Chemical Composition and Geographic Variation of Cold Pressed Balanites aegyptiaca Kernel Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Cold Pressing Process
2.3. Determination of the Fatty Acid Composition
2.4. Determination of the Triacylglecerol Composition
2.5. Determination of the Tocochromanol Composition
2.6. Determination of the Phytosterol Composition
2.7. Statistical Analysis
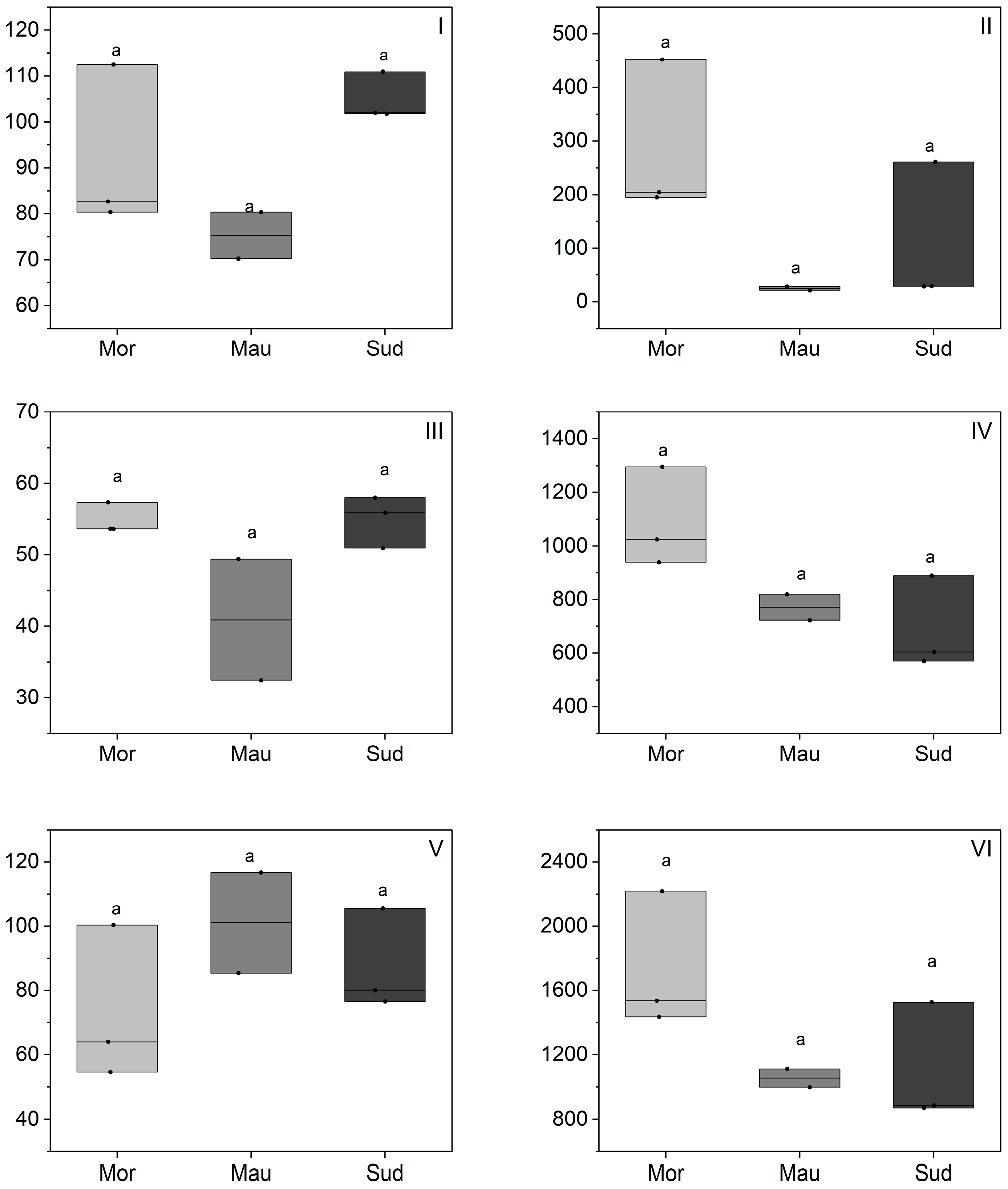
3. Results and Discussion
3.1. Fatty Acid Composition
3.2. Triacylglycerol Composition
3.3. Tocochromanol Composition
3.4. Phytosterol Composition
3.5. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdelaziz, S.M.; Lemine, F.M.M.; Tfeil, H.O.; Filali-Maltouf, A.; Boukhary, A.O.M.S. Phytochemicals, Antioxidant Activity and Ethnobotanical Uses of Balanites aegyptiaca (L.) Del. Fruits from the Arid Zone of Mauritania, Northwest Africa. Plants 2020, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Okia, C.A.; Agea, J.G.; Kimondo, J.M.; Refaat Atalla, A.A.; Obua, J.; Teklehaimanot, Z. Harvesting and processing of Balanites aegyptiaca leaves and fruits for local consumption by rural communities in Uganda. J. Food Technol. 2011, 9, 83–90. [Google Scholar] [CrossRef]
- Ahmed, A.A.O.; Kita, A.; Nemś, A.; Miedzianka, J.; Foligni, R.; Abdalla, A.M.A.; Mozzon, M. Tree-to-tree variability in fruits and kernels of a Balanites aegyptiaca (L.) Del. population grown in Sudan. Trees 2020, 34, 111–119. [Google Scholar] [CrossRef]
- Mariod, A.A. (Ed.) Wild Fruits: Composition, Nutritional Value and Products, 1st ed.; Springer: Cham, Switzerland, 2019; ISBN 9783030318840. [Google Scholar]
- Bellakhdar, J. La Pharmacopée Marocaine Traditionnelle: Médecine Arabe Ancienne et Savoirs Populaires; Ibis Press: Paris, France, 1997; ISBN 9782910728038. [Google Scholar]
- Abouri, M.; Mousadik, A.E.; Msanda, F.; Boubaker, H.; Saadi, B.; Cherifi, K. An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. Int. J. Med. Plant Res. 2012, 1, 99–123. [Google Scholar]
- Saini, M.K.; Prasad, J.; Raju, P.V.S.; Kothari, S.L.; Harish; Shukla, J.K.; Gour, V.S. Morphological Descriptors and Heritability as Markers for Oil Yield in Balanites aegyptiaca (L.) Del.: A Potential Biodiesel Xerophyte. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 695–706. [Google Scholar] [CrossRef]
- Chapagain, B.P.; Yehoshua, Y.; Wiesman, Z. Desert date (Balanites aegyptiaca) as an arid lands sustainable bioresource for biodiesel. Bioresour. Technol. 2009, 100, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Yadav, G.G.; Dewir, Y.H.; Ibrahim, A. Phytochemicals and Biological Activity of Desert Date (Balanites aegyptiaca (L.) Delile). Plants 2020, 10, 32. [Google Scholar] [CrossRef]
