2012, 5, 425–444. [Google Scholar] [CrossRef]
- Zhu, G.; Tian, C. Determining sugar content and firmness of ‘Fuji’ apples by using portable near-infrared spectrometer and diffuse transmittance spectroscopy. J. Food Process Eng. 2018, 41, e12810. [Google Scholar] [CrossRef]
- McGoverin, C.M.; Weeranantanaphan, J.; Downey, G.; Manley, M. The application of near infrared spectroscopy to the measurement of bioactive compounds in food commodities. J. Near Infrared Spectrosc. 2010, 18, 87–111. [Google Scholar] [CrossRef]
- Youwen, T.; Xin, M.; Yi, C. Advancement of Nondestructive Detection of Fruit Defects Based on Hyperspectral Imaging. J. Agric. Mech. Res. 2014, 36, 1–5. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, H.; Sun, D. Hyperspectral imaging technique for evaluating food quality and safety during various processes: A review of recent applications. Trends Food Sci. Technol. 2017, 69, 25–35. [Google Scholar] [CrossRef]
- Raki, H.; Aalaila, Y.; Taktour, A.; Peluffo-Ordonez, D.H. Combining AI Tools with Non-Destructive Technologies for Crop-Based Food Safety: A Comprehensive Review. Foods 2023, 13, 11. [Google Scholar] [CrossRef]
- Nasrabadi, N.M. Hyperspectral Target Detection. IEEE Signal Process. Mag. 2014, 31, 34–44. [Google Scholar] [CrossRef]
- Gao, H.M.; Zhang, Y.T.; Chen, Z.H.; Xu, S.F.; Hong, D.F.; Zhang, B. A Multidepth and Multibranch Network for Hyperspectral Target Detection Based on Band Selection. IEEE Trans. Geosci. Remote Sens. 2023, 61, 5506818. [Google Scholar] [CrossRef]
- Petry, R.; Schmitt, M.; Popp, J. Raman spectroscopy—A prospective tool in the life sciences. ChemPhysChem 2003, 4, 14–30. [Google Scholar] [CrossRef]
- Petersen, M.; Yu, Z.; Lu, X. Application of Raman spectroscopic methods in food safety: A review. Biosensors 2021, 11, 187. [Google Scholar] [CrossRef]
- Das, R.S.; Agrawal, Y.K. Raman spectroscopy: Recent advancements, techniques and applications. Vib. Spectrosc. 2011, 57, 163–176. [Google Scholar] [CrossRef]
- Xu, Y.; Zhong, P.; Jiang, A.; Shen, X.; Li, X.; Xu, Z.; Shen, Y.; Sun, Y.; Lei, H. Raman spectroscopy coupled with chemometrics for food authentication: A review. Trac-Trends Anal. Chem. 2020, 131, 116017. [Google Scholar] [CrossRef]
- Zhang, D.; Pu, H.; Huang, L.; Sun, D. Advances in flexible surface-enhanced Raman scattering (SERS) substrates for nondestructive food detection: Fundamentals and recent applications. Trends Food Sci. Technol. 2021, 109, 690–701. [Google Scholar] [CrossRef]
- Nilghaz, A.; Mahdi Mousavi, S.; Amiri, A.; Tian, J.; Cao, R.; Wang, X. Surface-enhanced Raman spectroscopy substrates for food safety and quality analysis. J. Agric. Food. Chem. 2022, 70, 5463–5476. [Google Scholar] [CrossRef]
- Pang, S.; Yang, T.; He, L. Review of surface enhanced Raman spectroscopic (SERS) detection of synthetic chemical pesticides. Trac-Trends Anal. Chem. 2016, 85, 73–82. [Google Scholar] [CrossRef]
- Corsetti, S.; McGloin, D.; Kiefer, J. Comparison of Raman and IR spectroscopy for quantitative analysis of gasoline/ethanol blends. Fuel 2016, 166, 488–494. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, P.; Chen, W.W.; Tang, Z.X.; Li, C.; Xiao, K.Y.; Jin, S.Z.; Ni, D.J.; Yu, Z. Rapid field trace detection of pesticide residue in food based on surface-enhanced Raman spectroscopy. Mikrochim. Acta 2021, 188, 370. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.T.; Ying, Y.B. Applications of Raman Spectroscopy in Agricultural Products and Food Analysis: A Review. Appl. Spectrosc. Rev. 2011, 46, 539–560. [Google Scholar] [CrossRef]
