Abstract
Sesame seeds (Sesamum indicum L.) have been cultivated for thousands of years and have long been celebrated for their culinary versatility. Beyond their delightful nutty flavor and crunchy texture, sesame seeds have also gained recognition for their remarkable health benefits. This article provides an in-depth exploration of the numerous ways in which sesame seeds contribute to overall well-being. Sesame seeds are a powerhouse of phytochemicals, including lignans derivatives, tocopherol isomers, phytosterols, and phytates, which have been associated with various health benefits, including the preservation of cardiovascular health and the prevention of cancer, neurodegenerative disorders, and brain dysfunction. These compounds have also been substantiated for their efficacy in cholesterol management. Their potential as a natural source of beneficial plant compounds is presented in detail. The article further explores the positive impact of sesame seeds on reducing the risk of chronic diseases thanks to their rich polyunsaturated fatty acids content. Nevertheless, it is crucial to remember the significance of maintaining a well-rounded diet to achieve the proper balance of n-3 and n-6 polyunsaturated fatty acids, a balance lacking in sesame seed oil. The significance of bioactive polypeptides derived from sesame seeds is also discussed, shedding light on their applications as nutritional supplements, nutraceuticals, and functional ingredients. Recognizing the pivotal role of processing methods on sesame seeds, this review discusses how these methods can influence bioactive compounds. While roasting the seeds enhances the antioxidant properties of the oil extract, certain processing techniques may reduce phenolic compounds.
1. Introduction
Sesame (Sesamum indicum L.) is a plant classified under the Pedaliaceae family and is often called the “seed of immortality.” This plant is an erect annual herb that has different names across various cultures, including ajonjoli (Spanish), hu ma (Chinese), gergelim (Portuguese), goma (Japanese), sesame (French), til (Hindi), and konjed (Persian) [1,2,3]. This crop is among the earliest to have been domesticated for oil production and also served as one of the first condiments utilized [4,5]. The precise location of sesame’s domestication remains uncertain; however, despite various assertions, it is widely believed that the crop originated in Africa and subsequently disseminated to West Asia, China, India, and Japan [5,6]. The harvested area (which has expanded from 5.0 million hectares in 1961 to 13.97 million ha in 2022) and the production of sesame have increased over the last few decades (1.4 million tonnes in 1961 to 7.4 million tonnes in 2022) [7,8]. Despite its widespread cultivation in various regions of the southern United States, Latin America, Asia, and Africa, the crop commonly known as the “queen of oilseeds” is considered an orphan crop and is not currently mandated by any International Crop Research Institute for Semi-Arid Tropics [7,9]. According to the Food and Agriculture Organization, most of the world’s sesame crop is cultivated in less developed nations like Uganda, Sudan, Nigeria, India, China, Burma, and Brazil. South Sudan ranks fifth in the world for area harvested for sesame seeds. The crop is mainly grown by small-holder farmers, while some commercial farmers are in Upper Nile State. In 2021, the total sesame production in South Sudan was 26,000 MT, and the yield was 0.3 tons/ha. Local demand for processed sesame seeds and byproducts, most of which are imported from neighboring countries, is growing. In 2021, South Sudan exported USD 253k in sesame oil or fractions not chemically modified, making it the 44th largest exporter in the world. Sesame is the 16th most exported product in South Sudan, the main destination of exports being the United Arab Emirates and France [7,8,10]. Sesame is regarded as one of China’s four most important traditional edible oil crops, along with soybean, peanut, and rape. Approximately 45% of sesame in China is allocated for producing sesame oil, while 22% is utilized for sesame paste, another 22% for sesame peeling, and a mere 5% for baked goods [11,12]. Sesame has long been a favorite among humans as a traditional medicinal plant with rich nutritional value and taste; also, it plays a crucial part in humans’ diet as a nutrient-dense food that is widely used in the food industry as an ingredient in various food products (e.g., bread, biscuits, burgers, cakes, dressings, dishes, snacks, and edible oil) due to its high oil content, pleasant scent, and resilience to oxidation [13]. In addition to their use as a food source, sesame seeds have extensive applications within the pharmaceutical and cosmetic sectors [14]. To produce different food products, animal feed, industrial supplies, lubricants, soaps, medicinal supplies, and co-products from sesame, the primary process of raw material is acquired [15].
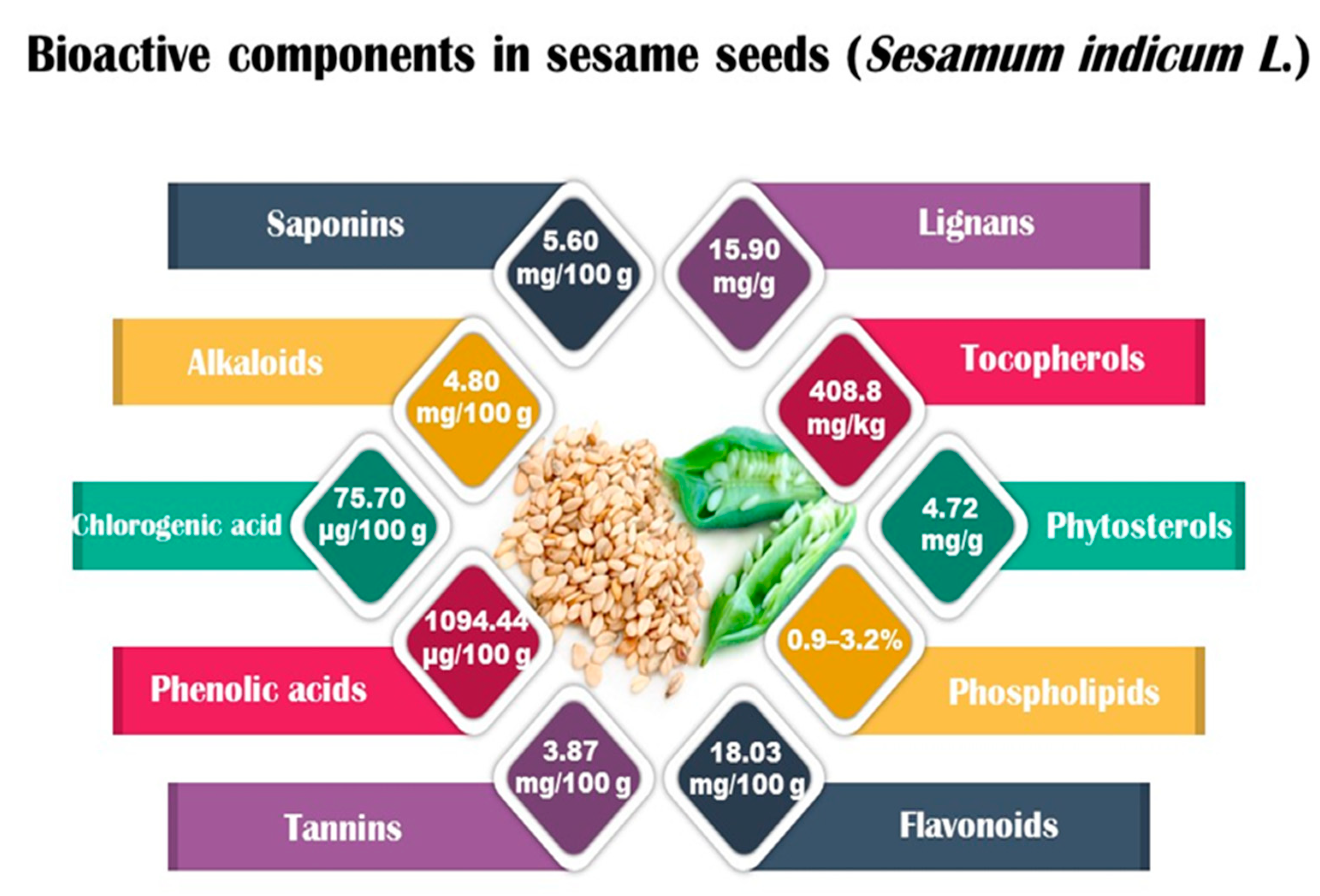
Sesame seeds are obtainable in three distinct colors: black, brown, and white. They contain several essential nutrients in varying proportions. The composition of sesame seeds comprises 45–65% oil, a noteworthy source of plant-based protein with content ranging from 19 to 35% per 100 g of seeds, 14 to 20% carbohydrates, and 15 to 20% hull material. Although the protein content of sesame seeds is lower than that of meat, it is comparable to or higher than many grains, such as rice or wheat [16,17]. These tiny seeds also contain measurable amounts of oxalic acid, dietary fiber, antioxidants, and minerals (iron, magnesium, and zinc). The fatty acid content in sesame seeds is predominantly unsaturated fatty acids, such as oleic and linoleic acids, with smaller amounts of saturated fatty acids, like palmitic and stearic acids. Sesame oil consists of unsaponifiable fractions such as sesamin, sesamolin, and sterols. Additionally, sesame seeds are an excellent source of calcium, containing essential amino acids like methionine, valine, and tryptophan. Sesame seeds also contain bioactive components like phenolics, vitamins, phytosterols, and polyunsaturated fatty acids (PUFAs), which benefit human health [18,19]. Figure 1 shows bioactive compounds that could be detected in sesame.
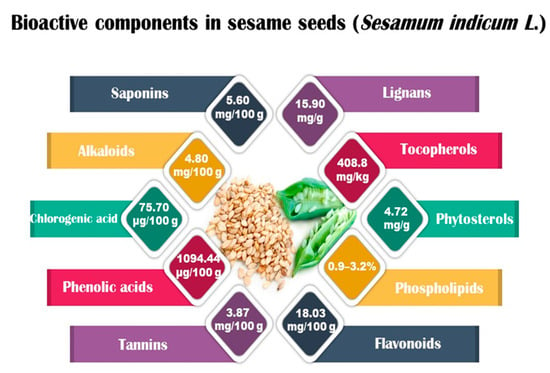
Figure 1.
Bioactive components in sesame (Sesamum indicum L.).
Sesame lignans may account for the seed’s popularity, including sesamin, sesamolin, and sesamol. Sesame oil has been shown to have antioxidant and health-promoting benefits due to its high concentration of tocopherol, phytosterol, lignan, and other components [20,21]. The protective effects of sesame on heart function, regulation of lipid metabolism, and prevention of mutations and cancer have been demonstrated in numerous studies [22,23]. According to Ahmad and Ghosh’s (2020) research, sesame seeds possess a high nutrient content that may benefit the immune system significantly and potentially mitigate the risk of health complications associated with COVID-19. They have reported that regular consumption of sesame may lessen the likelihood of contracting viruses and stave against health issues like malnutrition that might arise [24]. Hsu and Parthasarathy [25] have indicated that the consumption of sesame oil can reduce levels of low-density lipoprotein (LDL) and decrease the risk of atherosclerosis and cardiovascular diseases. Alzheimer’s disease is linked to the deposition of toxic cellular amyloid proteins, and the prolonged consumption of sesamol may efficiently hinder this buildup [26].
This review presents a comprehensive overview of the structure of various bioactive compounds found in sesame seeds and products. This review also covers recent advancements in studying the impact of food processing on sesame seed oil and the mechanisms by which these technologies affect the bioactivity of sesame compounds.
2. The Bioactive Compounds and Health Benefits of Sesame
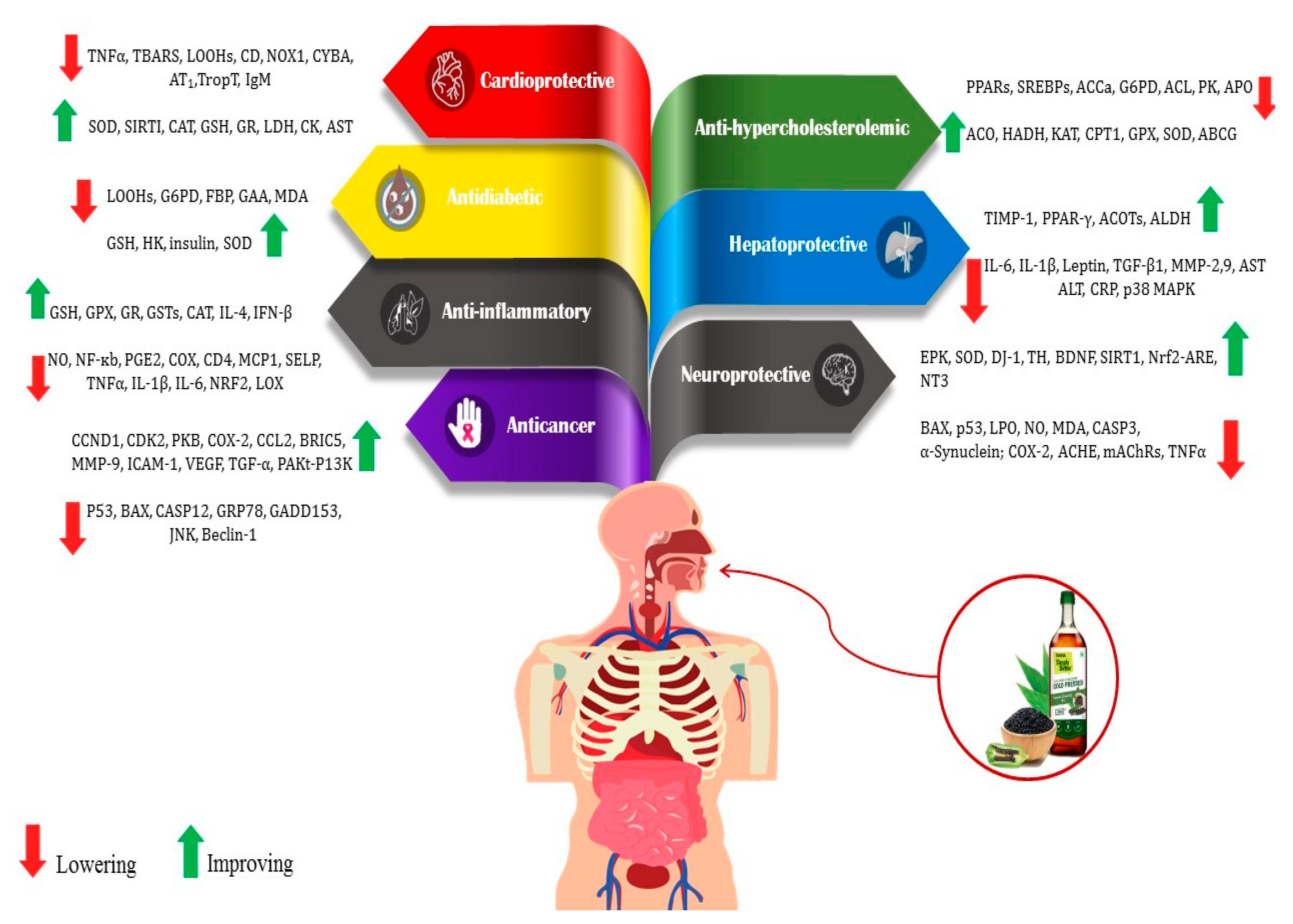
Bioactive compounds present in food are considered advantageous constituents that contribute to the prevention of diseases (Figure 2). The compounds in this category are diverse and consist of carotenoids, phenolics, phytosterols, and PUFAs [20,27,28]. The mentioned compounds are frequently employed as antioxidants and for various other functions, including but not limited to impeding cholesterol absorption and obstructing the activity of bacterial toxins [29,30,31]. PUFAs, lignans, tocopherols, and phytosterols are among the antioxidants and bioactive compounds that could be present in high levels of sesame. Compared to other edible oils, the high antioxidant content of sesame oil improves energy and resistance to aging [12,30].
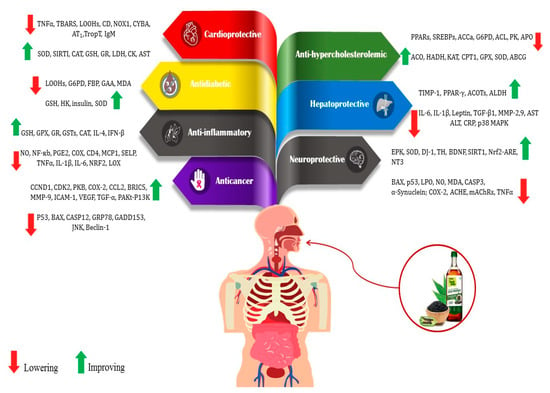
Figure 2.
Health benefits of bioactive compounds in sesame (Sesamum indicum L.) and its mechanism. ABCG: ATP-binding cassette, subfamily G; ACCa: acetyl-CoA carboxylase 1; ACHE: acetylcholinesterase; ACL: ATP citrate lyase; ACO: 1-aminocyclopropane-1-carboxylic acid oxidase; ACOTs: acyl-CoA thioesterases; ALDH: aldehyde dehydrogenases; ALT: alanine aminotransferase; APOA4: apolipoprotein; AST: aspartate aminotransferase; AT1: angiotensin type 1; BAX: apoptosis regulator; BDNF: brain-derived neurotrophic factor; BIRC5: survivin protein; CASP3: caspase 3; CASP12: caspase 12; CAT: catalase; CCL2: chemokine ligand 2; CCND1: cyclin D1; CD: conjugated dienes; CDK2: cyclin-dependent kinase 2; CD4: cluster of differentiation; CK: creatine kinase; CPT1: carnitine palmitoyl transferase I; COX: cyclooxygenase; CRP: C-reactive protein; CYBA: human neutrophil cytochrome b light chain; DJ-1: protein deglycase; ERK: extracellular signal-regulated kinase; FBP: fructose 1,6-bisphosphatase; GAA: alpha glucosidase; GADD153: DNA damage-inducible gene 153; GPx: glutathione peroxidase; GR: glutathione reductase; GRP78: glucose regulatory protein 78; GSH: reduced glutathione; GSTs: glutathione-S-transferases; G6P: glucose 6-phosphate; HADH: 3-hydroxy acyl-CoA dehydrogenase; HK: hexokinase; ICAM-1: Intercellular adhesion molecule-1; IFN-β: Interferon-β; IgM: anticardiolipin antibody; IL-1β: interleukin-1β; IL-6: interleukin 6; IL-4: interleukin-4; JNK: c-Jun N-terminal kinase; KAT: 3-ketoacyl-CoA thiolase; LDH: lactate dehydrogenase; LOOHs: lipid hydroperoxide; LOX: lipoxygenase; LPO: lipid peroxidation; mAChRs: muscarinic acetylcholine receptors; MCP1: monocyte chemoattractant protein-1; MMP2: metalloproteinase-2; MMP-9: matrix metallopeptidase 9; NF-κB: nuclear factor kappa B; NO: nitric oxide; NOX1: NADPH oxidase 1; NRF2: nuclear-factor-erythroid-2-related factor 2; Nrf2-ARE pathway: transcription factor Nrf2- antioxidant responsive element; NT3: neurotrophin-3; p38 MAPK: p38 mitogen-activated protein kinases; P53: yumor protein P53; PAkt-PI3K: phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway;PGE2: prostaglandin E2; PK: pyruvate kinase; PKB: protein kinase B; PPARs: peroxisome proliferate activated receptor α; PPAR-γ: peroxisome proliferator-activated receptor gamma; SELP: P-selectin; SIRT1: sirtuin 1; SREBPs: sterol regulatory element binding proteins; TBARS: thiobarbituric acid reactive substances; TH: tyrosine hydroxylase; TGF-β1: transforming growth factor beta 1; TIMP-1: tissue inhibitor of metalloproteinases-1; TropT: troponin T;TNFα: tumor necrosis factor α; VEGF: vascular endothelial growth factor [20].