- Al Ashaal, H.A.; Farghaly, A.A.; Abd El Aziz, M.M.; Ali, M.A. Phytochemical investigation and medicinal evaluation of fixed oil of Balanites aegyptiaca fruits (Balantiaceae). J. Ethnopharmacol. 2010, 127, 495–501. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Wolf, W.; Spiess, W.E.L. Physical, morphological and chemical characteristics, oil recovery and fatty acid composition of Balanites aegyptiaca Del. kernels. Plant Foods Hum. Nutr. 2002, 57, 179–189. [Google Scholar] [CrossRef]
- Saini, M.K.; Sharma, P.; Prasad, J.; Kothari, S.L.; Gour, V.S. Quality assessment of oil and biodiesel derived from Balanites aegyptiaca collected from different regions of Rajasthan. Biocatal. Agric. Biotechnol. 2019, 22, 101374. [Google Scholar] [CrossRef]
- Jauro, A.; Adams, M.H. Production and Biodegradability of Biodiesel from Balanites aegyptiaca Seed Oil. J. Korean Chem. Soc. 2011, 55, 680–684. [Google Scholar] [CrossRef]
- Kaoke, D.F.; Siryabe, E.; Iya-Sou, D.; Talla, E.; Kouotou, P.M. Physicochemical Potential of Balanites aegyptiaca Seed Kernel Oil from Northern Cameroon for Biodiesel valorization. J. Energy Res. Rev. 2021, 9, 21–31. [Google Scholar] [CrossRef]
- Bambara, L.; Sawadogo, M.; Roy, D.; Anciaux, D.; Blin, J.; Ouiminga, S. Biofuel from Balanites aegyptiaca: Optimization of the Feedstock Supply Chain. Sustainability 2018, 10, 4501. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Riyami, M.; Al-Haj, L.; Al-Muhtaseb, A.H.; Myint, M.T.Z.; Baawain, M.; Al-Abri, M. Waste Balanites aegyptiaca seed oil as a potential source for biodiesel production in the presence of a novel mixed metallic oxide catalyst. Int. J. Energy Res. 2021, 45, 17189–17202. [Google Scholar] [CrossRef]
- Huntington, J.L.; Hegewisch, K.C.; Daudert, B.; Morton, C.G.; Abatzoglou, J.T.; McEvoy, D.J.; Erickson, T. Climate Engine: Cloud Computing and Visualization of Climate and Remote Sensing Data for Advanced Natural Resource Monitoring and Process Understanding. Bull. Am. Meteorol. Soc. 2017, 98, 2397–2410. [Google Scholar] [CrossRef]
- DGF. Deutsche Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden und Verwandten Stoffen; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 1998. [Google Scholar]
- Li, C.; Yao, Y.; Zhao, G.; Cheng, W.; Liu, H.; Liu, C.; Shi, Z.; Chen, Y.; Wang, S. Comparison and analysis of fatty acids, sterols, and tocopherols in eight vegetable oils. J. Agric. Food Chem. 2011, 59, 12493–12498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, P.; Sun, X.; Wang, X.; Xu, B.; Wang, X.; Ma, F.; Zhang, Q.; Ding, X. Classification and adulteration detection of vegetable oils based on fatty acid profiles. J. Agric. Food Chem. 2014, 62, 8745–8751. [Google Scholar] [CrossRef]
- Elbadawi, S.M.; Ahmad, E.E.; Mariod, A.A.; Mathäus, B. Effects of thermal processing on physicochemical properties and oxidative stability of Balanities aegyptiaca kernels and extracted oil. Grasas Aceites 2017, 68, 184. [Google Scholar] [CrossRef]