- Khan, Z.H.; Ullah, M.H.; Rahman, B.; Talukder, A.I.; Wahadoszamen, M.; Abedin, K.M.; Haider, A. Laser-induced breakdown spectroscopy (LIBS) for trace element detection: A review. J. Spectrosc. 2022, 2022, 3887038. [Google Scholar] [CrossRef]
- Brunnbauer, L.; Gajarska, Z.; Lohninger, H.; Limbeck, A. A critical review of recent trends in sample classification using Laser-Induced Breakdown Spectroscopy (LIBS). Trac-Trends Anal. Chem. 2023, 159, 116859. [Google Scholar] [CrossRef]
- Laserna, J.; Vadillo, J.; Purohit, P. Laser-Induced Breakdown Spectroscopy (LIBS): Fast, Effective, and Agile Leading Edge Analytical Technology. Appl. Spectrosc. 2018, 72, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.S.; Zhao, N.J.; Ma, M.J.; Gu, Y.H.; Liu, J.G. Quantitative detection of Cu in different types of soils using laser induced breakdown spectroscopy combined with artificial neural network. J. Optoelectron. Laser 2015, 26, 1984–1989. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Lv, H.; Li, X.; Wu, Q.; Yang, X. Detection of heavy metals based on laser-induced breakdown spectroscopy under magnetic field constraints. J. Optoelectron. Laser 2023, 34, 422–428. [Google Scholar]
- Kirtil, E.; Oztop, M.H. 1H Nuclear Magnetic Resonance Relaxometry and Magnetic Resonance Imaging and Applications in Food Science and Processing. Food Eng. Rev. 2016, 8, 1–22. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Barrow, C.J. Compositional Information Useful for Authentication of Krill Oil and the Detection of Adulterants. Food Anal. Methods 2018, 11, 178–187. [Google Scholar] [CrossRef]
- Rudszuck, T.; Nirschl, H.; Guthausen, G. Perspectives in process analytics using low field NMR. J. Magn. Reson. 2021, 323, 106897. [Google Scholar] [CrossRef]
- Marcone, M.F.; Wang, S.A.; Albabish, W.; Nie, S.P.; Somnarain, D.; Hill, A. Diverse food-based applications of nuclear magnetic resonance (NMR) technology. Food Res. Int. 2013, 51, 729–747. [Google Scholar] [CrossRef]
- Agiomyrgianaki, A.; Petrakis, P.V.; Dais, P. Detection of refined olive oil adulteration with refined hazelnut oil by employing NMR spectroscopy and multivariate statistical analysis. Talanta 2010, 80, 2165–2171. [Google Scholar] [CrossRef]
- Meng, W.J.; Xu, X.N.; Cheng, K.K.; Xu, J.J.; Shen, G.P.; Wu, Z.D.; Dong, J.Y. Geographical Origin Discrimination of Oolong Tea (TieGuanYin, Camellia sinensis (L.) O. Kuntze) Using Proton Nuclear Magnetic Resonance Spectroscopy and Near-Infrared Spectroscopy. Food Anal. Methods 2017, 10, 3508–3522. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Akbari, E.; Toudeshki, A.; Homayouni, T.; Alizadeh, A.; Ehsani, R. Terahertz spectroscopy and imaging: A review on agricultural applications. Comput. Electron. Agric. 2020, 177, 105628. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Li, G.M.; Lv, M.; Ge, H.Y.; Zhang, Y. Determination of potassium sorbate and sorbic acid in agricultural products using THz time-domain spectroscopy*. Chin. Phys. B 2020, 29, 098705. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Li, G.M.; Ge, H.Y.; Wang, F.Y.; Li, L.; Chen, X.Y.; Lu, M.; Zhang, Y. Machine Learning and Application in Terahertz Technology: A Review on Achievements and Future Challenges. IEEE Access 2022, 10, 53761–53776. [Google Scholar] [CrossRef]