Secondary metabolites known as natural phenolic compounds are extensively found throughout the plant kingdom. Phenolic compounds found in plants have been recognized for their potential to mitigate oxidative-stress-related conditions, including but not limited to cardiovascular and neurodegenerative disorders, as well as cancer [32,33,34,35,36,37]. Phenolics are distinguished by an aromatic ring, typically with one or more hydroxyl groups attached. Phenolics exhibit a notable antioxidative capacity owing to their ability to generate stable radical intermediates through electron utilization. Owing to their capacity to act as antioxidants, they assume a significant function in stabilizing edible oils and safeguarding against the development of undesirable flavors [38,39,40,41,42]. Recent research indicates that phenolic compounds may significantly impact ailments related to oxidative stress. The antioxidant and diverse benefits associated with sesame seed and its oil are due to the existence of lignans, specifically sesamin, sesamolin, sesaminol, sesangolin, 2-episalatin, and tocopherol isomers [18,43]. The high resistance of sesame oil to oxidative rancidity can be attributed to the chemical constituents sesamol and sesamol dimer.
2.1. Lignans
Natural products are based on the fundamental structure of a C6C3 unit, which is identified as a phenylpropanoid skeleton or propylbenzene [29,44]. The first proposal to the categorization of compounds formed by the linkage of two C6C3 units with a β,β′ bond (8-8′ bond) as lignans was proposed by Haworth [45] in a study on natural resins. Lignan, derived from two p-hydroxyphenylpropane molecules, is a constituent of lignin, a substance with a general term. Sesame seeds contain two primary groups of lignans: (I) oil-soluble lignans (sesamin, sesamolin, sesaminol, sesamolinol, and pinoresinol) and (II) glycosylated water-soluble lignans (sesaminol triglucoside, pinoresinol triglucoside, sesaminol monoglucoside, pinoresinol monoglucoside, and two isomers of pinoresinol diglucoside and sesaminol diglucoside) [46,47]. They all demonstrate various biological characteristics (Table 1) [48,49,50].
The antimicrobial and antioxidant efficacy ranking among the three compounds is as follows: sesamolin, sesamin, and sesamol [51,52,53]. Egawa et al. [54] reported that stimulating sympathetic nerve activity by sesame lignans improves muscle blood flow. In addition, the antioxidative properties of lignans have been observed to affect multiple models of brain dysfunction and protect against age-related brain dysfunction [20,55]. Enhancing the lignan content of sesame has become a crucial objective in sesame breeding programs because of its health-promoting properties, which have made it a functional compound [48,56]. Sesamin and sesamolin are viable options for conducting quality control assessments on samples and determining their biological value [57,58,59].

Table 1.
Structures, quantities, biological activities, and mechanisms of different lignans detected from Sesamum indicum.
Table 1.
Structures, quantities, biological activities, and mechanisms of different lignans detected from Sesamum indicum.
| Lignans in Sesame | Name of Component | Molecular Structure | Quantity/Amount of Raw Sesame Seeds and Sesame Oil | Biological Characteristics | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Oil-soluble lignans | Sesamin | 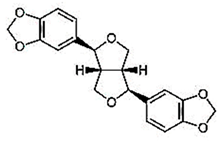 C20H18O6 | Sesame seed: 0.77–9.3 mg/g Sesame oil: 6.20 mg/g | Antioxidant Properties |
| [50,56,60,61,62,63,64,65] |
| Metabolic Health and Prevention of Diabetes |
| |||||
| Cytotoxic Activity |
| |||||
| Atherosclerosis |
| |||||
| Sesamol | 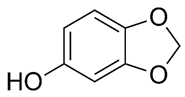 C7H6O3 | Sesame seed: 1.20 mg/g Sesame oil: 0.27–3.37 mg/g | Cardioprotective Properties |
| [66,67,68,69,70,71,72,73] | |
| Antioxidant Activity |
| |||||
| Anti-Inflammatory |
| |||||
| Antiangiogenic |
| |||||
| Apoptosis Induction |
| |||||
| Epigenetic Regulation |
| |||||
| Detoxification and Phase II Enzyme Induction |
| |||||
| Sesamolin | 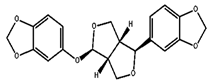 C20H18O7 | Sesame seed: 4.50 mg/g Sesame oil: 2.45 mg/g | Neuroprotective Activity |
| [51,59,74,75,76] | |
| Antileukemic Effects |
| |||||
| Antimelanogenesis in Skin Cancer |
| |||||
| Sesaminol | 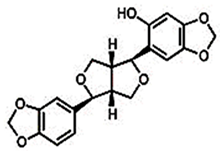 C20H18O7 | Sesame seed: 1.40 mg/g Sesame oil: 0.01 mg/g | Anticancer Effects |
| [77,78] | |
| Detoxification Pathways |
| |||||
| Preventing Parkinson’s Disease |
| |||||
| Sesamolinol | 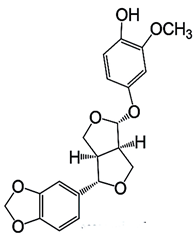 C20H20O7 | Cardioprotective Effects |
| [23,79,80] | ||
| Hormonal Modulation |
| |||||
| Antimicrobial Properties |
| |||||
| Pinoresinol | 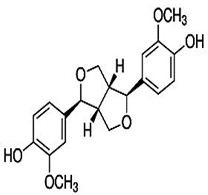 C20H22O6 | Sesame seed: 0.29–0.47 mg/g Sesame oil: - | Hypoglycemic agent |
| [31,81,82,83,84,85] | |
| Hepatoprotective |
| |||||
| Chemoprevention |
| |||||
| Enterolignan Formation |
| |||||
| Autophagy Induction |
| |||||
| Interaction with Gut Microbiota |
| |||||
| Glycosylated water-soluble lignans | Sesaminol Triglucoside (STG) | 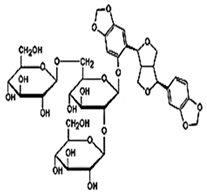 C38H48O22 | Sesame seed: 0.36–15.60 mg/g | Glucoside Hydrolysis |
| [86,87] |
| Pinoresinol Triglucoside (PTG) | 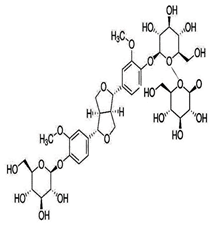 C34H42O18 | Not explicitly documented | Autophagy Induction | PTG induces autophagy in ovarian cancer cells (SKOV-3). This is associated with increased expression of LC3 II and Beclin and decreased expression of p62. | [84,88] | |
| Mitochondrial Membrane Potential (MMP) Loss | It reduces the MMP of SKOV-3 cells, affecting their mitochondrial function. | |||||
| Inhibition of Cell Invasion | It inhibits the invasion capacity of SKOV-3 cells. | |||||
| Raf/MEK/ERK Signaling Pathway Inhibition | PTG concentration-dependently inhibits the expression of phospho-MEK and phospho-ERK, key signaling pathway components. | |||||
| Tumor Growth Inhibition | In xenografted tumor models in mice, PTG significantly inhibits tumor growth, demonstrating its potential as an ovarian cancer treatment. | |||||
| Sesaminol Monoglucoside (SMG) | 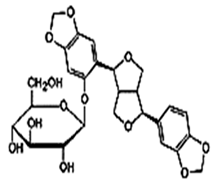 C26H28O12 | Not explicitly documented | Antioxidant Activity |
| [5,89] | |
| Anti-Inflammatory Effects | Modulating inflammatory pathways by inhibiting pro-inflammatory cytokines. | |||||
| Cardiovascular Health | Contributing to cardiovascular health by reducing oxidative damage and inflammation. | |||||
| Metabolic Regulation | It is impacting lipid metabolism and glucose homeostasis. | |||||
| Cancer Prevention | Some studies suggest that SMG may have anticancer potential, although further research is needed. | |||||
| Cell Signaling | Influencing cell signaling pathways related to cell growth and differentiation. | |||||
| Pinoresinol Monoglucoside (PMG) |  C26H32O11 | Not explicitly documented | Metabolism and Bioavailability | PMG would undergo metabolic processes, potentially in the digestive system or the liver, leading to the release of pinoresinol and glucose. The bioavailability of pinoresinol and its metabolites would influence their distribution and effects in the body. | [48,90] | |
| Antioxidant Effects | scavenging free radicals and reducing oxidative stress in cells. | |||||
| Impact on Lipid Metabolism | Modulating cholesterol levels and the promotion of cardiovascular health. | |||||
| Pinoresinol Diglucoside (PDG) |  C32H42O16 | Sesame seed: <5 to 232 mg/100 g Sesame oil: Not explicitly documented | Glucoside Hydrolysis | The glucoside structure of PDG may be hydrolyzed in vivo, leading to the release of pinoresinol. Enzymes often mediate this process, and the liberated pinoresinol can exert its biological effects. | [5,47,80] | |
| Antioxidant and Cytoprotective Effects |
| |||||
| Impact on Lipid Metabolism |
| |||||
| Sesaminol Diglucoside (SDG) | 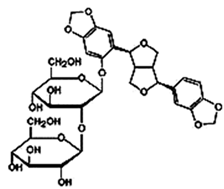 C32H38O17 | Sesame seeds: 98 mg/100 g Not explicitly documented | Anti-Inflammatory Properties |
| [5,41,91] | |
| Cellular Signaling Pathways |
|
2.1.1. Sesamol
Sesamol is a nutritional constituent and degradation byproduct of lignan derived from sesame. Identifying a novel antioxidative substance in sesame oil has highlighted its significance as a quality stabilizer and crucial aroma component [21,66,92]. Sesamol is a very-low-molecular-weight compound that is sufficiently volatile to be removed by deodorization. Consequently, it should be added to the fat after deodorization [93]. Sesamol has been documented as having various applications, including antioxidant, lipid-lowering, antidepressant, and therapeutic effects for diabetic nephropathy and neuropathy, among others [67,68]. Moreover, it has been extensively researched as an anti-inflammatory, antiatherosclerotic, and cardioprotective agent. Another research investigation has examined the potential of sesamol to enhance cognitive function and mitigate inflammation in elderly mice. The research has discovered that sesamol exhibited anti-inflammatory properties and diminished oxidative stress markers in mice, resulting in improved cognitive performance in diverse behavioral assessments. The results have indicated that sesamol could serve as a safeguard against mental deterioration and inflammatory processes associated with aging [66,69]. The study by Liu et al. [94] investigated the effects of sesamol on cognitive function in a mouse model with a high-fat- and high-fructose-diet-induced cognitive defect. They evaluated the potential of sesamol in ameliorating cognitive defects induced by the diet and investigated the insulin signaling pathways in the central nervous system. Sesamol administration significantly improved cognitive function, as confirmed by enhanced performance in the Morris water maze and novel object recognition tests. Sesamol also restored insulin signaling pathway activity in the central nervous system by increasing the expression of key proteins involved in insulin signaling. Sesamol can mitigate the production of amyloid and cognitive impairment caused by systemic inflammation by preventing neuronal injury. According to their study, sesamol has the potential to serve as a viable nutritional supplement for the prevention and treatment of obesity [94,95]. Chu et al. [70] conducted a study to investigate the potential protective effect of sesamol on endotoxemia-induced lung inflammation and injury in rats. They administered sesamol to rats and assessed its impact on endotoxemia-induced lung injury. Results indicated that sesamol administration significantly attenuated lung inflammation and injury, as demonstrated by reduced lung wet-to-dry weight ratio, decreased levels of proinflammatory cytokines, and reduced leukocyte infiltration in lung tissue. Sesamol was also found to inhibit the activation of nuclear factor-kappa B (NF-κB) and its downstream inflammatory mediators, including inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). These findings have suggested that sesamol has the potential to protect against endotoxemia-induced lung inflammation and injury in rats, potentially through its inhibitory effect on NF-κB activation and downstream inflammatory mediators. According to Thushara et al. [96], sesamol can effectively impede platelet aggregation, exhibiting antithrombotic and cardioprotective properties. Wang et al. [97] conducted a study to explore the potential of sesamol in preventing atherosclerosis that is mediated by renal injury. According to the research, sesamol could mitigate oxidative stress and inflammation, resulting in a decline in atherosclerosis among mice suffering from renal impairment. The inhibitory effect of Sesamol on IKKα and p53 activation, both implicated in atherosclerosis pathogenesis, has been reported. The results have indicated that sesamol could potentially serve as a therapeutic agent for preventing or treating atherosclerosis linked to renal damage [98]. The research has offered a potential mechanism through which sesamol can exert its protective impact, thereby providing a basis for further investigation into the development of focused treatments for atherosclerosis.
Chronic nephritis induces an irreversible decline in the glomerular filtration rate and the development of renal fibrosis, ultimately resulting in chronic kidney disease (CKD) and end-stage renal disease (ESRD) [99,100,101]. The infiltration of glomerular and interstitial macrophages is a hallmark of CKD, playing a pivotal role in renal injury [101,102]. Following kidney injury, damaged cells release cytokines or chemokines that recruit monocytes to inflammatory lesions, where they undergo activation and differentiation into macrophages [103,104]. Macrophages, functioning as crucial immunological regulators and inflammation mediators, are implicated in kidney damage and inflammation by secretion of inflammatory cytokines, such as interleukin (IL)-1 and IL-6 [71,105,106,107]. On-site activation and differentiation of inflammatory macrophages result in the release of IL-1, thereby triggering immune responses from Th1-type cells that contribute to tissue damage [108,109,110]. In CKD, immune cells infiltrating the kidneys play a deleterious role, actively participating in the progression of the disease and leading to nephron loss and fibrosis [111,112,113]. Tseng et al. [114] investigated the potential therapeutic effects of sesamol in addressing renal inflammation and reactive oxygen species (ROS)-mediated interleukin-1 beta (IL-1β) secretion. The study, in vivo and in vitro, focuses on the role of heme oxygenase-1 (HO-1) in inhibiting the IKKα/NFκB pathway. The findings indicated a protective role of sesamol, which revealed mitigation of renal inflammation and suppression of IL-1β secretion. The proposed mechanism involved the activation of HO-1, leading to the inhibition of the IKKα/NFκB pathway, recognized for its involvement in inflammatory processes. The study suggests the potential of sesamol as a therapeutic agent for addressing inflammatory conditions associated with renal disorders [114].
Sallam et al. [30] investigated the impact of incorporating sesame oil and sesamol as natural antimicrobial and antioxidant agents on the safety and shelf-life of meatballs. The findings of their research indicated that the incorporation of sesame oil and sesamol into meatballs resulted in a noteworthy antimicrobial impact on both Gram-positive and Gram-negative bacteria, encompassing Staphylococcus aureus, Escherichia coli, and Salmonella typhimurium. Moreover, the incorporation of sesame oil and sesamol enhanced the antioxidant potential of meatballs through the mitigation of lipid oxidation and elevation of the concentration of overall phenolic compounds. Furthermore, the sensory analysis conducted on meatballs incorporating sesame oil and sesamol indicated no statistically significant variation in flavor, appearance, and consistency compared to the control samples. The study’s results have suggested that sesame oil and sesamol possess the potential as natural preservatives for enhancing the safety and shelf-life of meat products due to their antimicrobial and antioxidant properties.
2.1.2. Sesamin
Sesamin is the most prominent lignan compound found in sesame seeds, one of the two highest sources of lignans (the other being flax) in the human diet. Sesamin is catered to be a nutritional supplement that is believed to possess various properties such as anti-inflammatory, pro-apoptotic, pro-angiogenic, antimetastatic, antiproliferative, and ant-oxidant effects, as well as pro-antiphagocytic activities (if touting its health properties) or possibly being an estrogen receptor modulator and fat burner (if targeting athletes or persons wishing to lose weight) [56,60]. Sesamin has several processes, which, when considered comprehensively, may be succinctly described as a modulator of fatty acid metabolism. It seems to hinder an enzyme called Δ5-desaturase, a key enzyme in the metabolism of fatty acids. By inhibiting this enzyme, it leads to decreased levels of eicosapentaenoic acid (EPA) and arachidonic acid, two types of fatty acids found in fish oil. This effect is observed when the substance is taken orally. Sesamin inhibits the process of tocopherol-ω-hydroxylation, which is the step that limits the metabolism of vitamin E. By inhibiting this enzyme, sesamin increases the levels of vitamin E in the body, especially the γ subset (γ-tocopherol and γ-tocotrienol). This mechanism has been confirmed to be active when sesamin is taken orally [61,115,116,117].
Although there are some potentially beneficial mechanisms at play, such as protection against Parkinson’s disease and promotion of bone mass, most of the mechanisms, including estrogen receptor modulation, fat burning from the liver, and activation of the antioxidant response element (ARE), have not been verified in humans. There are reasons to doubt their occurrence, such as the possibility of the concentration being too high to have an impact through oral supplementation or the fact that fat burning appears limited to rats. Ultimately, sesamin significantly enhances the metabolism of γ-tocopherol and γ-tocotrienol by inhibiting their degradation. This leads to increased levels of these vitamin E variants, which offer numerous therapeutic advantages. Given that these vitamin E supplements are costly, sesamin could serve as a cost-effective alternative or a means to dilute the vitamin E [62,118,119,120,121].
Episesamin, an isomer of sesamin, is produced during the refining of sesame oil. Additionally, it exerts a protective influence on the levels of lipid oxidation, blood glucose, and blood pressure [62,118,122]. According to Watanabe et al. [123], the consumption of sesamin is associated with reduced blood pressure. The impact of pure sesamin epimer on serum lipids was investigated by Peñalvo et al. [124]. The study findings demonstrated that administering stanol ester alone or combined with sesamin effectively mitigated the increase in cholesterol levels. The potential has been observed in sesamin as a phytochemical agent capable of effectively impeding the differentiation and function of osteoclasts [63]. Sesamin has been documented as a protective agent against acute lung injury induced by LPS. Research has investigated the potential of sesamin to suppress microglial activation induced by LPS. According to research findings, sesamin has demonstrated the ability to decrease the expression of TLR4, a receptor that plays a role in microglia activation. This results in decreased production of pro-inflammatory cytokines and nitric oxide. The results indicate that sesamin could potentially serve as a therapeutic agent for preventing or treating neuroinflammatory conditions linked to microglial activation. The research elucidates the mechanism through which sesamin confers its protective effect, thereby providing a potential direction for further investigation in developing precise therapeutic interventions for neuroinflammatory disorders [119,125]. Zhang et al. [126] found that sesamin administration in animal models and humans was associated with a reduction in serum levels of total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglycerides, as well as an increase in high-density lipoprotein cholesterol (HDL-C). Moreover, sesamin was observed to inhibit the activity of key enzymes involved in cholesterol synthesis and fatty acid metabolism, such as 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA reductase) and acetyl-CoA carboxylase (ACC). Zhang et al. [127] conducted a study to examine the potential beneficial effects of sesamin on high-fat-diet-induced dyslipidemia and kidney injury in rats. They investigated the role of oxidative stress in developing these conditions and assessed the impact of sesamin on oxidative stress markers. The results showed that rats fed a high-fat diet exhibited significant dyslipidemia, characterized by elevated levels of total cholesterol, triglycerides, and low-density lipoprotein cholesterol (LDL-C), as well as reduced levels of high-density lipoprotein cholesterol (HDL-C). In addition, these rats exhibited kidney injury, as evidenced by increased serum creatinine levels and blood urea nitrogen (BUN). However, supplementation with sesamin at both low and high doses was observed to ameliorate these effects. They have found that sesamin administration significantly reduced serum levels of total cholesterol, triglycerides, and LDL-C, as well as increased HDL-C. Furthermore, sesamin was found to decrease oxidative stress markers, including malondialdehyde (MDA) and reactive oxygen species (ROS), and increase the activity of antioxidant enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px). In another study, the results showed that sesamin increased the expression of key proteins involved in cholesterol efflux, such as ATP-binding cassette transporter A1 (ABCA1) and scavenger receptor class B type I (SR-BI), while decreasing the expression of proteins involved in cholesterol uptake, such as CD36 and scavenger receptor class A (SR-A) [128]. A study was conducted in HepG2 cells, a human liver cell line, and used various techniques to determine the effect of sesamin on lipid metabolism and gene expression. The results showed that sesamin inhibited lipid accumulation in HepG2 cells by downregulating the expression of key lipogenic genes, such as sterol regulatory element-binding protein 1c (SREBP-1c) and fatty acid synthase (FAS), through interaction with LXRα and PXR. The findings suggest that sesamin has the potential to be used as a therapeutic agent for preventing or treating lipogenesis-associated diseases such as steatosis [129]. Various studies have recognized Sesamin as a potent agent with promising immunomodulatory and anti-inflammatory effects. It has been shown to have the potential to modulate the immune response by regulating the production of cytokines, which are key players in the inflammatory response. Sesamin has been observed to inhibit the production of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), while promoting the production of anti-inflammatory cytokines, such as interleukin-10 (IL-10). This suggests that sesamin may have therapeutic potential in treating various inflammatory disorders. Moreover, sesamin has been reported to have potent antioxidant properties, which may contribute to its anti-inflammatory effects. The ability of sesamin to reduce oxidative stress and inflammation may make it a potential therapeutic candidate for conditions associated with chronic inflammation, such as cardiovascular disease, cancer, and neurodegenerative disorders [118]. Sesamin is effective as an adjuvant therapeutic agent in treating cardiovascular diseases (CVD) [62]. The anticancer potential of sesamin against non-small cell lung cancer (NSCLC) cells has been investigated by Chen et al. [130]. According to the study, sesamin has demonstrated the ability to impede the proliferation of NSCLC cells and trigger apoptosis, or programmed cell death, through the Akt/p53 pathway. The researchers employed various experimental techniques, including the MTT assay, flow cytometry, Western blotting, and immunofluorescence staining, to examine the mechanisms underlying the impact of sesamin on NSCLC cells. The results indicated that sesamin exhibits promise as a viable therapeutic intervention for treating non-small cell lung cancer. In summary, the research has presented compelling data regarding the potential antineoplastic effects of sesamin, indicating the need for additional exploration of this substance in the context of novel cancer treatments. Sesamin exhibited a significant inhibitory effect on tumor growth in vivo.