- Mehanni, A.E.S.; El-Reffaei, W.H.M.; Melo, A.; Casal, S.; Ferreira, I.M. Enzymatic Extraction of Oil from Balanites aegyptiaca (Desert Date) Kernel and Comparison with Solvent Extracted Oil. J. Food Biochem. 2017, 41, e12270. [Google Scholar] [CrossRef]
- Okia, C. Balanites aegyptiaca: A Resource for Improving Nutrition and Income of Dryland Communities in Uganda. Ph.D. Thesis, Bangor University, Bangor, UK, 2010. [Google Scholar]
- Diedhiou, D.; Faye, M.; Candy, L.; Vandenbossche, V.; Vilarem, G.E.; Sock, O.; Rigal, L. Composition and balance of the analytical fractionation of desert date (Balanites aegyptiaca L.) seeds harvested in Senegal. Afr. J. Biotechnol. 2021, 20, 150–158. [Google Scholar]
- Gardette, J.-L.; Baba, M. FTIR and DSC studies of the thermal and photochemical stability of Balanites aegyptiaca oil (Toogga oil). Chem. Phys. Lipids 2013, 170–171, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Lu, X.; Sun, H.; Wang, F. Effect of oilseed roasting on the quality, flavor and safety of oil: A comprehensive review. Food Res. Int. 2021, 150, 110791. [Google Scholar] [CrossRef] [PubMed]
- Chbani, M.; El Harkaoui, S.; Willenberg, I.; Matthäus, B. Review: Analytical Extraction Methods, Physicochemical Properties and Chemical Composition of Cactus (Opuntia ficus-indica) Seed Oil and Its Biological Activity. Food Rev. Int. 2023, 39, 4496–4512. [Google Scholar] [CrossRef]
- Hajib, A.; El Harkaoui, S.; Choukri, H.; Khouchlaa, A.; Aourabi, S.; El Menyiy, N.; Bouyahya, A.; Matthaeus, B. Apiaceae Family an Important Source of Petroselinic Fatty Acid: Abundance, Biosynthesis, Chemistry, and Biological Proprieties. Biomolecules 2023, 13, 1675. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.; Gholizadeh, A.; Rauf, S.; Shariati, F. Drought-stress induced changes of fatty acid composition affecting sunflower grain yield and oil quality. Food Sci. Nutr. 2023, 11, 7718–7731. [Google Scholar] [CrossRef]
- Porokhovinova, E.A.; Matveeva, T.V.; Khafizova, G.V.; Bemova, V.D.; Doubovskaya, A.G.; Kishlyan, N.V.; Podolnaya, L.P.; Gavrilova, V.A. Fatty acid composition of oil crops: Genetics and genetic engineering. Genet. Resour. Crop Evol. 2022, 69, 2029–2045. [Google Scholar] [CrossRef]
- Nguyen, Q.-H.; Talou, T.; Evon, P.; Cerny, M.; Merah, O. Fatty acid composition and oil content during coriander fruit development. Food Chem. 2020, 326, 127034. [Google Scholar] [CrossRef]
- Sidorov, R.A.; Tsydendambaev, V.D. Biosynthesis of fatty oils in higher plants. Russ. J. Plant Physiol. 2014, 61, 1–18. [Google Scholar] [CrossRef]
- Wei, W.; Sun, C.; Jiang, W.; Zhang, X.; Hong, Y.; Jin, Q.; Tao, G.; Wang, X.; Yang, Z. Triacylglycerols fingerprint of edible vegetable oils by ultra-performance liquid chromatography-Q-ToF-MS. LWT 2019, 112, 108261. [Google Scholar] [CrossRef]