- Fu, X.J.; Liu, Y.J.; Chen, Q.; Fu, Y.; Cui, T.J. Applications of Terahertz Spectroscopy in the Detection and Recognition of Substances. Front. Phys. 2022, 10, 869537. [Google Scholar] [CrossRef]
- Wang, K.Q.; Pu, H.B.; Sun, D.W. Emerging Spectroscopic and Spectral Imaging Techniques for the Rapid Detection of Microorganisms: An Overview. Compr. Rev. Food. Sci. Food Saf. 2018, 17, 256–273. [Google Scholar] [CrossRef]
- Gowen, A.A.; O’Sullivan, C.; O’Donnell, C.P. Terahertz time domain spectroscopy and imaging: Emerging techniques for food process monitoring and quality control. Trends Food Sci. Technol. 2012, 25, 40–46. [Google Scholar] [CrossRef]
- Wang, C.; Qin, J.Y.; Xu, W.D.; Chen, M.; Xie, L.J.; Ying, Y.B. Terahertz Imaging Applications in Agriculture and Food Engineering: A Review. Trans. Asabe 2018, 61, 411–424. [Google Scholar] [CrossRef]
- Anitha, V.; Beohar, A.; Nella, A. THz Imaging Technology Trends and Wide Variety of Applications: A Detailed Survey. Plasmonics 2023, 18, 441–483. [Google Scholar] [CrossRef]
- Shi, S.J.; Tang, Z.H.; Ma, Y.Y.; Cao, C.G.; Jiang, Y. Application of spectroscopic techniques combined with chemometrics to the authenticity and quality attributes of rice. Crit. Rev. Food. Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef]
- Tarapoulouzi, M.; Mironescu, M.; Drouza, C.; Mironescu, I.D.; Agriopoulou, S. Insight into the Recent Application of Chemometrics in Quality Analysis and Characterization of Bee Honey during Processing and Storage. Foods 2023, 12, 473. [Google Scholar] [CrossRef]
- Varzakas, T. Implementation of Chemometrics and Other Techniques as Means of Authenticity and Traceability to Detect Adulteration in Foods for the Protection of Human Health. Foods 2023, 12, 652. [Google Scholar] [CrossRef]
- Wang, T.; Feng, G.; Zhu, Y. Rapid Determination of Soluble Solids and Vitamin C in Blueberry by Near Infrared Spectroscopy Combined with Chemometrics. Sci. Technol. Food Ind. 2023, 44, 297–305. [Google Scholar] [CrossRef]
- Xiao-Lu, L.; Lu, X.; Xiao-Xiang, L.U.; Peng, Z.; Shao-Hui, C.; Jiang-Kuo, L.I. Fast non-destructive testing of total flavonoids and anthocyanins in blueberries by near-infrared spectroscope. Sci. Technol. Food Ind. 2015, 36, 58–61. [Google Scholar] [CrossRef]
- Sahamishirazi, S.; Zikeli, S.; Fleck, M.; Claupein, W.; Graeff-Hoenninger, S. Development of a near-infrared spectroscopy method (NIRS) for fast analysis of total, indolic, aliphatic and individual glucosinolates in new bred open pollinating genotypes of broccoli (Brassica oleracea convar. botrytis var. italica). Food Chem. 2017, 232, 272–277. [Google Scholar] [CrossRef]
- Pedro, A.; Ferreira, M. Nondestructive determination of solids and carotenoids in tomato products by near-infrared spectroscopy and multivariate calibration. Anal. Chem. 2005, 77, 2505–2511. [Google Scholar] [CrossRef]
- Lakade, A.J.; Venkataraman, V.; Ramasamy, R.; Shetty, P.H. NIR spectroscopic method for the detection of calcium carbide in artificial ripening of mangoes (Magnifera indica). Food Addit. Contam. Part A 2019, 36, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, B.; Mohajerani, E.; Jamshidi, J. Developing a Vis/NIR spectroscopic system for fast and non-destructive pesticide residue monitoring in agricultural product. Measurement 2016, 89, 1–6. [Google Scholar] [CrossRef]
- Xue, L.; Cai, J.; Li, J.; Liu, M. Application of Particle Swarm Optimization (PSO) Algorithm to Determine Dichlorvos Residue on the Surface of Navel Orange with Vis-NIR Spectroscopy. Procedia Eng. 2012, 29, 4124–4128. [Google Scholar] [CrossRef]