2.1.3. Sesamolin
Sesamolin is a well-known lignan in sesame oil that exhibits noteworthy antimutagenic, antiaging, and antioxidant characteristics. The results of a study by Nagarajan and Lee [74] have suggested that sesamolin could be a promising antileukemic agent in vivo based on its efficacy in a wehi-3B-induced leukemia model. According to Tsai et al. [131] research, it has been demonstrated that the antioxidant activity of related derivatives of sesame lignans may surpass that of endogenous lignans present in sesame oil. In another research, the effectiveness of sesamolin in reducing serum and liver lipid levels, along with a simultaneous increase in liver fatty acid oxidation, was observed [132]. Yu et al. [133] conducted a study investigating the therapeutic effects of sesamolin on nonalcoholic fatty liver disease (NAFLD) in mice fed a diet high in fat and fructose. The research aimed to investigate sesamolin’s capacity to regulate the gut microbiota and metabolites in individuals with NAFLD. The study involved the partitioning of 40 C57BL/6J mice into four distinct groups, namely, normal chow diet (NCD), high-fat and high-fructose diet (HFD), HFD with low-dose sesamolin (HFD+L-SES), and HFD with high-dose sesamolin (HFD+H-SES). The experimental subjects were mice that underwent a 16-week treatment regimen involving sesamolin. The study findings indicate that administering sesamolin resulted in a decrease in body weight, liver weight, and serum levels of alanine transaminase (ALT) and aspartate transaminase (AST) in the HFD+H-SES group as compared to the HFD group. Sesamolin exhibited a mitigating effect on hepatic lipid accumulation and inflammation induced by a high-fat diet. Moreover, the examination of gut microbiota demonstrated that administering sesamolin augmented the prevalence of advantageous bacteria, such as Akkermansia, and reduced the prevalence of detrimental bacteria, such as Desulfovibrio. According to the results of the metabolic analysis, the administration of sesamolin resulted in an elevation of short-chain fatty acids (SCFAs), bile acids, and amino acids, alongside a decrease in branched-chain amino acids (BCAAs) and lipids. The research findings suggest that administering sesamolin may have a beneficial effect on NAFLD in mice subjected to a diet high in fat and fructose. This effect is believed to be achieved by regulating gut microbiota and metabolites. The research presents a novel therapeutic strategy for managing NAFLD using sesamolin as a dietary adjunct. The potential of Sesamolin as a therapeutic agent for tumors lies in its ability to regulate the differentiation and activation of dendritic cells, leading to the efficient activation of natural killer cells [134]. According to Lee and Lee’s findings [135], it was observed that the cytolytic activity of natural killer cells is stimulated by sesamolin. The objective of the research conducted by Mohamed et al. [136] was to examine the potential neuroprotective properties of sesame oil in Alzheimer’s disease (AD) and explore the molecular mechanisms that may be involved in this protective effect. The AD rat model was created by administering amyloid-β (Aβ) 1–42 through intracerebroventricular injection, a recognized feature of AD pathogenesis. The study’s findings indicate that the administration of sesame oil had a notable effect in reducing memory impairment caused by Aβ, as demonstrated by the Morris water maze test results. In addition, the administration of sesame oil demonstrated a reduction in oxidative stress within the brain, as indicated by a decrease in malondialdehyde (MDA) levels and an increase in reduced glutathione levels. (GSH). The study found that using sesame oil resulted in a neuroprotective effect linked to suppressing the nuclear factor kappa B (NF-κB)/p38 mitogen-activated protein kinase (MAPK) signaling pathway. The observed reduction in the levels of p-NF-κB and p-p38 MAPK supported this. In addition, the administration of sesame oil increased brain-derived neurotrophic factor (BDNF) and peroxisome proliferator-activated receptor gamma (PPAR-γ) levels. These neurotrophic factors play a crucial role in neuroprotection and neuronal plasticity. The study’s results suggest that sesame oil can be utilized as a therapeutic intervention for AD. This may be due to its ability to regulate the NF-κB/p38MAPK/BDNF/PPAR-γ pathways [136]. Sesamolin has the potential to effectively stop the death of primary cortical cells brought on by hypoxia [137]. Sesamolin exhibits potential as a bioactive compound in vivo and may serve as a viable therapeutic agent for various diseases [59,89]. Katayama et al. [138] have investigated the impact of sesaminol, a substance obtained from sesame seeds, on the buildup of amyloid beta (Aβ) in the brains of senescence-accelerated mouse-prone 8 (SAMP8) mice, which serve as a model for Alzheimer’s disease. The study involved administering a diet containing sesaminol or a control diet to mice for 10 weeks. The researchers subsequently assessed the levels of Aβ in the brain and the activity of enzymes responsible for its production and degradation. The findings have indicated that the sesamol regimen had a notable impact on the reduction of Aβ buildup in the murine brain, as well as a concomitant decrease in the enzymatic activity responsible for Aβ production and an increase in the enzymatic activity that facilitates its degradation. Furthermore, consuming a sesamol-enriched diet resulted in elevated levels of BDNF. This protein plays a crucial role in supporting the survival and proliferation of neurons while concurrently decreasing the levels of oxidative stress markers in the brain. The results of this study indicated that sesaminol could serve as a viable therapeutic option for the prevention or treatment of Alzheimer’s disease. This is due to its ability to decrease the accumulation of Aβ and enhance the survival of neurons. Keowkase et al. [139] conducted a study to examine the impact of sesamin and sesamolin, two primary lignans found in sesame seeds, on the toxicity of amyloid-β (Aβ) in a transgenic Caenorhabditis elegans model of Alzheimer’s disease. The investigation was carried out by evaluating the paralysis of worms, their lifespan, markers of oxidative stress, and accumulation of Aβ. The findings indicate that sesamin and sesamolin can mitigate the toxicity induced by Aβ, prolong the lifespan of nematodes, and delay paralysis. Furthermore, the two lignans exhibited a reduction in the accumulation of Aβ in transgenic nematodes and a decline in oxidative stress markers, including reactive oxygen species (ROS) and malondialdehyde (MDA) levels. The authors suggested that the observed outcomes could be attributed to sesamin and sesamolin’s antioxidative and neuroprotective characteristics. Hence, it is probable that sesamin and sesamolin possess therapeutic properties that could be employed in treating Alzheimer’s disease. Nevertheless, additional research is necessary to ascertain their precise mechanism of operation and clinical effectiveness.
2.2. Tocopherols
Tocochromanols exhibit amphipathic characteristics, possessing both hydrophobic and hydrophilic components. Typically, these biomolecules comprise a lipophilic isoprenoid side chain attached to the membrane lipids and a polar chromanol ring oriented towards the membrane surface. Tocochromanols can impede the membrane lipid peroxidation process and function as scavengers of reactive oxygen species. Antioxidants have been widely recognized for their ability to counteract the harmful effects of free radicals, which helps mitigate DNA damage. Tocopherols function as scavengers of reactive oxygen species, thereby mitigating the impact of free radical attack and disrupting lipid peroxidation. These molecules safeguard cell membranes, facilitate lipid restoration and substitution, and exhibit utility in preventing cancer and cardiovascular ailments [140,141,142,143,144]. Tocopherols have been identified as having a significant function in plant metabolism, specifically in transporting sugar from leaves to phloem [145,146,147].
Tocopherols are a significant plant phenolic compound class that possesses both antioxidative properties and nutritional benefits. The molecules in question pertain to a family characterized by the presence of a chromanol ring, which is a type of chroman ring featuring an alcoholic hydroxyl group, as well as a 12-carbon aliphatic side chain that includes two methyl groups located centrally and two additional methyl groups situated at the terminus [148,149]. Plants can produce eight distinct forms of vitamin E, which encompass α-, β-, γ-, and δ-tocopherols and α-, β-, γ-, and δ-tocotrienols. Both tocopherols and tocotrienols comprise a chromanol ring and a variable quantity of methyl groups on the chromanol ring. Tocols have two main components: the chromanol ring and the hydrophobic side chain. Tocopherols and tocotrienols differ based on the acyl side chain they possess. The tocopherols have hydrophobic side chains that consist of saturated isoprenoid chains, whereas the tocotrienols have hydrophobic side chains that consist of isoprenyl chains with three double bonds. The chromanol ring can provide a hydrogen atom to reduce free radicals, while the hydrophobic side chain enables the molecule to permeate biological membranes [138,139] effectively. Tools’ metabolic outcomes and physiological effects are contingent upon their inherent structural characteristics. All the isoforms function as lipid antioxidants, with α-tocopherol exhibiting the greatest vitamin E activity [150,151,152,153]. Tocopherols are present in various plant organs of dicotyledonous species, encompassing roots, stems, leaves, flowers, fruits, and seeds. Nevertheless, a significant disparity exists in the overall tocopherol concentration and various tocopherol forms present in these biological tissues. The prevailing tocopherol variant in photosynthetic tissues, such as stems and leaves, is α-tocopherol. The prevalence of γ- and δ-tocopherols is typically higher than that of α-tocopherol in most seed crops [154,155,156]. The α-tocopherol content in sesame seeds varies but is usually present in trace amounts. Studies have reported levels ranging from 18.51 mg/100g to 49.63 mg/100g [157]. While sesame seeds contain other beneficial compounds, such as γ-tocopherol and δ-tocopherol, α-tocopherol is not the predominant form in seeds. The α-tocopherol content in sesame oil also varies depending on the extraction process and purity. Sesame oil may sometimes contain higher levels of α-tocopherol than the seeds. However, the exact amount can differ significantly. One study found that sesame seed oil extracts had a higher total phenolic content (TPC) than α-tocopherol. Specifically, the TPC in sesame oil was 26.00 mg GAE/g of extract, while α-tocopherol was 18.00 mg GAE/g [158].
Another study detected α-tocopherol concentrations in vegetable oils, including sesame oil, expressed as mg/kg. The values for α-tocopherol in sesame oil ranged from 71.3 ± 6.4 mg/kg to 432.3 ± 86.6 mg/kg [159]. The primary function of α-tocopherol as an antioxidant disrupts radical chains in lipoproteins and membranes. Its antioxidant potential and molecular functions help to mitigate the possibility of cardiovascular disease and cancer. Although in smaller amounts, other tools also have antioxidative and biological activity [160]. γ-tocopherol, for example, is more effective than α-tocopherol in reducing platelet aggregation, LDL oxidation, and intra-arterial thrombus formation. Tocotrienols can inhibit cholesterol biosynthesis and lower the risk of breast cancer. Tocopherols have been found to possess significant potential as antitumor agents, antioxidants, and hypocholesterolemic agents. The antiallergic properties of α-tocopherol were examined in one study using a mouse model of allergic rhinitis. α-tocopherol was administered to mice after they had been exposed to ovalbumin to create a sensitized state for the experiment. As seen by a drop in serum IgE levels and nasal symptom ratings, the findings demonstrated that α-tocopherol inhibited allergic reactions in the mice. The PI3K-PKB signaling pathway, which is essential for mast cell activation and degranulation, was shown to be blocked by α-tocopherol in in vitro tests using mouse mast cells. According to the research, α-tocopherol, a crucial receptor implicated in the allergic response, was also shown to reduce the expression of FcεRI on the surface of mast cells. The research indicated that α-tocopherol may have a therapeutic benefit for allergic rhinitis, presumably via inhibiting the mast cell PI3K-PKB signaling pathway [161,162,163,164].
Sesame seeds contain a combination of tocopherols and tocotrienols, with α-tocopherol and γ-tocopherol being the primary contributors to their beneficial properties (Table 2).
Tocotrienols are compounds belonging to the vitamin E group. Tocotrienols are less prevalent in nature compared to other types of vitamin E. The majority of our dietary intake consists of tocopherols rather than tocotrienols. Tocotrienols bioavailability varies, and they are often found in lower concentrations in the bloodstream. Tocotrienols have a similar basic structure to tocopherols, consisting of a chromanol ring and a phytyl tail. However, tocotrienols have three double bonds in their phytyl tail, distinguishing them from tocopherols. The four main tocotrienols are α, β, γ, and δ. γ -tocotrienol is the most abundant and studied form. Tocotrienols are found in certain plant-based oils, seeds, and grains. Notable sources include palm oil, rice bran oil, barley, wheat germ, and sesame seeds. However, some vegetable oils, such as palm oil, are rich in tocotrienols. The majority of vitamin E supplements often include tocopherols rather than tocotrienols. Research also indicates that tocotrienol is a more potent source of vitamin E than tocopherol. Research shows that tocotrienol has several health advantages [140,150]. Tocotrienols are known for their unique antioxidant properties that hinder plasma cholesterol levels and are linked with the prevention of cardiovascular disease [165]. Tocopherols were found to have the potential to mitigate stress-induced pathological alterations [166,167]. Dietary sesame seeds have been shown to increase both α-tocotrienol and γ-tocotrienol concentrations in specific tissues of rats. The research found that rats with a diet containing sesame seeds observed higher amounts of tocotrienols in their adipose tissue and skin. Nevertheless, these effects were not seen in plasma or any other tissue in the body. Remarkably, sesame seeds also increased the level of γ-tocopherol in different tissues despite its initial scarcity [168,169]. The combined application of tocotrienol and sesame lignans has been observed to exhibit a preventive effect against oxidative damage caused by UVB irradiation [170]. The tocopherol concentration in sesame oil ranges from 530 mg/kg to 1000 mg/kg. The predominant form of tocopherol found in sesame oil is γ-tocopherol, with concentrations ranging from 521 to 990 mg/kg. Additional tocopherols include δ (4 mg to 20 mg/kg) and α (up to 3 mg /kg). In addition, sesame oil may consist of a maximum of 20 mg /kg of γ-tocotrienol [157,171,172]. Morris et al. [173] have suggested that functional health foods can benefit from using δ- and γ-tocopherols within the concentration range of 214 to 239 μg/g. In addition to tocopherols and lignans, sesame has been found to contain small amounts of phenolic acids and naphthoquinone, which are also phenolic compounds with potential health benefits [5].

Table 2.
Structures, quantities, biological activities, and mechanisms of different tools detected from Sesamum indicum.
Table 2.
Structures, quantities, biological activities, and mechanisms of different tools detected from Sesamum indicum.
| Tocols in Sesame | Name of Component | Molecular Structure | Quantity/Amount of Raw Sesame Seeds and Sesame Oil | Biological Characteristics | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Tocopherols | α-Tocopherol | 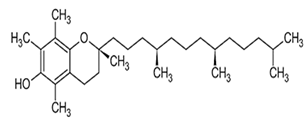 C29H50O2 | Sesame seeds: 18.51–49.63 mg/100 g Sesame oil: 71.3 ± 6.4–432.3 ± 86.6 mg/kg | Antioxidant Action |
| [157,158,159,174] |
| γ-Tocopherol | 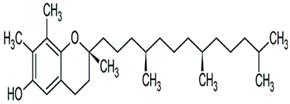 C28H48O2 | Sesame seeds: 169–577 mg/kg Sesmae oil: 329–1114 mg/L | Antioxidant properties | Guards against lipid oxidation. | [175] | |
| Potential Cancer Prevention | Maintains cell integrity | |||||
| Immune Support | Modulates gene expression | |||||
| δ-Tocopherol | 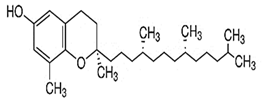 C27H46O2 | Sesame seeds: 0.1–1.5 mg/100 g Sesame oil: 5–10 mg/100 g | Antioxidant Properties |
| [29,167] | |
| Health benefits |
| |||||
| Tocotrienols | α-Tocotrienol | 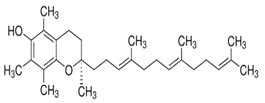 C29H44O2 | Sesame seeds: 0.134 mg/100 g Sesame oil: Not detected | Antioxidant Properties |
| [169] |
| Gene Modulation |
| |||||
| γ-Tocotrienol | 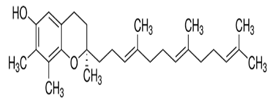 C28H42O2 | Sesame seeds: 0.415 mg/100 g Sesame oil: Up to 20 mg/kg | Specific Properties |
| [157] | |
| Neuroprotection |
|
2.3. Phytosterols
Phytosterols, which encompass sterols and stanols, are triterpenes derived from plants. They serve as crucial constituents of cell membranes and exhibit preventive properties against various diseases, notably cancer. Research has demonstrated that these entities exhibit characteristics of being antioxidants and anti-inflammatory and antibacterial agents [176,177,178,179]. Phytosterols, possessing an additional methyl group at the C-24 position, show a structural resemblance to cholesterol. Upon digestion, these plant sterols effectively compete with cholesterol for absorption in the small intestine, thereby reducing cholesterol levels in the bloodstream. Functional foods commonly recommended for their cholesterol-lowering properties often incorporate phytosterols derived from plant sources. Devaraj and Jialal [180], as well as Weingärtner et al. [168], have suggested that the incorporation of phytosterols in functional foods has been employed as a therapeutic measure for hypercholesterolemia and the mitigation of plasma cholesterol concentrations. Numerous studies have indicated that phytosterols can reduce blood cholesterol levels, enhance immune system function, and lower the incidence of particular cancers [181,182,183,184]. Alternatively, processed foods may be fortified with phytosterols and marketed as supplements for cholesterol management [185,186,187]. Phytosterols have the potential to serve as health-promoting constituents that may mitigate low-density lipoprotein and cholesterol levels, thereby averting the onset of cardiovascular disease [188,189]. Phytosterols have been shown to provide protection against prostate, breast, and colon cancer, as demonstrated in previous studies [190,191,192]. Meanwhile, Llop-Talaveron et al. [193] have recently published findings suggesting that phytosterolemia may protect against liver complications commonly associated with parenteral nutrition. In contrast, Nzekoue et al. [194] have observed that phytosterol oxidation products may form phytosterol oxidation products, which possess pro-inflammatory and pro-atherogenic properties. While phytosterols can be extracted from corn and legumes, it is noteworthy that sesame seeds contain the highest concentration of phytosterols, with a range of 400–413 mg per 100 g. Table 3 shows different classes of phytosterols that could be detected in sesamum indicum.