- Indelicato, S.; Bongiorno, D.; Pitonzo, R.; Di Stefano, V.; Calabrese, V.; Indelicato, S.; Avellone, G. Triacylglycerols in edible oils: Determination, characterization, quantitation, chemometric approach and evaluation of adulterations. J. Chromatogr. A 2017, 1515, 1–16. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Williams, M.; Chartzoulakis, K.; Harwood, J. Effect of irrigation on quality attributes of olive oil. J. Agric. Food Chem. 2009, 57, 7048–7055. [Google Scholar] [CrossRef] [PubMed]
- El Harkaoui, S.; Gharby, S.; Kartah, B.; El Monfalouti, H.; El-sayed, M.E.; Abdin, M.; Salama, M.A.; Charrouf, Z.; Matthäus, B. Lipid profile, volatile compounds and oxidative stability during the storage of Moroccan Opuntia ficus-indica seed oil. Grasas Aceites 2023, 74, e486. [Google Scholar] [CrossRef]
- Gharby, S.; Charrouf, Z. Argan Oil: Chemical Composition, Extraction Process, and Quality Control. Front. Nutr. 2021, 8, 804587. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Kelly, F.J.; Salonen, J.T.; Neuzil, J.; Zingg, J.-M.; Azzi, A. The European perspective on vitamin E: Current knowledge and future research. Am. J. Clin. Nutr. 2002, 76, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Kodad, O.; Socias i Company, R.; Alonso, J.M. Genotypic and Environmental Effects on Tocopherol Content in Almond. Antioxidants 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Carrera, C.S.; Seguin, P. Factors Affecting Tocopherol Concentrations in Soybean Seeds. J. Agric. Food Chem. 2016, 64, 9465–9474. [Google Scholar] [CrossRef]
- Romero, M.P.; Tovar, M.J.; Ramo, T.; Motilva, M.J. Effect of crop season on the composition of virgin olive oil with protected designation of origin “Les garrigues”. J. Am. Oil Chem. Soc. 2003, 80, 423–430. [Google Scholar] [CrossRef]
- Chaudhary, N.; Khurana, P. Vitamin E biosynthesis genes in rice: Molecular characterization, expression profiling and comparative phylogenetic analysis. Plant Sci. 2009, 177, 479–491. [Google Scholar] [CrossRef]
- Sattler, S.E.; Gilliland, L.U.; Magallanes-Lundback, M.; Pollard, M.; DellaPenna, D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 2004, 16, 1419–1432. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Semchuk, N.M. Tocopherol biosynthesis: Chemistry, regulation and effects of environmental factors. Acta Physiol. Plant 2012, 34, 1607–1628. [Google Scholar] [CrossRef]
- Aremu, M.O.; Andrew, C.; Oko, O.J.; Odoh, R.; Zando, C.; Usman, A.; Akpomie, T. Comparative Studies on the Physicochemical Characteristics and Lipid Contents of Desert Date (Balanites aegyptiaca (L.) Del) Kernel and Pulp Oils. Eur. J. Nutr. Food Saf. 2022, 14, 20–30. [Google Scholar] [CrossRef]
- Khan, Z.; Nath, N.; Rauf, A.; Emran, T.B.; Mitra, S.; Islam, F.; Chandran, D.; Barua, J.; Khandaker, M.U.; Idris, A.M.; et al. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chem. Biol. Interact. 2022, 365, 110117. [Google Scholar] [CrossRef] [PubMed]
- Kirkhus, B.; Lundon, A.R.; Haugen, J.-E.; Vogt, G.; Borge, G.I.A.; Henriksen, B.I.F. Effects of environmental factors on edible oil quality of organically grown Camelina sativa. J. Agric. Food Chem. 2013, 61, 3179–3185. [Google Scholar] [CrossRef]