- Shi, J.; Zou, X.; Zhao, J.; Mao, H.; Huang, X. Rapid and non-destructive diagnostics of nitrogen and magnesium deficiencies in cucumber plants by near-infrared spectroscopy. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2011, 27, 283–287. [Google Scholar]
- Petisco, C.; García-Criado, B.; Vázquez De Aldana, B.R.; Zabalgogeazcoa, I.; Mediavilla, S.; García-Ciudad, A. Use of near-infrared reflectance spectroscopy in predicting nitrogen, phosphorus and calcium contents in heterogeneous woody plant species. Anal. Bioanal. Chem. 2005, 382, 458–465. [Google Scholar] [CrossRef]
- Jiao, Y.P.; Li, Z.C.; Chen, X.S.; Fei, S.M. Preprocessing methods for near-infrared spectrum calibration. J. Chemom. 2020, 34, e3306. [Google Scholar] [CrossRef]
- Abrahamsson, C.; Löwgren, A.; Strömdahl, B.; Svensson, T.; Andersson-Engels, S.; Johansson, J.; Folestad, S. Scatter correction of transmission near-infrared spectra by photon migration data: Quantitative analysis of solids. Appl. Spectrosc. 2005, 59, 1381–1387. [Google Scholar] [CrossRef]
- Lv, J.P.; Chen, Z.H.; Luan, X.L.; Liu, F. Denoising stacked autoencoders-based near-infrared quality monitoring method via robust samples evaluation. Can. J. Chem. Eng. 2023, 101, 2693–2703. [Google Scholar] [CrossRef]
- Morales-Sillero, A.; Pierna, J.; Sinnaeve, G.; Dardenne, P.; Baeten, V. Quantification of protein in wheat using near infrared hyperspectral imaging: Performance comparison with conventional near infrared spectroscopy. J. Near Infrared Spectrosc. 2018, 26, 186–195. [Google Scholar] [CrossRef]
- Malegori, C.; Grassi, S.; Marques, E.; de Freitas, S.; Casiraghi, E. Vitamin C distribution in acerola fruit by near infrared hyperspectral imaging. J. Spectr. Imaging 2016, 5, a6. [Google Scholar] [CrossRef]
- Guo, L.; Yin, Y.; Yu, H.; Yuan, Y. Hyperspectral detection method of potato vitamin C content based on Fisher discriminant analysis separability information fusion. Food Sci. 2023, 1–12. Available online: https://link.cnki.net/urlid/11.2206.TS.20231123.1441.034 (accessed on 10 January 2024).
- Chen, H.Z.; Qiao, H.L.; Feng, Q.X.; Xu, L.L.; Lin, Q.Y.; Cai, K. Rapid Detection of Pomelo Fruit Quality Using Near-Infrared Hyperspectral Imaging Combined with Chemometric Methods. Front. Bioeng. Biotechnol. 2021, 8, 616943. [Google Scholar] [CrossRef]
- Kjær, A.; Nielsen, G.; Stærke, S.; Clausen, M.R.; Edelenbos, M.; Jorgensen, B. Detection of Glycoalkaloids and Chlorophyll in Potatoes (Solanum tuberosum L.) by Hyperspectral Imaging. Am. J. Potato Res. 2017, 94, 573–582. [Google Scholar] [CrossRef]
- Shi, J.Y.; Zou, X.B.; Zhao, J.W.; Wang, K.L.; Chen, Z.W.; Huang, X.W.; Zhang, D.T.; Holmes, M. Nondestructive diagnostics of nitrogen deficiency by cucumber leaf chlorophyll distribution map based on near infrared hyperspectral imaging. Sci. Hortic. 2012, 138, 190–197. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.H.; Xiao, H.; Gu, X.Z.; Pan, L.Q.; Tu, K. Hyperspectral imaging detection of decayed honey peaches based on their chlorophyll content. Food Chem. 2017, 235, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.; Rojas, M.Z.; Yang, Y.; Lee, L.; Hoagland, L. Classifying cadmium contaminated leafy vegetables using hyperspectral imaging and machine learning. Heliyon 2022, 8, e12256. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, C.J.; Sun, J.; Yao, K.S.; Xu, M.; Cheng, J.H. Nondestructive testing and visualization of compound heavy metals in lettuce leaves using fluorescence hyperspectral imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 291, 122337. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Kapoor, L.; Doss, C.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Gabbitas, A.; Ahlborn, G.; Allen, K.; Pang, S. Advancing Mycotoxin Detection: Multivariate Rapid Analysis on Corn Using Surface Enhanced Raman Spectroscopy (SERS). Toxins 2023, 15, 610. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, P.; Wang, M.; Zuo, M.; El-Seedi, H.R.; Chen, Q.; Shi, J.; Zou, X. Rapid enrichment detection of patulin and alternariol in apple using surface enhanced Raman spectroscopy with coffee-ring effect. LWT 2021, 152, 112333. [Google Scholar] [CrossRef]
- Lee, K.; Herrman, T.J.; Yun, U. Application of Raman spectroscopy for qualitative and quantitative analysis of aflatoxins in ground maize samples. J. Cereal Sci. 2014, 59, 70–78. [Google Scholar] [CrossRef]
- Huang, S.G.; Hu, J.P.; Wu, R.M.; Liu, M.H.; Fan, Y.; Wang, X.B.; Guo, P. Establishment of rapid detection method of phosalone residues in pakchoi by surface-enhanced Raman scattering spectroscopy. Spectr. Lett. 2016, 49, 128–134. [Google Scholar] [CrossRef]
- Xie, Y.; Mukamurezi, G.; Sun, Y.; Wang, H.; Qian, H.; Yao, W. Establishment of rapid detection method of methamidophos in vegetables by surface enhanced Raman spectroscopy. Eur. Food Res. Technol. 2012, 234, 1091–1098. [Google Scholar] [CrossRef]
- Yaseen, T.; Pu, H.; Sun, D. Rapid detection of multiple organophosphorus pesticides (triazophos and parathion-methyl) residues in peach by SERS based on core-shell bimetallic Au@Ag NPs. Food Addit. Contam. Part A 2019, 36, 762–778. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, H.; Sun, D. Fingerprinting and tagging detection of mycotoxins in agri-food products by surface-enhanced Raman spectroscopy: Principles and recent applications. Trends Food Sci. Technol. 2021, 110, 393–404. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, J.; Sachdev, T.; Basu, T.; Malhotra, B.D. Recent advances in mycotoxins detection. Biosens. Bioelectron. 2016, 81, 532–545. [Google Scholar] [CrossRef]
- Beldjilali, S.; Borivent, D.; Mercadier, L.; Mothe, E.; Clair, G.; Hermann, J. Evaluation of minor element concentrations in potatoes using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 727–733. [Google Scholar] [CrossRef]
- Rai, N.K.; Rai, P.K.; Pandhija, S.; Watal, G.; Rai, A.K.; Bicanic, D. Application of LIBS in Detection of Antihyperglycemic Trace Elements in Momordica charantia. Food Biophys. 2009, 4, 167–171. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, R.; Awasthi, S.; Singh, V.; Rai, A.K. Laser Induced breakdown spectroscopy: A rapid tool for the identification and quantification of minerals in cucurbit seeds. Food Chem. 2017, 221, 1778–1783. [Google Scholar] [CrossRef]
- Wu, D.; Meng, L.W.; Yang, L.; Wang, J.Y.; Fu, X.P.; Du, X.Q.; Li, S.J.; He, Y.; Huang, L.X. Feasibility of Laser-Induced Breakdown Spectroscopy and Hyperspectral Imaging for Rapid Detection of Thiophanate-Methyl Residue on Mulberry Fruit. Int. J. Mol. Sci. 2019, 20, 2017. [Google Scholar] [CrossRef]
- Zhao, X.D.; Zhao, C.J.; Du, X.F.; Dong, D.M. Detecting and Mapping Harmful Chemicals in Fruit and Vegetables Using Nanoparticle-Enhanced Laser-Induced Breakdown Spectroscopy. Sci. Rep. 2019, 9, 906. [Google Scholar] [CrossRef]