Gharby et al. [195] reported that β-sitosterol is a major component of phytosterols in sesame seed and oil, accompanied by campesterol and stigmasterol. β-sitosterol is the primary phytosterol present in sesame oil. A group of researchers discovered that the level of phytosterols present in brown sesame cultivars was comparatively greater than in white sesame cultivars [196,197]. β-Sitosterol has been studied extensively and shown to have significant potential for promoting human health. It exhibits cholesterol-lowering effects, boosts immunity, and exhibits anti-inflammatory properties [198,199,200,201]. Campesterol, another major sterol found in sesame oil, constitutes approximately 17.8% of total sterols, while Δ5-avenasterol and stigmasterol are present at around 10.2% and 6.4%, respectively. The minor sterols Δ7-stigmasterol and Δ7-avenasterol are also present. Sesame seed oil contains a total sterol content of about 540 mg/100 g oil [31,202].

Table 3.
Structures, quantities, biological activities, and mechanisms of different phytosterols detected from Sesamum indicum.
Table 3.
Structures, quantities, biological activities, and mechanisms of different phytosterols detected from Sesamum indicum.
| Phytosterols in Sesame | Molecular Structure | Quantity/Amount of Raw Sesame Seeds and Sesame Oil | Biological Characteristics | Mechanism | Reference |
|---|---|---|---|---|---|
| β-Sitosterol | 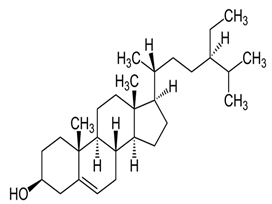 C29H50O | Sesame seed: 3.35 mg/g Sesame oil: 2.63 mg/g | Antitumor Effects |
| [29,179,203,204,205] |
| Anti-Inflammatory Properties |
| ||||
| Antidiabetic |
| ||||
| Ameliorative Effects on Prostatic Hyperplasia |
| ||||
| Hepatoprotective |
| ||||
| Campesterol | 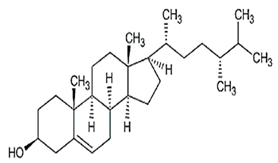 C28H48O | Sesame seed: 1.00 mg/g Sesame oil: 1.35 mg/g | Antioxidant Properties |
| [29,206,207] |
| Cardiovascular Health |
| ||||
| Cholesterol Regulation |
| ||||
| Stigmasterol | 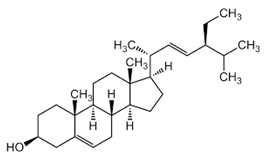 C29H48O | Sesame seed: 0.37 mg/g Sesame oil: 0.47 mg/g | Antiproliferative Activity |
| [29,208] |
| Mitochondrial Regulation and ROS Generation |
| ||||
| Autophagy Induction |
| ||||
| Sitostanol | 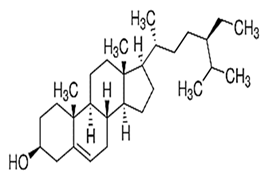 C29H52O | Sesame oil: 0.04 mg/g | Mitochondrial Respiration |
| [29,184,209] |
| Cholesterol Regulation |
| ||||
| Campestanol | 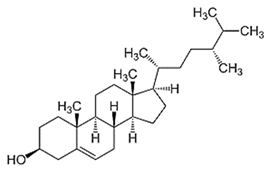 C28H50O | Sesame oil: 0.02 mg/g | Enzyme Inhibition |
| [187,210] |
| Antiatherogenic Effects |
| ||||
| ∆5-avenasterol | 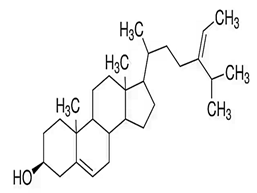 C29H48O | Sesame oil: 0.82 mg/g | Neutralization Stoichiometry |
| [211,212] |
| Antiviral Functions |
| ||||
| Δ5-Stigmasterol | 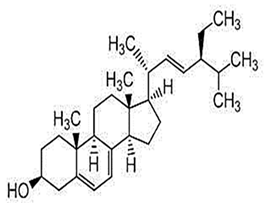 C29H48O | The specific amount was not detected | Antitumor Activity |
| [208,213] |
| Neuroprotection Against Oxidative Stress |
| ||||
| Δ7-avenasterol |  C29H48O | The specific amount was not detected | Protection Against Leishmania |
| |
| Improving Learning and Memory Ability |
| ||||
| Potential Antioxidant Function |
| ||||
| Δ7-Stigmastenol |  C29H50O | The specific amount was not detected | Gene Expression Analysis |
| [197,214] |
| Cholesterol Regulation |
|
2.4. Phytates
Phytic acid is a bioactive compound commonly present in various plant-based food sources. The molecular configuration of the substance enables it to form a complex with polyvalent cations, including minerals and trace elements. Phytic acid serves as a vital reservoir of phosphorus in plant seeds, with sesame seeds exhibiting a greater concentration of phytate than legumes [215]. Oil seeds, including linseed, rapeseed, sesame, soybean, and sunflower, display a phytic acid content that varies between 1% and 5.4%, whereas legumes typically contain 0.2% and 2.9%. The phytate concentration in sesame meal is higher than in soybean meal [216]. According to Touma et al. [217], the phytic acid content in sesame seeds is 5.36%. Phytates have also been discovered to possess hypocholesterolemic and anticancer characteristics.
2.5. Polyunsaturated Fatty Acids
Fatty acids are a type of organic molecule characterized by a lengthy hydrocarbon chain that is linked to a carboxyl group. Lipids, including fats, oils, and waxes, sourced from various organisms such as plants, animals, and microbes, exhibit a diverse range of carbon chain lengths from 1 to 30 [218,219,220]. Polyunsaturated fatty acids (PUFAs), which contain two or more double bonds in their carbon chain, are synthesized by specific desaturase enzymes. The health benefits of PUFAs have been extensively studied, including their potential to reduce the risk of chronic diseases such as heart disease, cancer, anti-inflammatory, hypolipidemic, vasodilatory, antithrombotic, and antiarrhythmic activities [196,221,222,223,224]. Vegetable oils abundant in PUFAs have garnered biomedical importance as potential dietary components for promoting normal human growth and development. LC-x-3-PUFAs are essential in regulating cholesterol synthesis, transportation, and eicosanoid synthesis, which is vital for maintaining cellular membrane integrity and overall human health. Namiki [225] proposed that combining sesamin and conjugated linoleic acid may have the potential as a weight-reducing agent. In sesame, linear acid formation and oleic desaturation ratio are limited [226].
Sesame seeds are a food source with high energy and oil content, constituting roughly 50% of the seed. Sesame oil shows a highly favorable fatty acid profile, comprising approximately 80–85% unsaturated and merely 15–20% saturated fatty acids. Sesame oil primarily contains linoleic (35–50%) and oleic (35–50%) acids, alongside minor quantities of palmitic (7–12%) and stearic (3.5–6%) acids and negligible levels of linolenic acid [227,228]. Recent scientific studies have shown that an excessive intake of n-6 fatty acids might cause a physiological condition that heightens platelet aggregation and blood clotting. Higher blood viscosity, vasospasm, vasoconstriction, and slower bleeding are all symptoms of this illness [229,230,231]. In contrast, it has been discovered that n-3 fatty acids offer several advantageous qualities, such as anti-inflammatory, antithrombotic, hypolipidemic, and vasodilatory actions. According to these results, a balanced diet with n-3 and n-6 fatty acids is essential for achieving and sustaining good cardiovascular health [232,233,234,235]. The concurrent usage of sesame and soybean oil can potentially enhance the vitamin E activity and nutritional efficacy of the lipid. Sesame oil predominantly comprises oleic and linoleic acids, constituting over 80% of the total fatty acid content [236,237]. A study was conducted by Uzun et al. [238] to investigate the variability in oil content, oil yield, and fatty acid composition among 77 sesame accessions that were gathered from various regions of Turkey. The findings indicate a significant disparity in the lipid composition of sesame accessions, with values ranging from 44.1% to 58.2%. The accession from Şanlıurfa exhibited the highest oil content, whereas the accession from Konya demonstrated the lowest. The fatty acid composition of sesame oil showed significant variability, with oleic acid being the predominant fatty acid, ranging from 32.3% to 48.2%. The results indicate that linoleic acid constituted the second most prevalent fatty acid, with a range of 36.6% to 47.4%, while palmitic acid was the most abundant, with a range of 8.3% to 9.9%. The investigation also found fluctuating quantities of additional marginal fatty acids, including stearic, arachidic, and behenic acid. The study found that the oil yield of various sesame accessions exhibited a range of 0.96 to 2.55 g per 100 seeds. Notably, the accession sourced from the Karaman region demonstrated the highest yield, while the accession obtained from the Kayseri region displayed the lowest yield. The research has identified certain favorable accessions, including a well-balanced fatty acid composition and high oil content. These accessions could be effectively employed in breeding programs to develop novel sesame cultivars with superior oil yield and quality. Therefore, based on this study, differences in the fatty acid profile of sesame seeds have been noted across various sesame cultivars. The acquired knowledge has the potential to facilitate the production of superior-grade edible oil in the forthcoming times. The germplasms of sesame originating from India exhibit a reduced concentration of saturated fatty acids, with palmitic acid being the most predominant [226]. C18:1 and C18:2 characterize Indian varieties as the primary unsaturated fatty acids. Sesame oil exhibits a considerable proportion of C18:1 and C18:2, comprising approximately 38% to 49% and 17% to 43%, respectively. Conversely, the content of C18:3 is comparatively low, measuring less than 1% [239]. A thorough evaluation of various cultivars is imperative to enhance the nutritional value of oil via manipulating the fatty acid biosynthesis pathway.
2.6. Short-Chain Peptides, Protein Hydrolysates, and Their Functional Properties
Bioactive polypeptides refer to chains of amino acids connected by peptide or amide bonds and have a molecular weight that does not exceed 20 kDa. Protein fragments exhibit distinct functional roles in diverse biological, physiological, or cellular activities. Unlike naturally occurring peptides, a wide range of peptides with varying composition and function are commonly utilized using synthetically derived protein hydrolysates. According to the literature, proteins can undergo digestion by proteases, or specific fragments can be generated through bio-fermenters utilizing microbes [240,241]. According to research, sesame food preparations are a noteworthy protein source. Protein hydrolysates have extensive applications as nutritional supplements, functional ingredients, food flavor enhancers, pharmaceuticals, and cosmetics. The substances in question exhibit characteristics similar to hormones or drugs and can be categorized according to their method of operation, including but not limited to antihypertensive, antioxidative, immunomodulatory, antithrombotic, opioid, mineral binding, and antimicrobial properties [242,243,244]. Sesame seeds are rich in oil, with a content ranging from 48–55%, but they are also a significant source of protein, with the seed coat accounting for approximately 20–25% of the total dry mass [245]. Dench et al. [246] reported that defatted sesame meal, containing 40–50% protein, can serve as an appropriate source of short-chain peptides that humans can easily digest. This is attributed to the presence of sulfur-containing amino acids [247]. Defatted sesame meal has been utilized in various food products, such as biscuits containing mixed grains and fortified table bread. Bandyopadhyay and Ghosh [248] investigated using papain to produce sesame protein hydrolysates with improved functional properties, longer shelf life, and better emulsifying properties than the original sesame protein lysate. A study was conducted by Aondona et al. [249] to investigate the antioxidant and antihypertensive properties of enzymatic protein hydrolysates and ultrafiltration peptide fractions obtained from sesame seeds. The research aimed to assess the prospective application of protein hydrolysates and peptides in the mitigation and control of oxidative stress and hypertension, both of which have been associated with cardiovascular ailments. They employed enzymatic hydrolysis and ultrafiltration methodologies to produce protein hydrolysates and peptide fractions, which were subsequently subjected to in vitro assays to evaluate their antioxidant and antihypertensive characteristics. The conducted assays encompassed the determination of total phenolic content, DPPH, and ABTS radical scavenging activities and ACE and renin inhibitory activities. The study’s findings have indicated that the hydrolysates and peptide fractions of sesame seed protein exhibited noteworthy antioxidant and antihypertensive properties [249]. The hydrolysates and peptides had a total phenolic content that varied between 10.56 and 31.12 mg GAE/g protein. Additionally, their DPPH and ABTS radical scavenging activities ranged from 17.31% to 63.82% and 20.26% to 68.96%, respectively. The hydrolysates and peptides exhibited ACE and renin inhibitory activities within the range of 23.56% to 69.83% and 28.64% to 76.44%, respectively. The investigation additionally recognized the peptide fractions exhibiting the most noteworthy levels of antioxidant and antihypertensive properties. These fractions could be used as potential functional ingredients in developing nutraceuticals and functional foods [249]. They proposed that the biological functionalities of sesame seed protein hydrolysates and peptide fractions are potentially attributed to bioactive peptides, including angiotensin-converting enzyme inhibitors and antioxidants, that exhibit advantageous impacts on cardiovascular well-being. Generally, the study’s results have offered significant insights into the potential application of sesame seed protein hydrolysates and peptide fractions as functional ingredients that possess antioxidant and antihypertensive properties [249].
3. Processing Technology of Sesame
Different processing techniques have been found to have varying effects on the bioactive compounds of sesame seeds. Roasting the seeds has been shown to increase the oil yield and improve the antioxidant properties of the oil extract [12]. However, it has also been observed that roasting and dehulling seeds can reduce the lignan and phenolic compounds content, which are important for the antioxidant activity of sesame extracts [250]. On the other hand, processing treatments such as soaking, cooking, germination, fermentation, and microwave heating have been found to reduce the phenolic compounds and tannins content in oilseeds, including sesame seeds [251]. Overall, the processing methods used for sesame seeds can have both positive and negative effects on the bioactive compounds, and further research is needed to optimize processing techniques to maximize the retention of these beneficial compounds [252]. The conventional technology for processing sesame primarily involves mechanical pressing, aqueous extraction, and solvent methods. On the other hand, the new processing and extraction techniques for sesame encompass supercritical (subcritical) CO2 extraction, microwave-assisted extraction, and water enzyme extraction [253,254,255]. The biochemical characteristics of proteins can be altered through various food processing techniques, including high pressure, thermal, radiation treatments, and ultrasound. This can lead to structural changes, such as aggregation, denaturation, loss of secondary and tertiary structures, formation/disruption of different types of bonds, and chemical reactions like glycation (Maillard reactions) [256,257,258,259].
3.1. Heating Method
Various heating techniques, such as blanching, boiling, autoclaving, roasting, and frying, are frequently employed in processing sesame-based food products. Heat application elicits various structural modifications in proteins, including but not limited to cleavage and reconfiguration of protein aggregation, disulfide bonds, and chemical reactions with other constituents such as carbohydrates and lipids. The modifications above lead to alterations in the epitopes of sesame, which can decrease or increase its allergenicity [260,261,262,263]. According to research findings, boiling sesame seeds at a temperature of 100 °C for a duration of 5 min resulted in an elevation of their antigenicity. However, subsequent boiling did not exhibit any further increase in antigenicity. The study found that subjecting sesame seeds to dry roasting at 150 °C for 7.5 min increased antigenicity. Furthermore, a greater increase in antigenicity was observed when the roasting time was extended to 15 min. The application of microwave heating at 1000 W for 3 min resulted in a significant reduction in the antigenicity of sesame seeds. However, no significant alterations in antigenicity were observed when microwave heating was administered for 1 min. Studies have shown that alterations in the antigenicity of sesame seeds are associated with the method of heating employed, as well as the duration and temperature of the process [264]. This is consistent with findings from research on the thermal treatment of soybeans [261]. The protein profile of various sesame proteins undergoes distinct alterations after heat treatment [264,265]. Further investigation is required to understand the mechanism underlying alterations in antigenicity. The heat treatment process can potentially lead to the creation of advanced glycation end products, thereby augmenting the IgE binding capacity. It is imperative to consider other constituents’ influence on sesame’s allergenicity [266].
3.2. Mechanical Pressing
The mechanical pressing technique is a commonly employed method for extracting oil from seeds with a high oil concentration, producing exceptional quality oil. This method is characterized by simplicity, safety, and cost-effectiveness [267]. Martínez et al. [268] employed Box–Behnken designs to optimize the screw-pressing process for sesame oil extraction, resulting in a maximum oil recovery of 71.1%. The optimal conditions were achieved with a seed moisture content of 12.3%, a 4 mm restriction die, and a pressing speed of 20 r/min. The study conducted by Yin et al. [269] revealed that the volatile compounds of mechanically pressed sesame oil exhibited elevated levels of sulfur heterocyclic compounds compared to those extracted through aqueous means.
3.3. Aqueous Extraction
The method of aqueous extraction can extract both protein and oil simultaneously. According to Xu et al. [270] and Lv and Wu [271], this approach presents numerous benefits, such as producing superior quality oil, utilizing uncomplicated equipment and production procedures, low initial investment, and adjusting production scale. The optimal conditions for sesame oil extraction have been determined by Hou et al. [272] to include a solid-to-water ratio of 0.8 g/mL (V/m), a temperature of 70 °C, and a pH of 5.0. The circumstances mentioned earlier have led to an extraction efficiency of 82.49%. The optimization of the method by Fasuan et al. [273] resulted in improved outcomes, with oil and protein recoveries of 73.60% and 75.12%, respectively. This was achieved using a solid-to-solvent ratio of 1:3 (m/V), a pH of 11, an extraction temperature of 47 °C, and a surfactant concentration of 0.1 mol/L NaCl.
3.4. Aqueous Enzymatic Extraction
High oil yield and good-quality oil can be obtained through enzymatic extraction, as indicated by Liu et al. [274]. Optimal conditions for the process included a liquid-to-material ratio of 7:1 (mL/g), a microwave power of 400 W, a treatment time of 4 min, the addition of alkaline protease at 0.1% (black sesame powder), a pH of 8.0, enzymatic hydrolysis temperature of 50 °C, and a hydrolysis time of 2 h. Furthermore, de Aquino et al. [275] demonstrated that adding a certain amount of water and a suitable temperature and enzyme dosage can also promote oil production.
3.5. Microwave/Ultrasonic-Assisted Extraction
Microwave-assisted extraction is a technique that uses electromagnetic waves to extract substances from cells. As the temperature increases, the solvent molecules inside the cell evaporate quickly, causing pressure to build up and eventually breaking the cell wall. This leads to the rapid outflow of cell contents. Lertbuaban and Muangrat [276] used microwave-assisted extraction to extract sesamin from black sesame seeds. They identified the optimal conditions as 90% ethanol as the extractant, a solid–liquid ratio of 1:8 (g/L), a microwave power of 700 W for 9 min, and a sesamin yield of 55.48 mg. On the other hand, Sarma et al. [277] focused on the effects of solvent-based microwave-assisted extraction of sesame phenolic compounds. They found that the highest total phenolic content was 206.14 mg GAE/100 g. These studies demonstrate that the effectiveness of microwave-assisted extraction varies depending on the target substance and extraction conditions.
3.6. Irradiation
The antigenicity of sesame seeds and protein solutions has been the subject of numerous research studies investigating the effects of γ-irradiation and high hydrostatic pressure. The survey conducted by Zoumpoulakis et al. [278] revealed that there were no statistically significant alterations in the antigenicity of sesame proteins when white sesame seeds were subjected to irradiation at 2.5, 5.0, and 10.0 kGy, with a p-value of less than 0.05. The acceptable level of irradiation for food items typically falls below 10 kGy [279]. Hence, the irradiation process aimed at mitigating the allergenic properties of sesame ought to concentrate on its protein solution instead of its seeds. Increasing doses of γ-irradiation lead to a decrease in antigenicity in solutions containing Ara h 2 and Ara h 6 [280].