- Roche, J.; Bouniols, A.; Mouloungui, Z.; Barranco, T.; Cerny, M. Management of environmental crop conditions to produce useful sunflower oil components. Eur. J. Lipid Sci. Technol. 2006, 108, 287–297. [Google Scholar] [CrossRef]
- Kumar, M.S.S.; Mawlong, I.; Ali, K.; Tyagi, A. Regulation of phytosterol biosynthetic pathway during drought stress in rice. Plant Physiol. Biochem. 2018, 129, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Szakiel, A. The role of sterols in plant response to abiotic stress. Phytochem. Rev. 2020, 19, 1525–1538. [Google Scholar] [CrossRef]
- Yamaya, A.; Endo, Y.; Fujimoto, K.; Kitamura, K. Effects of genetic variability and planting location on the phytosterol content and composition in soybean seeds. Food Chem. 2007, 102, 1071–1075. [Google Scholar] [CrossRef]
- Roche, J.; Alignan, M.; Bouniols, A.; Cerny, M.; Mouloungui, Z.; Vear, F.; Merah, O. Sterol content in sunflower seeds (Helianthus annuus L.) as affected by genotypes and environmental conditions. Food Chem. 2010, 121, 990–995. [Google Scholar] [CrossRef]





| Sample Code | Harvest Location | Temperature Min–Max (°C) | Rainfall (mm/Year) |
|---|---|---|---|
| Mo1 | Tata–Morocco | 3.98–41.04 | 69.7 |
| Mo2 | Tata–Morocco | 6.08–41.54 | 64.0 |
| Mo3 | Tata–Morocco | 6.02–41.49 | 205.4 |
| Mau1 | Guidimakha–Mauritania | 18.03–43.49 | 352.5 |
| Mau2 | Guidimakha–Mauritania | 18.03–43.49 | 352.5 |
| Su1 | El Fulah–Sudan | 11.03–39.90 | 533.2 |
| Su2 | Al Fashir–Sudan | 16.76–40.67 | 466.6 |
| Su3 | Al ‘Abbasiyah–Sudan | 18.91–38.12 | 591.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Harkaoui, S.; El Kaourat, A.; El Monfalouti, H.; Kartah, B.E.; Mariod, A.A.; Charrouf, Z.; Rohn, S.; Drusch, S.; Matthäus, B. Chemical Composition and Geographic Variation of Cold Pressed Balanites aegyptiaca Kernel Oil. Foods 2024, 13, 1135. https://doi.org/10.3390/foods13071135
El Harkaoui S, El Kaourat A, El Monfalouti H, Kartah BE, Mariod AA, Charrouf Z, Rohn S, Drusch S, Matthäus B. Chemical Composition and Geographic Variation of Cold Pressed Balanites aegyptiaca Kernel Oil. Foods. 2024; 13(7):1135. https://doi.org/10.3390/foods13071135
Chicago/Turabian StyleEl Harkaoui, Said, Asma El Kaourat, Hanae El Monfalouti, Badr Eddine Kartah, Abdalbasit Adam Mariod, Zoubida Charrouf, Sascha Rohn, Stephan Drusch, and Bertrand Matthäus. 2024. "Chemical Composition and Geographic Variation of Cold Pressed Balanites aegyptiaca Kernel Oil" Foods 13, no. 7: 1135. https://doi.org/10.3390/foods13071135
APA StyleEl Harkaoui, S., El Kaourat, A., El Monfalouti, H., Kartah, B. E., Mariod, A. A., Charrouf, Z., Rohn, S., Drusch, S., & Matthäus, B. (2024). Chemical Composition and Geographic Variation of Cold Pressed Balanites aegyptiaca Kernel Oil. Foods, 13(7), 1135. https://doi.org/10.3390/foods13071135