- Martino, L.J.; D’Angelo, C.A.; Marinelli, C.; Cepeda, R. Identification and detection of pesticide in chard samples by laser-induced breakdown spectroscopy using chemometric methods. Spectrochim. Acta Part B At. Spectrosc. 2021, 177, 106031. [Google Scholar] [CrossRef]
- Shen, T.T.; Kong, W.W.; Liu, F.; Chen, Z.H.; Yao, J.D.; Wang, W.; Peng, J.Y.; Chen, H.Z.; He, Y. Rapid Determination of Cadmium Contamination in Lettuce Using Laser-Induced Breakdown Spectroscopy. Molecules 2018, 23, 2930. [Google Scholar] [CrossRef]
- Yang, L.; Meng, L.W.; Gao, H.Q.; Wang, J.Y.; Zhao, C.; Guo, M.M.; He, Y.; Huang, L.X. Building a stable and accurate model for heavy metal detection in mulberry leaves based on a proposed analysis framework and laser-induced breakdown spectroscopy. Food Chem. 2021, 338, 127886. [Google Scholar] [CrossRef]
- Xu, F.; Jin, X.; Zhang, L.; Chen, X.D. Investigation on water status and distribution in broccoli and the effects of drying on water status using NMR and MRI methods. Food Res. Int. 2017, 96, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hatzakis, E.; Agiomyrgianaki, A.; Dais, P. Detection and Quantification of Free Glycerol in Virgin Olive Oil by 31P-NMR Spectroscopy. J. Am. Oil. Chem. Soc. 2010, 87, 29–34. [Google Scholar] [CrossRef]
- Siciliano, C.; Belsito, E.; De Marco, R.; Di Gioia, M.L.; Leggio, A.; Liguori, A. Quantitative determination of fatty acid chain composition in pork meat products by high resolution 1H NMR spectroscopy. Food Chem. 2013, 136, 546–554. [Google Scholar] [CrossRef]
- Capitani, D.; Mannina, L.; Proietti, N.; Sobolev, A.P.; Tomassini, A.; Miccheli, A.; Di Cocco, M.E.; Capuani, G.; De Salvador, R.; Delfini, M. Monitoring of metabolic profiling and water status of Hayward kiwifruits by nuclear magnetic resonance. Talanta 2010, 82, 1826–1838. [Google Scholar] [CrossRef] [PubMed]
- Clausen, M.R.; Edelenbos, M.; Bertram, H.C. Mapping the Variation of the Carrot Metabolome Using 1H NMR Spectroscopy and Consensus PCA. J. Agric. Food. Chem. 2014, 62, 4392–4398. [Google Scholar] [CrossRef]
- Zhong, S.C. Progress in terahertz nondestructive testing: A review. Front. Mech. Eng. 2019, 14, 273–281. [Google Scholar] [CrossRef]
- Liu, J.J. Terahertz spectroscopy and chemometric tools for rapid identification of adulterated dairy product. Opt. Quantum Electron. 2017, 49, 1. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z. Terahertz spectroscopy applied to quantitative determination of harmful additives in medicinal herbs. Optik 2018, 156, 834–840. [Google Scholar] [CrossRef]
- Zhang, J.N.; Feng, X.P.; Jin, J.; Fang, H. Concise Cascade Methods for Transgenic Rice Seed Discrimination using Spectral Phenotyping. Plant Phenomics 2023, 5, 0071. [Google Scholar] [CrossRef]
- Liu, W.; Liu, C.H.; Hu, X.H.; Yang, J.B.; Zheng, L. Application of terahertz spectroscopy imaging for discrimination of transgenic rice seeds with chemometrics. Food Chem. 2016, 210, 415–421. [Google Scholar] [CrossRef]
- Liu, J.J. Determination of transgenic organisms from non-transgenic using terahertz spectroscopy and chemometrics. Optik 2017, 131, 885–891. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, X.Z.; Wang, Y.F.; Hu, L.; Wang, P. Detection of tomato water stress based on terahertz spectroscopy. Front. Plant Sci. 2023, 14, 1095434. [Google Scholar] [CrossRef]
- Baek, S.H.; Kang, J.H.; Hwang, Y.H.; Ok, K.M.; Kwak, K.; Chun, H.S. Detection of Methomyl, a Carbamate Insecticide, in Food Matrices Using Terahertz Time-Domain Spectroscopy. J. Infrared Millim. Terahertz Waves 2016, 37, 486–497. [Google Scholar] [CrossRef]