3.7. High Hydrostatic Pressure
High hydrostatic pressure is a novel technological approach that impacts non-covalent bonds, such as hydrophobic, hydrogen, ionic bonds, and salt bridges, instead of covalent bonds. This results in the denaturation of proteins and consequential structural modifications, ultimately leading to the masking or destruction of epitopes and a decrease in allergenicity. Achouri and Boye [264] observed a reduction in the antigenicity of sesame protein solutions after exposure to high hydrostatic pressure (ranging from 100 to 500 MPa) across all pH levels tested over 10 min. The decrease in antigenicity observed can be attributed to the impact of high hydrostatic pressure on the protein’s conformation, which resulted in a compact and densely packed structure that obscured the allergen epitopes. The potential decrease in antigenicity of sesame protein solution may be attributed to the diminished allergenicity of certain sesame allergens, excluding Sei i 1 and Sei 2, which could be resistant to high hydrostatic pressure owing to their disulfide bonds.
3.8. Supercritical (Subcritical) Extraction
Supercritical carbon dioxide extraction is a technique that demonstrates efficacy in preserving oil’s nutritional and physiological properties while circumventing the deleterious effects of high-temperature oxidation [281,282]. Shi et al. [283] conducted a comparative analysis of sesame oil’s chemical properties, antioxidant capacity, and oxidative stability obtained through supercritical and subcritical techniques. The results of the study indicated that the processing methodologies had a negligible impact on the oil’s fatty acid and triacylglycerol composition. The suitability of compressed propane as a solvent for sesame oil extraction was demonstrated by Corso et al. [91], who found that this method required less time and pressure than carbon dioxide extraction. Liu et al. [284] optimized the extraction conditions, resulting in a 95.56% yield of sesame oil while maintaining the nutrient content.
4. Conclusions and Future Perspective
The comprehensive findings from recent studies highlight the numerous health advantages associated with the intake of sesame seeds, which are notably abundant in bioactive components. Lignans derived from sesame seeds offer various potential therapeutic applications, ranging from cognitive health to cardiovascular disease, cancer, and inflammation-related disorders. Functional health foods can also benefit from including tocopherols derived from sesame seeds because of their great antioxidant properties. These compounds function as antioxidants and can counteract the effects of reactive oxygen species, playing a role in safeguarding cell membranes and preventing conditions such as cancer and cardiovascular diseases. Phytosterols are also commonly incorporated into functional foods aimed at managing cholesterol levels. Numerous studies have demonstrated their ability to reduce blood cholesterol, enhance the immune system, and lower the risk of specific cancers. Although phytates are known to hinder mineral absorption, they also have potential hypocholesterolemic and anticancer properties. Numerous studies also indicate the richness of unsaturated fatty acids, mainly linoleic and oleic acid, in sesame oil, increasing its health-promoting potential. Sesame seeds, rich in oil and protein, are a valuable source of protein hydrolysates with diverse applications, including their potential as functional ingredients in nutraceuticals and functional foods, thanks to their antioxidant and antihypertensive properties as bioactive peptides. These findings underscore the significance of incorporating sesame seeds into diet and the potential for their utilization in developing dietary supplements and functional food products. Encouraging the adoption of sesame usage among consumers and food manufacturers is crucial. Additional research is essential to delve deeper into the beneficial health effects of the phytochemicals present in sesame, to understand how they work thoroughly, and to assess their clinical efficacy in treating various health conditions.
Author Contributions
Conceptualization, A.M.K. and P.M.; methodology, A.M.K. and P.M.; validation, A.M.K. and P.M.; investigation, P.M. and A.M.K., writing—original draft preparation, P.M.; writing—review and editing, P.M. and A.M.K.; visualization, P.M.; supervision, A.M.K.; project administration, P.M.; funding acquisition, A.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data generated from the study are clearly presented and discussed in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ABCA1 | Binding cassette transporter A1 |
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), radical cation |
| ACC | Acetyl-CoA carboxylase |
| ACE | Antioxidant capacity equivalent |
| AD | Alzheimer’s disease |
| ALT | Alanine transaminase |
| AMPK | AMP-activated protein kinase |
| ARE | Antioxidant response element |
| AST | Aspartate transaminase |
| Aβ | Amyloid-β |
| BBB | Blood–brain barrier |
| BCAAs | Branched-chain amino acids |
| Bcl-2 | B-cell lymphoma 2 |
| BDNF | Brain-derived neurotrophic factor |
| BUN | Blood urea nitrogen |
| CAT | Catalase |
| C57BL/6J | Strain of mice used as a universal model for studying diet-induced obesity |
| CD36 | Scavenger receptor involved in cholesterol uptake |
| CDKs | Cyclin-dependent kinases |
| CKD | In chronic kidney disease |
| COX-2 | Cyclooxygenase-2 |
| DHT | Dihydrotestosterone |
| DNA | Deoxyribonucleic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl, radical |
| ESRD | End-stage renal disease |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| ER | Endoplasmic reticulum |
| FAO | Food and Agriculture Organization |
| FAS | Fatty acid synthase |
| FcεRI | The high-affinity receptor for the Fc region of immunoglobulin E |
| FoxO | Forkhead box O |
| GAE | Gallic acid equivalent |
| GSH-Px | Glutathione peroxidase |
| HDL-C | High-density lipoprotein cholesterol |
| HepG2 | Human liver cell line |
| HMG-CoA reductase | 3-hydroxy-3-methylglutaryl-CoA reductase |
| HO-1 | Heme oxygenase-1 |
| IgE | Immunoglobulin E |
| IKKα | Kinase α |
| IL-1 | Interleukin 1 |
| IL-1β | Interleukin-1 beta |
| IL-10 | Interleukin-10 |
| IL-6 | Interleukin 6 |
| iNOS | Nitric oxide synthase |
| JNK | c-Jun N-terminal kinase |
| LCAT | Lecithin:cholesterol acyltransferase |
| LDL | Low-density lipoprotein |
| LDL-C | Low-density lipoprotein cholesterol |
| LOX | Lipoxygenase |
| LPS | Lipopolysaccharides |
| LXRα | Liver X receptor alpha |
| MAPK | p38 mitogen-activated protein kinase |
| MDA | Malondialdehyde |
| MMP | Mitochondrial membrane potential |
| mTOR | Mechanistic target of rapamycin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide |
| NAFLD | Non-alcoholic fatty liver disease |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NSCLC | Non-small cell lung cancer |
| p53 | Tumor protein, regulatory protein that is often mutated in human cancers |
| PI3K-PKB | Signaling pathway |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| PUFAs | Polyunsaturated fatty acids |
| PXR | Pregnane X receptor |
| ROS | Reactive oxygen species |
| SAMP8 | Senescence-accelerated mouse-prone 8 |
| SCFAs | Short-chain fatty acids |
| SOD | Superoxide dismutase |
| SR-A | Scavenger receptor class A |
| SR-BI | Scavenger receptor class B type I |
| SREBP-1c | Sterol regulatory element-binding protein 1c |
| SIRT1 | Sirtuin 1 |
| TLR4 | S gene-encoding Toll-like receptor 4 in humans |
| TNF-α | Tumor necrosis factor-alpha |
| UVB | Ultraviolet B |
References
- Anilakumar, K.R.; Pal, A.; Khanum, F.; Bawa, A.S. Nutritional, medicinal and industrial uses of sesame (Sesamum indicum L.) seeds-an overview. Agric. Conspec. Sci. 2010, 75, 159–168. [Google Scholar]
- Nagar, P.; Agrawal, M.A.K. Sesame (Sesamum indicum L.) seed as a functional food: A review. Pharma Innov. 2022, 519, 507–565. [Google Scholar]
- Yaseen, G.; Ahmad, M.; Zafar, M.; Akram, A.; Sultana, S.; Ahmed, S.N.; Kilic, O. Sesame (Sesamum indicum L.). In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 253–269. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Zitoun, A. Sesame (Sesamum indicum L.) seeds in food, nutrition, and health. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1029–1036. [Google Scholar] [CrossRef]
- Sharma, L.; Saini, C.S.; Punia, S.; Nain, V.; Sandhu, K.S. Sesame (Sesamum indicum) seed. In Oilseeds: Health Attributes and Food Applications; Tanwar, B., Goyal, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 305–330. [Google Scholar]
- Shah, N. Sesamum indicum (Sesame or Til): Seeds and Oil-A Historical and Scientific Evaluation from Indian Perspective. Asian Agri-History 2016, 20, 3–19. [Google Scholar]
- FAO. Special Report 2022 FAO Crop and Food Supply Assesment Mission (CFSAM) to the Republic of the Sudan; FAO: Rome, Italy, 2022. [Google Scholar]
- FAOSTAT. Statistical Data on Crops, Sesame Seeds, Area, Production Quantity of Tanzania, Africa and the World; FAOSTAT: Rome, Italy, 2022. [Google Scholar]
- Lukurugu, G.A.; Nzunda, J.; Kidunda, B.R.; Chilala, R.; Ngamba, Z.S.; Minja, A.; Kapinga, F.A. Sesame production constraints, variety traits preference in the Southeastern Tanzania: Implication for genetic improvement. J. Agric. Food Res. 2023, 14, 100665. [Google Scholar] [CrossRef]
- Weldemichael, M.Y.; Gebremedhn, H.M. Research advances and prospects of molecular markers in sesame: A review. Plant Biotechnol. Rep. 2023, 17, 585–603. [Google Scholar] [CrossRef]
- Rahman, A.; Akbar, D.; Trotter, T.; Thomson, M.; Timilsina, S.; Bhattarai, S. The prospect of developing sesame industry in Northern Australia through analysing market opportunity. Aust. J. Reg. Stud. 2020, 26, 347–378. [Google Scholar]
- Ma, X.; Wang, Z.; Zheng, C.; Liu, C. A comprehensive review of bioactive compounds and processing technology of sesame seed. Oil Crop Sci. 2022, 7, 88–94. [Google Scholar] [CrossRef]
- Torricelli, M.; Pierboni, E.; Rondini, C.; Altissimi, S.; Haouet, N. Sesame, pistachio, and macadamia nut: Development and validation of new allergenic systems for fast real-time PCR application. Foods 2020, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Langham, D.R.; Miao, H. Economic and academic importance of sesame. In The Sesame Genome. Compendium of Plant Genomes; Miao, H., Zhang, H., Kole, C., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–18. [Google Scholar] [CrossRef]
- Myint, D.; Gilani, S.A.; Kawase, M.; Watanabe, K.N. Sustainable sesame (Sesamum indicum L.) production through improved technology: An overview of production, challenges, and opportunities in Myanmar. Sustainability 2020, 12, 3515. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; AlJuhaimi, F.; Özcan, M.M.; Ghafoor, K.; Şimşek, Ş.; Babiker, E.E.; Osman, M.A.; Gassem, M.A.; Salih, H.A. Evaluation of chemical properties, amino acid contents and fatty acid compositions of sesame seed provided from different locations. J. Oleo Sci. 2020, 69, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, Y.; Hüseyin, G. Sesame Seed Protein: Amino Acid, Functional, and Physicochemical Profiles. Foods Raw Mater. 2023, 11, 72–83. [Google Scholar] [CrossRef]
- Agidew, M.G.; Dubale, A.A.; Atlabachew, M.; Abebe, W. Fatty acid composition, total phenolic contents and antioxidant activity of white and black sesame seed varieties from different localities of Ethiopia. Chem. Biol. Technol. Agric. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Wei, P.; Zhao, F.; Wang, Z.; Wang, Q.; Chai, X.; Hou, G.; Meng, Q. Sesame (Sesamum indicum L.): A comprehensive review of nutritional value, phytochemical composition, health benefits, development of food, and industrial applications. Nutrients 2022, 14, 4079. [Google Scholar] [CrossRef]
- Langyan, S.; Yadava, P.; Sharma, S.; Gupta, N.C.; Bansal, R.; Yadav, R.; Kumar, A. Food and nutraceutical functions of Sesame oil: An underutilized crop for nutritional and health benefits. Food Chem. 2022, 389, 132990. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, P.; Narasimhulu, C.A.; Rajagopalan, S.; Parthasarathy, S.; Desikan, R. Sesamol: A powerful functional food ingredient from sesame oil for cardioprotection. Food Funct. 2020, 11, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Chandra, P.; Sachan, N. Sesame seed in controlling human health and nutrition. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 183–210. [Google Scholar] [CrossRef]
- Singletary, K.W. Sesame: Potential Health Benefits. Nutr. Today 2022, 57, 271–287. [Google Scholar] [CrossRef]
- Ahmad, S.; Ghosh, P. Benefits of dietary sesame seed and flaxseed to strengthen immune system during COVID-19 pandemic and prevent associated comorbidities related health risks. Ann. Phytomed. 2020, 9, 50–61. [Google Scholar] [CrossRef]
- Hsu, E.; Parthasarathy, S. Anti-inflammatory and antioxidant effects of sesame oil on atherosclerosis: A descriptive literature review. Cureus 2017, 9, e1438. [Google Scholar] [CrossRef]
- Murata, J.; Ono, E.; Yoroizuka, S.; Toyonaga, H.; Shiraishi, A.; Mori, S.; Tera, M.; Azuma, T.; Nagano, A.J.; Nakayasu, M. Oxidative rearrangement of (+)-sesamin by CYP92B14 co-generates twin dietary lignans in sesame. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Hu, W.; Hu, Z.; Guan, Y.; Zhang, R. A review of nutritional implications of bioactive compounds of Ginger (Zingiber officinale Roscoe), their biological activities and nano-formulations. Ital. J. Food Sci. 2022, 34, 1–12. [Google Scholar] [CrossRef]
- Wani, S.A.; Naik, H.; Wagay, J.A.; Ganie, N.A.; Mulla, M.Z.; Dar, B. Mentha: A review on its bioactive compounds and potential health benefits. Qual. Assur. Saf. Crops Foods 2022, 14, 154–168. [Google Scholar] [CrossRef]
- Pathak, N.; Bhaduri, A.; Rai, A.K. Sesame: Bioactive compounds and health benefits. In Bioactive Molecules in Food; Springer: Berlin/Heidelberg, Germany, 2019; pp. 181–200. [Google Scholar] [CrossRef]
- Sallam, K.I.; Abd-Elghany, S.M.; Imre, K.; Morar, A.; Herman, V.; Hussein, M.A.; Mahros, M.A. Ensuring safety and improving keeping quality of meatballs by addition of sesame oil and sesamol as natural antimicrobial and antioxidant agents. Food Microbiol. 2021, 99, 103834. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Luo, J.; Nawaz, M.A.; Stockmann, R.; Buckow, R.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Phytochemistry, Bioaccessibility, and Bioactivities of Sesame Seeds: An Overview. Food Rev. Int. 2023, 40, 1–27. [Google Scholar] [CrossRef]
- Esmaeilzadeh Kenari, R.; Razavi, R. Phenolic profile and antioxidant activity of free/bound phenolic compounds of sesame and properties of encapsulated nanoparticles in different wall materials. Food Sci. Nutr. Today 2022, 10, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Geyik, Ö.G.; Tekin-Cakmak, Z.H.; Shamanin, V.P.; Karasu, S.; Pototskaya, I.V.; Shepelev, S.S.; Chursin, A.S.; Morgounov, A.I.; Yaman, M.; Sagdic, O. Effects of phenolic compounds of colored wheats on colorectal cancer cell lines. Qual. Assur. Saf. Crops Foods 2023, 15, 21–31. [Google Scholar] [CrossRef]
- Mohamadi, N.; Meraghni, M.; Meradci, F.; Necib, A.; El Arbi, M.; Elhadef, K.; Smaoui, S.; Bouaziz, M. Investigation and quantification of the potential antioxidant, inflammatory, and antibacterial bioactive molecules of the extracts of Algerian black and green table olive brine. Qual. Assur. Saf. Crops Foods 2023, 15, 92–106. [Google Scholar] [CrossRef]
- Alzubaidi, A.N.; Sekoulopoulos, S.; Pham, J.; Walter, V.; Fuletra, J.G.; Raman, J.D. Incidence and Distribution of New Renal Cell Carcinoma Cases: 27-Year Trends from a Statewide Cancer Registry. J. Kidney Cancer VHL 2022, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Gideon, M.; Rajendran, R.; Mathew, G.; Nair, K. Advancing Treatment Frontiers: Radiofrequency Ablation for Small Renal Mass—Intermediate-Term Results. J. Kidney Cancer VHL 2023, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lv, J.; Han, T.; Kan, J.; Jin, C.-H.; Liu, J. Hepatic antioxidant and gut ecological modulation properties of long-term intake of tea (Camellia sinensis L.) flower extract in vivo. Qual. Assur. Saf. Crops Foods 2023, 15, 11–21. [Google Scholar] [CrossRef]
- Jurasova, L.; Jurikova, T.; Baron, M.; Sochor, J. Content of selected polyphenolic substances in parts of grapevine. Ital. J. Food Sci. 2023, 35, 17–43. [Google Scholar] [CrossRef]
- Kaşıkçı, M.B.; Bağdatlıoğlu, N. Assessment of the bioaccessibility of phenolic compounds and antioxidant activity in raw and pickled white cabbage and gherkins. Ital. J. Food Sci. 2022, 34, 1–10. [Google Scholar] [CrossRef]
- Zeb, A.; Muhammad, B.; Ullah, F. Characterization of sesame (Sesamum indicum L.) seed oil from Pakistan for phenolic composition, quality characteristics and potential beneficial properties. J. Food Meas. Charact. 2017, 11, 1362–1369. [Google Scholar] [CrossRef]
- Ramos-Sotelo, H.; Figueroa-Pérez, M.G. Use of salicylic acid during cultivation of plants as a strategy to improve its metabolite profile and beneficial health effects. Ital. J. Food Sci. 2023, 35, 79–90. [Google Scholar] [CrossRef]
- Zhao, C.-C.; Zhang, M.; Peng, J.-F.; Ma, Y.-Y.; Zhao, X.-N.; Wen, Z.-Y.; Wang, S.-S.; Shen, A.-L.; Shi, H. Polygonatum polysaccharide attenuates inflammation through inhibiting NLRP3 inflammasome in diabetic cardiomyopathy rats. Ital. J. Food Sci. 2023, 35, 10–18. [Google Scholar] [CrossRef]
- Mekky, R.H.; Abdel-Sattar, E.; Segura-Carretero, A.; Contreras, M.d.M. Phenolic compounds from sesame cake and antioxidant activity: A new insight for agri-food residues’ significance for sustainable development. Foods 2019, 8, 432. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Lv, Y.; Lu, C.; Zhang, B.; Fu, Z.; Huang, Y. Mechanism of Rhizoma Coptidis in epilepsy with network pharmacology. Allergol. Immunopathol. 2022, 50, 138–150. [Google Scholar] [CrossRef]
- Haworth, R. Constituents of natural phenolic resins. Nature 1941, 147, 255–257. [Google Scholar] [CrossRef]
- Aregay, M.G.; Kang, M.; Kim, B.-S.; Lee, Y.-W. Recovery of water-soluble bioactive components from defatted sesame meal using carbon dioxide assisted hydrothermal process. J. Supercrit. Fluids 2021, 168, 105069. [Google Scholar] [CrossRef]
- Moazzami, A.A.; Andersson, R.E.; Kamal-Eldin, A. HPLC analysis of sesaminol glucosides in sesame seeds. J. Agric. Food Chem. 2006, 54, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Andargie, M.; Vinas, M.; Rathgeb, A.; Möller, E.; Karlovsky, P. Lignans of sesame (Sesamum indicum L.): A comprehensive review. Molecules 2021, 26, 883. [Google Scholar] [CrossRef]
- Michailidis, D.; Angelis, A.; Aligiannis, N.; Mitakou, S.; Skaltsounis, L. Recovery of sesamin, sesamolin, and minor lignans from sesame oil using solid support-free liquid–liquid extraction and chromatography techniques and evaluation of their enzymatic inhibition properties. Front. Pharmacol. 2019, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Karrar, E.; Liu, R.; Chang, M.; Wang, X. Comparative effects of sesame lignans (sesamin, sesamolin, and sesamol) on oxidative stress and lipid metabolism in steatosis HepG2 cells. J. Food Biochem. 2022, 46, e14180. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.-C.; Wang, T.-Y.; Hwang, L.S.; Gavahian, M. Biotransformation of sesaminol triglycoside by intestinal microflora of swine supplemented with probiotic or antibiotic diet. Qual. Assur. Saf. Crops Foods 2022, 14, 19–29. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kim, M.-Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef] [PubMed]
- Plaha, N.S.; Awasthi, S.; Sharma, A.; Kaushik, N. Distribution, biosynthesis and therapeutic potential of lignans. 3 Biotech 2022, 12, 255. [Google Scholar] [CrossRef]
- Egawa, K.; Horii, Y.; Misonou, Y.; Yamasaki, I.; Takemoto, D.; Yoshiko, O.; Tomohiro, R.; Shibata, H.; Nagai, K. Sesame lignans increase sympathetic nerve activity and blood flow in rat skeletal muscles. Physiol. Res. 2020, 69, 253. [Google Scholar] [CrossRef] [PubMed]
- Shimoyoshi, S.; Takemoto, D.; Ono, Y.; Kitagawa, Y.; Shibata, H.; Tomono, S.; Unno, K.; Wakabayashi, K. Sesame lignans suppress age-related cognitive decline in senescence-accelerated mice. Nutrients 2019, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Dossou, S.S.K.; XU, F.-T.; Dossa, K.; Rong, Z.; Zhao, Y.-Z.; Wang, L.-H. Antioxidant lignans sesamin and sesamolin in sesame (Sesamum indicum L.): A comprehensive review and future prospects. J. Integr. Agric. 2022, 22, 14–30. [Google Scholar] [CrossRef]
- Anju, V.; Busi, S.; Ranganathan, S.; Ampasala, D.R.; Kumar, S.; Suchiang, K.; Kumavath, R.; Dyavaiah, M. Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors. Microb. Pathog. 2021, 155, 104912. [Google Scholar] [CrossRef]
- Oikawa, D.; Yamashita, S.; Takahashi, S.; Waki, T.; Kikuchi, K.; Abe, T.; Katayama, T.; Nakayama, T. (+)-Sesamin, a sesame lignan, is a potent inhibitor of gut bacterial tryptophan indole-lyase that is a key enzyme in chronic kidney disease pathogenesis. Biochem. Biophys. Res. Commun. 2022, 590, 158–162. [Google Scholar] [CrossRef]
- Rosalina, R.; Weerapreeyakul, N. An Insight into sesamolin: Physicochemical properties, pharmacological activities, and future research prospects. Molecules 2021, 26, 5849. [Google Scholar] [CrossRef] [PubMed]
- Hadipour, E.; Emami, S.A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Effects of sesame (Sesamum indicum L.) and bioactive compounds (sesamin and sesamolin) on inflammation and atherosclerosis: A review. Food Sci. Nutr. 2023, 22, 3729–3757. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Jin, S.W.; Lee, G.H.; Park, J.S.; Kim, J.Y.; Thai, T.N.; Han, E.H.; Jeong, H.G. Sesamin induces endothelial nitric oxide synthase activation via transient receptor potential vanilloid type 1. J. Agric. Food Chem. 2020, 68, 3474–3484. [Google Scholar] [CrossRef] [PubMed]
- Dalibalta, S.; Majdalawieh, A.F.; Manjikian, H. Health benefits of sesamin on cardiovascular disease and its associated risk factors. Saudi Pharm. J. 2020, 28, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Wanachewin, O.; Pothacharoen, P.; Kongtawelert, P.; Phitak, T. Inhibitory effects of sesamin on human osteoclastogenesis. Arch. Pharm. Res. 2017, 40, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Keratibumrungpong, T.; Srisuthtayanont, W.; Wanachewin, O.; Klangjorhor, J.; Phitak, T.; Pothacharoen, P.; Shwe, T.H.; Kongtawelert, P. Sesamin Attenuates VEGFA-Induced Angiogenesis via Inhibition of Src and FAK Signaling in Chick Chorioallantoic Membrane Model and Human Endothelial EA. hy926 Cells. Biomedicines 2023, 11, 188. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, S.; Liu, Y.; Deng, P.; Huang, J.; He, G. Sesamin induces melanogenesis by microphthalmia-associated transcription factor and tyrosinase up-regulation via cAMP signaling pathway. Acta Biochim. Biophys. Sin. 2011, 43, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Bosebabu, B.; Cheruku, S.P.; Chamallamudi, M.R.; Nampoothiri, M.; Shenoy, R.R.; Nandakumar, K.; Parihar, V.K.; Kumar, N. An appraisal of current pharmacological perspectives of sesamol: A review. Mini Rev. Med. Chem. 2020, 20, 988–1000. [Google Scholar] [CrossRef]
- Gupta, B.; Dalal, P.; Rao, R. Cyclodextrin decorated nanosponges of sesamol: Antioxidant, anti-tyrosinase and photostability assessment. Food Biosci. 2021, 42, 101098. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hemavathy, J.; Gopala Krishna, A. Development of a rapid method for determination of lignans content in sesame oil. J. Food Sci. Technol. 2015, 52, 521–527. [Google Scholar] [CrossRef]
- Ren, B.; Yuan, T.; Zhang, X.; Wang, L.; Pan, J.; Liu, Y.; Zhao, B.; Zhao, W.; Liu, Z.; Liu, X. Protective effects of sesamol on systemic inflammation and cognitive impairment in aging mice. J. Agric. Food Chem. 2020, 68, 3099–3111. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.-Y.; Chien, S.-P.; Hsu, D.-Z.; Liu, M.-Y. Protective effect of sesamol on the pulmonary inflammatory response and lung injury in endotoxemic rats. Food Chem. Toxicol. 2010, 48, 1821–1826. [Google Scholar] [CrossRef]
- Nutakki, M.; Murhekar, K.V.; Sundersingh, S.; Raja, A. A Rare Site of Metachronous Metastases from Renal Cell Carcinoma. J. Kidney Cancer VHL 2024, 11, 1. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Mansour, Z.R. Sesamol, a major lignan in sesame seeds (Sesamum indicum): Anti-cancer properties and mechanisms of action. Eur. J. Pharmacol. 2019, 855, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bajaj, P.; Singh, B.; Paul, K.; Sharma, P.; Mehra, S.; Kaur, P.; Jasrotia, S.; Kumar, P.; Singh, V. Sesamol as a potent anticancer compound: From chemistry to cellular interactions. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Lee, J.K. Therapeutic effects of sesamolin on leukemia induced by WEHI-3B in model mice. Appl. Biol. Chem. 2021, 64, 1–12. [Google Scholar] [CrossRef]
- Li, Q.-Z.; Zuo, Z.-W.; Liu, Y. Recent status of sesaminol and its glucosides: Synthesis, metabolism, and biological activities. Crit. Rev. Food Sci. Nutr. 2023, 63, 12043–12056. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, S.J.; Srivastava, S.; Jahagirdar, A.; Rajendran, R.; Sukhdeo, S.V.; Rajakumari, S. Sesaminol induces brown and beige adipocyte formation through suppression of myogenic program. FASEB J. 2020, 34, 6854–6870. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H.; Matsui-Yuasa, I.; Matsumoto, K.; Omura, A.; Kiyomoto, K.; Kojima-Yuasa, A. Sesaminol prevents Parkinson’s disease by activating the Nrf2-ARE signaling pathway. Heliyon 2020, 6, e05342. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, R.; Lu, X.; Jia, C.; Sun, Q.; Huang, J.; Wei, S.; Ma, L. Enzymatic preparation and structure-activity relationship of sesaminol. J. Oleo Sci. 2021, 70, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Osawa, T.; Nagata, M.; Namiki, M.; Fukuda, Y. Sesamolinol, a novel antioxidant isolated from sesame seeds. Agric. Biol. Chem. 1985, 49, 3351–3352. [Google Scholar] [CrossRef]
- Moazzami, A.A.; Andersson, R.E.; Kamal-Eldin, A. Characterization and analysis of sesamolinol diglucoside in sesame seeds. Biosci. Biotechnol. Biochem. 2006, 70, 1478–1481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hernandez, E.M. Specialty oils: Functional and nutraceutical properties. In Functional Dietary Lipids; Elsevier: Amsterdam, The Netherlands, 2016; pp. 69–101. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Kim, J.-K.; Choi, J.-H.; Jung, J.-Y.; Oh, W.-Y.; Kim, D.C.; Lee, H.S.; Kim, Y.S.; Kang, S.S.; Lee, S.-H. Hepatoprotective effect of pinoresinol on carbon tetrachloride–induced hepatic damage in mice. J. Pharmacol. Sci. 2010, 112, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, C.; Liu, L.; Yang, B.; Zhang, Y. Structure identification and fermentation characteristics of pinoresinol diglucoside produced by Phomopsis sp. isolated from Eucommia ulmoides Oliv. Appl. Microbiol. Biotechnol. 2012, 93, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Fu, Y.L.; Zhang, Q.H.; Zhang, C.; Chen, Y. Inhibition of in vitro and in vivo ovarian cancer cell growth by pinoresinol occurs by way of inducing autophagy, inhibition of cell invasion, loss of mitochondrial membrane potential and inhibition Ras/MEK/ERK signalling pathway. JBUON 2019, 24, 709–714. [Google Scholar] [PubMed]
- Nair, A.; Kuwahara, A.; Nagase, A.; Yamaguchi, H.; Yamazaki, T.; Hosoya, M.; Omura, A.; Kiyomoto, K.; Yamaguchi, M.-A.; Shimoyama, T. Purification, gene cloning, and biochemical characterization of a β-glucosidase capable of hydrolyzing sesaminol triglucoside from Paenibacillus sp. KB0549. PLoS ONE 2013, 8, e60538. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, X.; Sun, Y.; Su, D.; Sun, Y.; Hu, B.; Zeng, X. Purification and fermentation in vitro of sesaminol triglucoside from sesame cake by human intestinal microbiota. J. Agric. Food Chem. 2013, 61, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, J.; Gao, Z.; Yangwu, R.; Jiang, H.; Che, J.; Liu, Y. Production of pinoresinol diglucoside, pinoresinol monoglucoside, and pinoresinol by Phomopsis sp. XP-8 using mung bean and its major components. Appl. Microbiol. Biotechnol. 2015, 99, 4629–4643. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Miao, H.; Ju, M.; Li, C.; Cao, H.; Zhang, H. Nutraceutomics of the Ancient Oilseed Crop Sesame (Sesamum indicum L.). In Compendium of Crop Genome Designing for Nutraceuticals; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 471–501. [Google Scholar] [CrossRef]
- Ryu, S.-N.; Kim, K.-S.; Bang, J.-K.; Lee, B.-H. Quantitative determination of sesaminol glucosides in sesame seed. Korean J. Crop Sci. 1998, 43, 209–213. [Google Scholar]
- Corso, M.P.; Fagundes-Klen, M.R.; Silva, E.A.; Cardozo Filho, L.; Santos, J.N.; Freitas, L.S.; Dariva, C. Extraction of sesame seed (Sesamun indicum L.) oil using compressed propane and supercritical carbon dioxide. J. Supercrit. Fluids 2010, 52, 56–61. [Google Scholar] [CrossRef]
- Castro-González, L.M.; Alvarez-Idaboy, J.R.L.; Galano, A. Computationally designed sesamol derivatives proposed as potent antioxidants. ACS Omega 2020, 5, 9566–9575. [Google Scholar] [CrossRef] [PubMed]
- Budowski, P.; Sesame oil. III. Antioxidant properties of sesamol. JAOCS J. Am. Oil Chem. Soc. 1950, 27, 264–267. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Qiao, Q.; Zhao, T.; Zhang, W.; Ren, B.; Liu, Q.; Liu, X. Sesamol ameliorates high-fat and high-fructose induced cognitive defects via improving insulin signaling disruption in the central nervous system. Food Funct 2017, 8, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Beegum, P.S.; Pandiselvam, R.; Ramesh, S.; Thube, S.H.; Pandian, T.P.; Khanashyam, A.C.; Manikantan, M.; Hebbar, K. A critical appraisal on the antimicrobial, oral protective, and anti-diabetic functions of coconut and its derivatives. Qual. Assur. Saf. Crops Foods 2022, 14, 86–100. [Google Scholar] [CrossRef]
- Thushara, R.; Hemshekhar, M.; Sunitha, K.; Kumar, M.; Naveen, S.; Kemparaju, K.; Girish, K. Sesamol induces apoptosis in human platelets via reactive oxygen species-mediated mitochondrial damage. Biochimie 2013, 95, 2060–2068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.-S.; Tsai, P.-H.; Tseng, K.-F.; Chen, F.-Y.; Yang, W.-C.; Shen, M.-Y. Sesamol ameliorates renal injury-mediated atherosclerosis via inhibition of oxidative stress/IKKα/p53. Antioxidants 2021, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wen, X.; Tang, M.; Peng, X.; Sheng, Q.; Liu, P. Gambogenic acid protects against high glucose-induced damage of renal tubular epithelial cells by inhibiting pyroptosis through regulating the AMPK–TXNIP pathway. Qual. Assur. Saf. Crops Foods 2022, 14, 40–46. [Google Scholar] [CrossRef]
- Ariafar, A.; Ahmed, F.; Khorshidi, A.; Torabi-Nezhad, S.; Hosseini, S.H. Inflammatory Myofibroblastic Tumor of the Right Kidney Mimicking a Locally Advanced Renal Carcinoma: A Case Report. J. Kidney Cancer VHL 2022, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Khaladkar, S.M.; Parripati, S.S.V.K.; Koganti, D.; Dhirawani, S.; Agarwal, U. Renal Cell Carcinoma Arising from Isthmus of Horseshoe K. J. Kidney Cancer VHL 2023, 10, 1. [Google Scholar] [CrossRef]
- Restrepo, J.C.Á.; Millan, D.A.C.; Sabogal, C.A.R.; Bernal, A.F.P.; Donoso, W.D. New trends and evidence for the management of renal angiomyolipoma: A comprehensive narrative review of the literature. J. Kidney Cancer VHL 2022, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yan, H.-R.; Wang, B.; Liu, B.-C. Macrophage heterogeneity in kidney injury and fibrosis. Front. Immunol. 2021, 12, 681748. [Google Scholar] [CrossRef] [PubMed]
- Larkin, K.; Chua, K.J.; Doppalapudi, S.K.; Smith, C.; Sadimin, E.; Fitzhugh, V.A.; Singer, E.A. Inflammatory Hibernoma of the Renal Hilum Mimicking a Renal Pelvis Tumor. J. Kidney Cancer VHL 2022, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xiao, L.; Cheng, G.; He, J.; Yin, C.; Wang, L.; Wang, Q.; Li, L.; Wei, B.; Weng, Y. Self-maintaining macrophages within the kidney contribute to salt and water balance by modulating kidney sympathetic nerve activity. Kidney Int. 2023, 104, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Drobner, J.; Portal, D.; Runcie, K.; Yang, Y.; Singer, E.A. Systemic Treatment for Advanced and Metastatic Non-Clear Cell Renal Cell Carcinoma: Examining Modern Therapeutic Strategies for a Notoriously Challenging Malignancy. J. Kidney Cancer VHL 2023, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Kong, S.; Ni, W.; Lu, Y.; Li, J.; Huang, Y.; Chen, J.; Lin, K.; Li, Y.; Ke, J. Association of the Systemic Immune-Inflammation Index with Outcomes in Acute Coronary Syndrome Patients with Chronic Kidney Disease. J. Inflamm. Res. 2023, 16, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Kusmartsev, S.; Kwenda, E.; Dominguez-Gutierrez, P.R.; Crispen, P.L.; O’Malley, P. High levels of PD-L1+ and HYAL2+ myeloid-derived suppressor cells in renal cell carcinoma. J. Kidney Cancer VHL 2022, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fu, P.; Ma, L. Kidney fibrosis: From mechanisms to therapeutic medicines. Signal Transduct. Target Ther. 2023, 8, 129. [Google Scholar] [CrossRef]
- Karayil, V.; Bhaskarashenoy, M.; Sukumaran, G. Leiomyoma of Kidney. J. Kidney Cancer VHL 2023, 10, 29. [Google Scholar] [CrossRef]
- Ramya, N.; Kanchan, M.; Anand, R.; Shirley, S. A Case Report: Mucinous Tubular and Spindle Cell Carcinoma of Kidney with Spindle Cell Predominance Mimicking Mesenchymal Tumour. J. Kidney Cancer VHL 2022, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wang, S.; Zhou, P.; Ding, F. New insights into immune cell diversity in acute kidney injury. Cell. Mol. Immunol. 2023, 20, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Mitsogiannis, I.C.; Mitsogianni, M.; Papathanassiou, M.; Anagnostou, M.; Tamposis, I.; Mitrakas, L.; Samara, M.; Tzortzis, V.; Vlachostergios, P.J. Current Options for Second-Line Systemic Therapy in Metastatic Renal Cell Carcinoma. J. Kidney Cancer VHL 2022, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Hou, Y.; Cai, A.; Xu, Y.; Yang, W.; Huang, M.; Mou, S. An integrated co-expression network analysis reveals novel genetic biomarkers for immune cell infiltration in chronic kidney disease. Front. Immunol. 2023, 14, 1129524. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.-F.; Tsai, P.-H.; Wang, J.-S.; Chen, F.-Y.; Shen, M.-Y. Sesamol Attenuates renal inflammation and arrests reactive-oxygen-species-mediated IL-1β secretion via the HO-1-induced inhibition of the IKKα/NFκB pathway in vivo and in vitro. Antioxidants 2022, 11, 2461. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, H.A.; Rios, D.C. Analysis of sesamin, asarinin, and sesamolin by HPLC with photodiode and fluorescent detection and by GC/MS: Application to sesame oil and serum samples. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 1943–1950. [Google Scholar] [CrossRef]
- Wang, M.; Liu, P.; Kong, L.; Xu, N.; Lei, H. Promotive effects of sesamin on proliferation and adhesion of intestinal probiotics and its mechanism of action. Food Chem. Toxicol. 2021, 149, 112049. [Google Scholar] [CrossRef] [PubMed]
- Tsiplakou, E.; Mitsiopoulou, C.; Karaiskou, C.; Simoni, M.; Pappas, A.C.; Righi, F.; Sotirakoglou, K.; Labrou, N.E. Sesame meal, vitamin E and selenium influence goats’ antioxidant status. Antioxidants 2021, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Yousef, S.M.; Abu-Yousef, I.A.; Nasrallah, G.K. Immunomodulatory and anti-inflammatory effects of sesamin: Mechanisms of action and future directions. Crit. Rev. Food Sci. Nutr. 2022, 62, 5081–5112. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zeng, L.; Jiang, G.; Liu, Q. Mechanism of the Protective Effect of Sesamin on Sepsis-Induced Acute Lung Injury. Curr. Top Nutraceutical Res. 2021, 19, 211. [Google Scholar] [CrossRef]
- Cai, J.; Tian, X.; Ren, J.; Lu, S.; Guo, J. Synergistic Effect of Sesamin and γ-Tocotrienol on Promoting Osteoblast Differentiation via AMPK Signaling. Nat. Prod. Commun. 2022, 17, 1934578X221074844. [Google Scholar] [CrossRef]