- Wang, Q.; Yin, S.; Shi, X.; Fan, J.; Huang, K.; Gao, W.; Xie, L.; Ying, Y. High-sensitivity detection of trace imidacloprid and tetracycline hydrochloride by multi-frequency resonance metamaterials. J. Food Meas. Charact. 2022, 16, 2041–2048. [Google Scholar] [CrossRef]
- Qin, B.; Li, Z.; Hu, F.; Hu, C.; Chen, T.; Zhang, H.; Zhao, Y. Highly Sensitive Detection of Carbendazim by Using Terahertz Time-Domain Spectroscopy Combined with Metamaterial. IEEE Trans. Terahertz Sci. Technol. 2018, 8, 149–154. [Google Scholar] [CrossRef]
- Chen, Z.W.; Zhang, Z.Y.; Zhu, R.H.; Xiang, Y.H.; Yang, Y.P.; Harrington, P.B. Application of terahertz time-domain spectroscopy combined with chemometrics to quantitative analysis of imidacloprid in rice samples. J. Quant. Spectrosc. Radiat. Transf. 2015, 167, 1–9. [Google Scholar] [CrossRef]
- Ma, Q.; Teng, Y.; Li, C.; Jiang, L. Simultaneous quantitative determination of low-concentration ternary pesticide mixtures in wheat flour based on terahertz spectroscopy and BPNN. Food Chem. 2022, 377, 132030. [Google Scholar] [CrossRef]

| Target of Detection | Component | Preprocessing | Variable Selecting | Optimal Model | Effect | Reference |
|---|---|---|---|---|---|---|
| Blueberry | SSC, Vitamin C | MSC + 2-DER | CARS + RF | PLSR | SSC: RMSEP = 0.9673 RP = 0.7376 Vitamin C: RMSEP = 3.6885 RP = 0.7021 | [66] |
| Blueberry | Total Flavonoids, Anthocyanins | 1-DER | / | PLSR | Total Flavonoids: RP2 = 0.7968 Anthocyanins: RP2 = 0.7902 | [67] |
| Broccoli | Glucosinolates | / | / | PLSR | R2 = 0.5–0.78 RPD = 1.35–2.19 | [68] |
| Tomato | Lycopene, β-carotene | MSC + 2-DER | / | PLS-1 | Lycopene: RP2 = 0.9996 β-carotene: RP2 = 0.9981 | [69] |
| Mango | As | / | PCA + SPA | PLS | R2 ranged from 0.9 to 0.96 for different arsenic concentrations | [70] |
| Cucumber | Diazinon Residue | MSC + 1-DER | PSO | PLS-DA | RCV = 0.91 SECV = 3.22 | [71] |
| Navel Oranges | Dichlorvos | / | PSO | PLS | R2 = 0.8732 | [72] |
| Cucumber | N, Mg | SNV | GA | KNN | Training Set Recognition Rate: 98% Prediction Set Recognition Rate: 96% | [73] |
| Woody Plant | N, P, Ca | data | / | PLSR | Ca: R2 = 0.91 P: R2 = 0.74 N: R2 = 0.95 | [74] |
| Target of Detection | Component | Preprocessing | Variable Selecting | Optimal Model | Effect | Reference |
|---|---|---|---|---|---|---|
| Acerolas | Vitamin C | SNV+SG | PCA | CLS | The amount of vitamin C gradually decreases during growth. | [79] |
| Potato | Vitamin C | MSC | CARS | FDA + BPNN | R2 = 0.999 REMSP = 0.1727 | [80] |
| Pomelo | Vitamin C | / | / | RBF + PLS | RMSEV = 41.381 mg/kg | [81] |
| Potato | Chlorophyll | / | / | PLSR | R2 = 0.93~0.97 | [82] |
| Cucumber | Chlorophyll | SNV | PCA | MLR | R2 = 0.8712 | [83] |
| Honey Peach | Chlorophyll | / | SPA | PLS, SPA-PLS | PLS: RP2 = 0.904 RMSEP = 0.633% SPA-PLS: RP2 = 0.858 RMSEP = 0.751% | [84] |
| Kale and Basil | Cadmium | / | RF+PCA | ANN | Ability to classify plants according to cadmium concentration above or below 0.2 mg/kg. | [85] |
| Lettuce Leaves | Cadmium | 1-DER | WT-ST | MLR | RP2 = 0.7905 RMSEP = 0.0096 | [86] |
| Target of Detection | Component | Preprocessing | Variable Selecting | Optimal Model | Effect | Reference |
|---|---|---|---|---|---|---|
| Maize | AFB1, ZEN, and OTA | / | / | PLS | The correlation coefficients for AFB1, ZEN, and OTA were 0.74, 0.89, and 0.72, respectively, by PLS model. | [88] |