- Farajbakhsh, A.; Mazloomi, S.M.; Mazidi, M.; Rezaie, P.; Akbarzadeh, M.; Ahmad, S.P.; Ferns, G.; Ofori-Asenso, R.; Babajafari, S. Sesame oil and vitamin E co-administration may improve cardiometabolic risk factors in patients with metabolic syndrome: A randomized clinical trial. Eur. J. Clin. Nutr. 2019, 73, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, F.; Lin, Y.; Li, L.; Chen, M.; Ni, L. A Comprehensive Review on Distribution, Pharmacological Properties, and Mechanisms of Action of Sesamin. J. Chem. 2022, 2022, 4236525. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamaori, S.; Kamijo, S.; Aikawa, K.; Ohmori, S. In vitro inhibitory effects of sesamin on CYP4F2 activity. Biol. Pharm. Bull. 2020, 43, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Peñalvo, J.L.; Hopia, A.; Adlercreutz, H. Effect of sesamin on serum cholesterol and triglycerides levels in LDL receptor-deficient mice. Eur. J. Nutr. 2006, 45, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Udomruk, S.; Kaewmool, C.; Pothacharoen, P.; Phitak, T.; Kongtawelert, P. Sesamin suppresses LPS-induced microglial activation via regulation of TLR4 expression. J. Funct. Foods 2018, 49, 32–43. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, C.; Wu, H.; Sheng, L.; Su, Y.; Zhang, X.; Luan, H.; Sun, G.; Sun, X.; Tian, Y. Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich Pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS ONE 2013, 8, e61922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yu, Y.; Deng, J.; Zhang, C.; Zhang, J.; Cheng, Y.; Luo, X.; Han, B.; Yang, H. Sesamin ameliorates high-fat diet–induced dyslipidemia and kidney injury by reducing oxidative stress. Nutrients 2016, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Ro, H.-S. The anti-atherogenic properties of sesamin are mediated via improved macrophage cholesterol efflux through PPARγ1-LXRα and MAPK signaling. Int. J. Vitam. Nutr. Res. 2014, 84, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.-S.; Tien, N.; Shen, H.-Y.; Chu, F.-Y.; Wang, C.C.; Lu, C.-H.; Yu, H.-I.; Kung, F.-P.; Chuang, H.-H.; Lee, Y.-R. Sesamin, a naturally occurring lignan, inhibits ligand-induced lipogenesis through interaction with liver X receptor alpha (LXRα) and pregnane X receptor (PXR). Evid. Based Complement. Altern. Med. 2019, 2019, 9401648. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Zhang, W.; Qi, W.; Lu, C.; Huang, H.; Yang, Z.; Liu, B.; Zhang, L. Sesamin suppresses NSCLC cell proliferation and induces apoptosis via Akt/p53 pathway. Toxicol. Appl. Pharmacol. 2020, 387, 114848. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Lee, W.-J.; Chu, I.-H.; Hung, W.-C.; Su, N.-W. Formation of samin diastereomers by acid-catalyzed transformation of sesamolin with hydrogen peroxide. J. Agric. Food Chem. 2020, 68, 6430–6438. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, V.R.M.; Chien, S.-P.; Hsu, D.-Z.; Liu, M.-Y. Anti-hepatotoxic effects of 3, 4-methylenedioxyphenol and N-acetylcysteine in acutely acetaminophen-overdosed mice. Hum. Exp. Toxicol. 2011, 30, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sun, H.; Yang, Y.; Yan, Y. Sesamolin alleviates nonalcoholic fatty liver disease through modulating gut microbiota and metabolites in high-fat and high-fructose diet-fed mice. Int. J. Mol. Sci. 2022, 23, 13853. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K. Sesamolin promotes cytolysis and migration activity of natural killer cells via dendritic cells. Arch. Pharm. Res. 2020, 43, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lee, J.K. Sesamolin affects both natural killer cells and cancer cells in order to create an optimal environment for cancer cell sensitization. Int. Immunopharmacol. 2018, 64, 16–23. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Ahmed, H.I.; Zaky, H.S.; Badr, A.M. Sesame oil mitigates memory impairment, oxidative stress, and neurodegeneration in a rat model of Alzheimer’s disease. A pivotal role of NF-κB/p38MAPK/BDNF/PPAR-γ pathways. J. Ethnopharmacol. 2021, 267, 113468. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.C.W.; Huang, H.M.; Tzen, J.T.; Jeng, K.C.G. Protective effects of sesamin and sesamolin on hypoxic neuronal and PC12 cells. J. Neurosci. Res. 2003, 74, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Sugiyama, H.; Kushimoto, S.; Uchiyama, Y.; Hirano, M.; Nakamura, S. Effects of sesaminol feeding on brain Aβ accumulation in a senescence-accelerated mouse-prone 8. J. Agric. Food Chem. 2016, 64, 4908–4913. [Google Scholar] [CrossRef] [PubMed]
- Keowkase, R.; Shoomarom, N.; Bunargin, W.; Sitthithaworn, W.; Weerapreeyakul, N. Sesamin and sesamolin reduce amyloid-β toxicity in a transgenic Caenorhabditis elegans. Biomed. Pharmacother. 2018, 107, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and tocotrienols—Bioactive dietary compounds; what is certain, what is doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Saito, Y.; Jones, L.S.; Shigeri, Y. Chemical reactivities and physical effects in comparison between tocopherols and tocotrienols: Physiological significance and prospects as antioxidants. J. Biosci. Bioeng. 2007, 104, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, S.; Wang, Y.; Luo, S.; Yao, W.; He, H.; Tian, Y.; Li, H.; Zhang, F.; Sun, B. Variation of chlorophyll and carotenoids in different varieties and organs of Chinese kale. Qual. Assur. Saf. Crops Foods 2022, 14, 136–145. [Google Scholar] [CrossRef]
- Patel, R.; Patel, M.; Patel, D.; Yang, C.; Onyechi, A.; Ohemeng-Dapaah, J.; Patel, Z.; Shaikh, S. Impact of Palliative Care Utilization among Kidney Cancer Patients in US Hospitals: A National-Scale Analysis. J. Kidney Cancer VHL 2024, 11, 24–32. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Codini, M.; Cataldi, S.; Fioretti, B.; Brecchia, G.; Albi, E.; Beccari, T. Licium Barbarum cultivated in Italy: Chemical characterization and nutritional evaluation. Ital. J. Food Sci. 2022, 34, 59–65. [Google Scholar] [CrossRef]
- Barouh, N.; Bourlieu-Lacanal, C.; Figueroa-Espinoza, M.C.; Durand, E.; Villeneuve, P. Tocopherols as antioxidants in lipid-based systems: The combination of chemical and physicochemical interactions determines their efficiency. Compr. Rev. Food Sci. Food Saf. 2022, 21, 642–688. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.S.; Rocha, J.C.B.; Arellano, D.B.; Pallone, J.A.L. Discrimination of South American grains based on fatty acid. Qual. Assur. Saf. Crops Foods 2022, 14, 30–42. [Google Scholar] [CrossRef]
- El Chami, A.; Conte, P.; Hassoun, G.; Piga, A. Effect of region of cultivation, tree age, and harvest time on the quality of Lebanese virgin olive oil. Ital. J. Food Sci. 2023, 35, 57–71. [Google Scholar] [CrossRef]
- Azzi, A. Many tocopherols, one vitamin E. Mol. Aspects Med. 2018, 61, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Tocopherols and tocotrienols in plants and their products: A review on methods of extraction, chromatographic separation, and detection. Food Res. Int. 2016, 82, 59–70. [Google Scholar] [CrossRef]
- Azzi, A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019, 26, 101259. [Google Scholar] [CrossRef] [PubMed]
- Irías-Mata, A.; Sus, N.; Flory, S.; Stock, D.; Woerner, D.; Podszun, M.; Frank, J. α-Tocopherol transfer protein does not regulate the cellular uptake and intracellular distribution of α-and γ-tocopherols and-tocotrienols in cultured liver cells. Redox Biol. 2018, 19, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Papas, A.M. Vitamin E: Tocopherols and tocotrienols. In Antioxidant Status, Diet, Nutrition, and Health; CRC Press: Boca Raton, FL, USA, 2019; pp. 188–210. [Google Scholar]
- Nartea, A.; Lucci, P.; Loizzo, M.R.; Tundis, R.; Leporini, M.; Gervasi, L.; Fanesi, B.; Núñez, O.; Frega, N.G.; Fiorini, D. Is coffee powder extract a possible functional ingredient useful in food and nutraceutical industries? Ital. J. Food Sci. 2022, 34, 140–148. [Google Scholar] [CrossRef]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (α-, β+ γ-and δ-tocopherol) levels in plant oils. Flavour. Fragr. J. 2020, 35, 504–510. [Google Scholar] [CrossRef]
- Ali, E.; Hussain, S.; Hussain, N.; Kakar, K.U.; Shah, J.M.; Zaidi, S.H.R.; Jan, M.; Zhang, K.; Khan, M.A.; Imtiaz, M. Tocopherol as plant protector: An overview of Tocopherol biosynthesis enzymes and their role as antioxidant and signaling molecules. Acta Physiol. Plant 2022, 44, 20. [Google Scholar] [CrossRef]
- Siger, A.; Górnaś, P. Free tocopherols and tocotrienols in 82 plant species’ oil: Chemotaxonomic relation as demonstrated by PCA and HCA. Food Res. Int. 2023, 164, 112386. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B.; Özcan, M.M. Fatty acid composition and tocopherol contents of some sesame seed oils. Iran. J. Chem. Chem. Eng. (IJCCE) 2018, 37, 151–155. [Google Scholar]
- Bopitiya, D.; Madhujith, T. Antioxidant activity and total phenolic content of sesame (Sesamum indicum L.) seed oil. Trop. Agric. Res. 2013, 24, 296–302. [Google Scholar] [CrossRef]
- Grilo, E.C.; Costa, P.N.; Gurgel, C.S.S.; Beserra, A.F.d.L.; Almeida, F.N.d.S.; Dimenstein, R. Alpha-tocopherol and gamma-tocopherol concentration in vegetable oils. Food Sci. Technol. 2014, 34, 379–385. [Google Scholar] [CrossRef]
- Arai, H.; Kono, N. α-Tocopherol transfer protein (α-TTP). Free Radic. Biol. Med. 2021, 176, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhu, H.; Wu, X.; Liu, L.; Ma, X.; Yuan, Y.; Fu, X.; Zhang, L.; Lv, Y.; Li, D. Anti-allergic function of α-Tocopherol is mediated by suppression of PI3K-PKB activity in mast cells in mouse model of allergic rhinitis. Allergol. Immunopathol. 2020, 48, 395–400. [Google Scholar] [CrossRef] [PubMed]
- da Silva Andrade, R.; de Souza, F.I.S.; Aranda, C.S.; Mallozi, M.C.; Ferreira, A.C.; Barreto, T.L.N.; Fonseca, F.L.A.; Sarni, R.O.S.; Solé, D. Antioxidant defense of children and adolescents with atopic dermatitis: Association with disease severity. Allergol. Immunopathol. 2024, 52, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Lin, J.; Wang, Y.; Yan, L.; Ying, L.; Dai, J.; Fu, Z.; Liu, J. Vitamin A–regulated ciliated cells promote airway epithelium repair in an asthma mouse model. Allergol. Immunopathol. 2023, 51, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, D.; Yin, W. lnc-THRIL and miR-125b relate to disease risk, severity, and imbalance of Th1 cells/Th2 cells in allergic rhinitis. Allergol. Immunopathol. 2022, 50, 15–23. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Xu, F.; Min, M.-H.; Chu, S.-H.; Kim, K.-W.; Park, Y.-J. Genome-wide association study of vitamin E using genotyping by sequencing in sesame (Sesamum indicum). Genes Genom. 2019, 41, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Azlina, M.F.; Qodriyah, M.S.; Kamisah, Y. Tocopherol and tocotrienol: Therapeutic potential in animal models of stress. Curr. Drug Targets 2018, 19, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Tzen, J.T. Beneficial components in sesame proteins and oil. In The Sesame Genome; Miao, H., Zhang, H., Kole, C., Eds.; Springer: Cham, Switzerland, 2021; pp. 59–78. [Google Scholar] [CrossRef]
- Ikeda, S.; Toyoshima, K.; Yamashita, K. Dietary sesame seeds elevate α-and γ-tocotrienol concentrations in skin and adipose tissue of rats fed the tocotrienol-rich fraction extracted from palm oil. J. Nutr. 2001, 131, 2892–2897. [Google Scholar] [CrossRef] [PubMed]
- Comitato, R.; Ambra, R.; Virgili, F. Tocotrienols: A family of molecules with specific biological activities. Antioxidants 2017, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K. Enhancing effects on vitamin E activity of sesame lignans. J. Clin. Biochem. Nutr. 2004, 35, 17–28. [Google Scholar] [CrossRef]
- Nagendra Prasad, M.; Sanjay, K.; Prasad, D.; Vijay, N.; Kothari, R.; Nanjunda Swamy, S. A review on nutritional and nutraceutical properties of sesame. J. Nutr. Food Sci. 2012, 2, 1–6. [Google Scholar]
- Moazzami, A.; Kamal-Eldin, A. Sesame seed oil. In Gourmet and Health-Promoting Specialty Oils; Elsevier: Amsterdam, The Netherlands, 2009; pp. 267–282. [Google Scholar] [CrossRef]
- Morris, J.B.; Wang, M.L.; Tonnis, B.D. Variability for oil, protein, lignan, tocopherol, and fatty acid concentrations in eight sesame (Sesamum indicum L.) genotypes. Ind. Crops Prod. 2021, 164, 113355. [Google Scholar] [CrossRef]
- Sadiq, M.; Akram, N.A.; Ashraf, M.; Al-Qurainy, F.; Ahmad, P. Alpha-tocopherol-induced regulation of growth and metabolism in plants under non-stress and stress conditions. J. Plant Growth Regul. 2019, 38, 1325–1340. [Google Scholar] [CrossRef]
- Jannat, B.; Oveisi, M.R.; Sadeghi, N.; Hajimahmoodi, M.; Behzad, M.; Nahavandi, B.; Tehrani, S.; Sadeghi, F.; Oveisi, M. Effect of roasting process on total phenolic compounds and γ-tocopherol contents of Iranian sesame seeds (Sesamum indicum). Iran J. Pharm. Res. IJPR 2013, 12, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Pera, J.; Parladé, J.; Castellari, M. Fungal bioconversion of brewery by-products: Assessment of fatty acids and sterols profiles. Qual. Assur. Saf. Crops Foods 2022, 14, 202–211. [Google Scholar] [CrossRef]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W. Phytosterols: From preclinical evidence to potential clinical applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef] [PubMed]
- Vecka, M.; Staňková, B.; Kutová, S.; Tomášová, P.; Tvrzická, E.; Žák, A. Comprehensive sterol and fatty acid analysis in nineteen nuts, seeds, and kernel. SN Appl. Sci. 2019, 1, 1531. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, I. The role of dietary supplementation with plant sterols and stanols in the prevention of cardiovascular disease. Nutr. Rev. 2006, 64, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, P.; Bustamante, A.; Echeverría, F.; Sambra, V.; Rincón-Cervera, M.Á.; Farías, C.; Valenzuela, R. Metabolic Benefits of Phytosterols: Chemical, Nutritional, and Functional Aspects. Food Rev. Int. 2023, 1–23. [Google Scholar] [CrossRef]
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896. [Google Scholar] [CrossRef] [PubMed]
- Colakerol, A.; Sahin, S.; Yazar, R.O.; Temiz, M.Z.; Yuruk, E.; Kandirali, E.; Semercioz, A.; Muslumanoglu, A.Y. The Significance of Serum C-Reactive Protein and Neutrophil–Lymphocyte Ratio in Predicting the Diagnostic Outcomes of Renal Mass Biopsy Procedure. J. Kidney Cancer VHL 2023, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Mehtiev, A.; Misharin, A.Y. Biological activity of phytosterols and their derivatives. Biochem. Moscow Suppl. Ser. B 2008, 2, 1–17. [Google Scholar] [CrossRef]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, Y.; McClements, D.J.; Xu, D.; Chen, S. Production, characterization, delivery, and cholesterol-lowering mechanism of phytosterols: A review. J. Agric. Food Chem. 2022, 70, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Zhang, Y.; Yu, K. Phytosterol compositions of enriched products influence their cholesterol-lowering efficacy: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2019, 73, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Makhmudova, U.; Schulze, P.C.; Lütjohann, D.; Weingärtner, O. Phytosterols and cardiovascular disease. Curr. Atheroscler. Rep. 2021, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Chailangka, A.; Leksawasdi, N.; Ruksiriwanich, W.; Jantanasakulwong, K.; Rachtanapun, P.; Sommano, S.R.; Khaneghah, A.M.; Castagnini, J.M.; Barba Orellana, F.J.; Kumar, A. Natural ingredients and probiotics for lowering cholesterol and saturated fat in dairy products: An updated review. Qual. Assur. Saf. Crops Foods 2023, 15, 140–160. [Google Scholar] [CrossRef]
- Blanco-Vaca, F.; Cedó, L.; Julve, J. Phytosterols in cancer: From molecular mechanisms to preventive and therapeutic potentials. Curr. Med. Chem. 2019, 26, 6735–6749. [Google Scholar] [CrossRef] [PubMed]
- Patidar, S.; Menon, A.R.; Sundersingh, S.; Seshadri, R.A.; Raja, A. Sarcomatoid Carcinoma Metastasis to the Colon from a Small Renal Mass: Case Report with Review of Literature. J. Kidney Cancer VHL 2023, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Al-Ezzi, E.; Mittal, A.; Veitch, Z.W.; Zahralliyali, A.; Mejia, N.M.D.; Abdeljalil, O.; Alqaisi, H.; Kumar, V.; Hansen, A.R.; Fallah-Rad, N.; et al. The Survival Outcomes of the Metastatic Nonclear Cell Renal Cell Carcinoma in the Immunotherapy Era: Princess Margaret cancer centre experience. J. Kidney Cancer VHL 2024, 11, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Llop-Talaveron, J.; Leiva-Badosa, E.; Alia-Ramos, P.; Rigo-Bonnin, R.; Virgili-Casas, N.; Farran-Teixidor, L.; Miró-Martín, M.; Garrido-Sanchez, L.; Suárez-Lledó, A.; Badía-Tahull, M.B. Genetic factors associated with alterations in liver function test results in adult hospitalized patients treated with parenteral nutrition: A substudy of a clinical trial. Nutrition 2022, 93, 111507. [Google Scholar] [CrossRef] [PubMed]
- Nzekoue, F.K.; Borsetta, G.; Navarini, L.; Abouelenein, D.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G.; Angeloni, S. Coffee silverskin: Characterization of B-vitamins, macronutrients, minerals and phytosterols. Food Chem. 2022, 372, 131188. [Google Scholar] [CrossRef] [PubMed]
- Gharby, S.; Harhar, H.; Bouzoubaa, Z.; Asdadi, A.; El Yadini, A.; Charrouf, Z. Chemical characterization and oxidative stability of seeds and oil of sesame grown in Morocco. J. Saudi Soc. Agric. Sci. 2017, 16, 105–111. [Google Scholar] [CrossRef]
- Pathak, N.; Rai, A.; Kumari, R.; Bhat, K. Value addition in sesame: A perspective on bioactive components for enhancing utility and profitability. Pharmacogn. Rev. 2014, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Miedes, D.; Makran, M.; Cilla, A.; Barberá, R.; Garcia-Llatas, G.; Alegría, A. Aging-related gastrointestinal conditions decrease the bioaccessibility of plant sterols in enriched wholemeal rye bread: In vitro static digestion. Food Funct. 2023, 14, 6012–6022. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Katsouli, M.; Tzia, C. β-Sitosterol as a functional bioactive. In A Centum of Valuable Plant Bioactives; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–212. [Google Scholar] [CrossRef]
- Chen, S.; Wang, R.; Cheng, M.; Wei, G.; Du, Y.; Fan, Y.; Li, J.; Li, H.; Deng, Z. Serum cholesterol-lowering activity of β-sitosterol laurate is attributed to the reduction of both cholesterol absorption and bile acids reabsorption in hamsters. J. Agric. Food Chem. 2020, 68, 10003–10014. [Google Scholar] [CrossRef] [PubMed]
- Benkiran, S.; Zinedine, A.; Aziz, T.; Rocha, J.M.; Ayam, I.M.; Raoui, S.M.; Chabir, R.; Errachidi, F.; Alharbi, M.; Albekairi, T.H. Wound-healing potentiation in mice treated with phenolic extracts of Moringa oleifera leaves planted at different climatic areas. Ital. J. Food Sci. 2024, 36, 28. [Google Scholar] [CrossRef]
- Deme, T.; Haki, G.D.; Retta, N.; Woldegiorgis, A.; Geleta, M.; Mateos, H.; Lewandowski, P.A. Sterols as a biomarker in tracing niger and sesame seeds oils adulterated with palm oil. J. Heliyon. 2021, 7, e06797. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, H.; Thomas, J.V.; Shyamprasad, K. A double blind, placebo-controlled randomized comparative study on the efficacy of phytosterol-enriched and conventional saw palmetto oil in mitigating benign prostate hyperplasia and androgen deficiency. BMC Urol. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Wong, H.-S.; Chen, J.-H.; Leong, P.-K.; Leung, H.-Y.; Chan, W.-M.; Ko, K.-M. β-Sitosterol protects against carbon tetrachloride hepatotoxicity but not gentamicin nephrotoxicity in rats via the induction of mitochondrial glutathione redox cycling. Molecules 2014, 19, 17649–17662. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, H.; Eslahi, A.; Ariafar, A.; Ahmed, F.; Monabati, A. Primary Rhabdomyosarcoma of Kidney with Local Recurrence and Liver Metastasis in Adults: A Case Report. J. Kidney Cancer VHL 2022, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Sarwat, N.; Ahmad, I.; Mobashar, A.; Sharif, A.; Shabbir, A.; Waqas, A.C. Mechanistic evaluation of antiarthritic and anti-inflammatory effect of campesterol ester derivatives in complete freund’s adjuvant-induced arthritic rats. Front. Pharmacol. 2023, 14, 1346054. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in Stigmasterol on its anti-tumor effect and mechanism of action. Front. Oncol. 2022, 12, 1101289. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.B.; Konings, M.; Schaart, G.; Groen, A.K.; Lütjohann, D.; van Marken Lichtenbelt, W.D.; Schrauwen, P.; Plat, J. In vitro effects of sitosterol and sitostanol on mitochondrial respiration in human brown adipocytes, myotubes and hepatocytes. Eur. J. Nutr. 2020, 59, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The bioavailability and biological activities of phytosterols as modulators of cholesterol metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R. Antiviral neutralizing antibodies: From in vitro to in vivo activity. Nat. Rev. Immunol. 2023, 23, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Nokhsorov, V.V.; Dudareva, L.V.; Semenova, N.V.; Sofronova, V.E. The Composition and the Content of ∆-5 Sterols, Fatty Acids, and the Activity of Acyl-Lipid Desaturases in the Shoots of Ephedra monosperma, Introduced in the Botanical Garden of the Cryolithozone of Yakutia. Horticulturae 2023, 9, 858. [Google Scholar] [CrossRef]
- Pratiwi, R.; Nantasenamat, C.; Ruankham, W.; Suwanjang, W.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Mechanisms and neuroprotective activities of stigmasterol against oxidative stress-induced neuronal cell death via sirtuin family. Front. Nutr. 2021, 8, 648995. [Google Scholar] [CrossRef] [PubMed]
- Drira, M.; Jabeur, H.; Marrakchi, F.; Bouaziz, M. Delta-7-stigmastenol: Quantification and isomeric formation during chemical refining of olive pomace oil and optimization of the neutralization step. Eur. Food Res. Technol. 2018, 244, 2231–2241. [Google Scholar] [CrossRef]
- Nassar, M.; Nassar, R.; Maki, H.; Al-Yagoob, A.; Hachim, M.; Senok, A.; Williams, D.; Hiraishi, N. Phytic acid: Properties and potential applications in dentistry. Front. Mater. 2021, 8, 638909. [Google Scholar] [CrossRef]
- Phescatcha, T.; Tukkeeree, S.; Rohrer, J. Determination of Phytic Acid in Soybeans and Black Sesame Seeds. Dionex Application Note, 25 July 2012. [Google Scholar]
- Touma, J.; Dominguez, S.; La Vieille, S.; Remington, B.C.; Baumert, J.L.; Théolier, J.; Godefroy, S.B. Sesame as an allergen in Lebanese food products: Occurrence, consumption and quantitative risk assessment. Food Chem. Toxicol. 2021, 156, 112511. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, E.B.; Li-Beisson, Y. Plant unusual fatty acids: Learning from the less common. Curr. Opin. Plant Biol. 2020, 55, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Gunstone, F.D. Fatty Acid and Lipid Chemistry; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Mukhametov, A.; Mamayeva, L.; Yerbulekova, M.; Aitkhozhayeva, G. Fatty acid profile of functional emulsion-based food products containing conventional and unconventional ingredients. Ital. J. Food Sci. 2022, 34, 89–97. [Google Scholar] [CrossRef]
- Horrillo, A.; Díaz-Caro, C.; Crespo-Cebada, E.; Tejerina, D.; Mesías, F.J.; Rodríguez-Ledesma, A.; García-Torres, S. Perceptions of Spanish consumers towards novel lamb burgers enriched with natural antioxidants and healthy fatty acids. Ital. J. Food Sci. 2022, 34, 11–24. [Google Scholar] [CrossRef]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary polyunsaturated fatty acids (PUFAs): Uses and potential health benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.; Theobald, H. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Arevalo, A.; Patel, N.; Muraki, P.; Ohtake, S.; Bratslavsky, G.; Clark, C.; Mann, J.; Iliopoulos, O.; Jonasch, E.; Srinivasan, R. Understanding the Impact of Belzutifan on Treatment Strategies for Patients with VHL. J. Kidney Cancer VHL 2022, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Namiki, M. Nutraceutical functions of sesame: A review. Crit. Rev. Food Sci. Nutr. 2007, 47, 651–673. [Google Scholar] [CrossRef]
- Mondal, N.; Bhat, K.; Srivastava, P. Variation in fatty acid composition in Indian germplasm of sesame. J. Am. Oil Chem. Soc. 2010, 87, 1263–1269. [Google Scholar] [CrossRef]
- Trad, S.; Chaabani, E.; Aidi Wannes, W.; Dakhlaoui, S.; Nait Mohamed, S.; Khammessi, S.; Hammami, M.; Bourgou, S.; Saidani Tounsi, M.; Fabiano-Tixier, A.-S. Quality of Edible Sesame Oil as Obtained by Green Solvents: In Silico versus Experimental Screening Approaches. Foods 2023, 12, 3263. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.; Stamatelopoulos, K.; Lykka, M.; Mantzouratou, P.; Skalidi, S.; Zakopoulos, N.; Papamichael, C.; Sidossis, L.S. Sesame oil consumption exerts a beneficial effect on endothelial function in hypertensive men. Eur. J. Prev. Cardiol. 2013, 20, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Agostoni, C.; Visioli, F. Dietary Fatty Acids and Inflammation: Focus on the n-6 Series. Int. J. Mol. Sci. 2023, 24, 4567. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Polyunsaturated fatty acids and metabolic health: Novel insights. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Antonogeorgos, G.; Mandrapylia, M.; Liakou, E.; Koutsokera, A.; Drakontaeidis, P.; Thanasia, M.; Ellwood, P.; García-Marcos, L.; Sardeli, O.; Priftis, K.N. Hierarchical analysis of Mediterranean Dietary pattern on atopic diseases’ prevalence in adolescence: The Greek Global Asthma Network study. Allergol. Immunopathol. 2022, 50, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Nieminen, L.R.; Blasbalg, T.L.; Riggs, J.A.; Lands, W.E. Healthy intakes of n− 3 and n–6 fatty acids: Estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006, 83, 1483S–1493S. [Google Scholar] [CrossRef] [PubMed]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of dietary n–3 and n–6 polyunsaturated fatty acids in inflammation and cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Faurot, K.R.; MacIntosh, B.; Horowitz, M.; Keyes, G.S.; Yuan, Z.-X.; Miller, V.; Lynch, C.; Honvoh, G. Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: Randomized controlled trial. BMJ 2021, 374. [Google Scholar] [CrossRef]
- Yang, L.; Yang, C.; Chu, C.; Wan, M.; Xu, D.; Pan, D.; Xia, H.; Wang, S.K.; Shu, G.; Chen, S. Beneficial effects of monounsaturated fatty acid-rich blended oils with an appropriate polyunsaturated/saturated fatty acid ratio and a low n-6/n-3 fatty acid ratio on the health of rats. J. Sci. Food Agric. 2022, 102, 7172–7185. [Google Scholar] [CrossRef] [PubMed]
- Hama, J.R. Comparison of fatty acid profile changes between unroasted and roasted brown sesame (Sesamum indicum L.) seeds oil. Int. J. Food Prop. 2017, 20, 957–967. [Google Scholar] [CrossRef]
- Kurt, C. Variation in oil content and fatty acid composition of sesame accessions from different origins. Grasas Aceites 2018, 69, e241. [Google Scholar] [CrossRef]
- Uzun, B.; Arslan, Ç.; Furat, Ş. Variation in fatty acid compositions, oil content and oil yield in a germplasm collection of sesame (Sesamum indicum L.). J. Am. Oil Chem. Soc. 2008, 85, 1135–1142. [Google Scholar] [CrossRef]
- Bhunia, R.K.; Chakraborty, A.; Kaur, R.; Gayatri, T.; Bhat, K.; Basu, A.; Maiti, M.K.; Sen, S.K. Analysis of fatty acid and lignan composition of Indian germplasm of sesame to evaluate their nutritional merits. J. Am. Oil Chem. Soc. 2015, 92, 65–76. [Google Scholar] [CrossRef]
- Deming, T.J. Synthetic polypeptides for biomedical applications. Prog. Polym. Sci. 2007, 32, 858–875. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Ding, J.; Chen, X. Controlled synthesis of polypeptides. Chin. Chem. Lett. 2020, 31, 3001–3014. [Google Scholar] [CrossRef]
- Kiewiet, M.B.; Faas, M.M.; De Vos, P. Immunomodulatory protein hydrolysates and their application. Nutrients 2018, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Li-Chan, E.C. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Silva, E.M.; Guzmán-Maldonado, S.H.; Cano-Medinal, A.; González-Alatorre, G. Simplified process for the production of sesame protein concentrate. Differential scanning calorimetry and nutritional, physicochemical and functional properties. J. Sci. Food Agric. 2003, 83, 972–979. [Google Scholar] [CrossRef]
- Dench, J.E.; Rivas, R.N.; Caygill, J.C. Selected functional properties of sesame (Sesamum indicum L.) flour and two protein isolates. J. Sci. Food Agric. 1981, 32, 557–564. [Google Scholar] [CrossRef]
- Bhattacharya, C.; Deshpande, B.; Pandey, B.; Paroha, S. Effect of ageing on storage in biochemical and antioxidant characterization of sesame (Sesamum indicum L.) seeds. Indo Am. J. Pharm. Res. 2014, 4, 384–389. [Google Scholar]
- Bandyopadhyay, K.; Ghosh, S. Preparation and characterization of papain-modified sesame (Sesamum indicum L.) protein isolates. J. Agric. Food Chem. 2002, 50, 6854–6857. [Google Scholar] [CrossRef] [PubMed]
- Aondona, M.M.; Ikya, J.K.; Ukeyima, M.T.; Gborigo, T.W.J.; Aluko, R.E.; Girgih, A.T. In vitro antioxidant and antihypertensive properties of sesame seed enzymatic protein hydrolysate and ultrafiltration peptide fractions. J. Food Biochem. 2021, 45, e13587. [Google Scholar] [CrossRef] [PubMed]
- Hamitri-Guerfi, F.; Ouahrani, S.; Benbouriche, A.; Bey, M.B.; Boulekbache-Makhlouf, L.; Madani, K. Impact of the extraction method on physico-chemical proprieties, phytochemicals and biological activity of sesame seeds oil. Ann. Univ. Dunarea De Jos Galati. Fascicle VI-Food Technol. 2020, 44, 82–103. [Google Scholar] [CrossRef]
- El-Geddawy, M.; Sorour, M.; Abou-El-Hawa, S.; Taha, E. Effect of domestic processing and microwave heating on phenolic compounds and tannins in some oil seeds. SVU-Int. J. Agric. Sci. 2019, 1, 23–32. [Google Scholar] [CrossRef]
- Konsoula, Z.; Liakopoulou-Kyriakides, M. Effect of endogenous antioxidants of sesame seeds and sesame oil to the thermal stability of edible vegetable oils. LWT 2010, 43, 1379–1386. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Uslu, N.; Özcan, M.M.; Juhaimi, F.A.; Ghafoor, K.; Babiker, E.E.; Osman, M.A.; Alqah, H.A. Effect of conventional oven roasting treatment on the physicochemical quality attributes of sesame seeds obtained from different locations. Food Chem. 2021, 338, 128109. [Google Scholar] [CrossRef] [PubMed]
- Elkhaleefa, A.; Shigidi, I. Optimization of sesame oil extraction process conditions. Adv. Chem. Eng. Sci. 2015, 5, 305. [Google Scholar] [CrossRef]
- Eom, S.J.; Zu, H.D.; Lee, J.; Kang, M.-C.; Park, J.; Song, K.-M.; Lee, N.H. Development of an ultrasonic system for industrial extraction of unheated sesame oil cake. Food Chem. 2021, 354, 129582. [Google Scholar] [CrossRef] [PubMed]
- Dasanayaka, B.P.; Li, Z.; Pramod, S.N.; Chen, Y.; Khan, M.U.; Lin, H. A review on food processing and preparation methods for altering fish allergenicity. Crit. Rev. Food Sci. Nutr. 2022, 62, 1951–1970. [Google Scholar] [CrossRef] [PubMed]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 2020, 60, 3367–3386. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.C.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Besler, M.; Steinhart, H.; Paschke, A. Stability of food allergens and allergenicity of processed foods. J. Chromatogr. B Biomed. Appl. 2001, 756, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Sun, Y.; Fu, G.; Wu, Z.; Cheng, J. Effect of processing on soybean allergens and their allergenicity. Trends Food Sci. Technol. 2021, 118, 316–327. [Google Scholar] [CrossRef]
- Pi, X.; Sun, Y.; Guo, X.; Chen, Q.; Cheng, J.; Guo, M. Effects of thermal sterilization on the allergenicity of soybeans. LWT 2022, 154, 112678. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Li, K.; Li, X.; Yang, A.; Tong, P.; Chen, H. Allergenicity assessment on thermally processed peanut influenced by extraction and assessment methods. Food Chem. 2019, 281, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Achouri, A.; Boye, J.I. Thermal processing, salt and high pressure treatment effects on molecular structure and antigenicity of sesame protein isolate. Food Res. Int. 2013, 53, 240–251. [Google Scholar] [CrossRef]
- Ahmadian-Kouchaksaraei, Z.; Varidi, M.; Varidi, M.J.; Pourazarang, H. Influence of processing conditions on the physicochemical and sensory properties of sesame milk: A novel nutritional beverage. LWT 2014, 57, 299–305. [Google Scholar] [CrossRef]
- Chung, S.-Y.; Champagne, E.T. Association of end-product adducts with increased IgE binding of roasted peanuts. J. Agric. Food Chem. 2001, 49, 3911–3916. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhang, G.; Fu, J.; Zhang, B. Advancement of the preparation methods and biological activity of peptides from sesame oil byproducts: A review. Int. J. Food Prop. 2020, 23, 2189–2200. [Google Scholar] [CrossRef]
- Martínez, M.L.; Bordón, M.G.; Lallana, R.L.; Ribotta, P.D.; Maestri, D.M. Optimization of sesame oil extraction by screw-pressing at low temperature. Food Bioproc. Tech. 2017, 10, 1113–1121. [Google Scholar] [CrossRef]
- Yin, W.-T.; Ma, X.-T.; Li, S.-J.; Liu, H.-M.; Shi, R. Comparison of key aroma-active compounds between roasted and cold-pressed sesame oils. Food Res. Int. 2021, 150, 110794. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yang, R.; Hua, X.; Zhao, W.; Tong, Y.; Zhang, W. Improvement of the yield and flavour quality of sesame oil from aqueous extraction process by moisture conditioning before roasting. Int. J. Food Sci. Technol. 2019, 54, 471–479. [Google Scholar] [CrossRef]
- Lv, M.; Wu, W. Optimization of an improved aqueous method for the production of high quality white sesame oil and de-oiled meal. Grasas Aceites 2020, 71, e349. [Google Scholar] [CrossRef]
- Hou, L.X.; Shang, X.L.; Wang, X.; Liu, J. Application of enzyme in aqueous extraction of sesame oil. Eur. Food Res. Technol. 2013, 236, 1027–1030. [Google Scholar] [CrossRef]
- Fasuan, T.O.; Omobuwajo, T.O.; Gbadamosi, S.O. Optimization of simultaneous recovery of oil and protein from sesame (Sesamum indicum) seed. J. Food Process. Preserv. 2018, 42, e13341. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, Q.; Huang, L. Study on extraction of oil from black sesame by microwave-assisted aqueous enzymatic method. Storage Process 2019, 19, 95–101. [Google Scholar]
- de Aquino, D.S.; Fanhani, A.; Stevanato, N.; da Silva, C. Sunflower oil from enzymatic aqueous extraction process: Maximization of free oil yield and oil characterization. J. Food Process Eng. 2019, 42, e13169. [Google Scholar] [CrossRef]
- Lertbuaban, P.; Muangrat, R. Effect of Roasting and Vacuum Microwave Drying Pretreatment on the Yield and Chemical Properties of Black Sesame Seed Oil Extracted by using Screw Press. 2023. Available online: https://rsucon.rsu.ac.th/proceedings (accessed on 28 April 2023).
- Sarma, L.; Chakraborty, S.; Duary, R.K. Solvent-based microwave-assisted extraction and identification of bioactive compounds from Sesamum indicum leaves using particle swarm optimization-integrated response surface methodology. Pharmacogn. Mag. 2018, 14, 275–283. [Google Scholar] [CrossRef]
- Zoumpoulakis, P.; Sinanoglou, V.J.; Batrinou, A.; Strati, I.F.; Miniadis-Meimaroglou, S.; Sflomos, K. A combined methodology to detect γ-irradiated white sesame seeds and evaluate the effects on fat content, physicochemical properties and protein allergenicity. Food Chem. 2012, 131, 713–721. [Google Scholar] [CrossRef]
- Pi, X.; Yang, Y.; Sun, Y.; Wang, X.; Wan, Y.; Fu, G.; Li, X.; Cheng, J. Food irradiation: A promising technology to produce hypoallergenic food with high quality. Crit. Rev. Food Sci. Nutr. 2021, 62, 6698–6713. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Hu, C.; Gao, J.; Li, X.; Wu, Z.; Yang, A.; Chen, H. A potential practical approach to reduce Ara h 6 allergenicity by gamma irradiation. Food Chem. 2013, 136, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.R.S.; Medeiros, N.G.; Rodrigues, A.M.; Araujo, M.E.; Machado, N.T.; Santos, A.G.; Santos, I.R.; Gomes-Leal, W.; Junior, R.N.C. Black sesame (Sesamum indicum L.) seeds extracts by CO2 supercritical fluid extraction: Isotherms of global yield, kinetics data, total fatty acids, phytosterols and neuroprotective effects. J. Supercrit. Fluids 2014, 93, 49–55. [Google Scholar] [CrossRef]
- Mostashari, P.; Marszałek, K.; Aliyeva, A.; Mousavi Khaneghah, A. The Impact of Processing and Extraction Methods on the Allergenicity of Targeted Protein Quantification as Well as Bioactive Peptides Derived from Egg. Molecules 2023, 28, 2658. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.K.; Zheng, L.; Liu, R.J.; Chang, M.; Jin, Q.Z.; Wang, X.G. Chemical characterization, oxidative stability, and in vitro antioxidant capacity of sesame oils extracted by supercritical and subcritical techniques and conventional methods: A comparative study using chemometrics. Eur. J. Lipid Sci. Technol. 2018, 120, 1700326. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, K.; Qin, Y.; Yu, J. A simple and green ultrasonic-assisted liquid–liquid microextraction technique based on deep eutectic solvents for the HPLC analysis of sesamol in sesame oils. Anal. Methods 2017, 9, 4184–4189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).