| Apple | PAT, AOH | 2-DER | Si, GA | Si-PLS, GA-PLS | PAT: SI-PLS: RC = 0.9905 RP = 0.9759 AOH: GA-PLS: RC = 0.9829 RP = 0.9808 | [89] |
| Maize | Aflatoxin | Deconvolution | / | PLSR | RV2 = 0.990 | [90] |
| Pakchoi | PHO | MSC | / | PLS | RP2 = 0.9807 RMSECV = 0.886 mg/L | [91] |
| Vegetables | MAP | / | / | SPSS | R2 = 0.9852 | [92] |
| Peach | Organophosphorus Chemical Pesticides | / | / | / | The detection limit was 0.001 mg/kg. | [93] |
| Target of Detection | Component | Preprocessing | Variable Selecting | Optimal Model | Effect | Reference |
|---|---|---|---|---|---|---|
| Momordica Charantia | Na, K, Mg, Ca | / | / | SPSS | The LIBS technology test matches the AAS test results. | [97] |
| Melon Seed | Na, K, Mg, Ca | / | / | SPSS | p < 0.05 | [98] |
| Mulberry Fruit | Thiophanate-Methyl Residue | SNV | CARS | PLSR | RPD = 2.585 RMSEP = 7.09 × 10−4 RP2 = 0.921 | [99] |
| Fruits and Vegetables | P, S, CI | / | / | / | Detection limits are two orders of magnitude lower than typical detection limits. | [100] |
| Chard Leaves | P, S, C, Cl | / | PCA | LDA | Detection error rate of less than 9.5%. | [101] |
| Lettuce | Cd | Normalization | GA | PLS | Rp2 more than 0.94, LODs less than 5.5 mg/kg | [102] |
| Mulberry Leaves | Cu, Cr | / | SOM, SPA, UVE | PLS | Cu: RPD = 10.0494 RMSEP = 110.4550 Cr: RPD = 8.3874 RMSEP = 41.4561 | [103] |
| Detection Technology | Advantage | Disadvantage |
|---|---|---|
| Near-infrared Spectroscopy | Fast and inexpensive detection | Vulnerable to environmental disturbances, low stability |
| Hyperspectral Imaging | More comprehensive spectral data | Excessive volume and complexity of information, requiring time-consuming data analysis |
| Raman Spectroscopy | Rapid, accurate, nondestructive, good identification of molecular functional groups present within the substance | Expensive, weak spectral signal |
| Laser-induced Breakdown Spectroscopy | Real-time, simultaneous multi-element analysis, simple detection process, wide detection range | Low resolution, unstable signal strength, baseline drift, substrate effect phenomenon |
| Nuclear Magnetic Resonance | Simple operation, little damage to the sample, fast detection speeds | Lower resolution, higher environmental requirements |
| Terahertz Spectroscopy | Strong penetrating power, unique electromagnetic waves can detect the physicochemical information inside the material | Low sensitivity, difficult to detect low-level substances |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Guo, Y.; Liang, X.; Lu, H. Intelligent Rapid Detection Techniques for Low-Content Components in Fruits and Vegetables: A Comprehensive Review. Foods 2024, 13, 1116. https://doi.org/10.3390/foods13071116
Xu S, Guo Y, Liang X, Lu H. Intelligent Rapid Detection Techniques for Low-Content Components in Fruits and Vegetables: A Comprehensive Review. Foods. 2024; 13(7):1116. https://doi.org/10.3390/foods13071116
Chicago/Turabian StyleXu, Sai, Yinghua Guo, Xin Liang, and Huazhong Lu. 2024. "Intelligent Rapid Detection Techniques for Low-Content Components in Fruits and Vegetables: A Comprehensive Review" Foods 13, no. 7: 1116. https://doi.org/10.3390/foods13071116
APA StyleXu, S., Guo, Y., Liang, X., & Lu, H. (2024). Intelligent Rapid Detection Techniques for Low-Content Components in Fruits and Vegetables: A Comprehensive Review. Foods, 13(7), 1116. https://doi.org/10.3390/foods13071116





